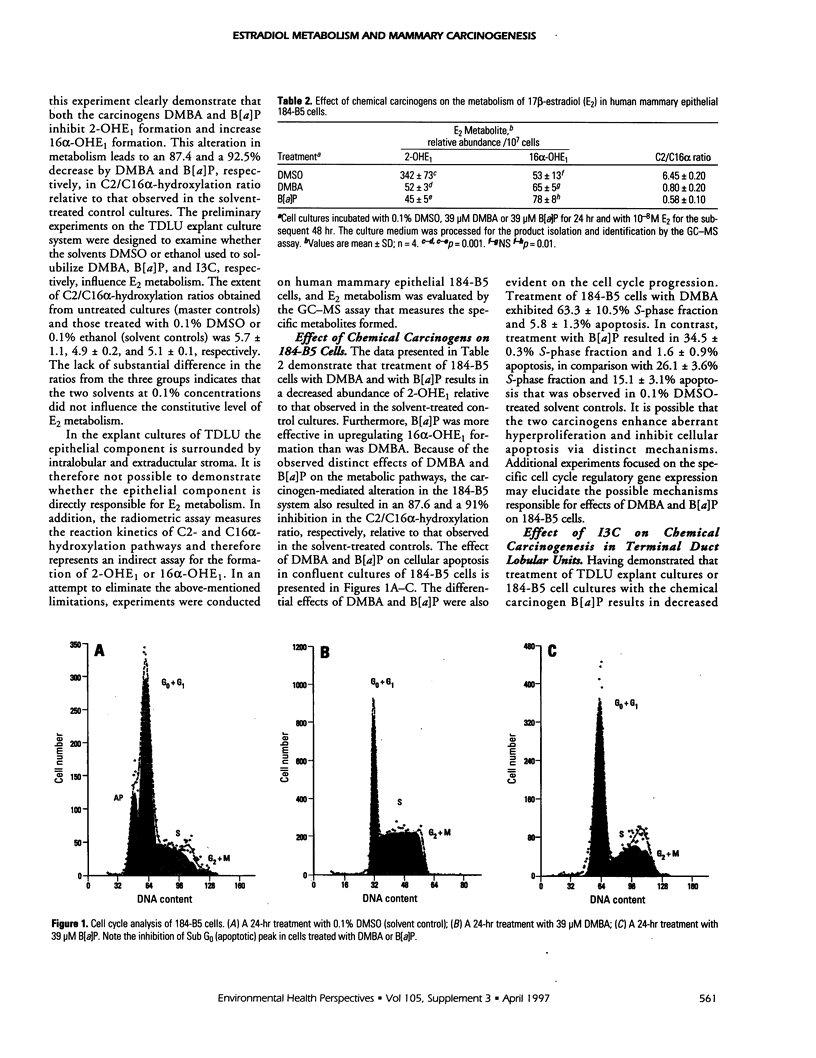
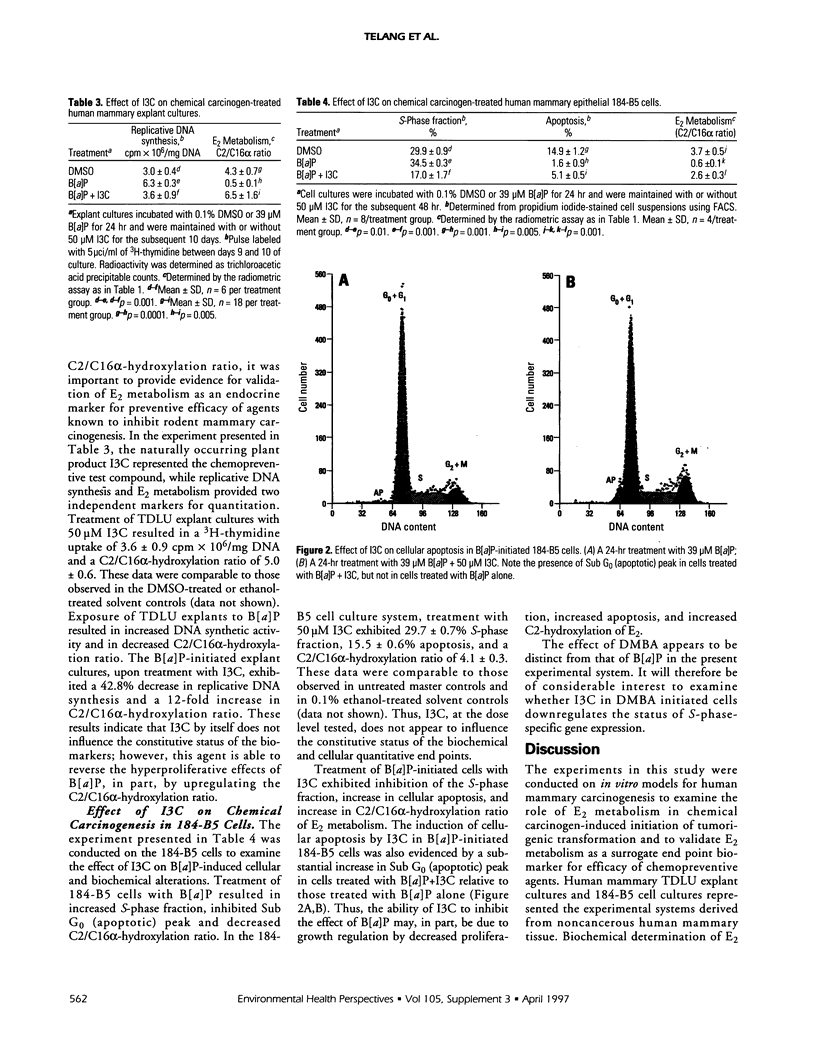
Abstract
The natural estrogen 17beta-estradiol (E2) has a profound influence on proliferation and neoplastic transformation of mammary epithelium. The role of cellular metabolism of E2 in mammary carcinogenesis, however, remains to be elucidated. Explant culture and cell culture models developed from noncancerous human mammary tissue were used to examine modulation of E2 metabolism in response to treatment with prototype rodent mammary carcinogens and the ability of the naturally occurring phytochemical indole-3-carbinol (13C) to influence E2 metabolism and regulate aberrant proliferation. In the two models, treatment with the chemical carcinogens 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene altered the metabolism of E2 as determined from the radiometric (tritium release) and gas chromatography-mass spectrometry (GC-MS) assays. This alteration in E2 metabolism was accompanied by aberrant proliferation and abrogation of apoptosis as determined by the extent of replicative DNA synthesis, S-phase fraction and Sub G0 (apoptotic) peak. Exposure of carcinogen-initiated cultures to 13C resulted in induction of C2-hydroxylation of E2 and of apoptosis and downregulation of hyperproliferation. Determination of altered cellular metabolism of E2 in response to initiators and modulators of carcinogenesis and evaluation of cell cycle related markers for proliferation and apoptosis may provide a mechanism-oriented approach to validate E2 metabolism as an endocrine biomarker for induction and prevention of human mammary carcinogenesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. B. Enzymatic regulation of estradiol-17 beta concentrations in human breast cancer cells. Breast Cancer Res Treat. 1992 Mar;20(3):145–154. doi: 10.1007/BF01834620. [DOI] [PubMed] [Google Scholar]
- Bernstein L., Ross R. K. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15(1):48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- Bradlow H. L., Michnovicz J., Telang N. T., Osborne M. P. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991 Sep;12(9):1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- Bradlow H. L., Sepkovic D. W., Telang N. T., Osborne M. P. Indole-3-carbinol. A novel approach to breast cancer prevention. Ann N Y Acad Sci. 1995 Sep 30;768:180–200. doi: 10.1111/j.1749-6632.1995.tb12121.x. [DOI] [PubMed] [Google Scholar]
- Davis D. L., Bradlow H. L., Wolff M., Woodruff T., Hoel D. G., Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993 Oct;101(5):372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubik D., Shiu R. P. Mechanism of estrogen activation of c-myc oncogene expression. Oncogene. 1992 Aug;7(8):1587–1594. [PubMed] [Google Scholar]
