Abstract
Leprosy is best understood as two conjoined diseases. The first is a chronic mycobacterial infection that elicits an extraordinary range of cellular immune responses in humans. The second is a peripheral neuropathy that is initiated by the infection and the accompanying immunological events. The infection is curable but not preventable, and leprosy remains a major global health problem, especially in the developing world, publicity to the contrary notwithstanding. Mycobacterium leprae remains noncultivable, and for over a century leprosy has presented major challenges in the fields of microbiology, pathology, immunology, and genetics; it continues to do so today. This review focuses on recent advances in our understanding of M. leprae and the host response to it, especially concerning molecular identification of M. leprae, knowledge of its genome, transcriptome, and proteome, its mechanisms of microbial resistance, and recognition of strains by variable-number tandem repeat analysis. Advances in experimental models include studies in gene knockout mice and the development of molecular techniques to explore the armadillo model. In clinical studies, notable progress has been made concerning the immunology and immunopathology of leprosy, the genetics of human resistance, mechanisms of nerve injury, and chemotherapy. In nearly all of these areas, however, leprosy remains poorly understood compared to other major bacterial diseases.
INTRODUCTION
Leprosy is best understood as two conjoined diseases. The first is a chronic mycobacterial infection that elicits an extraordinary range of cellular immune responses in humans. The second is a peripheral neuropathy that is initiated by the infection and its accompanying immunologic events, but whose course and sequelae often extend many years beyond the cure of the infection and may have severely debilitating physical, social, and psychological consequences. Both aspects must be considered by clinicians, researchers, and policymakers who deal with persons affected by this disease.
Leprosy is not going to disappear anytime soon. Effective multidrug regimens are now used worldwide, and the infection in individuals is curable. However, although the reported number of registered cases worldwide has declined in the last two decades, the reported number of new cases registered each year has remained the same (at 500,000 to 700,000) over the same interval (42, 237). In some countries where leprosy is endemic the number of new cases actually appears to be increasing, while in others decreasing trends are reported. Great caution must be used in reaching conclusions from these observations, however, because they are based entirely on operational data which reflect the intensity of ongoing work more than the extent of any given problem (112). Mathematical modeling of the potential decline in leprosy incidence and prevalence, using various premises regarding efficacy of treatment and prevention, suggests that the disease will remain a major public health problem for at least several decades (259).
The precise mechanism of transmission of Mycobacterium leprae is unknown. No highly effective vaccine has yet been developed, and extensive laboratory efforts have not yet produced any practical tools for early diagnosis of clinically unapparent disease.
The full genome of M. leprae was among the first to be sequenced, and this new knowledge is beginning to bear fruit. Molecular microbiology has begun to explain, for example, M. leprae's fastidious nature and predilection for an intracellular lifestyle. Similarly, recent human genetic studies have been highly informative, indicating that immunity to M. leprae is controlled at two fundamental levels: first, genetic determinants of overall susceptibility and resistance to this organism have now been described, and second, a range of HLA-D-related immune responses have been demonstrated among individuals who are infected.
Only recently has the probable mechanism of intracellular killing of M. leprae been identified. The regulation of cell-mediated immunity to M. leprae by cellular and cytokine interactions continues to be unraveled. The major animal models available are the nine-banded armadillo and footpad infection of normal or immunologically crippled (nu−/−) mice. These models, however, are seriously flawed in their ability to recapitulate many aspects of the human disease and are exceptionally slow, difficult, and expensive to employ. Leprosy therefore remains a medical and scientific challenge of the first order, even though support for research on this disease has declined substantially as other conditions have assumed greater global priority.
A great deal of important new information has been generated by recent research. Brief, authoritative overviews on progress in leprosy have been published in recent years, notably those of Jacobson and Krahenbuhl (167) and Britton and Lockwood (42). Specialized reviews of narrower scope are cited in the appropriate sections below. Here, we have attempted to provide a critical summary of current knowledge from basic and clinical research, focusing particularly on developments from 1990 to the present.
Basic Clinical and Immunopathological Features of Leprosy
Leprosy presents a wide range of clinical and histopathological manifestations. This great diversity puzzled and frustrated clinicians and investigators until it was appreciated that this diversity was based on the ability of the host to develop a cellular immune response to M. leprae. The first full formulation of this concept was described by Skinsnes as an “immunopathological spectrum” in 1964 (384). Soon thereafter, a practical classification scheme based on the same principles was proposed by Ridley and Jopling (319), enabling a degree of global uniformity in clinical practice that gave renewed impetus to research on this disease. In the same decade, the discovery by immunologists of functionally and phenotypically distinct T- and B-lymphocyte subsets and their respective roles in cell-mediated and antibody-mediated immune responses revolutionized immunology. Scientists rapidly developed an entirely new set of tools and simultaneously discovered leprosy as a challenging human disease that appeared to be an ideal model with which to examine theories and methods related to cellular immunity in humans. The convergence of these and other factors prompted an extraordinary burst of research on leprosy during the last three decades of the 20th century (355).
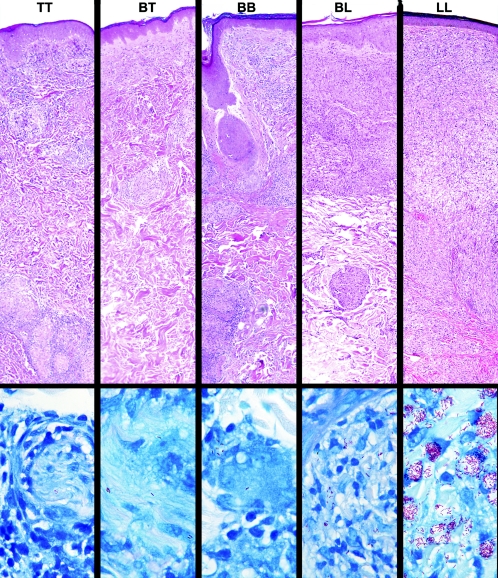
The five-part Ridley-Jopling classification identifies, at one extreme, patients with a high degree of cell-mediated immunity and delayed hypersensitivity, presenting with a single, well-demarcated lesion with central hypopigmentation and hypoesthesia. Biopsies of these reveal well-developed granulomatous inflammation and rare acid-fast bacilli demonstrable in the tissues; this is termed the polar tuberculoid (TT) (Fig. 1). At the other extreme, patients have no apparent resistance to M. leprae. These patients present with numerous, poorly demarcated, raised or nodular lesions on all parts of the body, biopsies of which reveal sheets of foamy macrophages in the dermis containing very large numbers of bacilli and microcolonies called globi. This nonresistant, highly infected form of the disease is termed polar lepromatous (LL). The majority of patients, however, fall into a broad borderline category between these two polar forms; this is subdivided into borderline lepromatous (BL), mid-borderline (BB), and borderline tuberculoid (BT).
FIG. 1.
Immunopathologic spectrum of leprosy. Representative fields from each of the histopathological types of leprosy in the Ridley-Jopling classification are presented in the upper panel, in hematoxylin- and eosin-stained sections (magnification, ×63). The well-formed epithelioid granulomatous infiltrates seen in polar tuberculoid (TT) lesions become increasingly disorganized in each successive increment in the scale until they become completely disorganized aggregates of foamy histiocytes, with only occasional lymphocytes, in polar lepromatous (LL) lesions. Representative fields of each classification are shown in Fite-stained sections in the lower panel (magnification, ×1,000). A search of more than 50 fields was required to find the two organisms shown in a cutaneous nerve in the TT sample, and organisms are often similarly difficult to find in BT lesions. This spectrum is the yardstick against which is measured each new hypothesis and discovery regarding immunological mechanisms proposed to be responsible for the wide range of human responses to M. leprae.
Very early lesions may present as relatively nonspecific perineural infiltrates in which rare acid-fast bacilli can be demonstrated, but without sufficient infiltrates to classify them; these are called indeterminate. This classification should be used only when the biopsy sample shows definite diagnostic evidence of leprosy (nerve involvement and acid-fast bacilli), since a diagnosis of leprosy may often have significant impact on a patient's family, employment, and psychological and social status.
In spite of nearly three decades of intensive research into the immunology of leprosy, the mechanism by which M. leprae is able to elicit the entire range of human cellular immune responses has still not been explained. Most clinical immunological inquiries have focused on the “immunologic defect” of lepromatous patients, i.e., their apparently specific anergy to M. leprae. The broad research efforts of recent years have, however, provided an increasingly detailed description of the immunological components in skin lesions across the leprosy spectrum, detailed below under Development of the Immune Response.
Lepromin Test
The lepromin test is often the cause of confusion and misplaced diagnostic expectations. The lepromin skin test is not diagnostic of leprosy or exposure to M. leprae. The test response is measured as induration (in mm) 4 weeks after injection and is ideally also evaluated by biopsy and histopathological examination of the test site. This test provides a measure of the individual's ability to mount a granulomatous response against the mixture of antigens present. Responses to lepromin are not leprosy specific; many individuals who have never been exposed to M. leprae will develop a positive lepromin reaction.
Leprosy bacilli, derived from different sources and subjected to different purification procedures, are the basis for different types of preparations used for intradermal skin testing (227). Themost frequently used preparation, and the one for which the response is best characterized, is Mitsuda lepromin. This is a suspension of whole, autoclaved leprosy bacilli (357) that is injected intradermally. Early studies used bacilli isolated directly from human lepromatous lesions, but armadillo-derived organisms have been used exclusively since the 1970s. In recent years, Mitsuda lepromin has been distributed for research applications by the World Health Organization. This skin test material is not approved by the Food and Drug Administration and is not recommended or provided for diagnostic use in the United States by the National Hansen's Disease Programs. Studies are under way to try to identify defined protein antigens that might be useful as diagnostic reagents (37, 94), but none of these has yet been determined to be satisfactorily sensitive or specific for this purpose.
Although the response to Mitsuda lepromin is not leprosy specific, a negative response is associated with lepromatous types of leprosy, i.e., with an inability to respond to M. leprae and to eliminate the bacilli. A positive lepromin test (at 4 weeks) is associated with the ability to develop a granulomatous response, involving antigen-presenting cells and CD4+ lymphocyte participation and, in leprosy patients, successful elimination of bacilli (121, 299).
Lepromin is probably the only widely studied skin test antigen that reflects the ability of an individual to generate a granulomatous response to mycobacterial antigens (as opposed to the 48- to 72-h delayed hypersensitivity response to tuberculin and other skin tests). For this reason, the possibility of genetic influences on lepromin responsiveness has been of interest to geneticists concerned with the inheritance of immunologic aspects of the granulomatous response (8, 30, 107).
Leprosy in Immunocompromised Individuals
Unlike tuberculosis, leprosy has not been observed to be more frequent in patients infected with human immunodeficiency virus (HIV) in regions where both diseases are endemic (162, 238, 303). It has been suggested that this may be due to the relatively low virulence of M. leprae or that HIV-infected individuals may die before leprosy (with its long incubation time) becomes clinically apparent (238). Nelson (286) has recently urged that investigators explore alternative explanations, however, since the apparent dissociation between the two diseases has continued even as the prevalence of AIDS has increased.
