Abstract
p53 is required for the induction of a G1 and/or G2 irreversible arrest after γ irradiation (IR), whereas blocked DNA replication causes a p53-independent S-phase arrest. We have examined the response to p53 when DNA synthesis is blocked by hydroxyurea (HU) or aphidicolin or when DNA is damaged by γ IR. Similarly to γ IR, blocked DNA synthesis induces high levels of phosphorylated nuclear p53. Surprisingly, several (but not all) p53 transcriptional targets that are rapidly induced by γ IR are weakly or not induced when DNA replication is blocked. Moreover, the p53 response to γ IR is inhibited by pretreatment of cells with HU or aphidicolin, suggesting that blocked DNA replication prevents p53 from being fully active as a transcription factor. HU-induced stabilization of p53 neither requires functional ATM (ataxia telangiectasia mutated), nor interferes with the γ IR-dependent activation of the ATM kinase. Thus, stalled replication forks activate kinases that modify and stabilize p53, yet act downstream of ATM to impair p53 transcriptional activity. The ramifications of this novel regulation of p53 are discussed.
The ability of cells to induce p53 in response to potentially mutagenic or oncogenic events has a crucial role in preventing malignant progression (1). In general, the induction of p53 is manifested by increased levels and activity of the protein through posttranscriptional mechanisms. Through its function as a transcriptional activator and repressor, a number of genes that control cell cycle, cell death, and other cellular functions are downstream targets of p53. Stress signals that can activate p53 are initiated by agents that generate DNA strand breaks, stalled DNA replication forks, ribonucleotide deprivation, hypoxia, and other forms of cellular trauma (2–4). Furthermore, p53 can be stabilized by viral (e.g., simian virus 40 large T antigen and adenovirus E1A protein) and cellular (e.g., ras and myc) oncogenes as a consequence of enhanced p19/14ARF activity (5).
Signals from these varied pathways all appear to converge on the inhibition of p53 by its negative regulator, Mdm-2, and the subsequent stabilization of p53 protein. In nonstressed cells p53 protein levels usually are maintained very low as a consequence of the interaction with Mdm-2 protein (6–11). After stress signals such as γ irradiation (IR), p53 is phosphorylated at a number of sites including S15, S20, and S33, and such phosphorylation disfavors its interaction with Mdm-2 (12–20). Additionally, there are multiple alternate mechanisms by which cells regulate the Mdm2–p53 feedback loop (3–5, 21–26).
Some transcriptional targets of p53 play crucial roles in both G1 and G2 checkpoints (e.g., see refs. 27–32). Less well characterized is the involvement of p53 in an S-phase checkpoint. In this regard, it has been reported that low levels of p53 that are incapable of triggering the accumulation of cells in G1 and G2 can induce a reversible S-phase arrest after ribonucleotide deprivation (33). Generally, however, when p53 is stabilized at higher levels, cells arrest irreversibly in G1 and/or G2 but not in S phase. Compounds such as hydroxyurea (HU), which inhibits the activity of ribonucleotide reductase (34), and aphidicolin (APH), which blocks DNA polymerase α (35), can cause a reversible late G1/early S-phase arrest independent of p53 (36). Interestingly, p53 levels do increase after treatment with these compounds, and it was suggested that one function of p53 in this scenario is to protect from uncoupling between completion of DNA synthesis and entry into mitosis (37). We have compared the downstream response to p53 in cells subjected to γ IR or inhibitors of DNA replication. Our results suggest that when DNA synthesis is blocked a process is triggered by which p53 is at least partially held in check.
Materials and Methods
Cell Lines.
RKO cells (human colorectal carcinoma; kindly provided by M. Kastan, St. Jude Children's Research Hospital, Memphis, TN) were maintained in DMEM with 10% FBS. HCT116 cells (human colorectal cancer) containing (+/+; clone 40.16) or lacking (−/−; clone 379.2) wild-type p53 (31) were generously provided by B. Vogelstein (Johns Hopkins, Baltimore) and were maintained in McCoy medium supplemented with 10% FCS. SAOS cells were maintained in DMEM and 10% FBS. GM02184D (normal) and GM01526E (ATM−/−) lymphoblasts (Coriell Institute, Camden, NJ) were grown in RPMI supplemented with 15% heat-inactivated FBS. For p53 induction, exponentially growing cells were treated either with γ IR (10 Gy for adherent cells; 3.5 Gy for suspension cultures) or with the following compounds: HU (1.5 mM) from Sigma), APH (5 μg/ml), N-acetyl-l-leucinyl-l-leucinyl-N-norleucinal (LLnL; 50 μM), and wortmannin (20 and 50 μM), all of which were from CalBiochem. For mitotic shake-off experiments cells were treated and collected as described (38).
Immunoblotting.