- Fishman J., Osborne M. P., Telang N. T. The role of estrogen in mammary carcinogenesis. Ann N Y Acad Sci. 1995 Sep 30;768:91–100. doi: 10.1111/j.1749-6632.1995.tb12113.x. [DOI] [PubMed] [Google Scholar]
- Goldberg Y., Nassif I. I., Pittas A., Tsai L. L., Dynlacht B. D., Rigas B., Shiff S. J. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene. 1996 Feb 15;12(4):893–901. [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C. Toward the primary prevention of cancer. Science. 1991 Nov 22;254(5035):1131–1138. doi: 10.1126/science.1957166. [DOI] [PubMed] [Google Scholar]
- Kelloff G. J., Boone C. W., Steele V. E., Crowell J. A., Lubet R., Doody L. A., Greenwald P. Development of breast cancer chemopreventive drugs. J Cell Biochem Suppl. 1993;17G:2–13. doi: 10.1002/jcb.240531103. [DOI] [PubMed] [Google Scholar]
- Kojima T., Tanaka T., Mori H. Chemoprevention of spontaneous endometrial cancer in female Donryu rats by dietary indole-3-carbinol. Cancer Res. 1994 Mar 15;54(6):1446–1449. [PubMed] [Google Scholar]
- Li J. J., Li S. A. Estrogen carcinogenesis in hamster tissues: a critical review. Endocr Rev. 1990 Nov;11(4):524–531. doi: 10.1210/edrv-11-4-524. [DOI] [PubMed] [Google Scholar]
- Lottering M. L., Haag M., Seegers J. C. Effects of 17 beta-estradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res. 1992 Nov 1;52(21):5926–5932. [PubMed] [Google Scholar]
- Martucci C. P., Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993 Feb-Mar;57(2-3):237–257. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis P., Kuttenn F., Gompel A. Estradiol/progesterone interaction in normal and pathologic breast cells. Ann N Y Acad Sci. 1986;464:152–167. doi: 10.1111/j.1749-6632.1986.tb16002.x. [DOI] [PubMed] [Google Scholar]
- McCormick D. L., Burns F. J., Albert R. E. Inhibition of benz[a]pyrene-induced mammary carcinogenesis by retinyl acetate. J Natl Cancer Inst. 1981 Mar;66(3):559–564. [PubMed] [Google Scholar]
- Michnovicz J. J., Bradlow H. L. Induction of estradiol metabolism by dietary indole-3-carbinol in humans. J Natl Cancer Inst. 1990 Jun 6;82(11):947–949. doi: 10.1093/jnci/82.11.947. [DOI] [PubMed] [Google Scholar]
- Moore C. J., Eldridge S. R., Tricomi W. A., Gould M. N. Quantitation of benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene binding to nuclear macromolecules in human and rat mammary epithelial cells. Cancer Res. 1987 May 15;47(10):2609–2613. [PubMed] [Google Scholar]
