Abstract
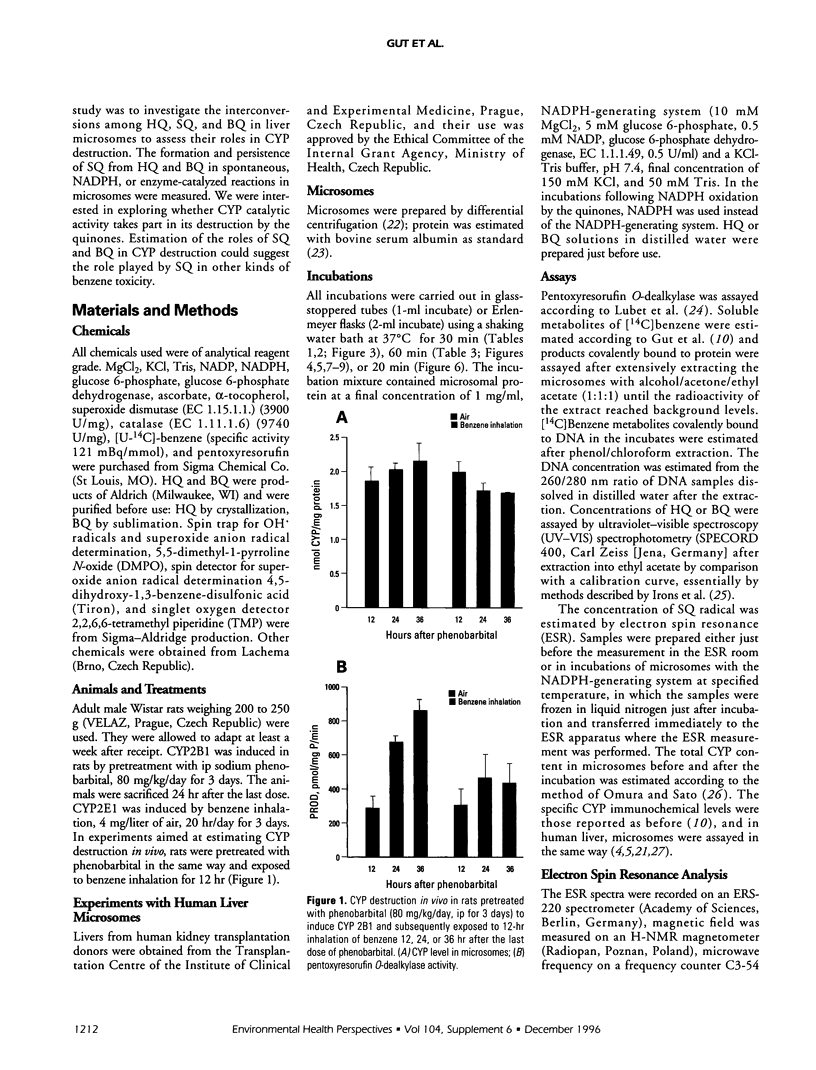
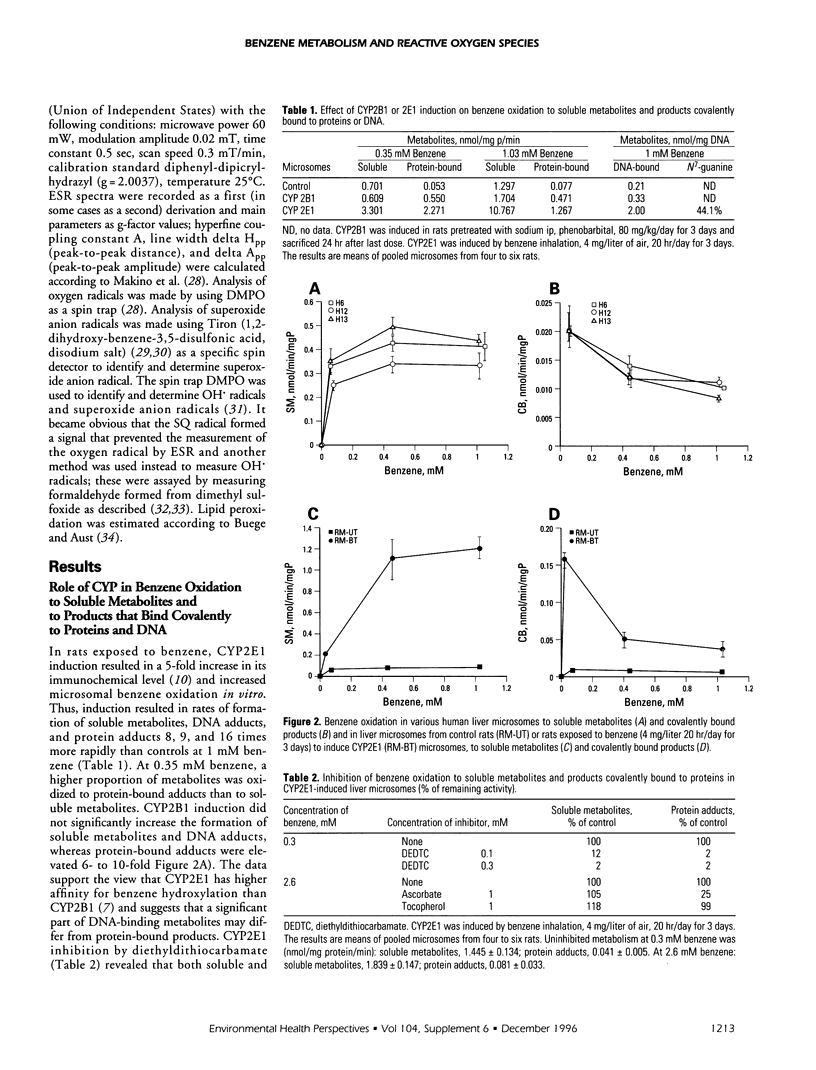
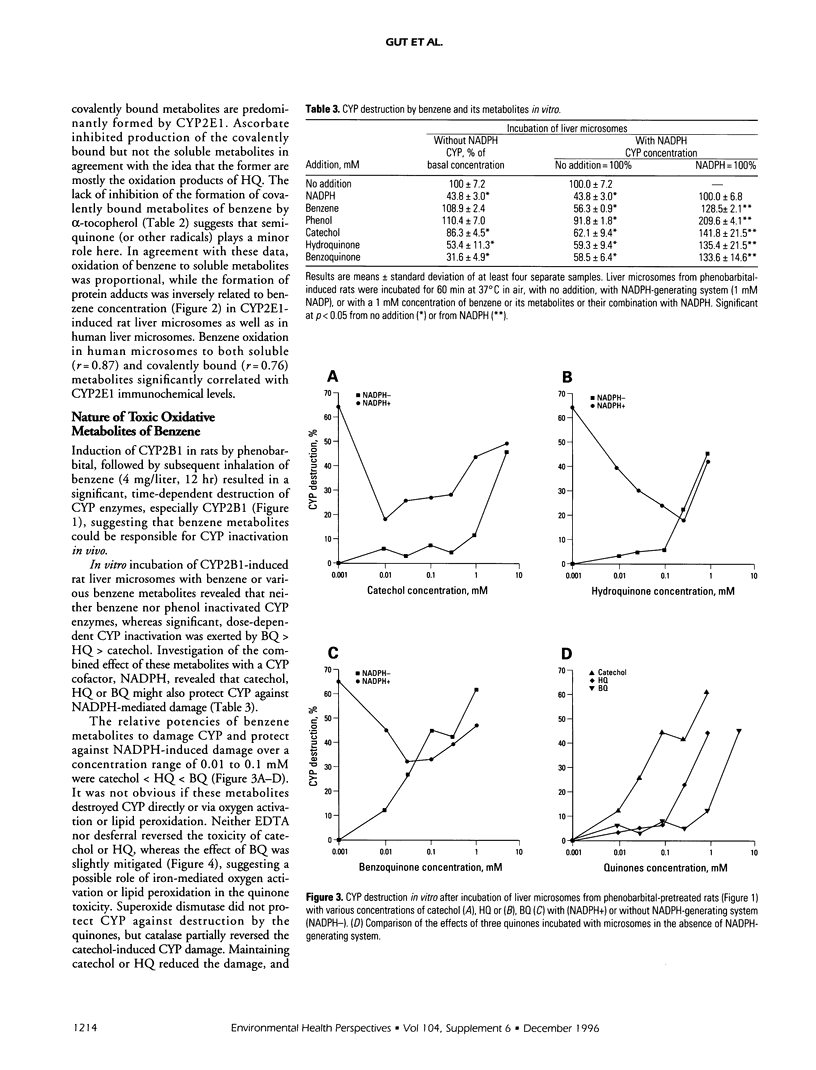
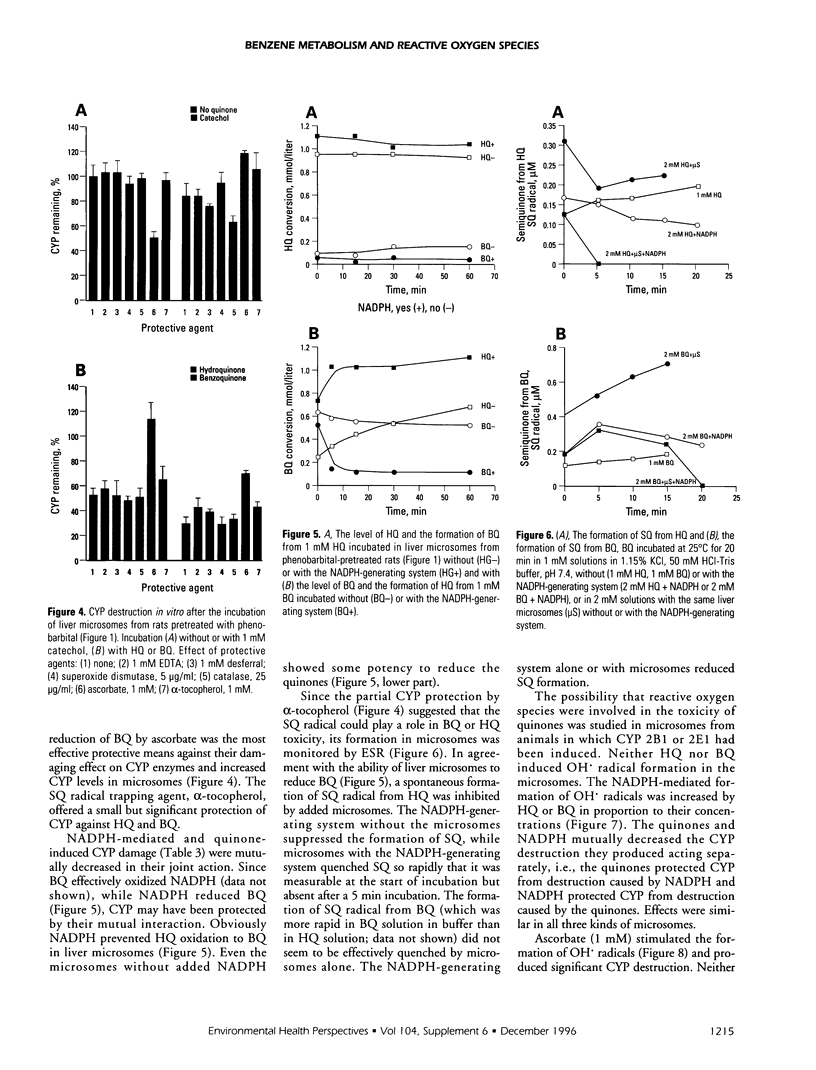
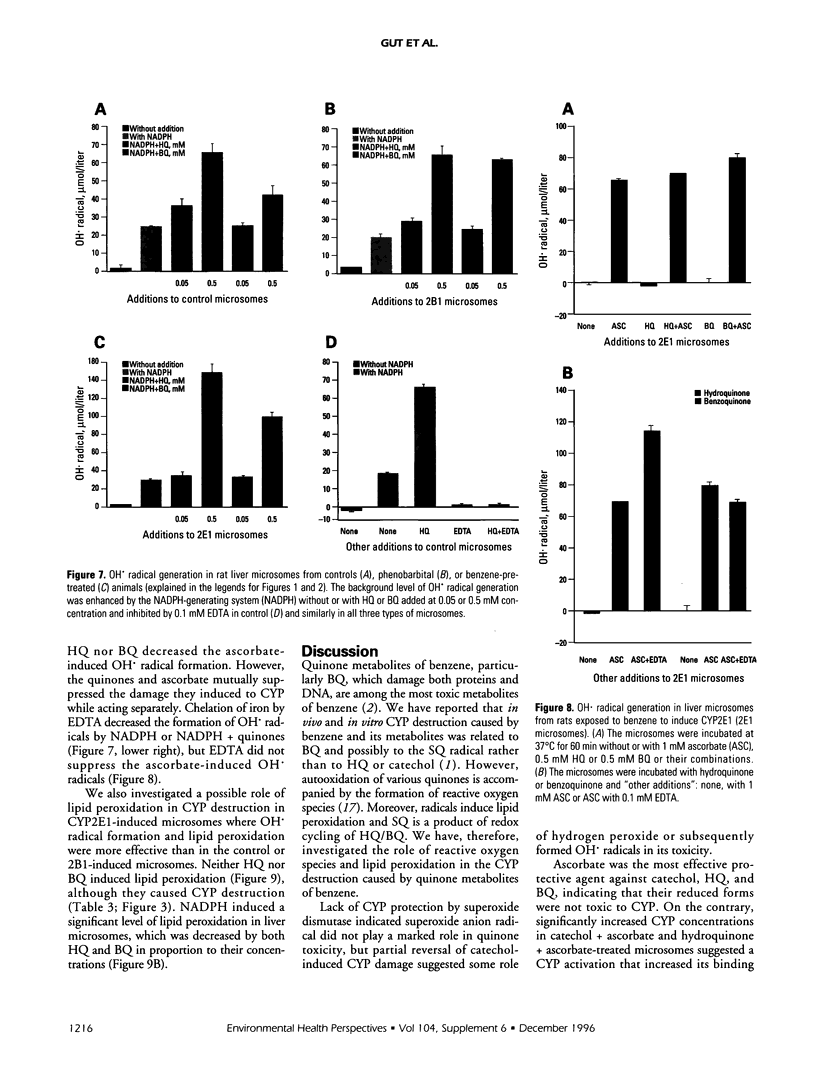
Cytochrome P450 (CYP) 2E1 was the most efficient CYP enzyme that oxidized benzene to soluble and covalently bound metabolites in rat and human liver microsomes. The covalent binding was due mostly to the formation of benzoquinone (BQ), the oxidation product of hydroquinone (HQ), and was inversely related to the formation of soluble metabolites. In rats, inhalation of benzene (4 mg/liter of air) caused a rapid destruction of CYP2B1 previously induced by phenobarbital. The ability of benzene metabolites to destroy liver microsomal CYP in vitro decreased in the order BQ > HQ > catechol > phenol. The destruction was reversed by ascorbate and diminished by alpha-tocopherol, suggesting that HQ was not toxic, whereas BQ and semiquinone radical (SQ) caused the effect. In the presence of nicotinamide adenine dinucleotide phosphate, reduced (NADPH) the microsomes did not oxidize HQ to BQ, while the formation of superoxide anion radical from both HQ and BQ was markedly quenched. Destruction of CYP in vitro caused by HQ or BQ was not mediated by hydroxyl radical formation or by lipid peroxidation. On the contrary, HQ and BQ inhibited NADPH-mediated lipid peroxidation. Ascorbate induced high levels of hydroxyl radical formation and lipid