Abstract
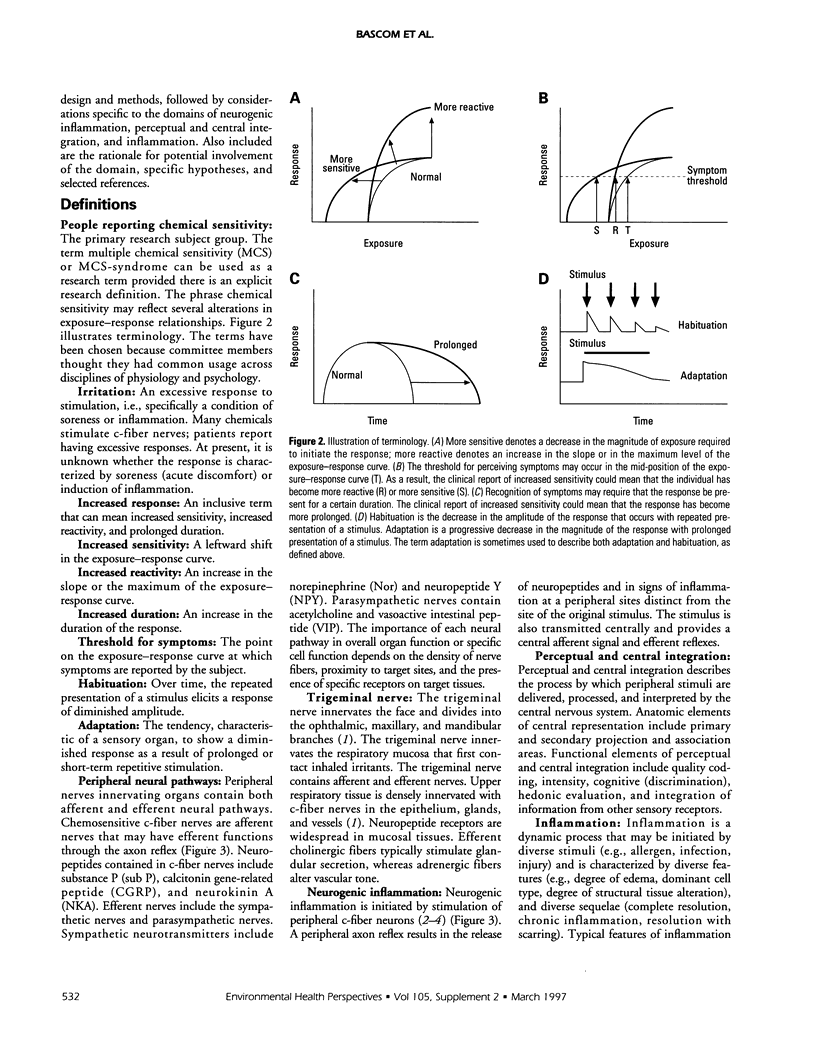
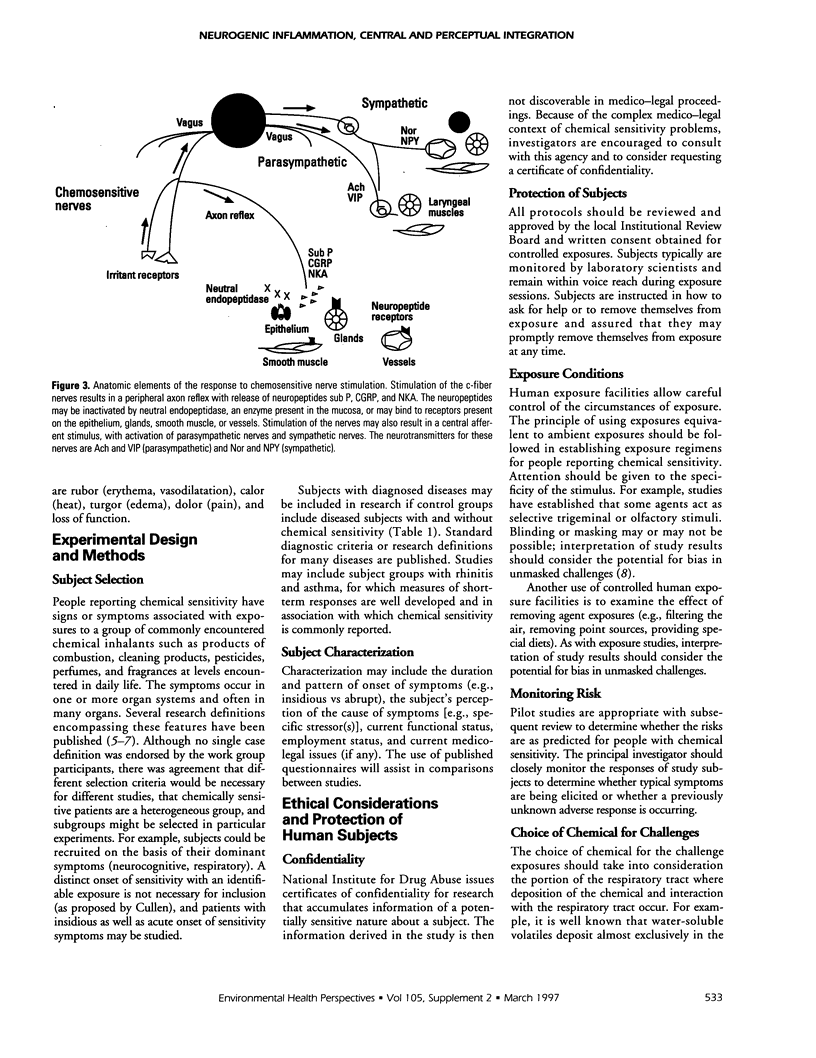
The Working Group on Neurogenic Inflammation proposed 11 testable hypotheses in the three domains of neurogenic inflammation, perceptual and central integration, and nonneurogenic inflammation. The working group selected the term people reporting chemical sensitivity (PRCS) to identify the primary subject group. In the domain of neurogenic inflammation, testable hypotheses included: PRCS have an increased density of c-fiber neurons in symptomatic tissues; PRCS produce greater quantities of neuropeptides and prostanoids than nonsensitive subjects in response to exposure to low-level capsaicin or irritant chemicals; PRCS have an increased and prolonged response to exogenously administered c-fiber activators such as capsaicin; PRCS demonstrate augmentation of central autonomic reflexes following exposure to agents that produce c-fiber stimulation; PRCS have decreased quantities of neutral endopeptidase in their mucosa; exogenous neuropeptide challenge reproduces symptoms of PRCS. In the domain of perceptual and central integration, testable hypotheses included: PRCS have alterations in adaptation, habituation, cortical representation, perception, cognition, and hedonics compared to controls; the qualitative and quantitative interactions between trigeminal and olfactory systems are altered in PRCS; higher integration of sensory inputs is altered in PRCS. In the domain of nonneurogenic inflammation, testable hypotheses included: increased inflammation is present in PRCS in symptomatic tissues and is associated with a heightened neurosensory response; PRCS show an augmented inflammatory response to chemical exposure. The working group recommended that studies be initiated in these areas.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baraniuk J. N., Castellino S., Lundgren J. D., Goff J., Mullol J., Merida M., Shelhamer J. H., Kaliner M. A. Neuropeptide Y (NPY) in human nasal mucosa. Am J Respir Cell Mol Biol. 1990 Aug;3(2):165–173. doi: 10.1165/ajrcmb/3.2.165. [DOI] [PubMed] [Google Scholar]
- Baraniuk J. N., Kaliner M. A. Neuropeptides and nasal secretion. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 2):620–627. doi: 10.1016/s0091-6749(05)80226-x. [DOI] [PubMed] [Google Scholar]