Cell were lysed in buffer containing 10 mM Tris (pH 7.5), 1 mM EDTA, 400 mM NaCL, 10% glycerol, 0.5% Nonidet P-40, and 5 mM NaF, 0.5 mM sodium orthovanadate, 1 mM DTT, 0.1 mM PMSF, and protease inhibitors. Monoclonal antibodies used for immunoblotting were: PAb 1801 vs. human p53; SMP14, 2A10, and 3F3 vs. MDM-2 (generously provided by A. Levine, Rockefeller University, New York); and WAF-1 vs. p21 (Calbiochem). A rabbit polyclonal vs. PIG 3 was kindly provided by D. Hill, Oncopene Research Products, Cambridge, MA. The p53 modification-specific antibodies directed against phospho-serine 15 (anti-P-S15), phospho-serine 46 (anti-P-S46), phospho-serine 392 (anti-p-S392), and acetyl-lysine 382 (anti-Ac-K382) were purified, characterized, and used as described (refs. 12 and 39; Y.T., unpublished work).
Northen Hybridization Blotting.
RNA was isolated by using Trizol reagent (GIBCO/BRL). 32P-dCTP-labeled hdm-2, p21, gadd45, PIG3, cyclin G, cyclin E, fos, or glyceraldehyde-3-phosphate dehydrogenase plasmid probes were used to assess specific mRNA levels.
Cell Cycle Analysis.
Cells were trypsinized, washed, and fixed with 5 ml of ice-cold methanol. For FACS analysis, fixed cells were suspended in PBS containing RNase I (50 μg/ml) and propidium iodide (25 μg/ml, Sigma). The stained cells were analyzed in a fluorescence-activated cell sorter (FACSCalibur, Becton Dickinson), and their cell cycle stage was analyzed by using the modfit lt program.
Immunofluorescence.
RKO cells, plated onto coverslips, were washed and then fixed in ice-cold methanol for 20 min at −20°C. After blocking, the coverslips were first incubated with PAb 1801 and then with anti-mouse-conjugated FITC antibody (1:100, Cappel) and PBS solution containing 1 mg/ml 4′,6-diamidino-2-phenylindole (Boehringer).
Results
After Release from a Block in DNA Synthesis Cells Progress Through the Cell Cycle in the Presence of High Levels of p53.
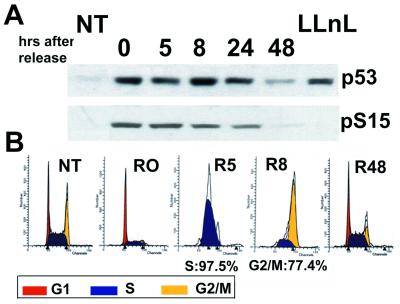
Treatment of cells with HU leads to rapid depletion of deoxyribnucleotide pools and results in the arrest of cells at the G1/S boundary or very early in S regardless of p53 status (36). To examine p53 under such conditions RKO cells expressing wild-type p53 were treated with and then released from HU (Fig. 1). As expected, treated cells accumulated in late G1/early S, and after release progressed rapidly although S and G2 (Fig. 1B). The levels of p53, which markedly increased within 6 h after of HU treatment (see Fig. 2A), remained high for at least 24 h after release. p53 protein also was phosphorylated at S15, a site modified after treatment with various DNA damaging agents (e.g., see refs. 12, 13, 15, 16, 20, and 25; Fig. 1A). Only by 48 h after removal of HU was their cell cycle profile similar to the untreated population, and levels of p53 were nearly reduced to those of untreated cells. Addition of APH to RKO cells led to similar kinetics of p53 accumulation, phosphorylation, and cell cycle profiles (data not shown). We also observed that the HCT116 (+/+) colorectal carcinoma cell line and a derivative (−/−) (31) showed virtually identical rates of progression through S to G2 after release from HU (data not shown). We thus conclude that after liberation from a DNA replication block cells re-enter the cell cycle rapidly in a p53-independent manner.
Figure 1.
Release from DNA replication block results in cell cycle progression in the presence of high p53 levels. (A) RKO cells were treated with HU (1.5 mM) for 17 h and then released by extensive washing. At the indicated time points cell extracts were prepared and analyzed by Western blotting using p53 antibody PAb 1801 (p53) or anti-p-S15 (pS15). As a control for pS15 specificity cells were treated with N-acetyl-l-leucinyl-l-leucinyl-N-norleucinal (LLnL) (50 μM) for 2 h. (B) RKO cells were treated as in A and at the indicated time points subjected to cell cycle analysis. NT, not treated; R, release.
