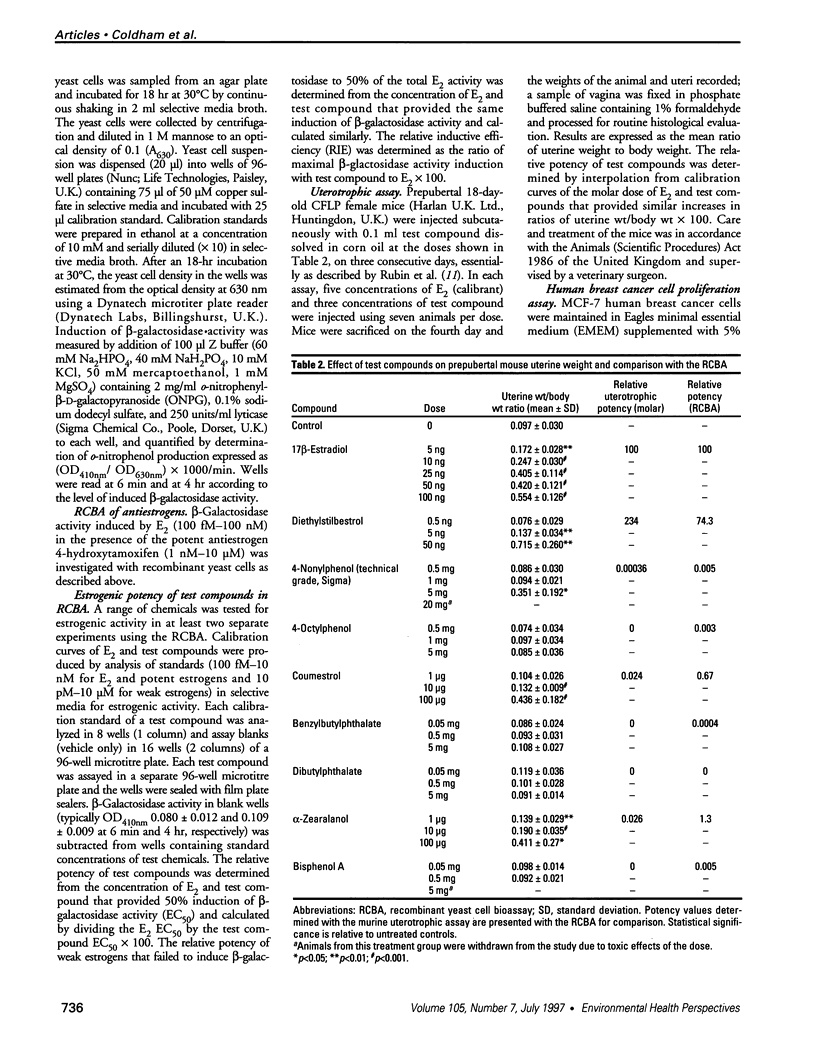
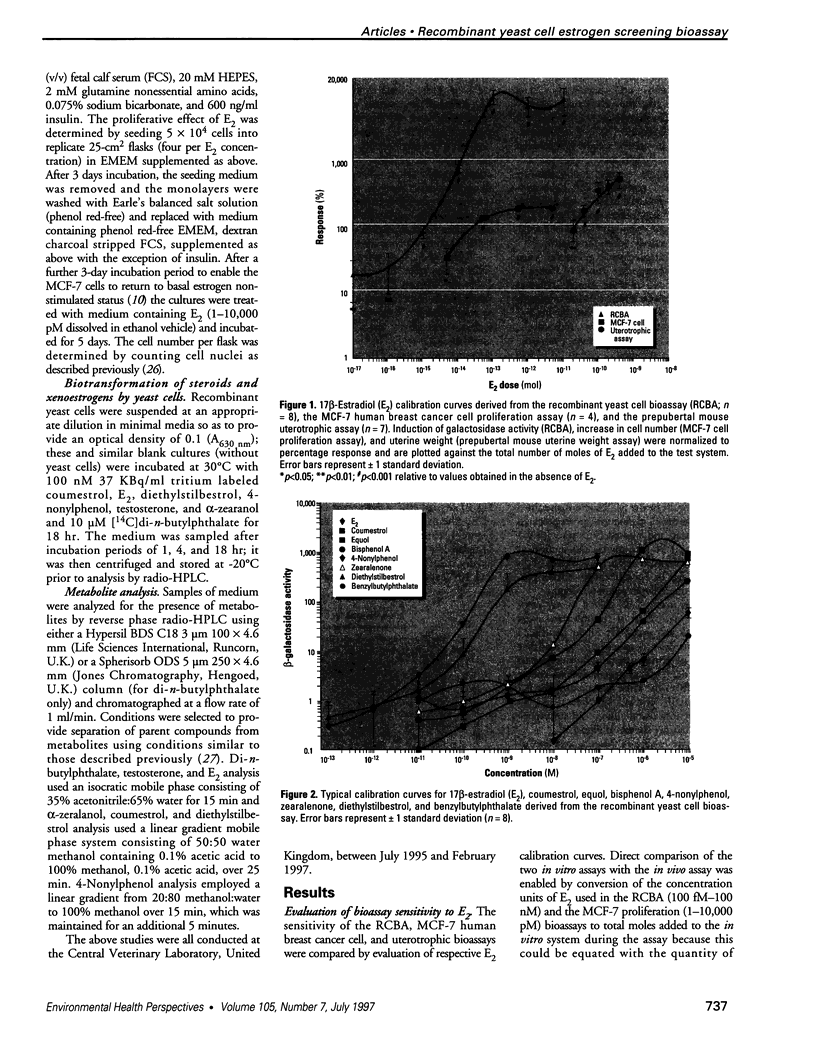
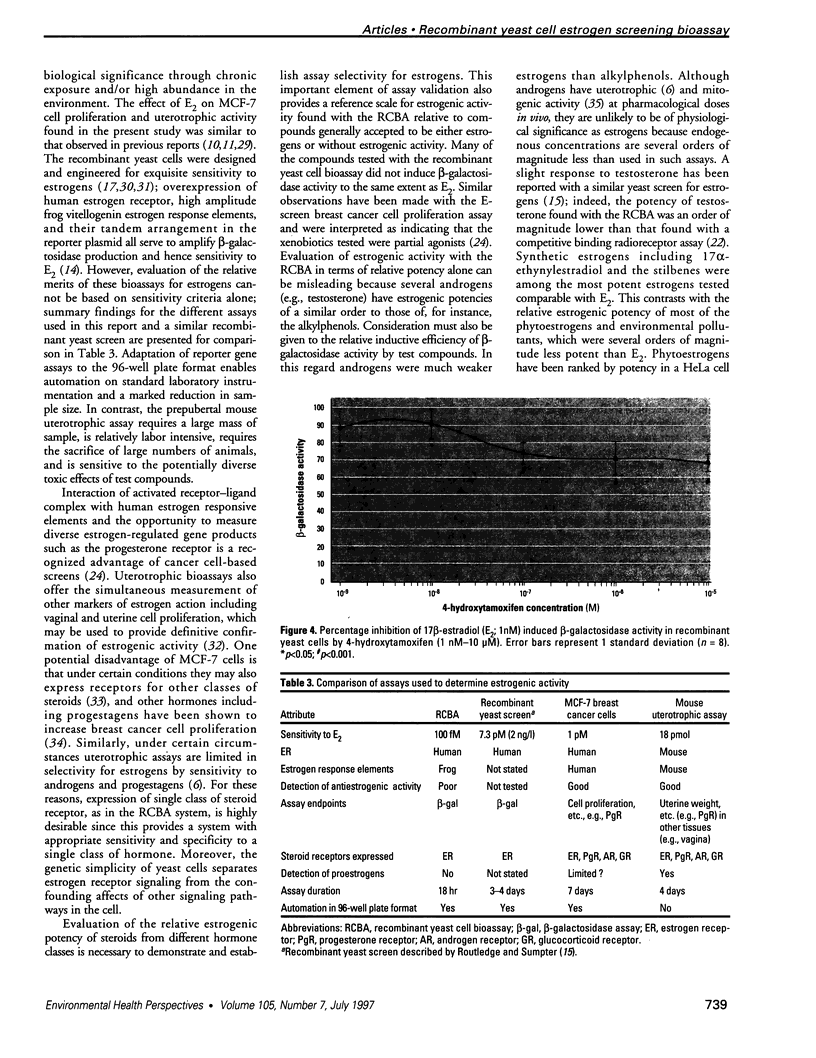
Abstract
A wide range of chemicals with diverse structures derived from plant and environmental origins are reported to have hormonal activity. The potential for appreciable exposure of humans to such substances prompts the need to develop sensitive screening methods to quantitate and evaluate the risk to the public. Yeast cells transformed with plasmids encoding the human estrogen receptor and an estrogen responsive promoter linked to a reporter gene were evaluated for screening compounds for estrogenic activity. Relative sensitivity to estrogens was evaluated by reference to 17 beta-estradiol (E2) calibration curves derived using the recombinant yeast cells, MCF-7 human breast cancer cells, and a prepubertal mouse uterotrophic bioassay. The recombinant yeast cell bioassay (RCBA) was approximately two and five orders of magnitude more sensitive to E2 than MCF-7 cells and the uterotrophic assay, respectively. The estrogenic potency of 53 chemicals, including steroid hormones, synthetic estrogens, environmental pollutants, and phytoestrogens, was measured using the RCBA. Potency values produced with the RCBA relative to E2 (100) included estrone (9.6), diethylstilbestrol (74.3), tamoxifen (0.0047), alpha-zearalanol (1.3), equol (0.085), 4-nonylphenol (0.005), and butylbenzyl phathalate (0.0004), which were similar to literature values but generally higher than those produced by the uterotrophic assay. Exquisite sensitivity, absence of test compound biotransformation, ease of use, and the possibility of measuring antiestrogenic activity are important attributes that argue for the suitability of the RCBA in screening for potential xenoestrogens to evaluate risk to humans, wildlife, and the environment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Robinson M. K., Notides A. C., Guillette L. J., Jr, McLachlan J. A. A yeast estrogen screen for examining the relative exposure of cells to natural and xenoestrogens. Environ Health Perspect. 1996 May;104(5):544–548. doi: 10.1289/ehp.96104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts C. J., van den Berg H. Multi-residue screening of bovine urine on xenobiotic oestrogens with an oestrogen radioreceptor assay. J Chromatogr. 1989 Apr 7;489(1):225–234. doi: 10.1016/s0378-4347(00)82900-x. [DOI] [PubMed] [Google Scholar]