In contrast to all other experience with mycobacterial infections in HIV-positive individuals, coinfection with M. leprae and HIV appears to have minimal effect upon the course of either leprosy or HIV/AIDS. This is best illustrated by studies following cohorts of infected patients (162; reviewed in reference 221).
The occurrence of leprosy reactions in HIV-positive patients with leprosy has been the focus of several reports (18, 31, 34, 50, 230, 298, 300), but since leprosy reactions affect a high percentage of all leprosy patients (see Leprosy Reactions, below), it is not clear that they are actually more frequent or more severe in HIV-positive individuals. Studies of cell phenotypes and cytokines in leprosy lesions in patients with and without HIV infection have found no significant differences between the two groups with respect to these immunologic parameters (136, 298, 329). It is possible that the very slow growth of M. leprae allows the host immune response to keep pace with this infection to a much greater degree than is the case with M. tuberculosis, M. avium, and the other mycobacterial pathogens in AIDS. Notably, however, infection with M. leprae may elicit antibodies cross-reactive with HIV screening assays (13, 15, 205, 372).
Treatment of HIV infection with highly active antiretroviral therapy has resulted in the emergence of previously unsuspected leprosy in a small number of reported cases (77, 230) and notably, many of these individuals have also developed type 1 reactions. This suggests that infection with M. leprae may be more common than has been documented among HIV-positive individuals in leprosy-endemic regions of the world, but that early leprosy lesions are overlooked when these patients are confronted by the other major, life-threatening complications of AIDS.
Interestingly, immunosuppression with humanized monoclonal antibodies to tumor necrosis factor alpha (TNF-α) has resulted in the rapid development of lepromatous leprosy in at least two individuals who were being treated for severe arthritis (355a).These patients are presumed to have had minor, undetected leprosy lesions prior to the administration of immunosuppressive treatment. They responded promptly to antimicrobial treatment for M. leprae with a multidrug regimen (see Chemotherapy,below), but did develop type 1 reactions during their recovery, as noted above with AIDS patients receiving highly active antiretroviral therapy. Similarly, a small number of patients who have been immunosuppressed for renal or heart transplantation have developed leprosy (266; Scollard, unpublished observations), and these have also responded well to antileprosy treatment.
Together, the evidence indicates that broad therapeutic immunosuppression does render individuals highly susceptible to infection with M. leprae, but that in HIV-positive individuals a degree of host response to M. leprae is maintained, comparable to that of non-HIV-infected individuals, even as HIV infection progresses and circulating CD4+ cell numbers decline. The experience with highly active antiretroviral therapy suggests that in leprosy-endemic areas, subclinical and early clinical infection with M. leprae may be more prevalent among HIV-positive individuals than has been generally recognized. In addition, it appears that the restoration of immune function after highly active antiretroviral therapy may be associated with the development of type 1 reactions.
Laboratory Tests for the Diagnosis of Leprosy
The “gold standard” for the diagnosis of leprosy is a full-thickness skin biopsy sample obtained from the advancing margin of an active lesion, fixed in neutral buffered formalin, embedded in paraffin, and examined by an experienced pathologist. The primary characteristics to be recognized are histological patterns of the host response in hematoxylin- and eosin-stained sections (described above), the involvement of cutaneous nerves, and the identification of acid-fast bacilli within nerves using the Fite-Faraco modification of the carbol fuchsin stain (70). In tuberculoid lesions, where bacilli may be rare and difficult to find, the differential diagnosis of the granulomatous response commonly includes cutaneous tuberculosis, sarcoidosis, and granuloma annulare. At the other extreme, bacilli are easily demonstrated in the infiltrates of polar lepromatous leprosy, but care must be exercised to identify bacilli within nerves because, in immunosuppressed individuals, cutaneous infections with other mycobacteria can mimic the florid infection of lepromatous leprosy.
An ancillary procedure, the slit-skin smear, can be used for the semiquantitative enumeration of acid-fast organisms in infected skin and is useful in follow-up of patients during and after treatment. This technique is reliable only when performed and interpreted by experienced technicians.
No serologic tests are available for the routine laboratory diagnosis of Hansen's disease, and no laboratories in the United States perform such assays routinely. Enzyme-linked immunosorbent assays and related immunoassays have been developed to detect antibodies to phenolic glycolipid 1 (PGL-1) of M. leprae (46, 97), and these have been used in epidemiological studies. Although they have some value in population follow-up studies, none of these assays has a satisfactory degree of sensitivity and specificity for diagnostic application. The greatest drawback to the serologic diagnosis of leprosy arises from the fact that patients in whom the diagnosis is most difficult, TT and BT patients with moderate to high-grade cellular immunity to M. leprae and only small numbers of organisms, do not reproducibly produce detectable, specific circulating antibodies.
Similarly, no skin test that enables the diagnosis of Hansen's disease has been developed. Intradermal injection of heat-killed M. leprae (the lepromin test, discussed above) reflects the ability of an individual to develop a granulomatous response to this organism, but it does not reflect infection by or even exposure to M. leprae. It has been used in epidemiological studies, but it has no diagnostic utility in individual cases, and it is not available in the United States.
M. leprae is not cultivable in vitro (see Basic Characteristics, below), and lack of growth on standard mycobacterial isolation media can be regarded as one laboratory criterion differentiating this organism from other mycobacterial pathogens. The major advance in the laboratory diagnosis of Hansen's disease in the last 15 years, however, has been the development of methods for the extraction, amplification, and identification of M. leprae DNA in clinical specimens using PCR and other molecular techniques. This is an invaluable addition to laboratory diagnosis and to studies of the basic microbiology of this uncultivable organism, although it is costly and has not yet been approved or become available as a routine clinical test. A detailed discussion of PCR evaluation of specimens for M. leprae DNA is presented below (see Molecular Identification by PCR, below).
MYCOBACTERIUM LEPRAE, THE ETIOLOGIC AGENT OF LEPROSY
Basic Characteristics
Cellular morphology.
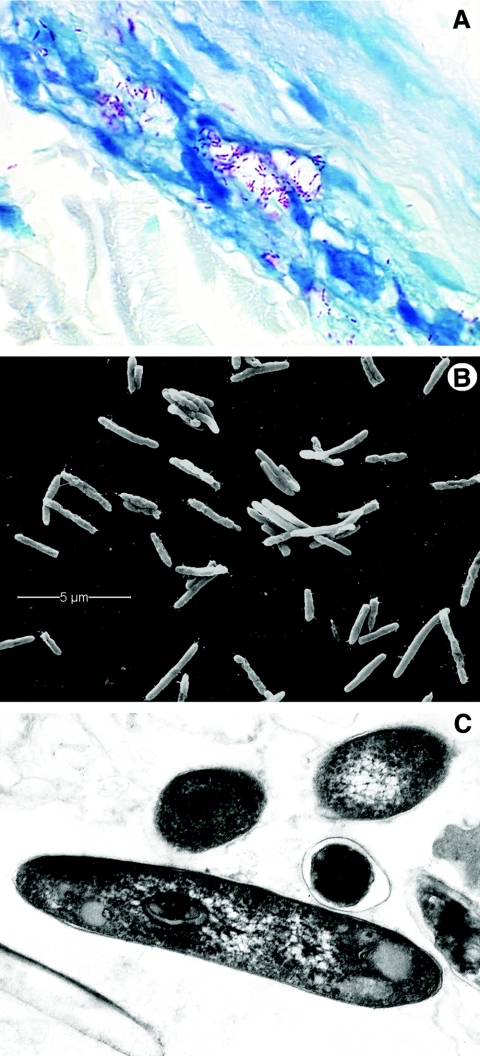
M. leprae is a nonmotile, non-spore-forming, microaerophilic, acid-fast-staining bacterium that usually forms slightly curved or straight rods (Fig. 2). A great deal has been learned about the nature of the mycobacterial cell wall through biochemical and genetic manipulation of cultivable strains such as M. tuberculosis, M. avium, M. smegmatis, and M. bovis BCG. Similar approaches with M. leprae have been meager by comparison, but basic chemical studies have concluded that the cell wall is a covalently linked peptidoglycan-arabinogalactan-mycolic acid complex similar in composition to all mycobacterial cell walls (79, 98, 425) (Fig. 3).
FIG. 2.
Morphology of M. leprae. A. M. leprae is weakly acid fast but, when stained with the Fite-Faraco method, it appears as red, rod-shaped organisms; shorter beaded or granular shapes are observed when the bacilli are dead or dying. The organisms are seen here within a human nerve, counterstained with methylene blue. Magnification, approximately ×800. B. A suspension of nude-mouse footpad-derived M. leprae under the scanning electron microscope, which reveals the surface of the organisms. M. leprae, like other mycobacteria, tends to cluster. Magnification, approximately ×12,000. C. Internal features of M. leprae are observed in this ultrathin section of the bacilli under a transmission electron microscope. The round and oval images seen in the upper portion of this photograph are bacilli that have been cut in cross section. Magnification, ×29,000.
FIG. 3.
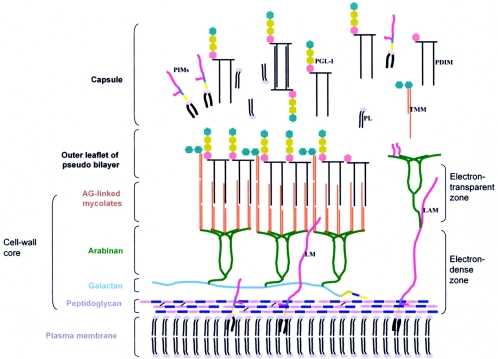
Schematic model of the cell envelope of M. leprae. The plasma membrane is covered by a cell wall core made of peptidoglycan covalently linked to the galactan by a linker unit of arabinogalactan. Three branched chains of arabinan are in turn linked to the galactan. Mycolic acids are linked to the termini of the arabinan chains to form the inner leaflet of a pseudolipid bilayer. An outer leaflet is formed by the mycolic acids of trehalose monomycolates (TMM) and mycocerosoic acids of phthiocerol dimycocerosates (PDIMs) and PGLs as shown. A capsule presumably composed largely of PGLs and other molecules such as PDIMs, phosphatidylinositol mannosides, and phospholipids surrounds the bacterium. Lipoglycans such as phosphatidylinositol mannosides, lipomannan (LM), and lipoarabinomannan (LAM), known to be anchored in the plasma membrane, are also found in the capsular layer as shown. (Reprinted from reference 425 with permission of the publisher.)