peroxidation, which were differentially affected by quinones, indicating different mechanisms. Despite reducing the toxicity of HQ and BQ, ascorbate appeared to induce its own toxicity, reflected in high levels of lipid peroxidation. Iron redox cycling played a significant role in the NADPH-induced hydroxyl radical formation but not in that caused by ascorbate; however, lipid peroxidation induced by NADPH or ascorbate was suppressed by ethylenediaminetraacetate, indicating a crucial role of iron. Thus, the data indicate that the quinones destroyed CYP directly and not via oxygen activation or lipid peroxidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar W. A., Au W. W., Legator M. S., Sadagopa Ramanujam V. M. Effect of dimethyl sulfoxide on the genotoxicity and metabolism of benzene in vivo. Carcinogenesis. 1989 Mar;10(3):441–445. doi: 10.1093/carcin/10.3.441. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Strickland T. W. Metabolism of vinyl chloride: destruction of the heme of highly purified liver Microsomal cytochrome P-450 by a metabolite. Mol Pharmacol. 1977 Nov;13(6):993–1004. [PubMed] [Google Scholar]
- Gut I., Terelius Y., Frantík E., Linhart I., Soucek P., Filipcová B., Klucková H. Exposure to various benzene derivatives differently induces cytochromes P450 2B1 and P450 2E1 in rat liver. Arch Toxicol. 1993;67(4):237–243. doi: 10.1007/BF01974342. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Johansson I., Penttilä K. E., Glaumann H., Lindros K. O. Centrilobular expression of ethanol-inducible cytochrome P-450 (IIE1) in rat liver. Biochem Biophys Res Commun. 1988 Nov 30;157(1):55–60. doi: 10.1016/s0006-291x(88)80010-x. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Neptun D. A., Pfeifer R. W. Inhibition of lymphocyte transformation and microtubule assembly by quinone metabolites of benzene: evidence for a common mechanism. J Reticuloendothel Soc. 1981 Nov;30(5):359–372. [PubMed] [Google Scholar]
- Irons R. D. Quinones as toxic metabolites of benzene. J Toxicol Environ Health. 1985;16(5):673–678. doi: 10.1080/15287398509530777. [DOI] [PubMed] [Google Scholar]
- Johansson I., Ekström G., Scholte B., Puzycki D., Jörnvall H., Ingelman-Sundberg M. Ethanol-, fasting-, and acetone-inducible cytochromes P-450 in rat liver: regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry. 1988 Mar 22;27(6):1925–1934. doi: 10.1021/bi00406a019. [DOI] [PubMed] [Google Scholar]
- Khan S., Krishnamurthy R., Pandya K. P. Generation of hydroxyl radicals during benzene toxicity. Biochem Pharmacol. 1990 Apr 15;39(8):1393–1395. doi: 10.1016/0006-2952(90)90017-f. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Cohen G., Cederbaum A. I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry. 1981 Oct 13;20(21):6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leanderson P., Tagesson C. Cigarette smoke-induced DNA-damage: role of hydroquinone and catechol in the formation of the oxidative DNA-adduct, 8-hydroxydeoxyguanosine. Chem Biol Interact. 1990;75(1):71–81. doi: 10.1016/0009-2797(90)90023-g. [DOI] [PubMed] [Google Scholar]
