Abstract
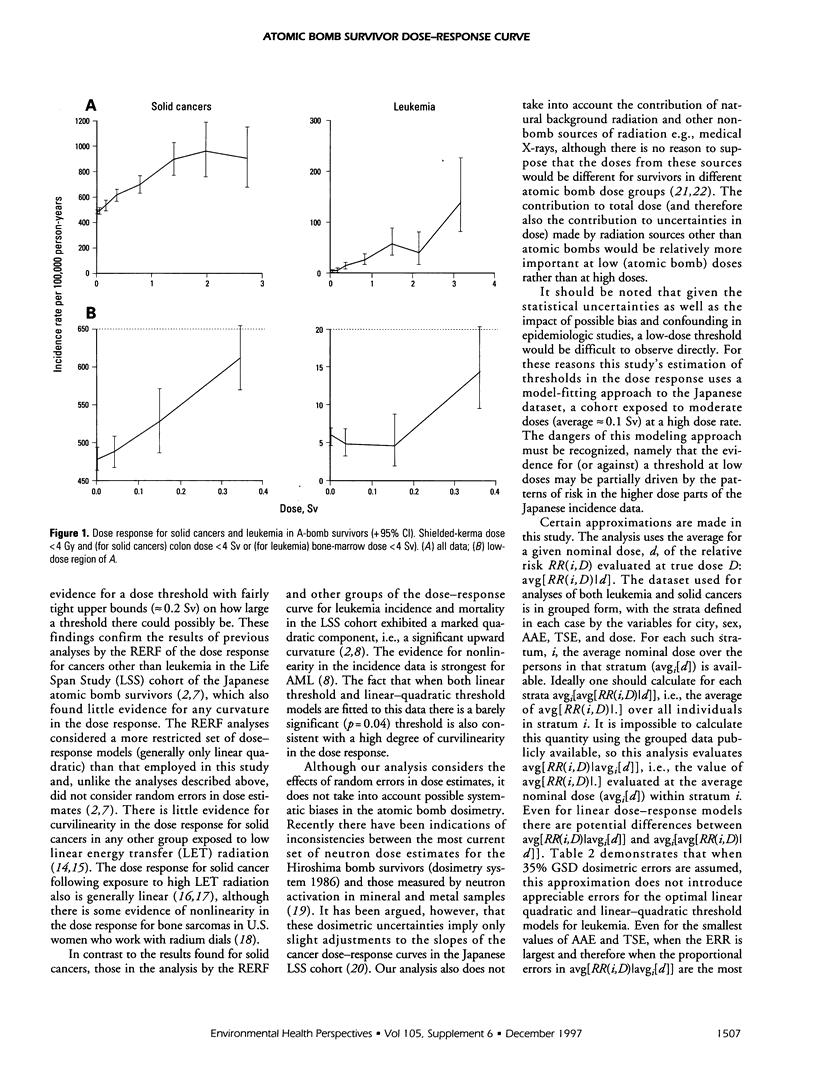
Recently released data on cancer incidence in Japanese atomic bomb survivors are analyzed using a variety of relative risk models that take account of errors in estimates of dose to assess the dose response at low doses. If a relative risk model with a threshold (the dose response is assumed linear above the threshold) is fitted to solid cancer data, a threshold of more than about 0.2 Sv is inconsistent with the data, whereas these data are consistent with there being no threshold. Among solid cancer subtypes there is strong evidence for a possible dose threshold only for nonmelanoma skin cancer. If a relative risk model with a threshold (the dose response is assumed linear above the threshold) is fitted to the leukemia data, a threshold of more than about 0.3 Sv is inconsistent with the data. In contrast to the estimates for the threshold level for solid cancer data, the best estimate for the threshold level in the leukemia data is significantly different from zero even when allowance is made for a possible quadratic term in the dose response, albeit at borderline levels of statistical significance (p = 0.04). There is little evidence for curvature in the leukemia dose response from 0.2 Sv upwards. However, possible underestimation of the errors in the estimates of the dose threshold as a result of confounding and uncertainties not taken into account in the analysis, together with the lack of biological plausibility of a threshold, makes interpretation of this finding questionable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardis E., Gilbert E. S., Carpenter L., Howe G., Kato I., Armstrong B. K., Beral V., Cowper G., Douglas A., Fix J. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995 May;142(2):117–132. [PubMed] [Google Scholar]
- Gilbert E. S. Some effects of random dose measurement errors on analyses of atomic bomb survivor data. Radiat Res. 1984 Jun;98(3):591–605. [PubMed] [Google Scholar]
- Goodhead D. T. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 1994 Jan;65(1):7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- Goodhead D. T., Nikjoo H. Track structure analysis of ultrasoft X-rays compared to high- and low-LET radiations. Int J Radiat Biol. 1989 Apr;55(4):513–529. doi: 10.1080/09553008914550571. [DOI] [PubMed] [Google Scholar]
- Kazuo K., Antoku S., Sawada S., Russell W. J. Organ doses received by atomic bomb survivors during radiological examinations at the Radiation Effects Research Foundation. Br J Radiol. 1991 Aug;64(764):720–727. doi: 10.1259/0007-1285-64-764-720. [DOI] [PubMed] [Google Scholar]
- Kneale G. W., Stewart A. M. Age variation in the cancer risks from foetal irradiation. Br J Cancer. 1977 Oct;36(4):501–510. doi: 10.1038/bjc.1977.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. P., Muirhead C. R. Evidence for curvilinearity in the cancer incidence dose-response in the Japanese atomic bomb survivors. Int J Radiat Biol. 1996 Jul;70(1):83–94. doi: 10.1080/095530096145364. [DOI] [PubMed] [Google Scholar]