- Baraniuk J. N., Lundgren J. D., Goff J., Mullol J., Castellino S., Merida M., Shelhamer J. H., Kaliner M. A. Calcitonin gene-related peptide in human nasal mucosa. Am J Physiol. 1990 Feb;258(2 Pt 1):L81–L88. doi: 10.1152/ajplung.1990.258.2.L81. [DOI] [PubMed] [Google Scholar]
- Baraniuk J. N., Lundgren J. D., Okayama M., Goff J., Mullol J., Merida M., Shelhamer J. H., Kaliner M. A. Substance P and neurokinin A in human nasal mucosa. Am J Respir Cell Mol Biol. 1991 Mar;4(3):228–236. doi: 10.1165/ajrcmb/4.3.228. [DOI] [PubMed] [Google Scholar]
- Baraniuk J. N., Lundgren J. D., Okayama M., Mullol J., Merida M., Shelhamer J. H., Kaliner M. A. Vasoactive intestinal peptide in human nasal mucosa. J Clin Invest. 1990 Sep;86(3):825–831. doi: 10.1172/JCI114780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. Neuropeptides in the lung: localization, function, and pathophysiologic implications. J Allergy Clin Immunol. 1987 Feb;79(2):285–295. doi: 10.1016/0091-6749(87)90143-6. [DOI] [PubMed] [Google Scholar]
- Bascom R., Kagey-Sobotka A., Proud D. Effect of intranasal capsaicin on symptoms and mediator release. J Pharmacol Exp Ther. 1991 Dec;259(3):1323–1327. [PubMed] [Google Scholar]
- Bascom R., Kesavanathan J., Fitzgerald T. K., Cheng K. H., Swift D. L. Sidestream tobacco smoke exposure acutely alters human nasal mucociliary clearance. Environ Health Perspect. 1995 Nov;103(11):1026–1030. doi: 10.1289/ehp.951031026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom R., Kulle T., Kagey-Sobotka A., Proud D. Upper respiratory tract environmental tobacco smoke sensitivity. Am Rev Respir Dis. 1991 Jun;143(6):1304–1311. doi: 10.1164/ajrccm/143.6.1304. [DOI] [PubMed] [Google Scholar]
- Bascom R., Pipkorn U., Lichtenstein L. M., Naclerio R. M. The influx of inflammatory cells into nasal washings during the late response to antigen challenge. Effect of systemic steroid pretreatment. Am Rev Respir Dis. 1988 Aug;138(2):406–412. doi: 10.1164/ajrccm/138.2.406. [DOI] [PubMed] [Google Scholar]
- Benignus V. A. Systematic considerations in the area of multiple chemical sensitivity. Environ Health Perspect. 1997 Mar;105 (Suppl 2):485–485. doi: 10.1289/ehp.97105s2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. R. The worker with multiple chemical sensitivities: an overview. Occup Med. 1987 Oct-Dec;2(4):655–661. [PubMed] [Google Scholar]
- Doty R. L., Deems D. A., Frye R. E., Pelberg R., Shapiro A. Olfactory sensitivity, nasal resistance, and autonomic function in patients with multiple chemical sensitivities. Arch Otolaryngol Head Neck Surg. 1988 Dec;114(12):1422–1427. doi: 10.1001/archotol.1988.01860240072027. [DOI] [PubMed] [Google Scholar]