The cell wall core contains peptidoglycan, composed of chains of alternating N-acetylglucosamine and N-glycolylmuramate linked by peptide cross-bridges, which is linked to the galactan layer by arabinogalactan. Three branched chains of arabinan are in turn linked to the galactan, forming, along with the peptidoglycan layer, an electron-dense zone around M. leprae. Mycolic acids are linked to the termini of arabinan chains to form the inner leaflet of a pseudolipid bilayer. The outer leaflet is composed of a rich array of intercalating mycolic acids of trehalose monomycolates and mycoserosoic acids of phthiocerol dimycocerosates as well as phenolic glycolipids (PGLs), forming the electron-transparent zone. It has been postulated that many of these same molecules together with phosphatidylinositol mannosides and phospholipids are released from the cell wall after synthesis, forming a capsule-like region. The dominant lipid in the cell wall which gives M. leprae immunological specificity is PGL-1. Recent studies suggest that PGL-1 is involved in the interaction of M. leprae with the laminin of Schwann cells, suggesting a role for PGL-1 in peripheral nerve-bacillus interactions (288).
Annotation of M. leprae's genome and comparative genomic studies with other bacterial genomes have produced insight into the putative genes needed to direct the synthesis of this complex cell wall biopolymer (38). Most of the genes necessary to build the peptidoglycan-arabinogalactan-mycolate polymer appear to be present in the M. leprae genome and fit a reasonable strategy for its construction. A few exceptions are two genes involved in polyprenyl-phosphate synthesis (dxs-II and idi), a gene (fabH) involved in meromycolate synthesis, and a glycosyltransferase gene (pimB) involved in the biosynthesis of phosphatidylinositol, phosphatidylinositol mannosides, lipomannan, and lipoarabinomannan. It should be noted that much of this comparative work, while speculative, provides an important framework from which to investigate the authenticity of these putative pathways.
Growth.
M. leprae has never been grown on artificial media but can be maintained in axenic cultures in what appears to be a stable metabolic state for a few weeks (414). As a result, propagation of M. leprae has been restricted to animal models, including the armadillo (415) and normal, athymic, and gene knockout mice (222). These systems have provided the basic resources for genetic, metabolic, and antigenic studies of the bacillus. Growth of M. leprae in mouse footpads also provides a tool for assessing the viability of a preparation of bacteria and testing the drug susceptibility of clinical isolates (364, 414). The viability of M. leprae harvested from several different sources is now known to vary greatly, and many standard laboratory practices, such as incubation at 37°C, rapidly reduce the viability of this organism (414). However, M. leprae stored at 33°C in 7H12 medium has been shown to remain viable for weeks.
Metabolism.
The primary reasons for investigating the metabolic aspects of M. leprae have been to determine whether special media could be formulated to support in vitro growth of the bacilli and to learn more about metabolic pathways that could potentially be exploited for developing new antileprosy drugs. Early work provided a picture of a bacterium with some basic anabolic and catabolic pathways needed for survival in the host, but a thorough assessment of M. leprae's metabolic potential was still lacking. With the completed sequencing and annotation of M. leprae's genome, an improved understanding of M. leprae's metabolic capabilities now exists (43, 105, 432).
Annotation of the genome identified genes showing that M. leprae has the capacity to generate energy by oxidizing glucose to pyruvate through the Embden-Meyerhof-Parnas pathway, supporting earlier biochemical observations. Acetyl-coenzyme A from glycolysis enters the Krebs cycle, producing energy in the form of ATP. In addition to glycolysis for energy production, genome analysis as well as biochemical studies in M. leprae and M. tuberculosis suggest that these organisms rely heavily upon lipid degradation and the glyoxylate shunt for energy production. In this regard, M. leprae contains a full complement of genes for B oxidation but, compared to M. tuberculosis, very few genes capable of lipolysis. Acetate has been lost to M. leprae as a carbon source since only pseudogenes are present for acetate kinase, phosphate acetyltransferase, and acetyl-coenzyme A synthase.
Overall, M. leprae has many fewer enzymes involved in degradative pathways for carbon and nitrogenous compounds than M. tuberculosis. This is reflected in the paucity of oxidoreductases, oxygenases, and short-chain alcohol dehydrogenases and their probable regulatory genes. In addition, other major problems associated with metabolism for M. leprae are that the bacilli have lost anaerobic and microaerophilic electron transfer systems and that the aerobic respiratory chain is severely curtailed, making it impossible for M. leprae to generate ATP from the oxidation of NADH. In contrast to the reduction in catabolic pathways, the anabolic capabilities of M. leprae appear relatively unharmed. For example, complete pathways are predicted for synthesis of purines, pyrimidines, most amino acids, nucleosides, nucleotides, and most vitamins and cofactors. The maintenance of these anabolic systems suggests that the intracellular niche that M. leprae has found for itself may not contain these compounds or transport systems.
Genome, Transcriptome, and Proteome
Genome.
M. leprae that was originally purified from the skin lesions of a multibacillary leprosy patient from Tamil Nadu, India (TN strain), and subsequently expanded in and purified from the liver of a nine-banded armadillo provided the source of DNA for sequencing of the M. leprae genome (74). The genome sequence was generated by sequencing a combination of Lorist6 cosmid library inserts (103) and sixfold whole-genome shotgun sequencing of M. leprae insert DNA in pUC18 (74). Annotation of M. leprae's genome has revived interest in basic investigations of its metabolic, biochemical, and pathogenic potential.
Comparison of M. leprae's genome with that of its close relative M. tuberculosis (Table 1) suggests that M. leprae has undergone an extreme case of reductive evolution (reviewed in references 74 and 104). This is reflected by its smaller genome (3.3 Mb for M. leprae versus 4.4 Mb for M. tuberculosis) and a major reduction in G+C content (58% for M. leprae versus 66% for M. tuberculosis). M. leprae's annotated genome contains only 1,614 open reading frames potentially encoding functional proteins, compared to 3,993 open reading frames predicted in M. tuberculosis (Table 1).
TABLE 1.
Comparative genomics of M. leprae and M. tuberculosis
| Parameter | M. leprae (strain TN)a | M. tuberculosis (strain H37Rv)b |
|---|---|---|
| EMBL/GenBank/DDBJ accession no. | AL450380 | AL123456 |
| Genome size (bp) | 3,268,203 | 4,411,532 |
| No. of protein genes | 1,614 | 3,993 |
| No. of unknown genes | 142 | 606 |
| No. of pseudogenes | 1,133 | 6 |
| No. of tRNA genes | 45 | 45 |
| No. of rRNA genes | 3 | 3 |
| No. of stable RNA genes | 2 | 2 |
| Gene density (bases/gene) | 2,024 | 1,106 |
| Avg gene length (bases) | 1,007 | 1,008 |
| % Protein coding | 49.5 | 91.2 |
| % G+C | 57.8 | 65.6 |
| SNPc frequency | 1 in 24,000 bpd | 1 in 3,000 bpe |
Data obtained from the Current Data Release (17 October 2003) for the M. tuberculosis genome (http://genolist.pasteur.fr/TubercuList/).
Data obtained from the Current Data Release (20 July 2005) for the M. leprae genome (http://genolist.pasteur.fr/Leproma/).
SNP, single-nucleotide polymorphism.
Data obtained from reference 272.
Data obtained from reference 114.
One of the most striking features of M. leprae's genome is that it possesses 1,133 inactivated genes (genes lost through mutation, or pseudogenes), compared to six pseudogenes in M. tuberculosis (72). In addition, a large number of genes apparently have been entirely deleted from the genome. The result of this massive gene loss leaves M. leprae with less than 50% of its genome encoding functional genes, compared to M. tuberculosis, in which 90% of the genome encodes functional genes, and 34% of M. leprae's proteins identified in silico appear to be the products of gene duplication events or share common domains (105).
Downsizing of the genome has resulted in the elimination of several metabolic pathways, leaving a pathogen with very specific growth requirements, as discussed above (Metabolism). The largest functional groups of genes in M. leprae are those involved in gene regulation, metabolism and modification of fatty acids and polyketides, cell envelope synthesis, and transport of metabolites (74, 104, 105). Defense against toxic radicals is severely degenerative, as neither katG nor the narGHJI cluster is functional. In addition, several other genes involved with detoxification are pseudogenes or are missing from the genome. Redundancy as seen in the M. tuberculosis genome is often lost in M. leprae, as most paralogues seen in M. tuberculosis are pseudogenes in M. leprae. None of the additional 142 genes found only in M. leprae appear to be associated with metabolic pathways.
M. leprae appears to have a major deficiency in its ability to acquire iron from its environment. The entire mbt operon is deleted, rendering it unable to make either the membrane-associated or excreted form of mycobactin T. While genes known to be involved in iron acquisition are not obvious in its genome, there is little doubt that M. leprae utilizes iron. Genes are present for cytochrome c (ccsAB), a ferredoxin (fdxCD), biosynthesis of the heme group (hem genes), a hemogloblin-like oxygen carrier (glbO), the iron storage bacterioferritin bfrA, and ideR, the iron regulation protein dependent on intracellular iron (432). There are no intact polymorphic G+C-rich sequence- or major polymorphic tandem repeat-related repetitive sequences in the genome of M. leprae and only a limited number of proline-proline-glutamic acid and proline-glutamic acid proteins (72). Annotation of M. leprae's genome has thus provided insight into why the leprosy bacillus is an obligate intracellular parasite and has provided the basis for future experimentation to better understand its pathogenicity.
Transcriptome.
While comparative genome analysis provides useful clues to identify deficits in general cellular metabolic potential and cellular composition, it offers only a starting point from which functional studies can proceed. Because of our inability to cultivate M. leprae axenically, extremely limited quantities of bacterial proteins can be purified for analysis. Transcriptional analysis of M. leprae genes provides a perspective that is complementary to protein analysis by identifying actively transcribed genes, thereby expanding our knowledge of genes expressed during infection that might otherwise be missed when examining M. leprae's proteome.
With less than 50% coding capacity and 1,133 pseudogenes, those genes that are expressed help define the minimal gene set necessary for in vivo survival of this mycobacterial pathogen as well as genes potentially required for infection and pathogenesis as seen in leprosy. To identify genes transcribed during infection, gene transcripts from M. leprae growing in athymic nude mice have been surveyed using reverse transcription-PCR and cross-species DNA microarray technologies with an M. tuberculosis microarray (440). Transcripts were detected for 221 open reading frames, which include genes involved in DNA replication, cell division, SecA-dependent protein secretion, energy production, intermediary metabolism, and iron transport and storage and genes associated with virulence (see supplemental Table S1 at http://www.leprosy-ila.org/Mycobacterium.html).