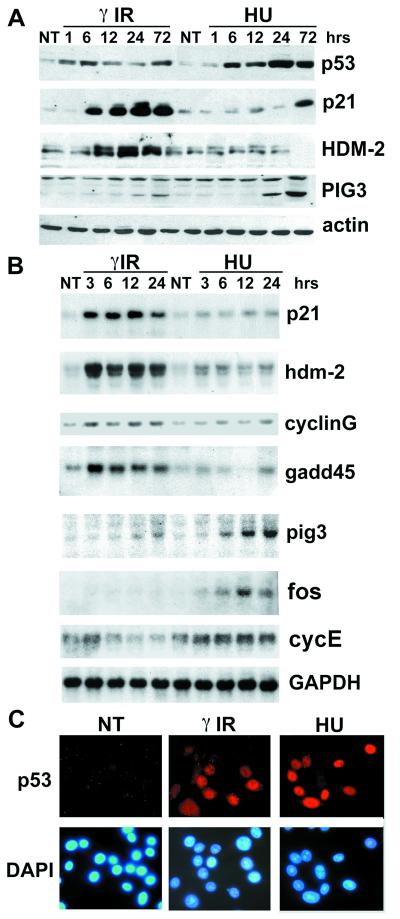
Figure 2.
Blocked DNA replication leads to a dysfunctional p53 response. (A) Exponentially growing RKO cells were γ-irradiated (10 Gy) or treated with HU (1.5 mM). Cells were lysed at the indicated time points, and total cell extracts were analyzed by Western blotting using the indicated antibodies. (B) RKO cells were treated as in A, and RNA samples were collected at the indicated time points. Northern blots were performed by using the indicated 32P-labeled plasmid probes. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) plasmid probe was used as normalizing control. (C) RKO cells were γ-irradiated (10 Gy) or treated with HU (1.5 mM) and fixed 24 h after treatment. Immunofluorescence staining was performed by using PAb 1801 (Upper). Cells were costained with 4′,6-diamidino-2-phenylindole (DAPI) to localize the nucleus (Lower). NT, not treated.
p53 Transcriptional Activity Is Impaired When DNA Replication Is Blocked.
Because p53 status and levels did not affect cell cycle progression after HU release, we examined some downstream targets of p53 in HU-treated cells. Strikingly, even though levels of p53 were higher than in γ IR-treated cells, we did not detect accumulation of p21 or Hdm-2 protein, both of which are commonly rapidly induced in response to increased levels of p53 (Fig. 2A). This is in line with a previous report investigating p53-dependent induction of p21 after APH and mimosine (ref. 40 and references therein). Similar results were obtained in HCT116 (+/+) cells (not shown). Northern blot analyses of these and other p53 target genes (cyclin G and GADD45) showed that the defect is at the transcriptional level, as RNA was either weakly or not induced after HU, in stark contrast to what was detected after γ IR (Fig. 2B). Similar results were obtained after treatment of cells with APH (see Fig. 3B). We do not believe that the impairment of p53 transcriptional activity is the result of a general shutdown of RNA synthesis when DNA replication is blocked. First, after 1-h pulse of 3H uridine to RKO cells there was no significant difference in the reduction in RNA synthesis after HU, APH, or γ IR treatments (in all cases the reduction was less than ≈80% of untreated cells). Second, HU treatment led to a significant increase in the expression of the transcription factor c-fos, as well as a modest increase in cyclin E mRNA levels (Fig. 2B). Therefore, we conclude that blockage of DNA replication does not cause a widespread inhibition of mRNA synthesis. When the subcellular localization of p53 protein was examined in RKO cells treated with HU (Fig. 2C) or APH (data not shown) it was extensively, if not exclusively, localized in the nucleus, thus eliminating nuclear exclusion as an explanation for the p53 defect in transactivation. Furthermore, PIG3, another p53 responsive gene, was induced markedly better by HU treatment than after γ IR. PIG3 also was induced in HCT116 (+/+) cells after HU, but not in the −/− derivative (data not shown), supporting the likelihood that PIG3 induction is p53-dependent. A similar experiment with HCT116 +/+ and −/− cells suggests that the delayed induction of p21 after treatment with HU (see Fig. 2A, 72 h) requires p53 as well (data not shown). We conclude that after treatment of a cell with compounds that block DNA synthesis, nuclear p53 is both impaired and altered in its transcriptional activity. Thus, even though both DNA damage and blocked DNA synthesis result in an accumulation of nuclear p53 there are striking differences in the p53 response to the two stress signals.
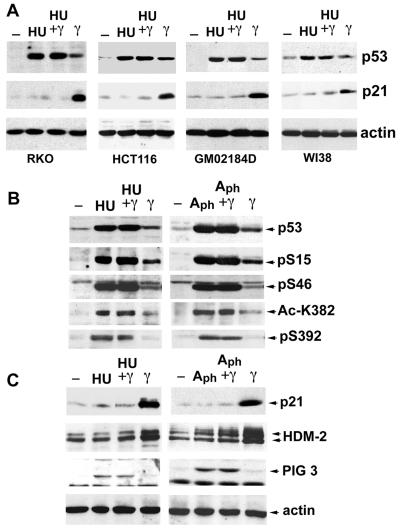
Figure 3.