- Ashby J., Lefevre P. A., Odum J., Harris C. A., Routledge E. J., Sumpter J. P. Synergy between synthetic oestrogens? Nature. 1997 Feb 6;385(6616):494–494. doi: 10.1038/385494a0. [DOI] [PubMed] [Google Scholar]
- Braunsberg H., Coldham N. G., Leake R. E., Cowan S. K., Wong W. Actions of a progestogen on human breast cancer cells: mechanisms of growth stimulation and inhibition. Eur J Cancer Clin Oncol. 1987 May;23(5):563–571. doi: 10.1016/0277-5379(87)90321-x. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Feil V. J., Kupfer D. Role of hepatic monooxygenases in generating estrogenic metabolites from methoxychlor and from its identified contaminants. Mol Pharmacol. 1985 Jan;27(1):115–124. [PubMed] [Google Scholar]
- Coldham N. G., James V. H. A possible mechanism for increased breast cell proliferation by progestins through increased reductive 17 beta-hydroxysteroid dehydrogenase activity. Int J Cancer. 1990 Jan 15;45(1):174–178. doi: 10.1002/ijc.2910450131. [DOI] [PubMed] [Google Scholar]
- Day B. W., Magarian R. A., Jain P. T., Pento J. T., Mousissian G. K., Meyer K. L. Synthesis and biological evaluation of a series of 1,1-dichloro-2,2,3-triarylcyclopropanes as pure antiestrogens. J Med Chem. 1991 Feb;34(2):842–851. doi: 10.1021/jm00106a052. [DOI] [PubMed] [Google Scholar]
- Galey F. D., Mendez L. E., Whitehead W. E., Holstege D. M., Plumlee K. H., Johnson B. Estrogenic activity in forages: diagnostic use of the classical mouse uterine bioassay. J Vet Diagn Invest. 1993 Oct;5(4):603–608. doi: 10.1177/104063879300500416. [DOI] [PubMed] [Google Scholar]
- Gyling M., Leclercq G. Estrogen and antiestrogen interaction with estrogen receptor of MCF-7 cells--relationship between processing and estrogenicity. J Steroid Biochem. 1988 Jan;29(1):1–8. doi: 10.1016/0022-4731(88)90368-8. [DOI] [PubMed] [Google Scholar]