These results support the view that M. leprae actively catabolizes fatty acids for energy, produces a wide array of secretory proteins, utilizes the limited array of sigma factors available, produces several proteins involved in iron transport, storage, and regulation (in the absence of recognizable genes encoding iron scavengers), and transcribes several genes associated with virulence in M. tuberculosis. Transcript levels of nine of these potential virulence genes (aceA, esat6, fbpA, relA, sigA, sigE, soda, aphC, and mce1A) were compared in M. leprae derived from the lesions of multibacillary leprosy patients and from infected nude mouse footpad tissue using quantitative real-time reverse transcription-PCR. Gene transcript levels were comparable in the two isolates for all but one of the genes studied (esat6), suggesting that profiling of M. leprae genes from animal models such as the nude mouse should be continued. Identifying genes associated with growth and survival during infection should lead to a more comprehensive understanding of M. leprae's ability to cause disease.
Proteome.
The functional complement of any genome is the proteome, which consists of the proteins expressed by a given organism under defined conditions. Understanding the basis of M. leprae's growth, virulence, and immunogenicity has always been the focus of proteomic discovery, with the goal of developing strategies for improved treatment and control of leprosy. Early work was driven by efforts to produce vaccines and diagnostic reagents. Prior to the publication of the full DNA sequence and annotation of the M. leprae genome in 2001 (74) protein analysis of M. leprae relied primarily on traditional subcellular fractionation of purified bacilli and genomic library screening using immunologic reagents. Of the two strategies, immunologic screening of genomic libraries proved more fruitful and expanded the number of purified and characterized proteins to over 40 (41, 131, 338, 407). Immunologic screening took advantage of the fact that serum and T cells from leprosy patients or healthy individuals previously sensitized to M. leprae were abundant, providing powerful probes for antigenic proteins of M. leprae expressed in Escherichia coli.
A major drawback to immunologic screening was that nonimmunogenic proteins were missed. Clark-Curtiss used an alternative genomic screening approach in which M. leprae genes were examined for expression in E. coli. This led to the discovery of a 46-kDa protein from M. leprae capable of complementing a citrate synthase mutant of E. coli (165) and the demonstration that M. leprae promoter activity was possible in E. coli (356). Unfortunately, while genetic complementation in E. coli had been very successful for identifying genes in other enteric bacteria, it appeared that problems associated with either M. leprae gene expression in E. coli or the relatively large evolutionary distance between the two bacteria limited further application of the approach.
Brennan and coworkers have combined subcellular fractionation of M. leprae with immunologic screening and chemical analysis of purified proteins using microsequencing and mass spectrometry to expand our understanding of the cellular location and function of many M. leprae proteins. Proteins have been identified from major cellular compartments, including cytosol, membrane, and cell wall, and a comprehensive list of these proteins and their characteristics was published earlier (248).
Much of this work has now been assimilated into a larger framework established from the completion of the M. leprae genome sequence. Annotation of the M. leprae genome indicates that approximately 1,600 open reading frames are scattered among a decaying genome, including approximately 1,100 pseudogenes, with gene remnants making up the remaining 23.5% of the genome. By comparative genomic analysis with M. tuberculosis and other known gene sequences, it appears that approximately 50% of M. leprae's open reading frames can be assigned putative functions. The other half of M. leprae's genes are considered to encode hypothetical proteins, with a small percentage designated unknown genes.
The power of this new portrait of M. leprae helps integrate what the bacterium can and cannot do. For example, bioinformatic analyses of metabolic pathways indicated that M. leprae has lost many metabolic pathways, together with their regulatory circuits, particularly those involved with catabolic potential (104). Aided by this metabolic picture, researchers can now investigate gene expression as it relates to various metabolic conditions either in limited culture with M. leprae or by studying gene function in cultivable mycobacteria into which specific mutations have been introduced. In this way it may be possible to determine the conditions that enhance or suppress M. leprae viability when it is held in axenic culture.
Another important element of establishing the proteome of M. leprae impacts earlier work involving antigenic analysis of M. leprae with the purpose of identifying proteins useful for diagnostics and vaccines. For example, a group of M. leprae proteins that have gone understudied until recently is secreted proteins. Purified bacilli from armadillo, mouse, and human tissues by definition lack (or are significantly reduced in their concentration of) secreted proteins. Bioinformatic approaches have identified the basic genes necessary for a functional SecA-dependent secretory system in M. leprae (74). In addition, Williams and coworkers (440) have shown that all genes in the SecA-dependent pathway are transcribed during growth of M. leprae in nude mouse footpads and that some 25 proteins with potential for secretion are transcribed (see Transcriptome, above). Therefore, M. leprae has the potential to produce secreted proteins, most of which have not been studied for immunogenic potential. A full assessment of these proteins may give rise to important antigens useful for developing much-needed early diagnostic tests as well as therapeutic and prophylactic vaccines.
Finally, by virtue of its relatively small gene set and possibly smaller proteome, M. leprae has become an important model for conceptualizing the minimal gene set needed for obligate intracellular parasitism. It is unlikely that all of M. leprae's open reading frames are expressed during all phases of growth and parasitism. Teasing apart these intricate relationships should provide important insights into mechanisms of virulence, such as nerve infection, as well as identifying proteins expressed during early infection, which could give rise to better diagnostic reagents and potentially a vaccine to improve our chances of managing leprosy.
Molecular Identification by PCR
Definitive identification of M. leprae is sometimes problematic, since the organism is not cultivable. This problem is confounded today by the increased prevalence of other mycobacterial infections of the skin. Rapid molecular-type assays have been developed for detection of M. leprae directly from patient specimens using available genetic data (reviewed in references 132, 208, and 348).
These assays have been based primarily on the amplification of M. leprae-specific sequences using PCR and identification of the M. leprae DNA fragment. This technique has been applied not only to skin biopsy samples but also to several different types of specimens, as indicated in Table 2. However, one should not infer from this that any tissue or specimen is suitable for PCR-based detection of M. leprae (see Table 3).
TABLE 2.
Application of PCR for the detection of M. leprae in human specimens
TABLE 3.
M. leprae genes used in the development of PCR assays
Many different M. leprae genes have been utilized in the development of PCR assays for detection of M. leprae in clinical specimens, as summarized in Table 4. RNA analysis using 16S rRNA and reverse transcription-PCR has the added benefit of measuring viability posttreatment (146, 228). PCR has thus generated new approaches to the detection and identification of M. leprae and, coupled with mutation detection analyses, has the ability to provide rapid drug susceptibility results from specimens taken directly from the patient.
TABLE 4.
Indications and suitable specimens for M. leprae PCR
| Category | Description |
|---|---|
| PCR indicated | Identification of acid-fast organisms when bacilli are numerous but tissue site, clinical history, or other circumstances are questionable; bacilli are sparse and tissue site, clinical history, or other circumstances are questionable |
| PCR not indicated | To find bacilli that are not identifiable in good-quality Fite-stained sections |
| Biopsies suitable | Specimens from newly diagnosed, untreated, or relapsed leprosy patients not yet retreated |
| Suitable specimens | Freshly acquired and processed immediately; frozen at −80°C indefinitely; frozen in over-the-counter cryopreservative at −80°C; fixed in 10% formalin for ≤24 h; fixed in 10% formalin for ≤24 h and paraffin embedded; fixed in 70% ethanol and stored at room temperature for up to 2 yr |
| Unsuitable specimens | Biopsy samples from treated leprosy patients; refrigerated specimens; unfixed (and unfrozen) specimens |
On the basis of extensive assessment of these tests in field studies, PCR-based and reverse transcription-PCR-based techniques have shown a specificity of 100% and a sensitivity ranging from 34 to 80% in patients with paucibacillary forms of the disease to greater than 90% in patients with multibacillary forms of the disease. Automation of PCR-based assays has allowed their implementation in many reference laboratories, chiefly in countries with endemic leprosy. Therefore, PCR can provide an excellent adjunct to clinical and histopathological diagnosis of leprosy.
Epidemiology and Strain Identification
Understanding the epidemiology of leprosy is a prerequisite for effective control of the disease. Since M. leprae cannot be cultured in vitro, it has been virtually impossible to assess exposure, onset of infection, and various aspects of disease progression. As a consequence, the sequence of events that must occur for successful transmission of leprosy is poorly understood. Genetic markers may hold the key to establishing species- and strain-specific markers for assessing exposure to M. leprae and tracing transmission patterns. These tools should be helpful for improving our understanding of the epidemiology of leprosy.
Over the last two decades, a wide range of molecular tests have been applied to reveal genotypic variation in M. leprae. The results of initial studies suggested that the genome of M. leprae was highly conserved. Restriction fragment length polymorphism analysis of M. leprae isolates using a combination of restriction enzymes and probes and sequencing of the internal transcribed spacer region of the 16S-23S rRNA operon yielded no polymorphic DNA sequences (69, 92, 436). A polymorphic structure in the polA gene (119) and variation in a GACATC repeat in the rpoT gene (252) have been described, but the value of these elements for differentiating possible M. leprae strains appears to be limited.
In completing the sequence of the M. leprae genome, Cole et al. (74) identified several tandem repeats that could prove useful for discriminating M. leprae strains. Recently, Shin et al. reported evidence for diversity among M. leprae isolates obtained from several patient biopsy samples in the Philippines, based on the frequency of TTC repeats located downstream of a putative sugar transporter pseudogene (371). In addition to the TTC locus, in silico analysis of the genome sequence indicates that M. leprae has several other tandem repeat loci which may provide the genetic diversity necessary for creating a typing scheme capable of answering important questions related to the epidemiology of leprosy. Recent studies employing four different variable-number tandem repeat markers have successfully differentiated M. leprae strains used in the laboratory and successfully grouped identical samples and passage samples tested in blind panels (412).
Far less diversity is seen with regard to single-nucleotide polymorphisms within the genome. The M. leprae single-nucleotide polymorphism frequency (∼1 per 28 kb) is among the lowest seen for a human pathogen, and only three informative single-nucleotide polymorphisms have been identified. Among the 64 possible permutations of different bases at each of these polymorphisms, only 4 are observed. The variation in single-nucleotide polymorphism genotype is highly correlated with the geographic origin of the strain, and analysis of strains from different continents has been useful in predicting the evolution and global spread of leprosy. The disease appears to have originated in eastern Africa or the Near East and spread with successive human immigrations (272). Europeans and North Africans appear to have introduced leprosy into West Africa and the Americas within the last 500 years.
EXPERIMENTAL MODELS OF LEPROSY
Overcoming Obstacles to Leprosy Research
Rees (315a) divided experimental leprosy research in animal models into two eras: 1874 to 1960, the dark ages, and post-1960, i.e., after the Shepard mouse footpad model. An exhaustive yet incomplete list of animal species tested as models for leprosy begins with rabbits infected by Hansen and includes dogs, cats, pigeons, chickens, paddy birds, canaries, parrots, lovebirds, eels, tadpoles, frogs, toads, pigs, turtles, snakes (including rattlesnakes), goldfish, rainbow perch, various saltwater fish, rats, black mice, white mice, “dancing” mice, chipmunks, golden hamsters, albino hamsters, gerbils, a variety of nonhuman primates, and guinea pigs (190). Experiments employed a confusing myriad of protocols with widely variant reports of “success,” but these were usually abortive or at best inconclusive and unconfirmed findings. No serial passage was reported and, in hindsight, because of the relative indestructibility of even dead M. leprae, the minimal successes reported could have been due to local lepromin-like responses.