- Moore C. J., Tricomi W. A., Gould M. N. Interspecies comparison of polycyclic aromatic hydrocarbon metabolism in human and rat mammary epithelial cells. Cancer Res. 1986 Oct;46(10):4946–4952. [PubMed] [Google Scholar]
- Nandi S., Guzman R. C., Yang J. Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3650–3657. doi: 10.1073/pnas.92.9.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M. P., Bradlow H. L., Wong G. Y., Telang N. T. Upregulation of estradiol C16 alpha-hydroxylation in human breast tissue: a potential biomarker of breast cancer risk. J Natl Cancer Inst. 1993 Dec 1;85(23):1917–1920. doi: 10.1093/jnci/85.23.1917. [DOI] [PubMed] [Google Scholar]
- Parker S. L., Tong T., Bolden S., Wingo P. A. Cancer statistics, 1996. CA Cancer J Clin. 1996 Jan-Feb;46(1):5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Spicer D. V., Dahmoush L., Press M. F. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- Russo J., Calaf G., Sohi N., Tahin Q., Zhang P. L., Alvarado M. E., Estrada S., Russo I. H. Critical steps in breast carcinogenesis. Ann N Y Acad Sci. 1993 Nov 30;698:1–20. doi: 10.1111/j.1749-6632.1993.tb17187.x. [DOI] [PubMed] [Google Scholar]
- Schneider J., Huh M. M., Bradlow H. L., Fishman J. Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J Biol Chem. 1984 Apr 25;259(8):4840–4845. [PubMed] [Google Scholar]
- Sekeris C. E. Hormonal steroids act as tumour promoters by modulating oncogene expression. J Cancer Res Clin Oncol. 1991;117(2):96–101. doi: 10.1007/BF01613131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkovic D. W., Bradlow H. L., Michnovicz J., Murtezani S., Levy I., Osborne M. P. Catechol estrogen production in rat microsomes after treatment with indole-3-carbinol, ascorbigen, or beta-naphthaflavone: a comparison of stable isotope dilution gas chromatography-mass spectrometry and radiometric methods. Steroids. 1994 May;59(5):318–323. doi: 10.1016/0039-128x(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R., Bartholomew J. C., Smith H. S., Bartley J. C. Metabolism of benzo[a]pyrene by human mammary epithelial cells: toxicity and DNA adduct formation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6251–6255. doi: 10.1073/pnas.78.10.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto A., Bradlow H. L., Wong G. Y., Osborne M. P., Telang N. T. Experimental down-regulation of intermediate biomarkers of carcinogenesis in mouse mammary epithelial cells. Breast Cancer Res Treat. 1993 Sep;27(3):193–202. doi: 10.1007/BF00665689. [DOI] [PubMed] [Google Scholar]
- Telang N. T., Axelrod D. M., Wong G. Y., Bradlow H. L., Osborne M. P. Biotransformation of estradiol by explant culture of human mammary tissue. Steroids. 1991 Jan;56(1):37–43. doi: 10.1016/0039-128x(91)90113-a. [DOI] [PubMed] [Google Scholar]
