Abstract
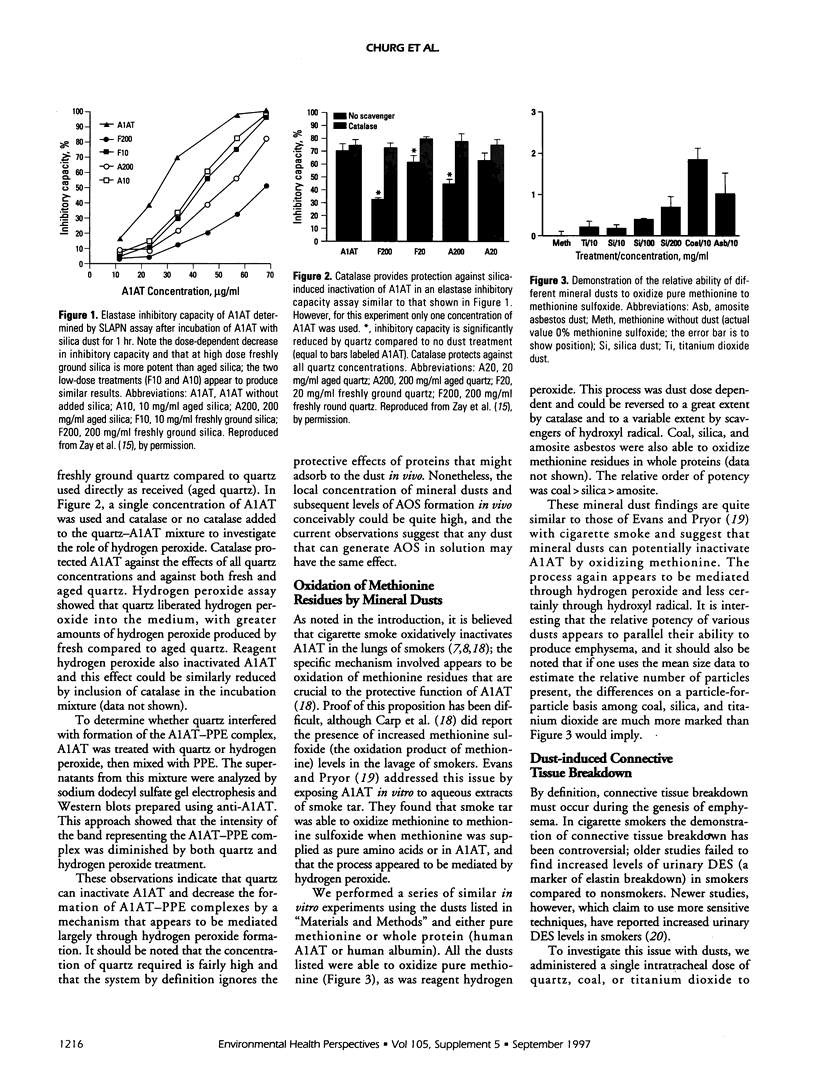
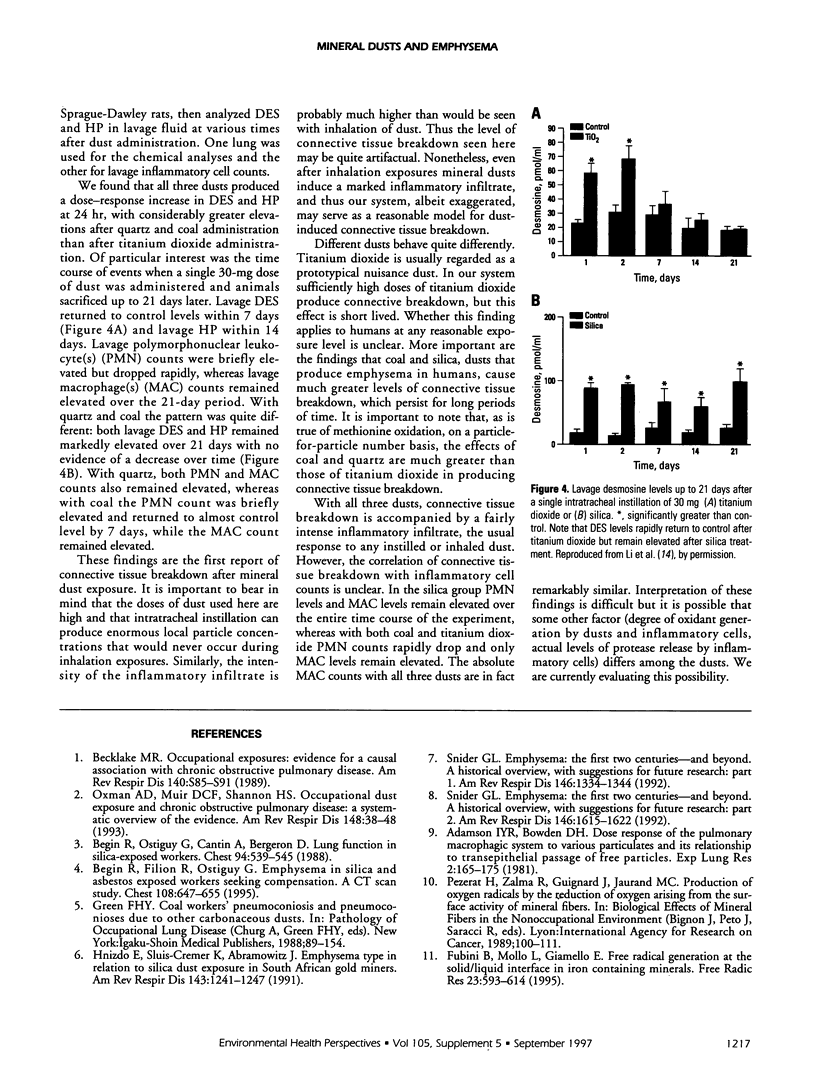
Mineral dust exposure can result in emphysema and chronic airflow obstruction. We postulated that dust-induced emphysema has a pathogenesis similar to that in cigarette smoke-induced emphysema, namely, excess release of proteolytic enzymes from dust-evoked inflammatory cells, and inactivation of alpha-1-antitrypsin (A1AT) by dust-catalyzed formation of oxidants. To test this theory we examined the antiproteolytic activity of A1AT exposed to quartz in vitro and found that it was decreased in a dose-response fashion. Catalase prevented this effect, which suggested that it was mediated by quartz-generated hydrogen peroxide. We also showed that a variety of dusts could oxidize methionine to methionine sulfoxide in vitro, using either pure amino acid or whole protein. The relative order of activity was coal > quartz > titanium dioxide. Lastly, we used a new high-performance liquid chromatography technique to demonstrate that quartz, coal, and titanium dioxide produced connective tissue breakdown in rat lungs, as determined by the appearance of desmosine and hydroxyproline in lavage fluid after dust instillation. On a particle-for-particle basis, the order of dust potency was similar to that for methionine oxidation. Connective tissue breakdown was associated with elevations of both polymorphonuclear leukocytes and macrophages in lavage fluid, and it is unclear whether one or both of these types of inflammatory cell mediates this process. These observations support our theory that dust-induced emphysema and smoke-induced emphysema occur through similar mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Dose response of the pulmonary macrophagic system to various particulates and its relationship to transepithelial passage of free particles. Exp Lung Res. 1981 Aug;2(3):165–175. doi: 10.3109/01902148109052312. [DOI] [PubMed] [Google Scholar]