One obstacle to the development of an animal model for leprosy has been the poor quality of the M. leprae inoculum, which usually consisted of fresh or frozen homogenates of nodules and lesions from untreated human lepromas. An important research emphasis in the National Hansen's Disease Program laboratories over the past few years has been the production, characterization, and provision of viable Mycobacterium leprae for our own researchers and qualified investigators around the world. A large (>200 mice) colony of M. leprae-infected athymic nu/nu mice is maintained for this purpose. A protocol of rigorously programmed passage of M. leprae, with radiorespirometry (the oxidation of 14C-labeled palmitic acid) as a measure of viability, has enabled the harvest, on a routine (weekly) schedule, of 4 to 6 billion bacilli that are 80 to 90% viable, a resource unprecedented in almost 130 years of leprosy research. With these organisms we have confirmed the preference of M. leprae for cooler temperatures (4°C for storage, 26°C to 33°C for metabolic activity) and the rapidly deleterious effects of incubation at 37°C or a single freezing-thawing cycle (414).
Whereas radiorespirometry measures the metabolic activity of a suspension of M. leprae, and this has been shown to be correlated with growth in the mouse footpad, a novel, two-color fluorescence viability staining assay (Molecular Probes BacLight bacterial viability kit) has recently been adapted to provide a rapid (∼1-h), reliable, quantitative, direct-count viability assay that measures the cell wall integrity of individual bacilli (229). This confirms previous findings regarding biophysical optima for maintaining M. leprae.
The second impediment in the early attempts to develop a leprosy model in animals was failure to recognize the prolonged growth cycle of M. leprae and not acknowledging its preference for cooler body sites. A new era was entered with description of the mouse footpad model by Shepard in 1960 (363). Passage of M. leprae infection was achieved, drug evaluation and rudimentary immunology studies became feasible, and the basis for subsequent exploration of M. leprae infection in various immunocompromised, transgenic, and knockout murine models was established.
Mouse Footpads and Nude Mice
Shepard's demonstration of the multiplication of M. leprae in the footpads of Carworth Farms white mice (363) opened new opportunities for investigation into basic immunological mechanisms of host resistance as well as screening of antileprosy drugs and drug combinations and detection of drug-resistant strains of M. leprae.
The importance of the T lymphocyte in host resistance was revealed in experimental M. leprae infection of neonatally thymectomized or congenitally athymic mice and rats (75, 208, 315). In immunocompetent mice, an inoculum of a few thousand bacilli grows locally and plateaus at approximately 1 million organisms per footpad. There is virtually no disease in these footpads, and the histopathological changes are minor, consisting of small granulomas containing a few lymphocytes and very few bacilli. Furthermore, there is essentially no dissemination of infection. In athymic nu/nu mice, however, local footpad multiplication of M. leprae appears to be unimpeded, reaching 1010 or more bacilli per footpad.
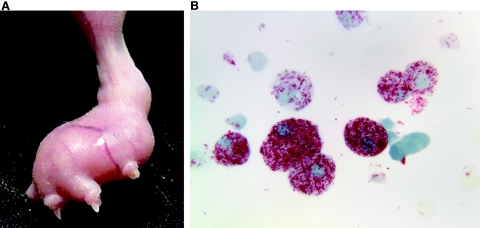
Histopathologically, the infected footpad tissue becomes an enormous foreign body-type macrophage (Mφ) granuloma, or leproma, and the cells are engorged with bacilli (61) (Fig. 4). Unlike the course in an immunologically intact mouse, some dissemination does occur, and if observed for a long enough interval, evidence of growth in the opposite hind footpad or forefeet is seen. Thus, development of this mouse model was a second major milestone in leprosy research for, in addition to its immunological significance, the athymic nu/nu mouse footpad allowed the routine culture of large numbers of highly viable M. leprae for experimental use (208, 229, 414) (see Basic Characteristics, above). More recently, other immunosuppressed strains of mice and rats have been reported to allow enhanced growth of M. leprae, including severe combined immunodeficiency mice, which lack both T and B cells (22).
FIG. 4.
Cultivationof M. leprae in mouse footpads. A. Enlarged nude-mouse footpad 6 months after infection with 5 × 107 live M. leprae. B. Heavily infected macrophages harvested from mouse footpad (magnification, ×1,000).
Gene Knockout Mice
The importance of the production of Th-1 cytokine responses in host resistance to intracellular infections, including M. tuberculosis and opportunistic mycobacteria, was confirmed with the recently identified human genetic deficiencies in cytokine receptors (420). Clinical evidence for an altered course of leprosy in such individuals has not been demonstrated, although this is most probably due to the protracted course of the disease and the relative avirulence of M. leprae. However, experimental M. leprae infections in cytokine knockout (KO) mice have substantiated the immunological importance of these cytokines across the leprosy spectrum and revealed compensatory mechanisms of host resistance to M. leprae.
An invaluable means for studying immunological parameters of host defense is via gene transfer technology in the murine system. Through a variety of mechanisms a specific gene can be rendered either totally or conditionally inactive (244). The resulting knockout models allow investigation at the level of the direct effects due to the loss of the functional gene as well as the compensatory mechanisms operational in its absence. Numerous targeted gene KO strains are now commercially available, including those unable to produce specific cytokines and chemokines, their receptors, immune modulators, or cell surface markers. In addition, new generations of mice, including tissue-specific KO, conditional KO, multiple KO, and knockin mutants, are continually being developed.
We have examined several strains of KO mice in our studies on M. leprae growth and granuloma development in experimental leprosy. Perhaps the best characterized is the inducible nitric oxide synthase (iNOS) KO (NOS2−/−) strain. Mφ isolated from NOS2−/− mice are incapable of producing reactive nitrogen products and they cannot inhibit M. leprae metabolic activity in vitro, although they are fully competent in producing reactive oxygen products (3). When inoculated into NOS2−/− mouse footpads, M. leprae initially grew to slightly higher levels than seen in wild-type controls, but thereafter the course of infection was similar. The granulomas formed in wild-type mice in response to M. leprae infection consisted of only small, focal collections of mononuclear cells; in contrast, the granulomas formed in NOS2−/− mice contained large, dense, organized collections of epithelioid cells and lymphocytes which infiltrated the perineurium and destroyed muscle bundles (6). In addition, Th-1-type cytokine expression was significantly augmented in NOS2−/− mice, and granulomas in liver tissue demonstrated a pattern similar to that seen in human leprosy lesions (268) with CD4+ T cells distributed throughout the lesion, surrounded by CD8+ T cells (D. A. Hagge et al., submitted for publication). Overall, the M. leprae-infected NOS2−/− mouse model exhibits findings similar to those of BT leprosy in humans. Interestingly, in contrast to NOS2−/− mice, macrophages from mice that are superoxide deficient due to a defective gp91 subunit of the phagocyte oxidase (phox91−/−) (93) efficiently kill M. leprae in vitro, and infected phox91−/− mice develop a footpad induration similar to that of wild-type mice (Adams, Scollard, and Krahenbuhl, unpublished).
Gamma interferon (IFN-γ) KO (IFN-γ−/−) mice also exhibited enlarged footpads and enhanced cellular infiltration upon infection with M. leprae (7). The footpad, however, contained epithelioid Mφ and scattered lymphocytes that were not assembled into organized granulomas. Growth of M. leprae was enhanced approximately 1 log over that in wild-type mice and a Th-2-type cytokine profile was generated. Overall, the general features exhibited by the IFN-γ−/− model were similar to those of BB-BL leprosy.
Studies in other KO mouse models are currently under way. Growth of M. leprae in mice deficient in interleukin-10 (IL-10) was similar to that seen in immunocompetent mice, whereas bacillary growth was augmented in mice deficient in IL-12, TNF-α, tumor necrosis factor receptor, lymphotoxin α, CD4, and CD8 (Adams, unpublished data). It is important to note, however, that M. leprae growth in these KO footpad models, including the IFN-γ−/− model, did not reach the enormous levels seen in the T-cell-deficient mouse models. Thus, even though these cytokines and cell types are important for the expression of cell-mediated immunity, compensatory mechanisms are able to limit bacillary growth. Immunoregulation in these mice is being studied further by additional “conditional” approaches, such as treatment with competitive inhibitors to create a second KO or restoration of the KO in infected mice. These immunoregulatory mechanisms may be crucial in the manifestation of the unstable borderline areas of the leprosy spectrum.
Nine-Banded Armadillo
Armadillos were originally adapted to captivity in order to study their unusual reproductive traits, which include both diapausic development and polyembrony (394). In 1968, Kirchheimer and Storrs began experimenting with armadillos to potentially exploit their cool body temperature (30 to 35°C) and showed that armadillos are uniquely susceptible to M. leprae (216, 217).
The nine-banded armadillo (Dasypus novemcintus) is the only immunologically intact animal that regularly develops fully disseminated M. leprae infections. Intravenous inoculation with 108 to 109 bacilli regularly results in 10,000-fold increases in the number of M. leprae over a span of about 18 months, and armadillos have been the hosts of choice for in vivo propagation of M. leprae for more than 30 years. With high burdens of bacilli in their reticuloendothelial tissues, armadillos can yield gram quantities of M. leprae (185, 413).
Approximately 65% of all armadillos experimentally inoculated with M. leprae will develop a fully disseminated infection. Infected armadillos exhibit few discernible clinical signs. Histopathological examination of infected animals typically reveals heavy infiltration of M. leprae-laden macrophages throughout the liver, spleen, and lymph nodes, as well as notable involvement of the lips, tongue, nose, nasal mucosa, skin, bone marrow, eyes, lungs, peripheral nervous system, gonads, and other tissues. Like humans, armadillos can upgrade and downgrade their response to M. leprae over the course of infection, but 90% of the animals that exhibit signs of systemic dissemination will eventually succumb to their leprosy (181). Intravenous inoculation promotes the most rapid and severe infections. However, respiratory instillation and percutaneous and intraperitoneal inoculation are also known to be effective (413).
Free-ranging armadillos are exposed to a number of atypical mycobacterial species in the environment and may develop nonspecific antibody responses cross-reactive with antigens shared by M. leprae (413). Armadillo immunoglobulin M (IgM) is highly cross-reactive with human IgM, and armadillo IgG reacts well with protein A or G.
The granulomatous response of armadillos to M. leprae is histopathologically identical to that seen in humans. Armadillo-derived lepromin (lepromin-A) can be used effectively to index the cell-mediated response of individual animals and to classify their type of leprosy according to the Ridley-Jopling scale. The reactions of individual animals can range from polar lepromatous to polar tuberculoid. Lepromatous armadillos develop the very large burdens of bacilli needed to obtain M. leprae from their tissues with high purity and are selected most commonly for propagation purposes (182-186), However, both lepromin-positive and lepromin-negative armadillos can resist challenge with M. leprae, and armadillos are useful models for studying both susceptibility and the variable resistance to leprosy that may result from treatment or vaccination (185, 187-189).