- Telang N. T., Bradlow H. L., Osborne M. P. Molecular and endocrine biomarkers in non-involved breast: relevance to cancer chemoprevention. J Cell Biochem Suppl. 1992;16G:161–169. doi: 10.1002/jcb.240501128. [DOI] [PubMed] [Google Scholar]
- Telang N. T., Kurihara H., Wong G. Y., Bradlow H. L., Osborne M. P. Preneoplastic transformation in mouse mammary tissue: identification and validation of intermediate biomarkers for chemoprevention. Anticancer Res. 1991 May-Jun;11(3):1021–1027. [PubMed] [Google Scholar]
- Telang N. T., Narayanan R., Bradlow H. L., Osborne M. P. Coordinated expression of intermediate biomarkers for tumorigenic transformation in RAS-transfected mouse mammary epithelial cells. Breast Cancer Res Treat. 1991 Aug;18(3):155–163. doi: 10.1007/BF01990031. [DOI] [PubMed] [Google Scholar]
- Telang N. T. Oncogenes, estradiol biotransformation, and mammary carcinogenesis. Ann N Y Acad Sci. 1996 Apr 30;784:277–287. doi: 10.1111/j.1749-6632.1996.tb16242.x. [DOI] [PubMed] [Google Scholar]
- Telang N. T., Suto A., Wong G. Y., Osborne M. P., Bradlow H. L. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992 Apr 15;84(8):634–638. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- Tiwari R. K., Guo L., Bradlow H. L., Telang N. T., Osborne M. P. Selective responsiveness of human breast cancer cells to indole-3-carbinol, a chemopreventive agent. J Natl Cancer Inst. 1994 Jan 19;86(2):126–131. doi: 10.1093/jnci/86.2.126. [DOI] [PubMed] [Google Scholar]
- Vandewalle B., Lefebvre J. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cell Endocrinol. 1989 Feb;61(2):239–246. doi: 10.1016/0303-7207(89)90135-4. [DOI] [PubMed] [Google Scholar]
- Weisz A., Bresciani F. Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit Rev Oncog. 1993;4(4):361–388. [PubMed] [Google Scholar]
- Welsch C. W. Host factors affecting the growth of carcinogen-induced rat mammary carcinomas: a review and tribute to Charles Brenton Huggins. Cancer Res. 1985 Aug;45(8):3415–3443. [PubMed] [Google Scholar]
- Zhai Y. F., Beittenmiller H., Wang B., Gould M. N., Oakley C., Esselman W. J., Welsch C. W. Increased expression of specific protein tyrosine phosphatases in human breast epithelial cells neoplastically transformed by the neu oncogene. Cancer Res. 1993 May 15;53(10 Suppl):2272–2278. [PubMed] [Google Scholar]
- Zhu B. T., Bui Q. D., Weisz J., Liehr J. G. Conversion of estrone to 2- and 4-hydroxyestrone by hamster kidney and liver microsomes: implications for the mechanism of estrogen-induced carcinogenesis. Endocrinology. 1994 Nov;135(5):1772–1779. doi: 10.1210/endo.135.5.7956900. [DOI] [PubMed] [Google Scholar]


