Abstract
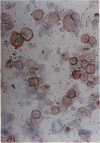
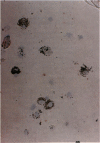
Intercellular adhesion molecule-1 (ICAM-1) is expressed on a variety of cells including endothelial cells, alveolar epithelial cells, and alveolar macrophages. Endothelial/epithelial cell ICAM-1 participates in the migration of leukocytes out of the blood in response to pulmonary inflammation, whereas alveolar macrophage ICAM-1 may represent cell activation. Our previous studies have shown that there is increased expression of ICAM-1 in lung tissue during acute inflammation following intratracheal injection with silica particles (2 mg/mouse). This increased expression was shown to play a role, in part, in the migration of neutrophils from the circulation into the tissue parenchyma. The aim of the current work is to localize expression of ICAM-1 during acute inflammation in lungs of mice exposed to either silica or the nuisance dust, titanium dioxide. In silica-exposed mice, a significant increase in ICAM-1 was detected on day-1 and localized by immunohistochemistry to aggregates of pulmonary macrophages and to type II epithelial cells. Areas of the lung with increased ICAM-1 expression also showed increased tumor necrosis factor alpha expression. Immunocytochemical staining of bronchoalveolar lavage (BAL) cells demonstrated increased ICAM-1 expression associated with alveolar macrophages 3, 5, and 7 days following silica exposure. Finally, soluble ICAM-1 levels in the BAL fluid were significantly increased in mice exposed to silica on the same days. Titanium dioxide exposure elicited a minimal increase in expression of ICAM-1 in the lungs. These data demonstrate that exposure to the toxic particle silica specifically increases ICAM-1 expression localized to pulmonary macrophages and type II epithelial cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Letourneau H. L., Bowden D. H. Comparison of alveolar and interstitial macrophages in fibroblast stimulation after silica and long or short asbestos. Lab Invest. 1991 Mar;64(3):339–344. [PubMed] [Google Scholar]
- Adamson I. Y., Prieditis H., Bowden D. H. Instillation of chemotactic factor to silica-injected lungs lowers interstitial particle content and reduces pulmonary fibrosis. Am J Pathol. 1992 Aug;141(2):319–326. [PMC free article] [PubMed] [Google Scholar]
- Barton R. W., Rothlein R., Ksiazek J., Kennedy C. The effect of anti-intercellular adhesion molecule-1 on phorbol-ester-induced rabbit lung inflammation. J Immunol. 1989 Aug 15;143(4):1278–1282. [PubMed] [Google Scholar]
- Brody A. R., Roe M. W., Evans J. N., Davis G. S. Deposition and translocation of inhaled silica in rats. Quantification of particle distribution, macrophage participation, and function. Lab Invest. 1982 Dec;47(6):533–542. [PubMed] [Google Scholar]
- Callis A. H., Sohnle P. G., Mandel G. S., Wiessner J., Mandel N. S. Kinetics of inflammatory and fibrotic pulmonary changes in a murine model of silicosis. J Lab Clin Med. 1985 May;105(5):547–553. [PubMed] [Google Scholar]
- Chanez P., Vignola A. M., Lacoste P., Michel F. B., Godard P., Bousquet J. Increased expression of adhesion molecules (ICAM-1 and LFA-1) on alveolar macrophages from asthmatic patients. Allergy. 1993 Nov;48(8):576–580. doi: 10.1111/j.1398-9995.1993.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Christensen P. J., Kim S., Simon R. H., Toews G. B., Paine R., 3rd Differentiation-related expression of ICAM-1 by rat alveolar epithelial cells. Am J Respir Cell Mol Biol. 1993 Jan;8(1):9–15. doi: 10.1165/ajrcmb/8.1.9. [DOI] [PubMed] [Google Scholar]