In 1973 Kirchheimer showed that 80% of the armadillos sensitized with heat-killed M. leprae could resist infectious challenge (213). Subsequent vaccination studies with BCG demonstrated effective protection of armadillos against M. leprae. The armadillo is the only animal model that can demonstrate effective protection against M. leprae with BCG, equivalent to rates observed for BCG in several human vaccination trials. Studies with these animals could potentially benefit our efforts to generate more efficacious antituberculosis and antileprosy vaccines (29, 188, 214, 215). Unfortunately, the number of armadillos and duration of time required (up to 1,140 days) for effective challenge studies with armadillos currently limit the utility of armadillos as vaccine models.
In addition to the armadillo's value as a source of organisms and an immunological model, the infection of peripheral nerves in the armadillo constitutes a unique model of lepromatous nerve involvement in humans (350, 351) (reviewed in Mechanisms of Nerve Injury, below).
Recently, the Human Genome Consortium completed the sequencing of the armadillo genome as part of a comparative genomics initiative. Armadillos are the most common modern representatives of Xenathra, a family of mammals that diverged from the rodent-primate tree in the Cretaceous period. The availability of extensive sequence information on the armadillo (http://www.ncbi.nlm.nih.gov/BLAST) is likely to rapidly expand the availability of new immunological probes and reagents for use with armadillos and will advance their use as models for resistance, vaccination, and nerve injury.
Wild nine-banded armadillos in the south central United States are highly susceptible natural hosts of Mycobacterium leprae.Surveys conducted over the last 30 years on more than 5,000 animals confirm that the infection is present among armadillos in Arkansas, Louisiana, Mississippi, and Texas. Little evidence for M. leprae infection is found among armadillos elsewhere in the U.S. range, and only a few reports relate finding the infection among animals in Central or South America. However, the issue has received only scant attention in other countries. Armadillos only recently expanded their range into the United States, and leprosy was present in Texas and Louisiana prior to the arrival of armadillos. The ecological relationship between humans and armadillos with M. leprae in this region remains unclear. However, infected armadillos constitute a large reservoir of M. leprae, and they may be a source of infection for some humans in this country, and perhaps in other locations across the animal's range (415).
The impact of armadillo leprosy on humans is difficult to measure. A number of anecdotal reports have associated handling armadillos with individuals' developing leprosy, and case-control studies have yielded conflicting results (45, 239). Among Louisiana residents developing leprosy, no association with armadillo contact was found (110), but among Mexican-born patients who presented in Los Angeles and who had lived in areas where they could have been exposed to armadillos, an increased likelihood for lepromatous-type leprosy was reported (408). Leprosy remains rare in the United States, and the degree of risk attributable to armadillos is quite low. Nonetheless, armadillos are a large natural reservoir and can be an effective vehicle for exposure to large numbers of M. leprae (112). However, understanding the actual impact of armadillos on human infection will likely require the evolution of better molecular techniques that can track transmission.
HOST RESPONSE TO M. LEPRAE
Genetic Influences on Leprosy in Humans
Before Hansen's discovery of the leprosy bacillus in 1874, leprosy was widely regarded as an inherited disease. Evidence from studies of twins with leprosy and of family clustering of cases continued to suggest that some inherited influence was a factor in susceptibility to this disease. An appreciation of the role of immunity in the different clinical and pathological manifestations of leprosy as well as subsequent advances in the field of immunology provided a foundation for focused inquiries concerning the genes that might influence susceptibility to M. leprae.
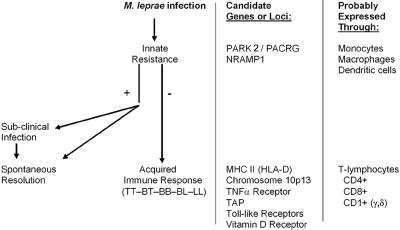
The idea that at least two different genes might control the human immune response to leprosy was proposed in the 1970s (89) and supported by subsequent investigations (1, 427). A convincing body of evidence now exists to indicate that different genes do influence the human immune response to M. leprae, operating at two levels (Fig. 5). The first level, overall susceptibility/resistance to the infection, is a manifestation of innate resistance mediated by cells of the monocyte lineage. If innate resistance is insufficient and infection becomes established, genetic influence is expressed at the second level, i.e., influencing the degree of specific cellular immunity and delayed hypersensitivity generated by the infected individual. Such acquired immunity is mediated primarily through the function of T lymphocytes, in cooperation with antigen-presenting cells (see Adaptive Immunity, below). The association of genes involved in both innate and acquired immunity to leprosy has recently been reviewed by Marquet and Schurr (249), and genetic defects in different components of the type 1 cytokine pathway that affect human resistance to mycobacteria have been reviewed by van de Vosse and colleagues (420).
FIG. 5.
Two-stage model of genetic influence on human immunity to M. leprae. Infection by M. leprae probably occurs through a skin or nasal route, by mechanisms that are not yet defined. The various genes and loci listed are discussed in the text under Genetic Influences, and the cells listed are discussed in the succeeding sections.
Genetic influences on innate resistance to M. leprae. (i) PARK2/PACRG.
One of the most extraordinary advances in the understanding of leprosy has been the identification by Mira and colleagues (262) of a locus within the gene PARK2/PACRG that is associated with overall susceptibility of human populations to M. leprae. This is the first example of the use of positional cloning to identify a human gene associated with susceptibility to an infectious disease (48). In their initial association scan of a linkage peak identified through a genome scan of a Vietnamese patient population, the investigators identified a locus within this gene that was highly associated with leprosy (leprosy per se), regardless of the subtype of this disease. These results were confirmed by a second analysis of Brazilian families with one or more persons affected by leprosy. The specific locus is a promoter region of PARK2 and a coregulated gene, PACRG, located on chromosome 6q25-q27. PARKIN was named for its association with an early-onset form of Parkinson's disease, and the finding that a locus within this gene is also associated with susceptibility to leprosy is very unexpected.
Functionally, the specific locus identified codes for the synthesis of a ligase in the ubiquitin-proteasome pathway of intracellular protein degradation (454). Recent work has revealed some mechanisms by which this pathway regulates the processing of protein antigens within macrophages, thereby affecting antigen presentation to lymphocytes and the resulting immune response (278, 429). The exact mechanism by which this gene influences overall susceptibility to leprosy, however, remains to be determined.
(ii) NRAMP1.
The first evidence of a genetic determinant of overall susceptibility or resistance that might relate to leprosy was the demonstration by Skamene and colleagues of a gene controlling susceptibility and resistance of mice to intracellular pathogens (383). Located at a single locus on mouse chromosome 1, this gene was initially designated BCG. Based on its function in mice it was termed natural resistance-associated macrophage protein 1 (NRAMP1) and is now designated SLC11A1. Functionally, murine NRAMP proteins influence pathogen viability and/or replication within macrophages by transporting iron and other divalent cations across the phagosomal membrane (139). Although the precise function of human NRAMP1 has not been definitely established, the human gene, located on chromosome 2q35, is highly homologous with the mouse gene.
An association of this gene with overall susceptibility to leprosy was first reported in a study of families with multiple cases of leprosy (2). Subsequent studies have also suggested that NRAMP1 may be associated with different leprosy types in some populations, possibly through its influence on the expression of major histocompatibility complex (MHC) class II molecules, regulation of expression of TNFA, and induction of nitric oxide synthase (33). The ability (or inability) of an individual to develop a granulomatous response to an intradermal injection of killed M. leprae (the Mitsuda skin test) has been linked to NRAMP1 in some studies (8, 108) but not in others (152), possibly due to the frequency of different polymorphisms in different populations or to methodological differences in the studies.
Genetic influences on acquired immune responses in leprosy. (i) HLA.
Early studies using serotyping techniques attempted to find associations between leprosy and human leukocyte antigens (HLAs) in major histocompatibility complex class II. These have been reviewed by Sergeantson (358) and Ottenhoff and de Vries (296). Overall, several of these serotyping studies suggested an association of HLA-DR2 and -DR3 with tuberculoid (paucibacillary) leprosy. Although some studies indicated an association of HLA-DR2 with both tuberculoid and lepromatous leprosy, no evidence convincingly demonstrated an association of the lepromatous response with any other HLA-D loci. Subsequent molecular genetic studies have borne out many of the early suggestions that the HLA region does play a determining role in the response to M. leprae. Advances in the technology of molecular genetics and in mathematical methods of interpretation of the data have extended these investigations far beyond the capabilities of the earlier techniques.
(ii) Chromosome 10p13.
A genomewide linkage scan of 244 families in southern India revealed significant linkage of a series of microsatellite markers on chromosome 10p13 with susceptibility to leprosy (377). Most of the patients in these families had tuberculoid (paucibacillary) leprosy, and it is not clear whether the loci that were identified are associated with overall susceptibility to leprosy or only with the tuberculoid type of leprosy.
(iii) TAP.
The transporter associated with antigen processing (TAP) is a protein composed of two polypeptides, TAP1 and TAP2. Their respective genes, located on chromosome 6p21, lie within the MHC class II region between HLA-DP and -DQ (388). Functionally, TAP proteins transport peptides to the endoplasmic reticulum in antigen-presenting cells, where they are joined to MHC class I molecules for antigen presentation. The TAP2 gene has been associated with tuberculoid leprosy (306), but because this gene is located so close to other HLA genes, interpretation of the results has been difficult and the significance of this finding is uncertain and awaits more detailed studies.
(iv) TNFA.
Tumor necrosis factor alpha, produced primarily by macrophages and causing activation of macrophages and T cells, plays a major role in nonspecific inflammation and innate resistance and is also one of the most powerful stimulants of cell-mediated immunity. In leprosy, TNF-α is generally associated with resistance to M. leprae. For example, serum levels of TNF-α are elevated in patients with resistant (tuberculoid) disease and with type 1 reactions, and expression of this cytokine is also increased locally in skin lesions in these manifestations of leprosy (see Leprosy Reactions, below).
The TNFA gene is located in the MHC class III region on chromosome 6p21. Several polymorphisms of this gene have been identified, especially in the promoter region. Because of the wide range of influence of TNF-α on cellular immunity, these promoter polymorphisms are of great interest as possible modulators of the degree of host response and therefore of clinical types of leprosy. Thus, several genetic studies have reported associations of TNFA alleles with different types of leprosy. In an Indian population, an association of one allele with lepromatous leprosy was observed (326); in a Brazilian population, another allele was associated with tuberculoid disease (335). The latter study also found this allele to be protective against leprosy per se, i.e., against the overall likelihood of acquiring leprosy of any type. Associations of some TNFA alleles with the strength of skin test responses to M. leprae (the Mitsuda test) have also been reported (108, 273). Together, the clinical, experimental, and genetic evidence suggests that the TNFA gene is involved in a complex manner in the regulation of human immune resistance to M. leprae.
