Abstract
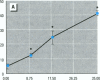
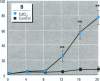
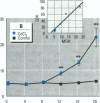
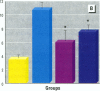
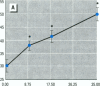
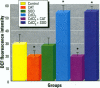
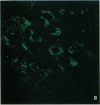
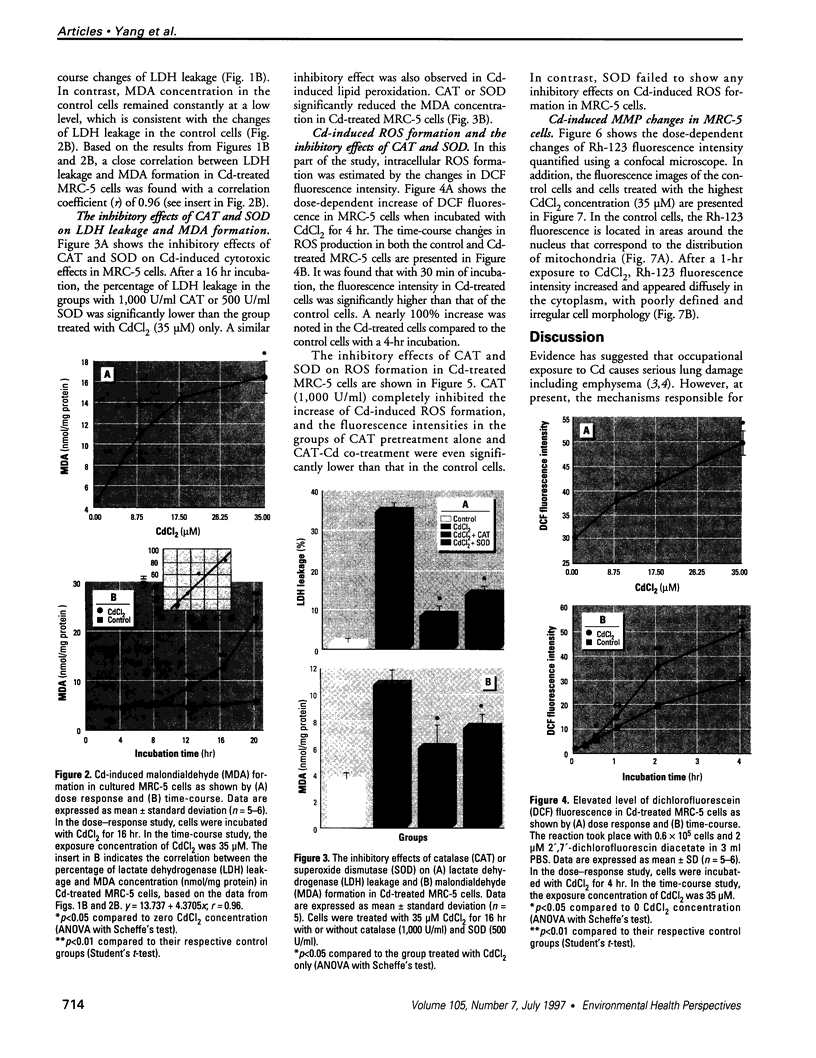
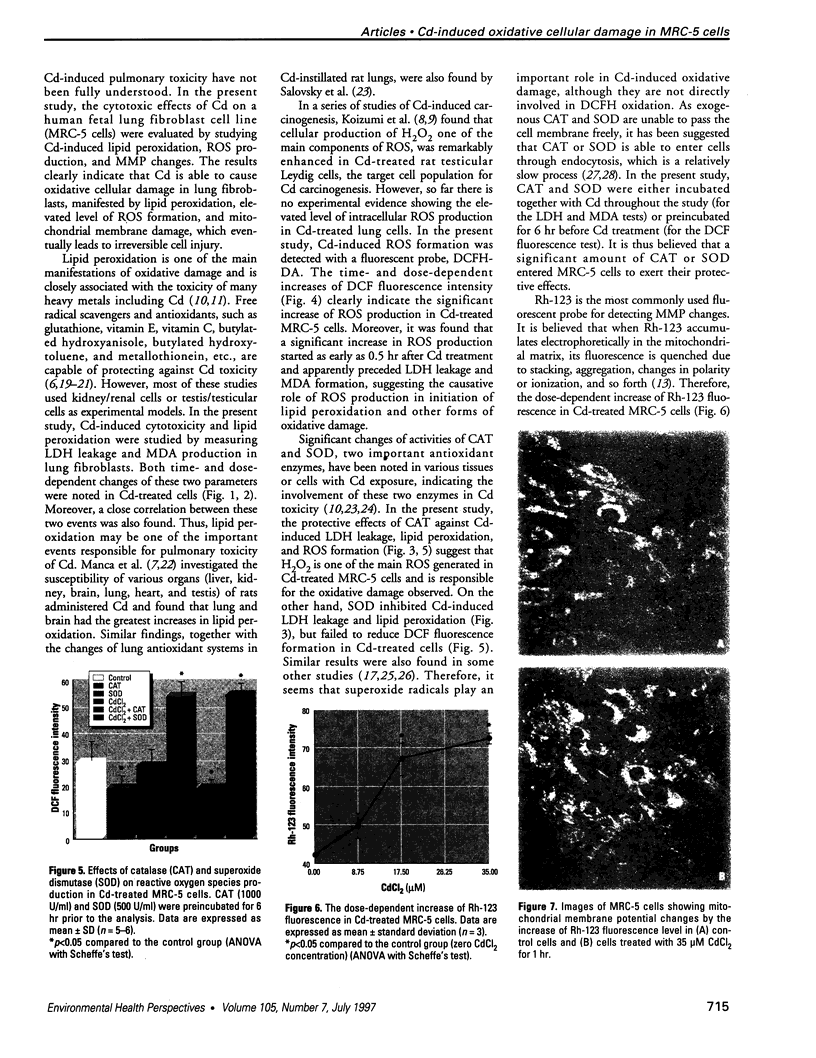
Epidemiological evidence suggests that cadmium (Cd) exposure causes pulmonary damage such as emphysema and lung cancer. However, relatively little is known about the mechanisms involved in Cd pulmonary toxicity. In the present study, the effects of Cd exposure on human fetal lung fibroblasts (MRC-5 cells) were evaluated by determination of lipid peroxidation, intra-cellular production of reactive oxygen species (ROS), and changes of mitochondrial membrane potential. A time- and dose-dependent increase of both lactate dehydrogenase leakage and malondialdehyde formation was observed in Cd-treated cells. A close correlation between these two events suggests that lipid peroxidation may be one of the main pathways causing its cytotoxicity. It was also noted that Cd-induced cell injury and lipid peroxidation were inhibited by catalase and superoxide dismutase, two antioxidant enzymes. By using the fluorescent probe 2',7'-dichlorofluorescin diacetate, a significant increase of ROS production in Cd-treated MRC-5 cells was detected. The inhibition of dichlorofluorescein fluorescence by catalase, not superoxide dismutase, suggests that hydrogen peroxide is the main ROS involved. Moreover, the significant dose-dependent changes of mitochondrial membrane potential in Cd-treated MRC-5 cells, demonstrated by increased fluorescence of rhodamine 123 examined using a laser-scanning confocal microscope, also indicate the involvement of mitochondrial damage in Cd cytotoxicity. These findings provide in vitro evidence that Cd causes oxidative cellular damage in human fetal lung fibroblasts, which may be closely associated with the pulmonary toxicity of Cd.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Minor R. L., Jr, White C. W., Repine J. E., Rosen G. M., Freeman B. A. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988 May 15;263(14):6884–6892. [PubMed] [Google Scholar]
- Carini R., Parola M., Dianzani M. U., Albano E. Mitochondrial damage and its role in causing hepatocyte injury during stimulation of lipid peroxidation by iron nitriloacetate. Arch Biochem Biophys. 1992 Aug 15;297(1):110–118. doi: 10.1016/0003-9861(92)90647-f. [DOI] [PubMed] [Google Scholar]