- Dalhoff K., Bohnet S., Braun J., Kreft B., Wiessmann K. J. Intercellular adhesion molecule 1 (ICAM-1) in the pathogenesis of mononuclear cell alveolitis in pulmonary sarcoidosis. Thorax. 1993 Nov;48(11):1140–1144. doi: 10.1136/thx.48.11.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll K. E., Lindenschmidt R. C., Maurer J. K., Higgins J. M., Ridder G. Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am J Respir Cell Mol Biol. 1990 Apr;2(4):381–390. doi: 10.1165/ajrcmb/2.4.381. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart S. G., Cartun R. W., Wyand D. S., Khairallah E. A., Cohen S. D. Immunohistochemical localization of acetaminophen in target tissues of the CD-1 mouse: correspondence of covalent binding with toxicity. Fundam Appl Toxicol. 1995 Feb;24(2):260–274. doi: 10.1006/faat.1995.1029. [DOI] [PubMed] [Google Scholar]
- Hubbard A. K., Vetrano K. M., Morris J. B., Thrall R. S. Acute NO2 exposure alters inflammatory cell activation and particle clearance in silica-injected mice. J Toxicol Environ Health. 1994 Mar;41(3):299–314. doi: 10.1080/15287399409531845. [DOI] [PubMed] [Google Scholar]
- Kang B. H., Crapo J. D., Wegner C. D., Letts L. G., Chang L. Y. Intercellular adhesion molecule-1 expression on the alveolar epithelium and its modification by hyperoxia. Am J Respir Cell Mol Biol. 1993 Oct;9(4):350–355. doi: 10.1165/ajrcmb/9.4.350. [DOI] [PubMed] [Google Scholar]
- Kang B. H., Manderschied B. D., Huang Y. C., Crapo J. D., Chang L. Y. Contrasting response of lung parenchymal cells to instilled TNF alpha and IFN gamma: the inducibility of specific cell ICAM-1 in vivo. Am J Respir Cell Mol Biol. 1996 Oct;15(4):540–550. doi: 10.1165/ajrcmb.15.4.8879188. [DOI] [PubMed] [Google Scholar]
- Kasper M., Koslowski R., Luther T., Schuh D., Müller M., Wenzel K. W. Immunohistochemical evidence for loss of ICAM-1 by alveolar epithelial cells in pulmonary fibrosis. Histochem Cell Biol. 1995 Nov;104(5):397–405. doi: 10.1007/BF01458134. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Kohno S., Morikawa N., Kadota J., Saito A., Hara K. Activation of macrophages and expansion of specific T lymphocytes in the lungs of mice intratracheally inoculated with Cryptococcus neoformans. Clin Exp Immunol. 1994 May;96(2):230–237. doi: 10.1111/j.1365-2249.1994.tb06547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. D., Sandberg E. T., Selvakumar A., Fang P., Beaudet A. L., Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J Immunol. 1995 Jun 1;154(11):6080–6093. [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Lesur O., Cantin A. M., Tanswell A. K., Melloni B., Beaulieu J. F., Bégin R. Silica exposure induces cytotoxicity and proliferative activity of type II pneumocytes. Exp Lung Res. 1992 Mar-Apr;18(2):173–190. doi: 10.3109/01902149209031679. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt R. C., Driscoll K. E., Perkins M. A., Higgins J. M., Maurer J. K., Belfiore K. A. The comparison of a fibrogenic and two nonfibrogenic dusts by bronchoalveolar lavage. Toxicol Appl Pharmacol. 1990 Feb;102(2):268–281. doi: 10.1016/0041-008x(90)90026-q. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Dame M. K., Lane C. L., Smith C. W., Anderson D. C., Ward P. A. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991 Oct;88(4):1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y., Munakata M., Ukita H., Takahashi T., Satoh A., Homma Y., Kawakami Y. Increased susceptibility to silicosis and TNF-alpha production in C57BL/6J mice. Am J Respir Crit Care Med. 1995 Dec;152(6 Pt 1):2144–2149. doi: 10.1164/ajrccm.152.6.8520788. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Sappino A. P., Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990 Mar 15;344(6263):245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Power C., Kobayashi K., Nishimura T., Yoshida T. CD11/CD18 and ICAM-1 expression in a murine foreign body granulomatous lung model. Clin Immunol Immunopathol. 1994 Dec;73(3):321–329. doi: 10.1006/clin.1994.1205. [DOI] [PubMed] [Google Scholar]