(v) TLRs.
Colorfully named after their counterpart in Drosophila melanogaster, human Toll-like receptors (TLRs) are cell surface molecules that play an important role in the recognition of pathogens. Because activation of TLRs results in the release of several chemical mediators of immunity, TLR genes also exert an important influence on the early events in specific immune responses (154). Evidence from studies of leprosy patients indicates that TLR2 controls the production of cytokines, cell signaling, and other aspects of resistance to M. leprae (35, 195, 196, 223).
(vi) VDR.
After earlier studies suggested that polymorphisms of the human vitamin D receptor gene (VDR) were associated with susceptibility to tuberculosis (323), a study of leprosy patients indicated that different alleles of this gene were associated with tuberculoid and lepromatous leprosy (325). The VDR gene, located on chromosome 12q12, encodes an intracellular receptor protein which binds the active metabolite of vitamin D, 1α,25(OH)2D3. Binding to this receptor leads to the activation of monocytes and influences the function of both CD4+ and CD8+ T lymphocytes (153).
Development of the Immune Response
Innate immunity.
The host defense events that operate early in infection during the indeterminate phase are perhaps the least understood aspects of the immunology of leprosy. An effective innate immune response in combination with the low virulence of the leprosy bacillus may underlie resistance to the development of clinical disease.
(i) Antigen-presenting cells and dendritic cells.
Dendritic cells (DC) likely play a key role in modulating the early innate immune response to M. leprae (87). At the site of M. leprae invasion of the host, e.g., the nasal mucosa or skin abrasion, and in the absence of an adaptive immune response, the DC may be the first cell to encounter the bacilli. Uptake of the bacilli by DC and subsequent local production of cytokines and chemokines could regulate inflammation and manipulate the ensuing course of the adaptive cell-mediated immunity into a Th-1 or Th-2 response to M. leprae. DC have been found to be very effective presenters of M. leprae antigen (242, 265, 336). MHC class I and II expression was downregulated in monocyte-derived DC infected with M. leprae bacilli (151), but DC stimulated with M. leprae membrane antigens upregulated MHC class II and CD40 ligand-associated IL-12 production (242). This suggests that whole bacilli may suppress the interaction of DC and T cells.
DC infected with M. leprae expressed PGL-1 on the cell surface. PGL-1 has exhibited immunosuppressive properties, and masking of DC-expressed PGL-1 with specific antibody upregulated both the proliferative response and IFN-γ production by T cells stimulated with M. leprae-infected DC (151).
Both IL-12 and IL-10 are produced by DC, and IL-10 and anti-IL-12 have been reported to inhibit the lymphoproliferative response following presentation of M. leprae by DC (336). Macrophage-derived DC have been shown to be even more efficient antigen-presenting cells; furthermore, they were highly susceptible to killing by M. leprae membrane-specific CD8+ cytotoxic T cells (212). Higher levels of CD1+ DC are found in TT lesions than in LL lesions (379).
Langerhans cells are a subset of DC that initiate immune responses in the skin. LL patients have significantly fewer Langerhans cells in the skin, regardless of whether the biopsy sample was taken from healthy skin or a lesion, compared to uninfected controls or TT patients (133). In contrast, patients with TT lesions have increased numbers of Langerhans cells in the lesions, suggesting an active infiltration of these cells to these sites. Langerhans cells found in the epidermis of leprosy lesions coexpress high levels of CD1a and langerin (161), and M. leprae-reactive, CD1a-restricted T-cell clones derived from leprosy patients responded to antigen presented by Langerhans cell-like DC. The antigen presented was likely arabinomycolate, a glycolipid component of the mycobacterial cell wall. Administration of recombinant cytokines such as granulocyte-macrophage colony-stimulating factor (201) and IL-2 (199) into LL lesions has been shown to induce an infiltration of Langerhans cells into the sites.
Examination of leprosy biopsy samples has revealed that monocytes and dendritic cells in tuberculoid lesions expressed Toll-like receptors TLR1 and TLR2 much more strongly than those in lepromatous lesions (223). In addition, in vitro studies showed that the M. leprae 19-kDa and 33-kDa lipoproteins could activate monocytes and monocyte-derived dendritic cells through TLR2. The cytokine profile present in the lesion also appeared to be correlated with TLR function: Th-1-type cytokines were generally associated with TLR1 and TLR2 activation, and Th-2-type cytokines were associated with inhibition of activation. Interestingly, specific cytokines could regulate the TLR through two independent mechanisms in vitro, via modulation of TLR expression or by affecting TLR activation.
(ii) Pattern recognition receptors.
During the innate immune response, pathogen-associated molecular patterns displayed on many microorganisms are recognized by pattern recognition receptors expressed on immune cells at the sites of initial exposure. One class of pattern recognition receptor contains the calcium-dependent or C-type lectins, which bind specific carbohydrate moieties on pathogens and facilitate internalization for antigen processing and presentation. A second category of pattern recognition receptor is comprised of Toll-like receptors. Engagement of these receptors can trigger release of antimicrobial products which can exert a preliminary assault on the pathogen as well as signal expression of costimulatory molecules and production of cytokines which induce the adaptive immune system. A third class of receptor important for mycobacterial uptake contains the complement receptors.
(iii) C-type lectin receptors.
The mannose receptor (also called CD206), a receptor belonging to the C-type lectin superfamily, binds carbohydrate moieties on a variety of pathogens (9). It is expressed primarily on cells of the myeloid lineage, especially mature Mφ, although not on monocytes, and on some subsets of dendritic cells. The Mφ has been shown to play a role in uptake of virulent mycobacteria (342), and a major mycobacterial ligand is likely lipoarabinomannan (304) which, on virulent strains of M. tuberculosis as well as M. leprae, contains terminal mannose caps on the arabinose side chains of the molecule (38). Mannose-capped lipoarabinomannan can modulate several effector functions of mononuclear phagocytes, including TNF-α, prostaglandin E2, and nitrite production (5, 14, 24, 59), as well as Mφ activation for microbicidal capacity (5). It has also been reported that uptake of mycobacteria via the mannose receptor does not elicit a respiratory burst (20).
Another C-type lectin is the dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN; also called CD209). DC-SIGN is expressed on dendritic cells and also recognizes pathogens via the binding of mannose-containing structures (422). In studies using M. tuberculosis, DC-SIGN was shown to be the major receptor on DC for the bacilli, with complement receptors and the mannose receptor playing a minor role (400). Again, the primary mycobacterial ligand for DC-SIGN was mannose-capped lipoarabinomannan (220, 242, 400, 422). Some investigators have proposed that virulent mycobacteria may subvert DC function via DC-SIGN by suppressing DC maturation, possibly through the inhibition of IL-12 production (290) and the induction of IL-10 (125). Engagement of DC-SIGN may also inhibit TLR signaling (422).
Langerin (CD207) is a C-type pattern recognition receptor expressed by Langerhans cells. Langerin oligomerizes as trimers at the cell surface and possesses a single calcium-dependent carbohydrate recognition domain with specificity for mannose, N-acetylglucosamine, and fucose (219).
Langerin is necessary for the formation of Birbeck granules, the pentalaminar endosomal structures found exclusively in Langerhans cells. Exogenous carbohydrate ligands are endocytosed via langerin and transported to the Birbeck granules for processing. Langerin may play a role in the uptake of nonpeptide mycobacterial antigens (161).
(iv) Toll-like receptors.
Mammalian Toll-like receptors are crucial for the recognition of microbial pathogens by Mφ and dendritic cells during innate immunity. TLRs are phylogenetically conserved transmembrane proteins that contain repeated leucine-rich motifs in their extracellular domains. The cytoplasmic signaling domain is linked to the IL-1 receptor-associated kinase, which activates transcription factors such as NF-kB to induce cytokine production. Ten TLRs have been identified, of which TLR2-TLR1 heterodimers, TLR2 homodimers, and TLR4 appear to be significant in the recognition of mycobacteria. TLRs have been found to be necessary for the optimal production of IL-12 (39), a proinflammatory cytokine responsible for the induction of Th-1-type immunity, as well as TNF-α (417), a cytokine important in cellular activation and granuloma formation but also implicated in the tissue destruction associated with leprosy reactions.
Recent work has substantiated an important role for TLRs in the recognition and subsequent immune response to M. leprae, particularly through dendritic cells (discussed above). Kang and Chae (193) first noted the correlation of a C-to-T substitution at nucleotide 2029 of TLR2, which resulted in the change of Arg to Trp at amino acid residue 667, with the lepromatous form of leprosy. Subsequently, upon stimulation of cells expressing this mutation with M. leprae or M. leprae antigens, this mutation was shown to be associated with a defective activation of NF-κB (35) and decreased production of IL-12, IL-2, IFN-γ, and TNF-α but increased generation of IL-10 (195, 196) compared to wild-type cells.
(v) C′ receptors.
Schlesinger and Horwitz (343) established that complement receptors 1 and 3 on the surface of monocytes and CR1, CR3, and CR4 on Mφ are key mediators of phagocytosis of M. leprae. In addition, uptake of PGL-1, a major surface glycolipid of M. leprae, was facilitated by complement component C3 (343). Uptake via complement receptors does not elicit a respiratory burst (see below); thus, this is one mechanism whereby pathogenic mycobacteria can elude the toxic oxygen radicals which can be generated during phagocytosis.
Adaptive immunity: development of cell-mediated immunity.
Cells of the T-cell lineage play an essential role in resistance to M. leprae, as evidenced by the prolific local footpad multiplication of the bacilli in neonatally thymectomized (315) and congenitally athymic (75) mice. However, LL patients are not immunocompromised hosts and are not prone to cancer or the opportunistic infections that afflict persons with immunodeficiency diseases. The immunological anergy associated with LL is specific for the antigens of M. leprae.
(i) Protective and destructive effects of cell-mediated immunity.
It has been estimated that >95% of persons are resistant to leprosy. Upon exposure, protection probably occurs early, with no overt signs of disease. There is no test currently available to reliably detect exposure to M. leprae or to diagnose preclinical infection. Individuals with clinical leprosy, even those classified with paucibacillary disease and having high levels of cell-mediated immunity, possess living organisms in their tissues. The protective aspects of cell-mediated immunity in paucibacillary leprosy are largely defined as controlling the multiplication of organisms. The collateral damage to tissues caused by the granulomatous inflammation accompanying cell-mediated immunity may have serious, long-term consequences, such as injury to peripheral nerves.
(ii) T-lymphocyte populations.