- Carter W. O., Narayanan P. K., Robinson J. P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994 Feb;55(2):253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- Chambers R. C., McAnulty R. J., Shock A., Campa J. S., Newman Taylor A. J., Laurent G. J. Cadmium selectively inhibits fibroblast procollagen production and proliferation. Am J Physiol. 1994 Sep;267(3 Pt 1):L300–L308. doi: 10.1152/ajplung.1994.267.3.L300. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- Chubatsu L. S., Gennari M., Meneghini R. Glutathione is the antioxidant responsible for resistance to oxidative stress in V79 Chinese hamster fibroblasts rendered resistant to cadmium. Chem Biol Interact. 1992 Mar;82(1):99–110. doi: 10.1016/0009-2797(92)90017-f. [DOI] [PubMed] [Google Scholar]
- Davison A. G., Fayers P. M., Taylor A. J., Venables K. M., Darbyshire J., Pickering C. A., Chettle D. R., Franklin D., Guthrie C. J., Scott M. C. Cadmium fume inhalation and emphysema. Lancet. 1988 Mar 26;1(8587):663–667. doi: 10.1016/s0140-6736(88)91474-2. [DOI] [PubMed] [Google Scholar]
- Elinder C. G., Kjellström T., Hogstedt C., Andersson K., Spång G. Cancer mortality of cadmium workers. Br J Ind Med. 1985 Oct;42(10):651–655. doi: 10.1136/oem.42.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariss M. W. Cadmium toxicity: unique cytoprotective properties of alpha tocopheryl succinate in hepatocytes. Toxicology. 1991;69(1):63–77. doi: 10.1016/0300-483x(91)90154-s. [DOI] [PubMed] [Google Scholar]
- Fox M. R., Fry B. E., Jr Cadmium toxicity decreased by dietary ascorbic acid supplements. Science. 1970 Sep 4;169(3949):989–991. doi: 10.1126/science.169.3949.989. [DOI] [PubMed] [Google Scholar]
- Ito Y., Hiraishi H., Razandi M., Terano A., Harada T., Ivey K. J. Role of cellular superoxide dismutase against reactive oxygen metabolite-induced cell damage in cultured rat hepatocytes. Hepatology. 1992 Jul;16(1):247–254. doi: 10.1002/hep.1840160136. [DOI] [PubMed] [Google Scholar]
- Koizumi T., Li Z. G. Role of oxidative stress in single-dose, cadmium-induced testicular cancer. J Toxicol Environ Health. 1992 Sep;37(1):25–36. doi: 10.1080/15287399209531654. [DOI] [PubMed] [Google Scholar]
- Koizumi T., Li Z. G., Tatsumoto H. DNA damaging activity of cadmium in Leydig cells, a target cell population for cadmium carcinogenesis in the rat testis. Toxicol Lett. 1992 Nov;63(2):211–220. doi: 10.1016/0378-4274(92)90013-a. [DOI] [PubMed] [Google Scholar]
- LeBel C. P., Ischiropoulos H., Bondy S. C. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992 Mar-Apr;5(2):227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Manca D., Ricard A. C., Tra H. V., Chevalier G. Relation between lipid peroxidation and inflammation in the pulmonary toxicity of cadmium. Arch Toxicol. 1994;68(6):364–369. doi: 10.1007/s002040050083. [DOI] [PubMed] [Google Scholar]