- Ledenev A. N., Konstantinov A. A., Popova E., Ruuge E. K. A simple assay of the superoxide generation rate with Tiron as an EPR-visible radical scavenger. Biochem Int. 1986 Aug;13(2):391–396. [PubMed] [Google Scholar]
- Levin W., Lu A. Y., Jacobson M., Kuntzman R., Poyer J. L., McCay P. B. Lipid peroxidation and the degradation of cytochrome P-450 heme. Arch Biochem Biophys. 1973 Oct;158(2):842–852. doi: 10.1016/0003-9861(73)90580-8. [DOI] [PubMed] [Google Scholar]
- Lubet R. A., Mayer R. T., Cameron J. W., Nims R. W., Burke M. D., Wolff T., Guengerich F. P. Dealkylation of pentoxyresorufin: a rapid and sensitive assay for measuring induction of cytochrome(s) P-450 by phenobarbital and other xenobiotics in the rat. Arch Biochem Biophys. 1985 Apr;238(1):43–48. doi: 10.1016/0003-9861(85)90138-9. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Elovaara E., Park S. S., Gelboin H. V., Hietanen E., Vainio H. Immunochemical characterization of cytochrome P-450 isozymes responsible for benzene oxidation in the rat liver. Carcinogenesis. 1989 Sep;10(9):1713–1717. doi: 10.1093/carcin/10.9.1713. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Schaefer W. H., Harris T. M., Guengerich F. P. Characterization of the enzymatic and nonenzymatic peroxidative degradation of iron porphyrins and cytochrome P-450 heme. Biochemistry. 1985 Jun 18;24(13):3254–3263. doi: 10.1021/bi00334a027. [DOI] [PubMed] [Google Scholar]