DNA replication block actively represses the p53 activating pathway. (A) RKO, HCT116, GM02186, and WI38 cells were not treated (−) or exposed to HU treatment for 24 h (HU and HU + γ) or subjected to γ IR for 12 h (HU + γ and γ) before lysis, and subjected to Western blot analysis using p53, p21, and actin probes. (B) RKO cells were treated as in A or exposed to APH treatment (5 μg/ml) for 24 h (Aph and Aph + γ) or subjected to γ IR (Aph+ γ and γ) for 12 h before lysis. Extracts were subjected to Western blot analysis of p53 protein using Pab 1801(p53) and modification-specific antibodies as described in the text. (C) The same extracts as in B were used for Western blot analysis of the p53 downstream targets: p21, HDM-2, and PIG3. Actin was used as a loading control.
γ IR Does Not Rescue p53 from a DNA Replication Block.
A possible explanation for the defect in response to p53 after HU or APH treatment could be the lack of one or more critical modifications of the p53 protein (i.e., a defect in the upstream signaling pathway) or the lack of a cofactor required for p53 transcriptional activity (i.e., a defect in a p53-independent signal pathway). If one or both of these hypotheses were true, then p53 induced by HU and APH might be viewed as partially latent and should be liberated by treatments known to fully activate the protein (41, 42). Alternately, p53 may be held in a state of active repression in cells that have stalled DNA replication forks. This would render p53 irreversibly incapable of eliciting at least some of its downstream programs. To distinguish between these two hypotheses we irradiated cells that had been treated first with HU or APH and examined modifications of p53 and its downstream targets, p21 and Hdm-2.
To detect in vivo-induced modifications of p53, antibodies that have been validated to recognize specifically phosphorylated or acetylated residues were used (12, 16, 39, 43). No cases were found where a specific phosphorylation event occurred with γ IR but not after HU or APH. In fact, one modification, phosphorylation at S392, was more prevalent after HU. This finding is not likely to be relevant to the transcriptional defect in p53 because it has been reported that UV, but not IR, causes phosphorylation at this site, and UV treatment induces a fully active form of p53 (43, 44). HU treatment also led to phosphorylation of S20 in CEM cells (data not shown), whose high levels of mutant p53 permit recognition by the relatively weak anti-P-S20 antibody (16).
Importantly, there was no γ IR-induced rescue of the transcriptional defects induced by HU and APH (Fig. 3 B and C). Although γ-IR treatment of RKO cells resulted in p21 and HDM-2 up-regulation, irradiation of the same cells that had been previously treated with HU or APH did not result in any increase in the level of these proteins. Similar results were obtained with HU and/or γ IR-treated HCT116 and GM02184D cells, as well as WI38 cells that more closely resemble normal diploid cells (Fig. 3A). These data show that despite the presence of modifications on p53 required for its activation and stabilization treatment of cells with inhibitors of DNA replication results in repression of the p53-activating pathway.
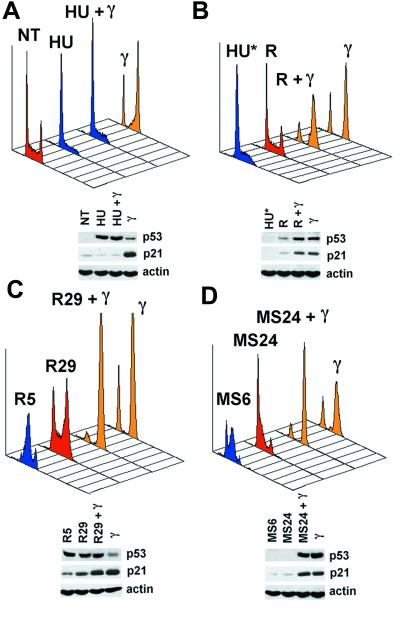
Consistent with the effects on p53 targets, cell-cycle analysis showed that cells treated with HU (Fig. 4A) and APH (data not shown), alone or in combination with γ IR, showed similar profiles, whereas γ IR alone resulted in the expected G1/G2 accumulation (Fig. 4A). This dominance of HU was observed even at very late time points (60 h; data not shown). Interestingly, different results were obtained when HU was washed out 5 h after cells have been irradiated (Fig. 4B, HU*). Here, removal of HU, even if performed several hours after IR, led to the acquisition of a typical γ IR-dependent cell cycle profile (compare R + γ and γ) as opposed to the distribution seen after the release of unirradiated cells that reassume normal growth (compare NT in Fig. 4A and R in Fig. 4B). This result shows that γ-irradiated HU-treated cells retain a signal that, even if unable to induce the expected p53 transcriptional activation in the presence of HU, is capable of triggering a full γ IR response whenever the repression is removed. This cellular “memory” indicates that arrest in S phase is required to block the response to γ IR. Further support for this comes from the following experiments. First, cells released from HU treatment were irradiated as they were passing through S phase (R5 in Fig. 4C). Although the released unirradiated population slowly reverted to a typical asynchronous distribution (R29) the irradiated population acquired a cell cycle profile similar to that induced by γ IR (compare R29 + γ and γ). Second, mitotic cells were collected, replated, and then irradiated while they were passing through S phase (MS6 in Fig. 4D). In contrast to the situation observed after release from DNA replication block where inactive p53 was already induced by HU, in this case p53 and p21 were induced by γ IR. Although the unirradiated populations continued cycling (MS24) the irradiated population acquired a cell cycle profile similar to the one resulting from irradiation of asynchronous cells (compare MS24 + γ and γ). From these experiments we conclude that cells passing through S phase can successfully activate p53-dependent transcription. On the other hand, irradiation of G1 or G2 arrested RKO cells results in the induction of p53 and p21 (data not shown). Taken together our data support the hypothesis that the repression of p53-dependent transcription is related to arrest specifically in S phase.