- Harris M., Zacharewski T., Safe S. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds on the occupied nuclear estrogen receptor in MCF-7 human breast cancer cells. Cancer Res. 1990 Jun 15;50(12):3579–3584. [PubMed] [Google Scholar]
- Imhof M. O., McDonnell D. P. Yeast RSP5 and its human homolog hRPF1 potentiate hormone-dependent activation of transcription by human progesterone and glucocorticoid receptors. Mol Cell Biol. 1996 Jun;16(6):2594–2605. doi: 10.1128/mcb.16.6.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995 Jun;103(6):582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Bhardwaj B., Fang H., Ince B. A., Pakdel F., Reese J. C., Schodin D., Wrenn C. K. Hormone binding and transcription activation by estrogen receptors: analyses using mammalian and yeast systems. J Steroid Biochem Mol Biol. 1993 Dec;47(1-6):39–48. doi: 10.1016/0960-0760(93)90055-2. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Katzenellenbogen J. A., Mordecai D. Zearalenones: characterization of the estrogenic potencies and receptor interactions of a series of fungal beta-resorcylic acid lactones. Endocrinology. 1979 Jul;105(1):33–40. doi: 10.1210/endo-105-1-33. [DOI] [PubMed] [Google Scholar]
- Klein K. O., Baron J., Colli M. J., McDonnell D. P., Cutler G. B., Jr Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest. 1994 Dec;94(6):2475–2480. doi: 10.1172/JCI117616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korach K. S., Sarver P., Chae K., McLachlan J. A., McKinney J. D. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988 Jan;33(1):120–126. [PubMed] [Google Scholar]
- Korenman S. G. Comparative binding affinity of estrogens and its relation to estrogenic potency. Steroids. 1969 Feb;13(2):163–177. doi: 10.1016/0039-128x(69)90004-x. [DOI] [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993 Jun;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Kupfer D. Critical evaluation of methods for detection and assessment of estrogenic compounds in mammals: strengths and limitations for application to risk assessment. Reprod Toxicol. 