Abstract
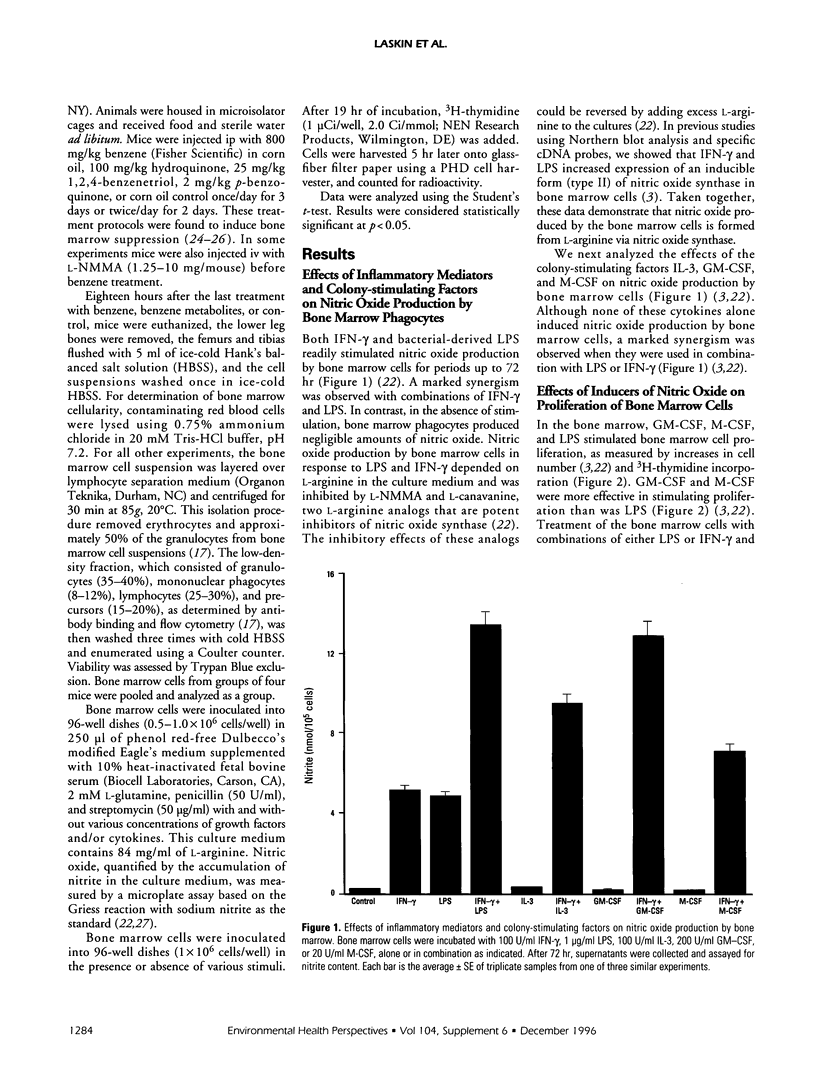
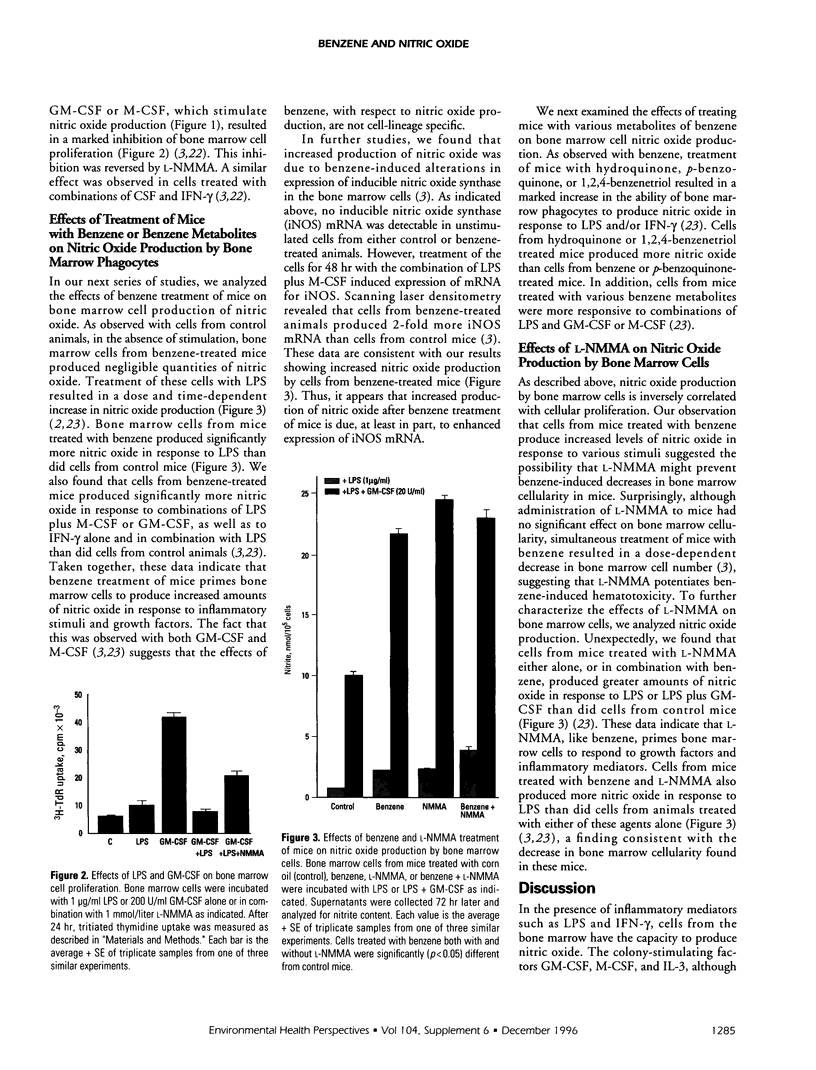
It is becoming increasingly apparent that nitric oxide plays a multifunctional role in regulating inflammatory processes in the body. Although nitric oxide and its oxidation products are cytotoxic toward certain pathogens, they can also cause tissue injury and suppress proliferation. Cytokines and growth factors released at sites of inflammation or injury stimulate both immune and nonimmume cells to produce nitric oxide. Nowhere in the body is this more detrimental than in the bone marrow, for the continuous production of hematopoietic precursors is essential for normal blood cell maturation. Our laboratories have discovered that, in response to inflammatory mediators, bone marrow cells readily produce nitric oxide. Nitric oxide production is enhanced by hematopoietic growth factors including interleukin-3, macrophage colony stimulating factor, and granulocyte-macrophage colony-stimulating factor. When bone marrow cells produce nitric oxide, hematopoiesis is impaired, an effect that is potentiated by colony-stimulating factors. Treatment of mice with benzene, which suppresses bone marrow cell development, was found to markedly enhance the ability of bone marrow cells to produce nitric oxide in response to inflammatory mediators alone and in combination with hematopoietic growth factors. Taken together, these data suggest that nitric oxide may be an important mediator of benzene-induced bone marrow suppression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Fujihara M., Ito N., Pace J. L., Watanabe Y., Russell S. W., Suzuki T. Role of endogenous interferon-beta in lipopolysaccharide-triggered activation of the inducible nitric-oxide synthase gene in a mouse macrophage cell line, J774. J Biol Chem. 1994 Apr 29;269(17):12773–12778. [PubMed] [Google Scholar]
- Gaido K. W., Wierda D. Modulation of stromal cell function in DBA/2J and B6C3F1 mice exposed to benzene or phenol. Toxicol Appl Pharmacol. 1985 Dec;81(3 Pt 1):469–475. doi: 10.1016/0041-008x(85)90418-1. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Wierda D. Suppression of bone marrow stromal cell function by benzene and hydroquinone is ameliorated by indomethacin. Toxicol Appl Pharmacol. 1987 Jul;89(3):378–390. doi: 10.1016/0041-008x(87)90157-8. [DOI] [PubMed] [Google Scholar]
- Gaido K., Wierda D. In vitro effects of benzene metabolites on mouse bone marrow stromal cells. Toxicol Appl Pharmacol. 1984 Oct;76(1):45–55. doi: 10.1016/0041-008x(84)90027-9. [DOI] [PubMed] [Google Scholar]
- Garnett H. M., Cronkite E. P., Drew R. T. Effect of in vivo exposure to benzene on the characteristics of bone marrow adherent cells. Leuk Res. 1983;7(6):803–810. doi: 10.1016/0145-2126(83)90074-7. [DOI] [PubMed] [Google Scholar]