Figure 4.
Impairment of p53 transcriptional activity requires arrest in S phase. (A) RKO cells were treated as described in Fig. 3A and subjected to cell cycle analysis. NT, not treated. (B) HU-treated cells were γ-irradiated 12 h post-HU treatment. Five hours after γ-IR cells were released by extensive washing (HU* represents the profile at the moment of release for both irradiated and unirradiated cells). Forty eight hours later the released population (R, release) and the released γ-irradiated population (R + γ) were compared with irradiated asynchronous cells (γ). (C) Cells released from HU treatment were irradiated at the moment they were progressing through S phase (R5, S phase: 98.8%). Twenty four hours postirradiation the released unirradiated populations (R29) and the released γ-irradiated populations (R29 + γ) were compared with irradiated asynchronous cells (γ). (D) Cells progressing through S phase after replating of mitotic cells were irradiated (MS6, S phase: 79.8%). Twenty four hours postirradiation the released unirradiated (MS29) and the released γ-irradiated populations (MS24 + γ) were compared with irradiated asynchronous cells (γ). The levels of p53 and p21 are shown. Actin was used as a loading control. In each case the red profile represents asynchronous cycling cells, the blue profile represents cells mainly in S phase, and the yellow profile represents cells arrested with a typical γ IR distribution.
ATM Kinase Is Functional but Is Not Required for p53 Accumulation When DNA Synthesis Is Blocked.
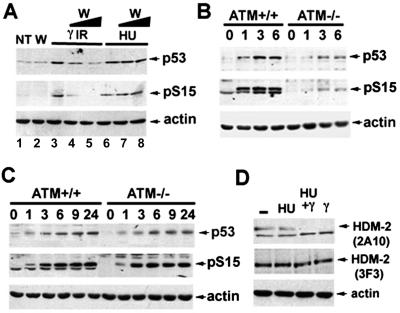
The ATM gene product is critical for p53 accumulation and arrest after γ IR, but is not required for the activation of p53 after other sources of DNA damage such as UV (13, 45–47). To gain insight into the role of ATM and other related kinases in the relative responses of p53 to DNA damage or blocked DNA synthesis we treated HCT116 cells with wortmannin, an inhibitor of phosphatidylinositol 3-kinase family members (ref. 48 and references therein). We observed that only after γ IR, but not after HU, treatment the levels of p53 and its phosphorylation at S15 were reduced in a dose-dependent manner (Fig. 5A). This finding suggests that kinases such as ATM, which are sensitive to this concentration of wortmannin, are not involved in phosphorylation of p53 at S15 after treatment with HU. To extend this observation normal lymphoblasts and ATM−/− lymphoblasts were compared. ATM−/− cells were defective in p53 accumulation and phosphorylation at S15 after γ IR, as shown (13, 46). By contrast, these cells displayed similar kinetics of p53 accumulation and S15 phosphorylation after HU treatment (Fig. 5C).
Figure 5.
HU neither requires functional ATM, nor interferes with the γ IR-dependent activation of ATM kinase. (A) HCT116 cells were pretreated with wortmannin in DMSO (W; lanes 4 and 7, 20 μM; lanes 2, 5 and 8, 50 μM) or DMSO (lanes 1, 3, and 6) for 2 h, then either exposed to γ IR (10 Gy) or HU (1.5 mM) for 6 h, and cell extracts were analyzed by Western blotting using either PAb 1801 (p53) or phospho-serine 15-specific antibodies (pS15). Actin was used as a loading control. NT, not treated. (B) Normal and AT−/− lymphoblasts were untreated (−) or treated with γ IR (3.5 Gy). Cells were harvested at the indicated hours after treatment, and lysates from each sample were analyzed as described in A. (C) Normal and AT−/− lymphoblasts were either untreated (−) or treated with HU (1.5 mM). Cells were harvested at the indicated hours and analyzed as in A. (D) SAOS-2 cells were grown without treatment (−) or exposed to HU treatment for 13 h (HU and HU + γ) or subjected to γ IR for 1 h (HU+ γ and γ). All samples were treated with MG132 proteosome inhibitor for 2 h before lysis to allow HDM-2 accumulation. Total cellular extracts were immunoblotted with the two indicated mAbs to HDM-2. Actin was used as a normalization control.