- Lloyd D. C., Edwards A. A., Leonard A., Deknudt G. L., Verschaeve L., Natarajan A. T., Darroudi F., Obe G., Palitti F., Tanzarella C. Chromosomal aberrations in human lymphocytes induced in vitro by very low doses of X-rays. Int J Radiat Biol. 1992 Mar;61(3):335–343. doi: 10.1080/09553009214551021. [DOI] [PubMed] [Google Scholar]
- Lubin J. H., Boice J. D., Jr, Edling C., Hornung R. W., Howe G. R., Kunz E., Kusiak R. A., Morrison H. I., Radford E. P., Samet J. M. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst. 1995 Jun 7;87(11):817–827. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- Mole R. H., Papworth D. G., Corp M. J. The dose-response for x-ray induction of myeloid leukaemia in male CBA/H mice. Br J Cancer. 1983 Feb;47(2):285–291. doi: 10.1038/bjc.1983.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake M., Schull W. J. Radiation-related small head sizes among prenatally exposed A-bomb survivors. Int J Radiat Biol. 1993 Feb;63(2):255–270. doi: 10.1080/09553009314550341. [DOI] [PubMed] [Google Scholar]
- Papworth D. G., Hulse E. V. Dose-response models for the radiation-induction of skin tumours in mice. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Nov;44(5):423–431. doi: 10.1080/09553008314551401. [DOI] [PubMed] [Google Scholar]
- Pierce D. A., Stram D. O., Vaeth M. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res. 1990 Sep;123(3):275–284. [PubMed] [Google Scholar]
- Pierce D. A., Vaeth M. The shape of the cancer mortality dose-response curve for the A-bomb survivors. Radiat Res. 1991 Apr;126(1):36–42. [PubMed] [Google Scholar]
- Pohl-Rüling J., Fischer P., Haas O., Obe G., Natarajan A. T., van Buul P. P., Buckton K. E., Bianchi N. O., Larramendy M., Kucerová M. Effect of low-dose acute X-irradiation on the frequencies of chromosomal aberrations in human peripheral lymphocytes in vitro. Mutat Res. 1983 Jun-Jul;110(1):71–82. doi: 10.1016/0027-5107(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Preston D. L., Kusumi S., Tomonaga M., Izumi S., Ron E., Kuramoto A., Kamada N., Dohy H., Matsuo T., Matsui T [corrected to Matsuo T. ]. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res. 1994 Feb;137(2 Suppl):S68–S97. [PubMed] [Google Scholar]
- Rabbitts T. H. Chromosomal translocations in human cancer. Nature. 1994 Nov 10;372(6502):143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Rowland R. E., Stehney A. F., Lucas H. F., Jr Dose-response relationships for female radium dial workers. Radiat Res. 1978 Nov;76(2):368–383. [PubMed] [Google Scholar]
- Shimizu Y., Kato H., Schull W. J. Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 2. Cancer mortality based on the recently revised doses (DS86). Radiat Res. 1990 Feb;121(2):120–141. [PubMed] [Google Scholar]
- Straume T., Egbert S. D., Woolson W. A., Finkel R. C., Kubik P. W., Gove H. E., Sharma P., Hoshi M. Neutron discrepancies in the DS86 Hiroshima dosimetry system. Health Phys. 1992 Oct;63(4):421–426. doi: 10.1097/00004032-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Thompson D. E., Mabuchi K., Ron E., Soda M., Tokunaga M., Ochikubo S., Sugimoto S., Ikeda T., Terasaki M., Izumi S. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958-1987. Radiat Res. 1994 Feb;137(2 Suppl):S17–S67. [PubMed] [Google Scholar]