- Manca D., Ricard A. C., Trottier B., Chevalier G. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology. 1991 May;67(3):303–323. doi: 10.1016/0300-483x(91)90030-5. [DOI] [PubMed] [Google Scholar]
- Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978 May;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Nordberg G. F. Application of the 'critical effect' and 'critical concentration' concept to human risk assessment for cadmium. IARC Sci Publ. 1992;(118):3–14. [PubMed] [Google Scholar]
- Palmeira C. M., Moreno A. J., Madeira V. M., Wallace K. B. Continuous monitoring of mitochondrial membrane potential in hepatocyte cell suspensions. J Pharmacol Toxicol Methods. 1996 Feb;35(1):35–43. doi: 10.1016/1056-8719(95)00131-x. [DOI] [PubMed] [Google Scholar]
- Peters J. M., Duncan J. R., Wiley L. M., Keen C. L. Influence of antioxidants on cadmium toxicity of mouse preimplantation embryos in vitro. Toxicology. 1995 May 5;99(1-2):11–18. doi: 10.1016/0300-483x(94)02989-8. [DOI] [PubMed] [Google Scholar]
- Salovsky P., Shopova V., Dancheva V., Marev R. Changes in antioxidant lung protection after single intra-tracheal cadmium acetate instillation in rats. Hum Exp Toxicol. 1992 May;11(3):217–222. doi: 10.1177/096032719201100310. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Yadav P., Trivedi R., Bansal A. K., Bhatnagar D. Cadmium-induced lipid peroxidation and the status of the antioxidant system in rat tissues. J Trace Elem Med Biol. 1995 Oct;9(3):144–149. doi: 10.1016/S0946-672X(11)80038-6. [DOI] [PubMed] [Google Scholar]
- Sharma G., Nath R., Gill K. D. Effect of ethanol on cadmium-induced lipid peroxidation and antioxidant enzymes in rat liver. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S9–16. doi: 10.1016/0006-2952(91)90386-j. [DOI] [PubMed] [Google Scholar]
- Shen H. M., Ong C. N., Shi C. Y. Involvement of reactive oxygen species in aflatoxin B1-induced cell injury in cultured rat hepatocytes. Toxicology. 1995 May 5;99(1-2):115–123. doi: 10.1016/0300-483x(94)03008-p. [DOI] [PubMed] [Google Scholar]
- Shen H. M., Shi C. Y., Shen Y., Ong C. N. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic Biol Med. 1996;21(2):139–146. doi: 10.1016/0891-5849(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Wu E. Y., Smith M. T., Bellomo G., Di Monte D. Relationships between the mitochondrial transmembrane potential, ATP concentration, and cytotoxicity in isolated rat hepatocytes. Arch Biochem Biophys. 1990 Nov 1;282(2):358–362. doi: 10.1016/0003-9861(90)90129-m. [DOI] [PubMed] [Google Scholar]