Abstract
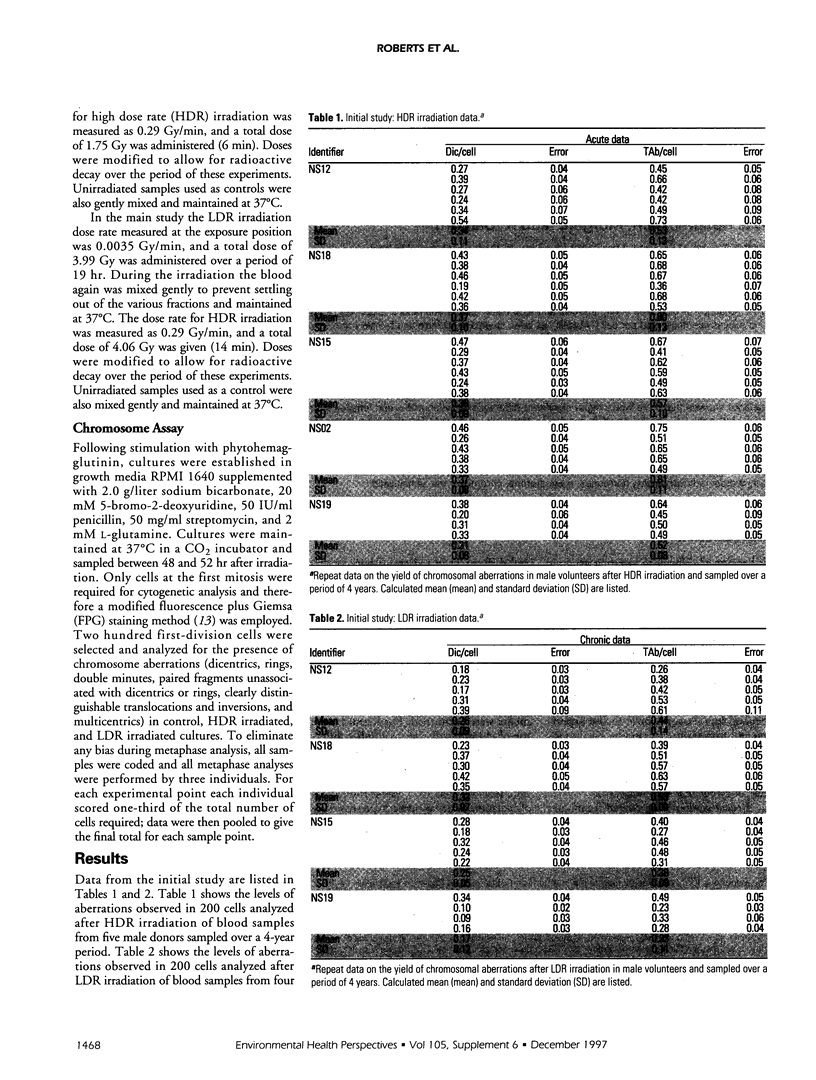
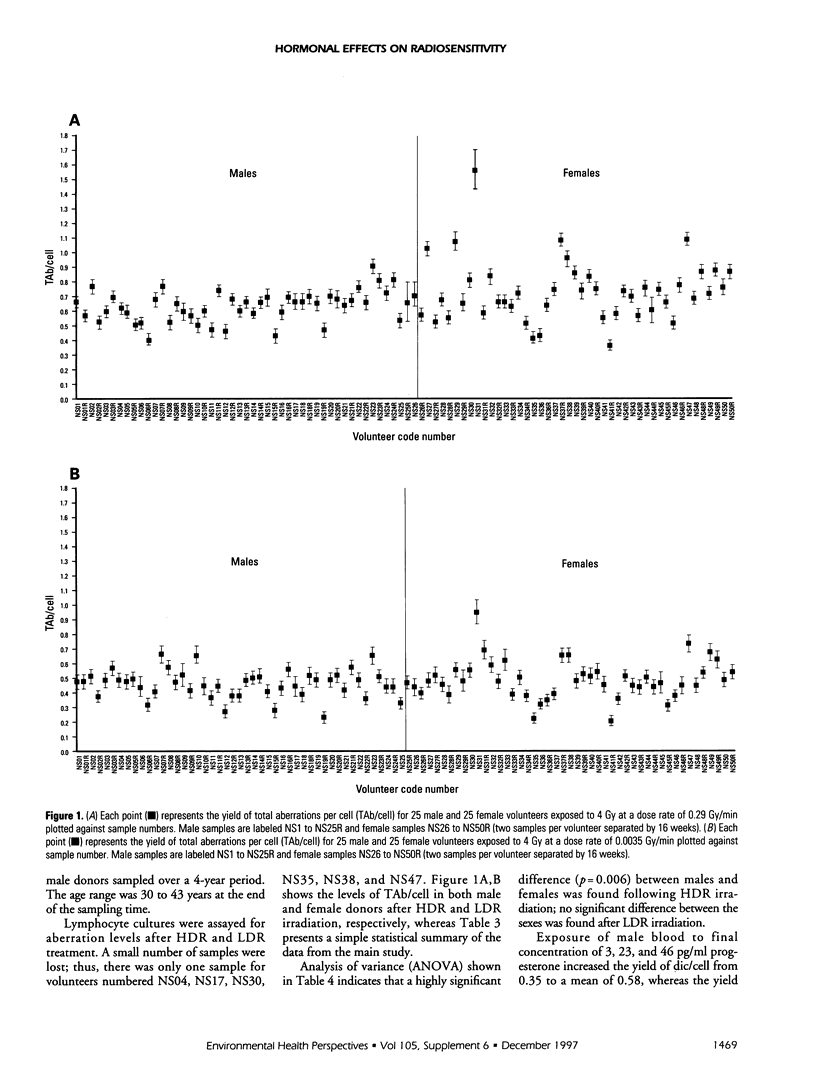
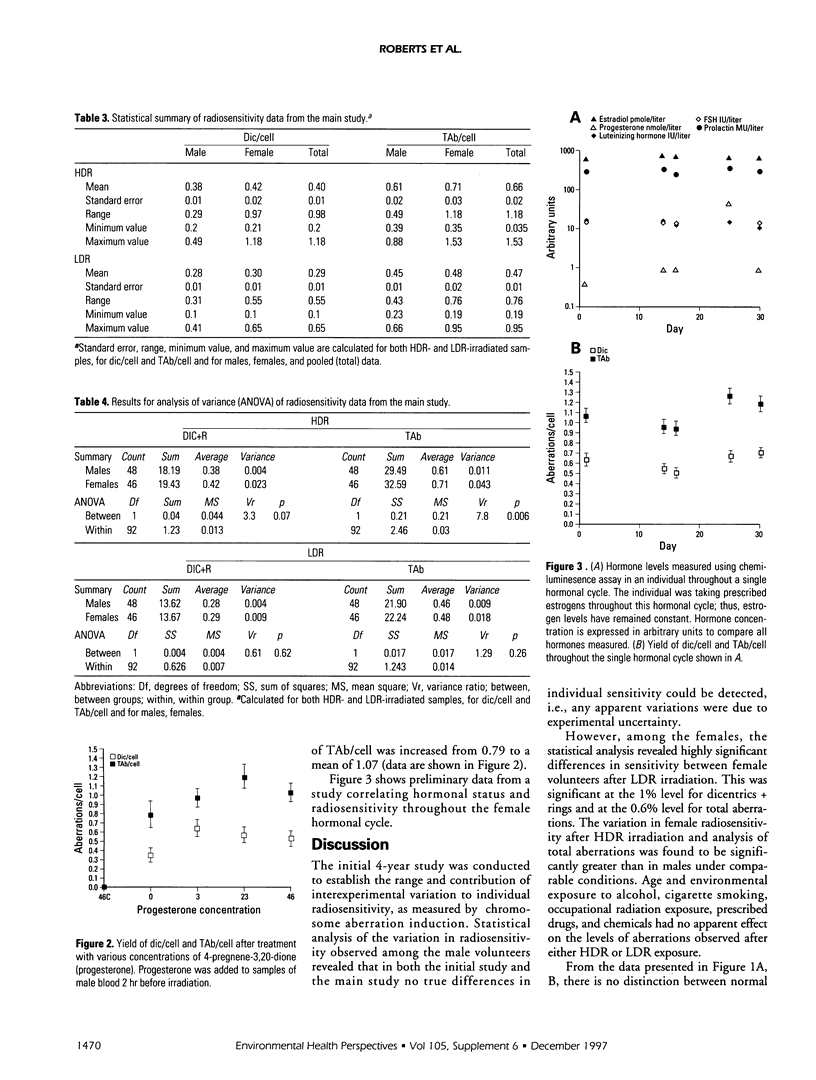
The frequency of dicentrics + ring (dic/cell) and total chromosome aberrations (dicentrics, rings and excess acentrics, etc.) per cell (TAb/cell) has been studied in 50 male and female volunteers after high or low dose rate (HDR, LDR) irradiation of peripheral blood lymphocytes. The mean male aberration frequencies per cell after HDR irradiation were 0.38 dic/cell and 0.61 TAb/cell; following LDR irradiation, the mean aberration frequencies were 0.28 dic/cell and 0.45 TAb/cell. Equivalent female values after HDR irradiation were 0.42 dic/cell and 0.71 TAb/cell; after LDR irradiation, the mean aberration frequencies were 0.30 dic/cell and 0.48 TAb/cell. Analysis of variance showed that there was a highly significant difference between males and females have a greater HDR, but not LDR, irradiation It is concluded from this study that females have a greater variability in their radioresponse, and that this variability is related to progesterone, which has a profound effect upon radiosensitivity, as measured by cytogenetic end points.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. K., Banerjee S., Li S. A., Li J. J. Induction of chromosome aberrations in Syrian hamster renal cortical cells by various estrogens. Mutat Res. 1994 Dec 1;311(2):191–197. doi: 10.1016/0027-5107(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Carrano A. V., Natarajan A. T. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC publication no. 14. Considerations for population monitoring using cytogenetic techniques. Mutat Res. 1988 Mar;204(3):379–406. doi: 10.1016/0165-1218(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Elyan S. A., West C. M., Roberts S. A., Hunter R. D. Use of low-dose rate irradiation to measure the intrinsic radiosensitivity of human T-lymphocytes. Int J Radiat Biol. 1993 Oct;64(4):375–383. doi: 10.1080/09553009314551561. [DOI] [PubMed] [Google Scholar]
- Huber R., Braselmann H., Bauchinger M. Intra- and inter-individual variation of background and radiation-induced micronucleus frequencies in human lymphocytes. Int J Radiat Biol. 1992 May;61(5):655–661. doi: 10.1080/09553009214551461. [DOI] [PubMed] [Google Scholar]
- Huber R., Braselmann H., Bauchinger M. Screening for interindividual differences in radiosensitivity by means of the micronucleus assay in human lymphocytes. Radiat Environ Biophys. 1989;28(2):113–120. doi: 10.1007/BF01210295. [DOI] [PubMed] [Google Scholar]
- Lloyd D. C., Edwards A. A., Leonard A., Deknudt G. L., Verschaeve L., Natarajan A. T., Darroudi F., Obe G., Palitti F., Tanzarella C. Chromosomal aberrations in human lymphocytes induced in vitro by very low doses of X-rays. Int J Radiat Biol. 1992 Mar;61(3):335–343. doi: 10.1080/09553009214551021. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Sposto R., Kushiro J., Akiyama M. Is interindividual variation of cellular radiosensitivity real or artifactual? Radiat Res. 1991 Mar;125(3):326–330. [PubMed] [Google Scholar]
- Perry P., Evans H. J. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature. 1975 Nov 13;258(5531):121–125. doi: 10.1038/258121a0. [DOI] [PubMed] [Google Scholar]