- Guy R. L., Dimitriadis E. A., Hu P. D., Cooper K. R., Snyder R. Interactive inhibition of erythroid 59Fe utilization by benzene metabolites in female mice. Chem Biol Interact. 1990;74(1-2):55–62. doi: 10.1016/0009-2797(90)90058-u. [DOI] [PubMed] [Google Scholar]
- Guy R. L., Hu P., Witz G., Goldstein B. D., Snyder R. Depression of iron uptake into erythrocytes in mice by treatment with the combined benzene metabolites p-benzoquinone, muconaldehyde and hydroquinone. J Appl Toxicol. 1991 Dec;11(6):443–446. doi: 10.1002/jat.2550110611. [DOI] [PubMed] [Google Scholar]
- Harigaya K., Miller M. E., Cronkite E. P., Drew R. T. The detection of in vivo hematotoxicity of benzene by in vitro liquid bone marrow cultures. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):346–353. doi: 10.1016/0041-008x(91)90237-9. [DOI] [PubMed] [Google Scholar]
- King A. G., Landreth K. S., Wierda D. Hydroquinone inhibits bone marrow pre-B cell maturation in vitro. Mol Pharmacol. 1987 Dec;32(6):807–812. [PubMed] [Google Scholar]
- Laskin D. L., MacEachern L., Snyder R. Activation of bone marrow phagocytes following benzene treatment of mice. Environ Health Perspect. 1989 Jul;82:75–79. doi: 10.1289/ehp.898275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin D. L., Pendino K. J. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995;35:655–677. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- Laskin D. L., Rodriguez del Valle M., Heck D. E., Hwang S. M., Ohnishi S. T., Durham S. K., Goller N. L., Laskin J. D. Hepatic nitric oxide production following acute endotoxemia in rats is mediated by increased inducible nitric oxide synthase gene expression. Hepatology. 1995 Jul;22(1):223–234. [PubMed] [Google Scholar]
- Laskin J. D., Rao N. R., Punjabi C. J., Laskin D. L., Synder R. Distinct actions of benzene and its metabolites on nitric oxide production by bone marrow leukocytes. J Leukoc Biol. 1995 Mar;57(3):422–426. doi: 10.1002/jlb.57.3.422. [DOI] [PubMed] [Google Scholar]
- MacEachern L., Laskin D. L. Increased production of tumor necrosis factor-alpha by bone marrow leukocytes following benzene treatment of mice. Toxicol Appl Pharmacol. 1992 Apr;113(2):260–266. doi: 10.1016/0041-008x(92)90123-a. [DOI] [PubMed] [Google Scholar]
- MacEachern L., Snyder R., Laskin D. L. Alterations in the morphology and functional activity of bone marrow phagocytes following benzene treatment of mice. Toxicol Appl Pharmacol. 1992 Dec;117(2):147–154. doi: 10.1016/0041-008x(92)90231-g. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994 May 13;269(19):13725–13728. [PubMed] [Google Scholar]
- Nguyen T., Brunson D., Crespi C. L., Penman B. W., Wishnok J. S., Tannenbaum S. R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendino K. J., Gardner C. R., Laskin J. D., Laskin D. L. Induction of functionally active platelet-activating factor receptors in rat alveolar macrophages. J Biol Chem. 1993 Sep 15;268(26):19165–19168. [PubMed] [Google Scholar]