- Schlosser P. M., Bond J. A., Medinsky M. A. Benzene and phenol metabolism by mouse and rat liver microsomes. Carcinogenesis. 1993 Dec;14(12):2477–2486. doi: 10.1093/carcin/14.12.2477. [DOI] [PubMed] [Google Scholar]
- Schrenk D., Ingelman-Sundberg M., Bock K. W. Influence of P-4502E1 induction on benzene metabolism in rat hepatocytes and on biliary metabolite excretion. Drug Metab Dispos. 1992 Mar-Apr;20(2):137–141. [PubMed] [Google Scholar]
- Seaton M. J., Schlosser P. M., Bond J. A., Medinsky M. A. Benzene metabolism by human liver microsomes in relation to cytochrome P450 2E1 activity. Carcinogenesis. 1994 Sep;15(9):1799–1806. doi: 10.1093/carcin/15.9.1799. [DOI] [PubMed] [Google Scholar]
- Singh V., Ahmad S., Rao G. S. Prooxidant and antioxidant properties of iron-hydroquinone and iron-1,2,4-benzenetriol complex. Implications for benzene toxicity. Toxicology. 1994 Mar 25;89(1):25–33. doi: 10.1016/0300-483x(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Smart R. C., Zannoni V. G. DT-diaphorase and peroxidase influence the covalent binding of the metabolites of phenol, the major metabolite of benzene. Mol Pharmacol. 1984 Jul;26(1):105–111. [PubMed] [Google Scholar]