- Dusser D. J., Djokic T. D., Borson D. B., Nadel J. A. Cigarette smoke induces bronchoconstrictor hyperresponsiveness to substance P and inactivates airway neutral endopeptidase in the guinea pig. Possible role of free radicals. J Clin Invest. 1989 Sep;84(3):900–906. doi: 10.1172/JCI114251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck C. Eye symptoms and signs in buildings with indoor climate problems ('office eye syndrome'). Acta Ophthalmol (Copenh) 1986 Jun;64(3):306–311. doi: 10.1111/j.1755-3768.1986.tb06925.x. [DOI] [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., St George J. A., Wilson D. W., Dungworth D. L. Response of macaque bronchiolar epithelium to ambient concentrations of ozone. Am J Pathol. 1993 Sep;143(3):857–866. [PMC free article] [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., St George J. A., Wilson D. W., Dungworth D. L. Response of the macaque nasal epithelium to ambient levels of ozone. A morphologic and morphometric study of the transitional and respiratory epithelium. Am J Pathol. 1987 Jul;128(1):29–44. [PMC free article] [PubMed] [Google Scholar]
- Heck H. D., Casanova M., Starr T. B. Formaldehyde toxicity--new understanding. Crit Rev Toxicol. 1990;20(6):397–426. doi: 10.3109/10408449009029329. [DOI] [PubMed] [Google Scholar]
- Hudnell H. K., Otto D. A., House D. E., Mølhave L. Exposure of humans to a volatile organic mixture. II. Sensory. Arch Environ Health. 1992 Jan-Feb;47(1):31–38. doi: 10.1080/00039896.1992.9935941. [DOI] [PubMed] [Google Scholar]
- Hummel T., Gruber M., Pauli E., Kobal G. Chemo-somatosensory event-related potentials in response to repetitive painful chemical stimulation of the nasal mucosa. Electroencephalogr Clin Neurophysiol. 1994 Sep;92(5):426–432. doi: 10.1016/0168-5597(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Hummel T., Hummel C., Friedel I., Pauli E., Kobal G. A comparison of the antinociceptive effects of imipramine, tramadol and anpirtoline. Br J Clin Pharmacol. 1994 Apr;37(4):325–333. doi: 10.1111/j.1365-2125.1994.tb04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T., Kobal G. Differences in human evoked potentials related to olfactory or trigeminal chemosensory activation. Electroencephalogr Clin Neurophysiol. 1992 Jan-Feb;84(1):84–89. doi: 10.1016/0168-5597(92)90070-r. [DOI] [PubMed] [Google Scholar]