- Becklake M. R. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989 Sep;140(3 Pt 2):S85–S91. doi: 10.1164/ajrccm/140.3_Pt_2.S85. [DOI] [PubMed] [Google Scholar]
- Brown G. M., Brown D. M., Slight J., Donaldson K. Persistent biological reactivity of quartz in the lung: raised protease burden compared with a non-pathogenic mineral dust and microbial particles. Br J Ind Med. 1991 Jan;48(1):61–69. doi: 10.1136/oem.48.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégin R., Filion R., Ostiguy G. Emphysema in silica- and asbestos-exposed workers seeking compensation. A CT scan study. Chest. 1995 Sep;108(3):647–655. doi: 10.1378/chest.108.3.647. [DOI] [PubMed] [Google Scholar]
- Bégin R., Ostiguy G., Cantin A., Bergeron D. Lung function in silica-exposed workers. A relationship to disease severity assessed by CT scan. Chest. 1988 Sep;94(3):539–545. doi: 10.1378/chest.94.3.539. [DOI] [PubMed] [Google Scholar]
- Carp H., Miller F., Hoidal J. R., Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2041–2045. doi: 10.1073/pnas.79.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. D., Pryor W. A. Damage to human alpha-1-proteinase inhibitor by aqueous cigarette tar extracts and the formation of methionine sulfoxide. Chem Res Toxicol. 1992 Sep-Oct;5(5):654–660. doi: 10.1021/tx00029a010. [DOI] [PubMed] [Google Scholar]
- Fubini B., Mollo L., Giamello E. Free radical generation at the solid/liquid interface in iron containing minerals. Free Radic Res. 1995 Dec;23(6):593–614. doi: 10.3109/10715769509065280. [DOI] [PubMed] [Google Scholar]
- Hannothiaux M. H., Scharfman A., Wastiaux A., Cornu L., van Brussel E., Lafitte J. J., Sebastien P., Roussel P. An attempt to evaluate lung aggression in monkey silicosis: hydrolases, peroxidase and antiproteases activities in serial bronchoalveolar lavages. Eur Respir J. 1991 Feb;4(2):191–204. [PubMed] [Google Scholar]