- Snyder R., Witz G., Goldstein B. D. The toxicology of benzene. Environ Health Perspect. 1993 Apr;100:293–306. doi: 10.1289/ehp.93100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek P., Filipcova B., Gut I. Cytochrome P450 destruction and radical scavenging by benzene and its metabolites. Evidence for the key role of quinones. Biochem Pharmacol. 1994 Jun 15;47(12):2233–2242. doi: 10.1016/0006-2952(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Tindberg N., Ingelman-Sundberg M. Cytochrome P-450 and oxygen toxicity. Oxygen-dependent induction of ethanol-inducible cytochrome P-450 (IIE1) in rat liver and lung. Biochemistry. 1989 May 16;28(10):4499–4504. doi: 10.1021/bi00436a056. [DOI] [PubMed] [Google Scholar]
- Tindberg N., Ingelman-Sundberg M. Cytochrome P-450 and oxygen toxicity. Oxygen-dependent induction of ethanol-inducible cytochrome P-450 (IIE1) in rat liver and lung. Biochemistry. 1989 May 16;28(10):4499–4504. doi: 10.1021/bi00436a056. [DOI] [PubMed] [Google Scholar]
- van de Straat R., Vromans R. M., Bosman P., de Vries J., Vermeulen N. P. Cytochrome P-450-mediated oxidation of substrates by electron-transfer; role of oxygen radicals and of 1- and 2-electron oxidation of paracetamol. Chem Biol Interact. 1988;64(3):267–280. doi: 10.1016/0009-2797(88)90102-0. [DOI] [PubMed] [Google Scholar]
- van der Hoeven T. A., Coon M. J. Preparation and properties of partially purified cytochrome P-450 and reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase from rabbit liver microsomes. J Biol Chem. 1974 Oct 10;249(19):6302–6310. [PubMed] [Google Scholar]