- Reiser K. M., Haschek W. M., Hesterberg T. W., Last J. A. Experimental silicosis. II. Long-term effects of intratracheally instilled quartz on collagen metabolism and morphologic characteristics of rat lungs. Am J Pathol. 1983 Jan;110(1):30–40. [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Kishimoto T. K., Mainolfi E. Cross-linking of ICAM-1 induces co-signaling of an oxidative burst from mononuclear leukocytes. J Immunol. 1994 Mar 1;152(5):2488–2495. [PubMed] [Google Scholar]
- Rothlein R., Mainolfi E. A., Czajkowski M., Marlin S. D. A form of circulating ICAM-1 in human serum. J Immunol. 1991 Dec 1;147(11):3788–3793. [PubMed] [Google Scholar]
- Schapira R. M., Ghio A. J., Effros R. M., Morrisey J., Almagro U. A., Dawson C. A., Hacker A. D. Hydroxyl radical production and lung injury in the rat following silica or titanium dioxide instillation in vivo. Am J Respir Cell Mol Biol. 1995 Feb;12(2):220–226. doi: 10.1165/ajrcmb.12.2.7865220. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S., Vallyathan V. ESR evidence for the hydroxyl radical formation in aqueous suspension of quartz particles and its possible significance to lipid peroxidation in silicosis. J Toxicol Environ Health. 1988;25(2):237–245. doi: 10.1080/15287398809531205. [DOI] [PubMed] [Google Scholar]
- Shijubo N., Imai K., Aoki S., Hirasawa M., Sugawara H., Koba H., Tsujisaki M., Sugiyama T., Hinoda Y., Yachi A. Circulating intercellular adhesion molecule-1 (ICAM-1) antigen in sera of patients with idiopathic pulmonary fibrosis. Clin Exp Immunol. 1992 Jul;89(1):58–62. doi: 10.1111/j.1365-2249.1992.tb06877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yu X. Y., Schofield B. H., Kleeberger S. R., Scott A. L., Hasegawa S., Spannhake E. W. Expression of ICAM-1 in airway epithelium after acute ozone exposure in the mouse. J Appl Physiol (1985) 1995 Nov;79(5):1753–1761. doi: 10.1152/jappl.1995.79.5.1753. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Mega J. F., Shi X., Dalal N. S. Enhanced generation of free radicals from phagocytes induced by mineral dusts. Am J Respir Cell Mol Biol. 1992 Apr;6(4):404–413. doi: 10.1165/ajrcmb/6.4.404. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Shi X. L., Dalal N. S., Irr W., Castranova V. Generation of free radicals from freshly fractured silica dust. Potential role in acute silica-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1213–1219. doi: 10.1164/ajrccm/138.5.1213. [DOI] [PubMed] [Google Scholar]
- Wegner C. D., Wolyniec W. W., LaPlante A. M., Marschman K., Lubbe K., Haynes N., Rothlein R., Letts L. G. Intercellular adhesion molecule-1 contributes to pulmonary oxygen toxicity in mice: role of leukocytes revised. Lung. 1992;170(5):267–279. doi: 10.1007/BF00566679. [DOI] [PubMed] [Google Scholar]
- Welty S. E., Rivera J. L., Elliston J. F., Smith C. V., Zeb T., Ballantyne C. M., Montgomery C. A., Hansen T. N. Increases in lung tissue expression of intercellular adhesion molecule-1 are associated with hyperoxic lung injury and inflammation in mice. Am J Respir Cell Mol Biol. 1993 Oct;9(4):393–400. doi: 10.1165/ajrcmb/9.4.393. [DOI] [PubMed] [Google Scholar]