- Sharma T., Das B. C. Higher incidence of spontaneous sister-chromatid exchanges (SCEs) and X-ray-induced chromosome aberrations in peripheral blood lymphocytes during pregnancy. Mutat Res. 1986 May;174(1):27–33. doi: 10.1016/0165-7992(86)90073-4. [DOI] [PubMed] [Google Scholar]
- Tates A. D., van Dam F. J., van Mossel H., Schoemaker H., Thijssen J. C., Woldring V. M., Zwinderman A. H., Natarajan A. T. Use of the clonal assay for the measurement of frequencies of HPRT mutants in T-lymphocytes from five control populations. Mutat Res. 1991 Oct;253(2):199–213. doi: 10.1016/0165-1161(91)90133-s. [DOI] [PubMed] [Google Scholar]
- Thierens H., Vral A., de Ridder L. Biological dosimetry using the micronucleus assay for lymphocytes: interindividual differences in dose response. Health Phys. 1991 Nov;61(5):623–630. doi: 10.1097/00004032-199111000-00005. [DOI] [PubMed] [Google Scholar]
- de Jong G., van Sittert N. J., Natarajan A. T. Cytogenetic monitoring of industrial populations potentially exposed to genotoxic chemicals and of control populations. Mutat Res. 1988 Mar;204(3):451–464. doi: 10.1016/0165-1218(88)90041-9. [DOI] [PubMed] [Google Scholar]
- van Buul P. P., Goudzwaard J. H. Effect of follicle-stimulating hormone (FSH) on radiation-induced chromosomal aberrations in mouse germ cells and Chinese hamster cells in vitro. Mutat Res. 1982 Dec;106(2):247–253. doi: 10.1016/0027-5107(82)90106-3. [DOI] [PubMed] [Google Scholar]
- van Buul P. P., van Buul-Offers S. C. Effects of hormone treatment on chromosomal radiosensitivity of somatic and germ cells of Snell's dwarf mice. Mutat Res. 1988 Apr;198(2):263–268. doi: 10.1016/0027-5107(88)90002-4. [DOI] [PubMed] [Google Scholar]