1987 1988;1(2):147–153. doi: 10.1016/0890-6238(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Kupfer D., Mani C., Lee C. A., Rifkind A. B. Induction of tamoxifen-4-hydroxylation by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), beta-naphthoflavone (beta NF), and phenobarbital (PB) in avian liver: identification of P450 TCDDAA as catalyst of 4-hydroxylation induced by TCDD and beta NF. Cancer Res. 1994 Jun 15;54(12):3140–3144. [PubMed] [Google Scholar]
- Lyttle C. R., Damian-Matsumura P., Juul H., Butt T. R. Human estrogen receptor regulation in a yeast model system and studies on receptor agonists and antagonists. J Steroid Biochem Mol Biol. 1992 Aug;42(7):677–685. doi: 10.1016/0960-0760(92)90108-u. [DOI] [PubMed] [Google Scholar]
- MARTIN L., CLARINGBOLD P. J. The mitogenic action of oestrogens in the vaginal epithelium of the ovariectomized mouse. J Endocrinol. 1960 May;20:173–186. doi: 10.1677/joe.0.0200173. [DOI] [PubMed] [Google Scholar]
- Magarian R. A., Avor K. S., Overacre L. B., Kunchandy J., Paxton T. R. Synthesis and biological evaluation of basic side chain derivatives of Analog II as pure antiestrogens and antitumor agents. Anticancer Drug Des. 1995 Jun;10(4):311–331. [PubMed] [Google Scholar]
- McDonnell D. P., Nawaz Z., Densmore C., Weigel N. L., Pham T. A., Clark J. H., O'Malley B. W. High level expression of biologically active estrogen receptor in Saccharomyces cerevisiae. J Steroid Biochem Mol Biol. 1991 Sep;39(3):291–297. doi: 10.1016/0960-0760(91)90038-7. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Nawaz Z., O'Malley B. W. In situ distinction between steroid receptor binding and transactivation at a target gene. Mol Cell Biol. 1991 Sep;11(9):4350–4355. doi: 10.1128/mcb.11.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan J. A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993 Oct;101(5):386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek R. J. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994 Jun;49(2-3):153–160. doi: 10.1016/0960-0760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Moore M. R. Comment on 'Difference between R5020 and the anti progestin RU486 in antiproliferative effects on human breast cancer cells'. Breast Cancer Res Treat. 1988 Sep;12(1):81–82. doi: 10.1007/BF01805744. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Struck R. F., James R. Estrogenic activities of chlorinated hydrocarbons. J Toxicol Environ Health. 1978 Mar-May;4(2-3):325–339. doi: 10.1080/15287397809529664. [DOI] [PubMed] [Google Scholar]