- Hnizdo E., Sluis-Cremer G. K., Abramowitz J. A. Emphysema type in relation to silica dust exposure in South African gold miners. Am Rev Respir Dis. 1991 Jun;143(6):1241–1247. doi: 10.1164/ajrccm/143.6.1241. [DOI] [PubMed] [Google Scholar]
- Li K., Keeling B., Churg A. Mineral dusts cause elastin and collagen breakdown in the rat lung: a potential mechanism of dust-induced emphysema. Am J Respir Crit Care Med. 1996 Feb;153(2):644–649. doi: 10.1164/ajrccm.153.2.8564112. [DOI] [PubMed] [Google Scholar]
- Oxman A. D., Muir D. C., Shannon H. S., Stock S. R., Hnizdo E., Lange H. J. Occupational dust exposure and chronic obstructive pulmonary disease. A systematic overview of the evidence. Am Rev Respir Dis. 1993 Jul;148(1):38–48. doi: 10.1164/ajrccm/148.1.38. [DOI] [PubMed] [Google Scholar]
- Rom W. N. Basic mechanisms leading to focal emphysema in coal workers' pneumoconiosis. Environ Res. 1990 Oct;53(1):16–28. doi: 10.1016/s0013-9351(05)80127-6. [DOI] [PubMed] [Google Scholar]
- Snider G. L. Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 1. Am Rev Respir Dis. 1992 Nov;146(5 Pt 1):1334–1344. doi: 10.1164/ajrccm/146.5_Pt_1.1334. [DOI] [PubMed] [Google Scholar]
- Snider G. L. Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 2. Am Rev Respir Dis. 1992 Dec;146(6):1615–1622. doi: 10.1164/ajrccm/146.6.1615. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Gottlieb D. J., O'Connor G. T., Ciccolella D. E., Breuer R., Bryan-Rhadfi J., Shaw H. A., Franzblau C., Snider G. L. Elastin and collagen degradation products in urine of smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995 Apr;151(4):952–959. doi: 10.1164/ajrccm.151.4.7697272. [DOI] [PubMed] [Google Scholar]
- Zay K., Devine D., Churg A. Quartz inactivates alpha 1-antiproteinase: possible role in mineral dust-induced emphysema. J Appl Physiol (1985) 1995 Jan;78(1):53–58. doi: 10.1152/jappl.1995.78.1.53. [DOI] [PubMed] [Google Scholar]