Although ATM was shown not to be required for p53 stabilization and phosphorylation after HU treatment, it was not determined whether ATM is still functional in cells treated with HU. Because p53 becomes phosphorylated at S15 after both HU and γ IR, we assessed the phosphorylation state of another target of ATM, HDM-2. It recently was reported that ATM phosphorylates HDM-2 in vitro and that ATM activation after γ IR correlates with a reduction of reactivity with the Mdm2-specific mAb 2A10 (22). We confirmed the loss of mAb 2A10 reactivity after γ IR and observed that HU treatment does not affect its reactivity, confirming that this stress signal does not activate ATM. However, when HU-pretreated cells were γ-irradiated the reactivity of mAb 2A10 was reduced (see Fig. 5D, 2A10). Furthermore, the change in electrophoretic mobility of HDM-2 reported for γ IR treatment (22) also was observed after the irradiation of HU-treated cells (see Fig. 5D, 3F3). These findings suggest that whatever the means by which HU results in repressed p53, down-regulation of ATM is not required.
Discussion
We have discovered that when DNA replication is blocked p53 becomes extensively modified and stabilized but is impaired in inducing many of its target genes. Furthermore, this transcriptionally compromised p53 is incapable of being reactivated by a normally potent inducer of active p53, namely γ IR. A number of questions are posed by these observations, which should point to future directions for investigation.
What Is the Nature of the Signaling Event That Triggers p53 Accumulation When DNA Synthesis Is Arrested?
Pathways initiated when DNA replication is stalled are not as well understood at present as those initiated after γ IR. However, with respect to the observations made herein, the source of the block itself is likely to be irrelevant because HU and APH inhibit DNA synthesis through different mechanisms. Strand breaks, considered the main conduit for activation of p53 after γ IR, are also common events when DNA replication forks are stalled (49, 50). It is not understood whether a stalled replication fork without any breaks can initiate the signaling cascade, or whether the nature of the breaks or structures that ensue when DNA synthesis stops is different from those induced by γ IR (e.g., single- vs. double-strand breaks). Whatever the initiating signal, residues S15, S20, S46, and K382 are modified both after γ IR and when DNA synthesis is inhibited. However, the pathways leading to such modifications could be very different: we (this study) and others (45) have found that accumulation of p53 after DNA synthesis block is ATM-independent, whereas it is well documented that γ IR stabilization of p53 requires functional ATM kinase. A number of alternate candidates such as the ATR kinase might be involved in phosphorylation of S15 when DNA replication is stalled (51). Moreover, studies in fission yeast and mammalian systems have reported that activation of Cds1/Chk 2 occurs when DNA synthesis is blocked by HU (52, 53), and that hChk2 can phosphorylate p53 at multiple sites including S15 and S20 (19, 54). Whatever the pathway(s) involved, because γ IR fails to rescue the effect of HU or APH, this finding suggests that modifications of p53 are not likely to be the primary cause of its transcriptional defect.
How Is p53 Transcriptionally Impaired When DNA Synthesis Is Blocked?
A number of p53 targets are very poorly activated even in the presence of high levels of correctly modified and localized p53 after treatment of cells with either HU or APH. The inability of γ IR to rescue this impairment suggests strongly that stalled DNA synthesis actively represses p53. This is not likely due to an overall reduction in cellular mRNA synthesis because c-fos and cyclin E mRNA were actually up-regulated by HU. Interestingly as well, we observed that the transcriptional activity of NF-κB is partially impaired after HU treatment, suggesting that blocked DNA replication may coordinately down-regulate the transcriptional activity of stress-responsive genes (data not shown). Moreover, the fact that PIG3 is actually more strongly induced in HU than in γ IR-treated cells is another line of evidence against a general failure to synthesize RNA. There are a number of possibilities to consider as to how p53 might be partially impaired. First, upstream events activated by blocked DNA replication could lead to a repressing modifaction(s) of p53. Second, inhibition of DNA synthesis could prevent one or more critical kinases from phosphorylating p53. In this regard, we showed that HU treatment does not affect ATM kinase activity. Because both p53 and Chk kinases are regulated by ATM, our results suggest that this upstream pathway is intact. Studies in yeast, however, have led to the proposal that HU-induced activation of Cds1 leads to inactivation of Chk1 (55). If this were the case in mammalian cells, and if p53 function requires both hChk kinases for its full activity, then this could explain our results. The possibility exists that hChk1 and hChk2 may differ somewhat in their overall ability to phosphorylate p53 (54). If Chk1 could uniquely phosphorylate a key residue(s) required for transactivation of p21, etc. by p53, but was down-regulated when hChk2 was activated in S phase, this might provide the combination of events that are necessary for a partial repression of p53. Third, inhibition of DNA synthesis may result in an event that selectively inactivates p53, which is downstream of the signaling/modification pathways. This could be the result of an interaction with a corepressor such as m-Sin 3A (56) or the result of an as-yet-unidentified p53-specific repressor.