- Punjabi C. J., Laskin D. L., Heck D. E., Laskin J. D. Production of nitric oxide by murine bone marrow cells. Inverse correlation with cellular proliferation. J Immunol. 1992 Sep 15;149(6):2179–2184. [PubMed] [Google Scholar]
- Punjabi C. J., Laskin J. D., Hwang S. M., MacEachern L., Laskin D. L. Enhanced production of nitric oxide by bone marrow cells and increased sensitivity to macrophage colony-stimulating factor (CSF) and granulocyte-macrophage CSF after benzene treatment of mice. Blood. 1994 Jun 1;83(11):3255–3263. [PubMed] [Google Scholar]
- Renz J. F., Kalf G. F. Role for interleukin-1 (IL-1) in benzene-induced hematotoxicity: inhibition of conversion of pre-IL-1 alpha to mature cytokine in murine macrophages by hydroquinone and prevention of benzene-induced hematotoxicity in mice by IL-1 alpha. Blood. 1991 Aug 15;78(4):938–944. [PubMed] [Google Scholar]
- Rosenthal G. J., Snyder C. A. Modulation of the immune response to Listeria monocytogenes by benzene inhalation. Toxicol Appl Pharmacol. 1985 Sep 30;80(3):502–510. doi: 10.1016/0041-008x(85)90395-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. J., Snyder C. A. Modulation of the immune response to Listeria monocytogenes by benzene inhalation. Toxicol Appl Pharmacol. 1985 Sep 30;80(3):502–510. doi: 10.1016/0041-008x(85)90395-3. [DOI] [PubMed] [Google Scholar]
- Santiago M., Machado A., Cano J. Effect of L-arginine/nitric oxide pathway on MPP(+)-induced cell injury in the striatum of rats. Br J Pharmacol. 1994 Mar;111(3):837–842. doi: 10.1111/j.1476-5381.1994.tb14814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K., Klatt P., Mayer B. Characterization of endothelial cell amino acid transport systems involved in the actions of nitric oxide synthase inhibitors. Mol Pharmacol. 1993 Sep;44(3):615–621. [PubMed] [Google Scholar]
- Snyder R., Dimitriadis E., Guy R., Hu P., Cooper K., Bauer H., Witz G., Goldstein B. D. Studies on the mechanism of benzene toxicity. Environ Health Perspect. 1989 Jul;82:31–35. doi: 10.1289/ehp.898231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R., Witz G., Goldstein B. D. The toxicology of benzene. Environ Health Perspect. 1993 Apr;100:293–306. doi: 10.1289/ehp.93100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. J., Reasor M. J., Wierda D. Macrophage regulation of myelopoiesis is altered by exposure to the benzene metabolite hydroquinone. Toxicol Appl Pharmacol. 1989 Mar 1;97(3):440–453. doi: 10.1016/0041-008x(89)90249-4. [DOI] [PubMed] [Google Scholar]
- Warren J. B. Nitric oxide and human skin blood flow responses to acetylcholine and ultraviolet light. FASEB J. 1994 Feb;8(2):247–251. doi: 10.1096/fasebj.8.2.7509761. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Kasprzak K. S., Maragos C. M., Elespuru R. K., Misra M., Dunams T. M., Cebula T. A., Koch W. H., Andrews A. W., Allen J. S. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991 Nov 15;254(5034):1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]