- Nimrod A. C., Benson W. H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996 May;26(3):335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- Paris A., Dolo L., Debrauwer L., Rao D., Terqui M. Analysis of [3H]estradiol-17beta metabolites in calf perirenal fat. Analyst. 1994 Dec;119(12):2623–2626. doi: 10.1039/an9941902623. [DOI] [PubMed] [Google Scholar]
- Pham T. A., Hwung Y. P., McDonnell D. P., O'Malley B. W. Transactivation functions facilitate the disruption of chromatin structure by estrogen receptor derivatives in vivo. J Biol Chem. 1991 Sep 25;266(27):18179–18187. [PubMed] [Google Scholar]
- RUBIN B. L., DORFMAN A. S., BLACK L., DORFMAN R. I. Bioassay of estrogens using the mouse uterine response. Endocrinology. 1951 Oct;49(4):429–439. doi: 10.1210/endo-49-4-429. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C., Soto A. M., Fernandez M. F., Olea N., Olea-Serrano M. F., Ruiz-Lopez M. D. Development of a marker of estrogenic exposure in human serum. Clin Chem. 1995 Dec;41(12 Pt 2):1888–1895. [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. L., Watts C. K., Hall R. E., Ruenitz P. C. Mechanisms of growth inhibition by nonsteroidal antioestrogens in human breast cancer cells. J Steroid Biochem. 1987;27(4-6):891–897. doi: 10.1016/0022-4731(87)90165-8. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Rottinghaus G. E., Nonneman D. J., Dolan-Timpe M., Ross P. F. A sensitive bioassay for detection of dietary estrogens in animal feeds. J Vet Diagn Invest. 1990 Oct;2(4):268–273. doi: 10.1177/104063879000200403. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wild S. R., Harrad S. J., Jones K. C. The influence of sewage sludge applications to agricultural land on human exposure to polychlorinated dibenzo-p-dioxins (PCDDs) and -furans (PCDFs). Environ Pollut. 1994;83(3):357–369. doi: 10.1016/0269-7491(94)90158-9. [DOI] [PubMed] [Google Scholar]