Of the several p53 targets examined, only PIG3 was efficiently induced when cells were arrested in S phase. It was reported that transactivation of PIG3, but not p21 or Hdm2, by p53 requires its PXXP domain (57). This finding suggests that the PIG3 gene requires one or more different regulatory transcription cofactors that are not a target of signaling pathways initiated by stalled DNA replication. Several of the PIG genes were found to play a role in the metabolism of reactive oxygen species (ROS), and we might speculate that there is a need to deal with increased ROS when DNA synthesis is arrested. Whatever the mechanism of regulation of p53, this stimulus is novel in that it can both stabilize p53 and yet actively block the p53 transcriptional activating program that is induced by γ IR.
Why Is p53 Selectively Held in Check When DNA Replication Is Blocked?
Impairment of p53 transcriptional activity is an explanation for its inability to affect cell cycle redistribution after HU or APH treatment. It should be noted, however, that protective functions of p53 in S phase to prevent aberrant entrance into G2/M have been demonstrated by Taylor et al. (37). Whether or not this effect requires the full transcriptional repertoire of p53 was not reported. Nevertheless our data show that in several cell lines p53 appears to be dysfunctional when DNA synthesis is blocked. This observation leads to the question as to whether the repression of p53 is a normal consequence of its passing through S phase or rather requires that cells be actively stalled in this phase. We strongly favor the latter because the experiment shown in Fig. 4 demonstrates that both S phase and arrest must be combined to successfully repress γ IR signaling, and that the necessary and sufficient condition for releasing the repression of the γ IR pathway to p53 is the resumption of DNA replication. Taken together our data suggest that an essential component of the γ IR DNA damage-signaling pathway is retained in cells with incompletely synthesized DNA. However, this signal requires the reactivation of DNA synthesis to proceed toward a p53-dependent cell cycle arrest. Our results also provide cautionary implications for cancer treatment in that combination therapies may not always synergize and in some cases could result in noncooperative or even counteracting outcomes.
Perhaps the most elusive question is why do cells need to restrict p53 when DNA synthesis is stalled? We might consider the case of DNA tumor viruses such as adenovirus, which encodes products, E1A and E1B, that both stabilize and inactivate p53, respectively. It generally is assumed that such viruses need to disable the p53 apoptotic pathway for efficient infection to proceed (58). We might propose an analogous situation in which during the normal course of S phase there are likely to be strand breaks or stalled forks that can initiate the signaling events that stabilize p53. However, in S phase the E2F-1 protein is active and, when combined with a fully functional p53, is likely to induce cell death (59). To avoid a catastrophic response to what is likely to be a commonly occurring stall or break that would normally be repaired or resolved, the cell must disable its chief protector, the p53 tumor suppressor.
Acknowledgments
We are grateful for the expert technical assistance of Ella Freulich. We thank the members of the Prives laboratory for helpful suggestions. This work was supported by National Institutes of Health Grant CA 58316 to C.P.
Abbreviations
- IR
irradiation
- HU
hydroxyurea
- APH
aphidicolin
- ATM
ataxia telangiectasia mutated
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 781.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021282898.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021282898
References
- 1.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 2.Giaccia A J, Kastan M B. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 3.Prives C. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft M, Vousden K H. Oncogene. 1999;18:7637–7643. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- 5.Sherr C J. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 6.Haupt Y, Maya R, Kazaz A, Oren M. Nature (London) 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 7.Kubbutat M H, Jones S N, Vousden K H. Nature (London) 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 8.Honda R, Tanaka H, Yasuda H. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 9.Roth J, Dobbelstein M, Freedman D A, Shenk T, Levine A J. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lain S, Midgley C, Sparks A, Lane E B, Lane D P. Exp Cell Res. 1999;248:457–472. doi: 10.1006/excr.1999.4433. [DOI] [PubMed] [Google Scholar]
- 11.Tao W, Levine A J. Proc Natl Acad Sci USA. 1999;96:3077–3080. doi: 10.1073/pnas.96.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shieh S Y, Ikeda M, Taya Y, Prives C. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 13.Siliciano J D, Canman C E, Taya Y, Sakaguchi K, Appella E, Kastan M B. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi K, Herrera J E, Saito S, Miki T, Bustin M, Vassilev A, Anderson C W, Appella E. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chehab N H, Malikzay A, Stavridi E S, Halazonetis T D. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shieh S Y, Taya Y, Prives C. EMBO J. 1999;18:1815–1823. doi: 10.1093/emboj/18.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibbetts R S, Brumbaugh K M, Williams J M, Sarkaria J N, Cliby W A, Shieh S Y, Taya Y, Prives C, Abraham R T. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unger T, Juven-Gershon T, Moallem E, Berger M, Vogt Sionov R, Lozano G, Oren M, Haupt Y. EMBO J. 1999;18:1805–1814. doi: 10.1093/emboj/18.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chehab N H, Malikzay A, Appel M, Halazonetis T D. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor M, Hamm R, Yan W, Taya Y, Lozano G. Oncogene. 2000;19:358–364. doi: 10.1038/sj.onc.1203300. [DOI] [PubMed] [Google Scholar]