- Kehrl H. R., Vincent L. M., Kowalsky R. J., Horstman D. H., O'Neil J. J., McCartney W. H., Bromberg P. A. Ozone exposure increases respiratory epithelial permeability in humans. Am Rev Respir Dis. 1987 May;135(5):1124–1128. doi: 10.1164/arrd.1987.135.5.1124. [DOI] [PubMed] [Google Scholar]
- Kesavanathan J., Swift D. L., Fitzgerald T. K., Permutt T., Bascom R. Evaluation of acoustic rhinometry and posterior rhinomanometry as tools for inhalation challenge studies. J Toxicol Environ Health. 1996 Jun 28;48(3):295–307. doi: 10.1080/009841096161348. [DOI] [PubMed] [Google Scholar]
- Kipen H. M., Hallman W., Kelly-McNeil K., Fiedler N. Measuring chemical sensitivity prevalence: a questionnaire for population studies. Am J Public Health. 1995 Apr;85(4):574–577. doi: 10.2105/ajph.85.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. R., Carson J. L., Collier A. M., Gatzy J. T., Boucher R. C. Measurements of nasal transepithelial electric potential differences in normal human subjects in vivo. Am Rev Respir Dis. 1981 Oct;124(4):484–490. doi: 10.1164/arrd.1981.124.4.484. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-sensitive vagal neurons involved in control of vascular permeability in rat trachea. Acta Physiol Scand. 1982 Aug;115(4):521–523. doi: 10.1111/j.1748-1716.1982.tb07116.x. [DOI] [PubMed] [Google Scholar]
- Meggs W. J. Neurogenic inflammation and sensitivity to environmental chemicals. Environ Health Perspect. 1993 Aug;101(3):234–238. doi: 10.1289/ehp.93101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. J., Menzel D. B., Coffin D. L. Similarity between man and laboratory animals in regional pulmonary deposition of ozone. Environ Res. 1978 Aug;17(1):84–101. doi: 10.1016/0013-9351(78)90064-6. [DOI] [PubMed] [Google Scholar]
- Nielsen G. D. Mechanisms of activation of the sensory irritant receptor by airborne chemicals. Crit Rev Toxicol. 1991;21(3):183–208. doi: 10.3109/10408449109089879. [DOI] [PubMed] [Google Scholar]
- Thürauf N., Friedel I., Hummel C., Kobal G. The mucosal potential elicited by noxious chemical stimuli with CO2 in rats: is it a peripheral nociceptive event? Neurosci Lett. 1991 Jul 22;128(2):297–300. doi: 10.1016/0304-3940(91)90283-y. [DOI] [PubMed] [Google Scholar]
- Thürauf N., Hummel T., Kettenmann B., Kobal G. Nociceptive and reflexive responses recorded from the human nasal mucosa. Brain Res. 1993 Dec 3;629(2):293–299. doi: 10.1016/0006-8993(93)91333-n. [DOI] [PubMed] [Google Scholar]


