Abstract
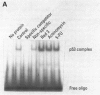
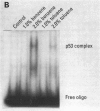
Benzene is carcinogenic, whereas toluene is thought to have little carcinogenic potential. Benzene and toluene were found to activate cyclin-dependent kinase 2 in rat liver epithelial (RLE) and HL60 cells. pRb105 was hyperphosphorylated in RLE cells treated with either solvent. Kinase activation and subsequent hyperphosphorylation of pRb105 and p53 by benzene or toluene may be responsible for their growth promotional effects, but it does not account for increased potential of benzene to induce cancer. Therefore, we examined the ability of these solvents to increase p53-DNA site-specific binding in RLE cells. Benzene increased p53-DNA site-specific DNA binding in RLE cells compared to control levels or the effects of toluene. Increased p53-DNA site-specific binding by benzene may be caused by damage to cellular DNA. If so, although both solvents appear to have promotional activity, the increased potential of benzene to damage DNA may be responsible to the difference in the ability of benzene to cause cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Da Silva C., Fan X. T., Castagna M. Benzene-mediated protein kinase C activation. Environ Health Perspect. 1989 Jul;82:91–95. doi: 10.1289/ehp.898291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees C., Travis C. C. Increased p53 site-specific DNA binding in cells producing mutant p53. Cancer Lett. 1995 Sep 25;96(2):225–231. doi: 10.1016/0304-3835(95)03936-q. [DOI] [PubMed] [Google Scholar]
- Dees C., Travis C. Hyperphosphorylation of p53 induced by benzene, toluene, and chloroform. Cancer Lett. 1994 Sep 15;84(2):117–123. doi: 10.1016/0304-3835(94)90365-4. [DOI] [PubMed] [Google Scholar]
- Foster J. S., Wimalasena J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol Endocrinol. 1996 May;10(5):488–498. doi: 10.1210/mend.10.5.8732680. [DOI] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber T. G., Peterson J. F., Tsai M., Monica K., Fornace A. J., Jr, Giaccia A. J. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994 Sep;14(9):6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Shimura M., Omatsu T., Okaichi K., Majima H., Ohnishi T. p53 proteins accumulated by heat stress associate with heat shock proteins HSP72/HSC73 in human glioblastoma cell lines. Cancer Lett. 1994 Nov 25;87(1):39–46. doi: 10.1016/0304-3835(94)90407-3. [DOI] [PubMed] [Google Scholar]
- Roghani M., Da Silva C., Castagna M. Tumor promoter chloroform is a potent protein kinase C activator. Biochem Biophys Res Commun. 1987 Feb 13;142(3):738–744. doi: 10.1016/0006-291x(87)91476-8. [DOI] [PubMed] [Google Scholar]
- Roghani M., Da Silva C., Guvelli D., Castagna M. Benzene and toluene activate protein kinase C. Carcinogenesis. 1987 Aug;8(8):1105–1107. doi: 10.1093/carcin/8.8.1105. [DOI] [PubMed] [Google Scholar]
- Tishler R. B., Calderwood S. K., Coleman C. N., Price B. D. Increases in sequence specific DNA binding by p53 following treatment with chemotherapeutic and DNA damaging agents. Cancer Res. 1993 May 15;53(10 Suppl):2212–2216. [PubMed] [Google Scholar]
- Yatsunami J., Komori A., Ohta T., Suganuma M., Fujiki H. Hyperphosphorylation of retinoblastoma protein and p53 by okadaic acid, a tumor promoter. Cancer Res. 1993 Jan 15;53(2):239–241. [PubMed] [Google Scholar]
- Zhang W., McClain C., Gau J. P., Guo X. Y., Deisseroth A. B. Hyperphosphorylation of p53 induced by okadaic acid attenuates its transcriptional activation function. Cancer Res. 1994 Aug 15;54(16):4448–4453. [PubMed] [Google Scholar]