- 21.Kamijo T, Weber J D, Zambetti G, Zindy F, Roussel M F, Sherr C J. Proc Natl Acad Sci USA. 1998;95:8292–8297. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Proc Natl Acad Sci USA. 1999;96:14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stommel J M, Marchenko M D, Jimenez G S, Moll U M, Hope T J, Wahl G M. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber J D, Taylor L J, Roussel M F, Sherr C J, Bar-Sagi D. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft M, Taya Y, Vousden K H. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/mcb.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohrum M A, Ashcroft M, Kubbutat M H, Vousden K H. Nat Cell Biol. 2000;2:179–181. doi: 10.1038/35004057. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal M L, Agarwal A, Taylor W R, Stark G R. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 29.Waldman T, Kinzler K W, Vogelstein B. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 30.Hermeking H, Lengauer C, Polyak K, He T C, Zhang L, Thiagalingam S, Kinzler K W, Vogelstein B. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 31.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 32.Wang X W, Zhan Q, Coursen J D, Khan M A, Kontny H U, Yu L, Hollander M C, O'Connor P M, Fornace A J, Jr, Harris C C. Proc Natl Acad Sci USA. 1999;96:3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal M L, Agarwal A, Taylor W R, Chernova O, Sharma Y, Stark G R. Proc Natl Acad Sci USA. 1998;95:14775–14780. doi: 10.1073/pnas.95.25.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timson J. Mutat Res. 1975;32:115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- 35.Ikegami S, Taguchi T, Ohashi M, Oguro M, Nagano H, Mano Y. Nature (London) 1978;275:458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- 36.Linke S P, Clarkin K C, Di Leonardo A, Tsou A, Wahl G M. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- 37.Taylor W R, Agarwal M L, Agarwal A, Stacey D W, Stark G R. Oncogene. 1999;18:283–295. doi: 10.1038/sj.onc.1202516. [DOI] [PubMed] [Google Scholar]
- 38.Nagasawa H, Keng P, Maki C, Yu Y, Little J B. Cancer Res. 1998;58:2036–2041. [PubMed] [Google Scholar]
- 39.Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, Taya Y. Cell. 2000;102:849–862. doi: 10.1016/s0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 40.Ji C, Marnett L J, Pietenpol J A. Oncogene. 1997;15:2749–2753. doi: 10.1038/sj.onc.1201441. [DOI] [PubMed] [Google Scholar]
- 41.Lutzker S G, Levine A J. Nat Med. 1996;2:804–810. doi: 10.1038/nm0796-804. [DOI] [PubMed] [Google Scholar]
- 42.Chernov M V, Ramana C V, Adler V V, Stark G R. Proc Natl Acad Sci USA. 1998;95:2284–2289. doi: 10.1073/pnas.95.5.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu H, Taya Y, Ikeda M, Levine A J. Proc Natl Acad Sci USA. 1998;95:6399–6402. doi: 10.1073/pnas.95.11.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoor M, Lozano G. Proc Natl Acad Sci USA. 1998;95:2834–2837. doi: 10.1073/pnas.95.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khanna K K, Lavin M F. Oncogene. 1993;8:3307–3312. [PubMed] [Google Scholar]
- 46.Canman C E, Lim D S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siliciano J D. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 47.Banin S, Moyal L, Shieh S, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 48.Price B D, Youmell M B. Cancer Res. 1996;56:246–250. [PubMed] [Google Scholar]
- 49.Brox L, Hunting D, Belch A. Biochem Biophys Res Commun. 1984;120:959–963. doi: 10.1016/s0006-291x(84)80200-4. [DOI] [PubMed] [Google Scholar]
- 50.Li J C, Kaminskas E. Cancer Res. 1987;47:2755–2758. [PubMed] [Google Scholar]
- 51.Sarkaria J N, Tibbetts R S, Busby E C, Kennedy A P, Hill D E, Abraham R T. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- 52.Matsuoka S, Huang M, Elledge S J. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 53.Chaturvedi P, Eng W K, Zhu Y, Mattern M R, Mishra R, Hurle M R, Zhang X, Annan R S, Lu Q, Faucette L F, et al. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 54.Shieh S Y, Ahn J, Tamai K, Taya Y, Prives C. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 55.Brondello J M, Boddy M N, Furnari B, Russell P. Mol Cell Biol. 1999;19:4262–4269. doi: 10.1128/mcb.19.6.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy M, Ahn J, Walker K K, Hoffman W H, Evans R M, Levine A J, George D L. Genes Dev. 1999;13:2490–2501. doi: 10.1101/gad.13.19.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venot C, Maratrat M, Dureuil C, Conseiller E, Bracco L, Debussche L. EMBO J. 1998;17:4668–4679. doi: 10.1093/emboj/17.16.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debbas M, White E. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 59.Wu X, Levine A J. Proc Natl Acad Sci USA. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]