(a) MHC-restricted CD4+ and CD8+ cells. By immunohistological staining, TT lesions exhibited mostly CD4+ helper cells with a CD4+/CD8+ ratio of 1.9:1 (269). Although the CD4+/CD8+ ratio in normal peripheral blood is also 2:1, there appeared to be a preferential migration into, proliferation in, or retention of selected cells in the various types of leprosy lesions in that cells of the T helper/memory phenotype outnumbered the naive phenotype 14-fold in TT lesions. T cytotoxic cells were numerous in TT lesions. These cells may play a role in mediating the Mφ localization, activation, and maturation that lead to restriction or elimination of the pathogen. Interestingly, CD4+ cells were distributed throughout the lesion, whereas CD8+ cells were stationed at the periphery in the TT lesion (268).
LL lesions, in contrast, displayed a CD4+/CD8+ ratio of 0.6:1, and unlike TT lesions, the CD8+ T cells were distributed throughout the lesion rather than at the periphery. Using monoclonal antibodies which could distinguish T-cell subsets, the authors found that the CD4+ cells present were primarily of a naïve phenotype and the CD8+ cells were predominantly of a suppressor subset; thus, they proposed that these CD8+ suppressor cells may serve to downregulate Mφ activation and suppress cell-mediated immunity. However, a role for the recently described Foxp3-expressing CD4+ CD25+ regulatory T cells (159) in the various forms of leprosy has yet to be determined but may prove critical in the development of LL.
(b) CD1-restricted T cells. CD1 molecules bind ligands via hydrophobic interactions in a structurally unique, deep antigen-binding pocket that is designed to accommodate the hydrocarbon chains of lipids. There are two distinct groups of CD1 molecules. Group I, comprised of CD1s a, b, c, and e, is found in human systems but not in rodents. Group II contains CD1d of humans and CD1 of rodents. All have evolved to present lipid and glycolipid antigens rather than peptides, and human CD1 molecules present nonpeptide components of mycobacteria to specific CD1-restricted T cells.
In vitro and in vivo studies have indicated an important role for the CD1 system of mycobacterial lipid antigen presentation in immunity to M. leprae. Mycobacterium-reactive double-negative T-cell lines derived from a skin lesion of a leprosy patient responded to subcellular fractions of mycobacteria in the presence of CD1-expressing antigen-presenting cells (378). Lipoarabinomannan-depleted soluble cell wall fraction did not induce detectable T-cell proliferation. Recognition of purified lipoarabinomannan from M. leprae was restricted by CD1b, and T cells lysed lipoarabinomannan-pulsed monocytes in a CD1b-restricted manner. Lipoarabinomannan also induced these T cells to secrete large amounts of IFN-γ. Upon examination of leprosy patients, few CD1+ cells were found in LL leprosy lesions. In contrast, there was a strong upregulation of CD1+ cells in the granulomatous lesions of patients with TT leprosy or reversal reaction (324). These cells were also CD83+, a marker for dendritic cells, indicating a strong correlation between CD1 expression and cell-mediated immunity in leprosy. Interestingly, administration of granulocyte-macrophage colony-stimulating factor, a cytokine which can promote dendritic cell activation, to LL leprosy patients induced infiltration of CD1+ cells into the lesions (332).
(iii) Cytotoxic cells.
(a) T cells. CD8+ and CD4+ T cells can function as class I- and class II-restricted cytotoxic T cells, respectively, and both are capable of lysing M. leprae-infected Mφ (65, 149, 192). Lysis of target cells by cytotoxic T lymphocytes is mediated by perforin and cytotoxic granules such as granzyme B, a serine protease located in cytotoxic T cells and NK cells (369). Upon contact with the target cell, perforin is released by cytotoxic T cells and forms pores in the target cell membrane, allowing granzyme B to enter the cell, where it activates caspases and leads to target cell death. Granulysin is another defensive antimicrobial protein used by T lymphocytes and is expressed in tuberculosis (393) and leprosy (293). In leprosy lesions the presence of granulysin correlated with the polar forms of the disease and was observed more frequently in TT skin lesions than in LL skin lesions. Perforin was equally distributed in cells across the spectrum. Neither NK cells, Mφ, nor dendritic cells expressed granulysin.
Lysis of M. leprae-infected Mφ target cells may contribute to protection in leprosy as an adjunct to the ongoing attempts at intracellular killing or inhibition by IFN-γ-activated Mφ. Ex vivo and in vitro data from mice have demonstrated that the long-term intracellular presence of live M. leprae impaired the afferent and efferent functions of the infected Mφ, especially their ability to become activated upon stimulation with IFN-γ (373, 375, 376). M. leprae released from these heavily infected Mφ after lysis by T cells would be phagocytized by newly arrived activated Mφ, which are more able to cope with the bacilli than the previous host cells, and subjected to another round of attack by powerful antimicrobial mechanisms.
(b) Natural killer cells. NK cells exert spontaneous non-MHC-restricted cytotoxicity against a variety of neoplastic and pathogen-bearing target cells, and although CD3−, they share many characteristics with cytotoxic CD3+ T cells. While NK cell numbers in the blood were similar across the leprosy spectrum, a marked decrease in circulating NK has been reported when patients were undergoing erythema nodosum leprosum (ENL) reactions (see below) (160). This situation reversed when the ENL reaction subsided. NK cells appear to be recruited to LL lesions injected with IL-2, where they may be responsible for the subsequent local clearance of the bacilli (199). The cytotoxicity of NK cells and their more active IL-2-stimulated lymphokine-activated killer (LAK) cell lacks antigen specificity but is directed against M. leprae-infected macrophages (66) and Schwann cells (392).
(iv) Macrophages.
The Mφ is the primary host cell for M. leprae. In the absence of an effective adaptive immune response, these relatively nontoxic bacilli can multiply in Mφ to over 100 organisms per cell (145). In vitro, M. leprae can be maintained in a metabolically active state for weeks in Mφ supplemented with IL-10 and cultured at 33°C (122). The Mφ also plays an important role in the host's defense against M. leprae, being a key player in both the afferent and efferent limbs of the immune response. Antigen processing and presentation and monokine secretion are three major functions of the Mφ in the afferent stage. The primary efferent function of Mφ is killing this intracellular pathogen.
TLRs have also recently been shown to be important in the differentiation of monocytes into macrophages capable of antimicrobial functions or dendritic cells having primarily antigen-presenting capabilities (224). Activation of TLR2, using mycobacterial antigen, on monocytes isolated from TT patients induced differentiation into both (DC-SIGN+) Mφ and CD1b+ dendritic cells. In contrast, when peripheral blood monocytes from LL patients were stimulated in such a manner, the cells differentiated into DC-SIGN+ Mφ but not CD1b+ dendritic cells. This pattern of expression was likewise observed in leprosy lesions. The implication of these findings is that upon initial M. leprae stimulation of monocytes via TLRs, both tuberculoid and lepromatous patients may generate similar innate responses to M. leprae, but lepromatous patients are unable to proceed to the adaptive response seen in tuberculoid patients. If confirmed and expanded, this may offer important insight into the mechanisms that underlie the broad spectrum of human immune response in this disease.
(a) Mechanisms of macrophage killing of M. leprae. Although the viability of M. leprae is supported in normal mouse Mφ, IFN-γ-activated Mφ can drastically inhibit or kill M. leprae in vitro (307). These findings confirmed the demonstration that in normal Mφ, phagosome-lysosome fusion was blocked by live, but not dead, M. leprae (373) and, more importantly, that in activated Mφ, phagosomes harboring M. leprae fused with secondary lysosomes. Two important antimicrobial pathways by which Mφ can inhibit or kill invading pathogens are the generation of reactive oxygen intermediates and of reactive nitrogen intermediates.
Upon phagocytosis of microorganisms by Mφ, a respiratory burst ensues in which there is a great increase in the consumption of oxygen catalyzed by NADPH oxidase and the production of superoxide. Other reactive oxygen intermediates, including hydrogen peroxide, hydroxyl radical, and singlet oxygen, are subsequently generated. These toxic oxygen products are an important antimicrobial defense mechanism of phagocyte cells, especially against extracellular pathogens. M. leprae, however, is only a weak stimulus of the Mφ oxidative burst (155), possibly due to a downregulation of superoxide generation by PGL-1 (58). M. leprae also possesses a superoxide dismutase (406) and expresses both SodC and SodA by reverse transcription-PCR (440). Thus, leprosy bacilli appear to be well equipped to handle antimicrobial reactive oxygen intermediates generated by the host Mφ.
Reactive nitrogen intermediates, primarily nitric oxide, are derived from the terminal guanidino nitrogen of l-arginine by a high-output, inducible form of nitric oxide synthase (iNOS) produced by activated Mφ. The ability of activated murine Mφ to inhibit M. leprae metabolic activity is dependent on the generation of such reactive nitrogen intermediates, as activated Mφ cultured in the presence of competitive inhibitors of the enzyme, such as l-monomethylarginine or aminoguanidine, had no detrimental effect on bacterial metabolism (4). Furthermore, activated Mφ from NOS2 knockout mice could not kill M. leprae (6).
The role of reactive nitrogen intermediates as a Mφ effector mechanism in humans is somewhat controversial as it has been difficult to get in vitro-cultured cells to generate high levels of nitrite in as consistent a manner as in murine cells (430). However, several studies have shown that iNOS is expressed, as detected by immunohistochemistry, at the site of disease in patients infected with intracellular pathogens, including M. leprae. Khanolkar-Young et al. (210), using antibodies against iNOS, found that iNOS was highly expressed in the lesions of tuberculoid leprosy patients and was increased to even higher levels during the reversal reaction (see below). Moreover, the levels of iNOS subsided over the course of prednisolone treatment (233).
Because detection of iNOS by immunohistochemistry does not necessarily indicate actual production of reactive nitrogen intermediates, lesions have also been stained for nitrotyrosine, the stable end product of the nitrosylation of tyrosine residues in proteins by peroxynitrite (345). It was found that iNOS and nitrotyrosine were expressed in borderline leprosy patients both with and without the reversal reaction. In addition, elevated levels of nitrates were measured in the urine of leprosy patients undergoing reversal reactions, and these levels decreased upon high-dose prednisolone treatment (344, 345).
Methods have recently been developed to isolate granuloma Mφ from the footpads of M. leprae-infected mice (145, 373). This model, which enables the study of Mφ from the actual site of infection in experimental leprosy, has allowed determination of culture characteristics, cytokine production, and cell surface phenotypic markers by flow cytometry of these granuloma-derived cells. Initial studies of footpad granuloma Mφ from M. leprae-infected athymic nu/nu mice indicated that the Mφ were phenotypically indistinguishable from normal peritoneal Mφ except that they contained enormous numbers of M. leprae (374, 375). These M. leprae-infected footpad-derived granuloma Mφ were also functionally similar to peritoneal Mφ except for one notable defect: they were refractory to activation by IFN-γ for both microbicidal and tumoricidal activity. In addition, there was no IFN-γ-induced augmentation of class II MHC expression or phorbol myristate acetate-induced superoxide production in the M. leprae-engorged granuloma Mφ. These results provide further support that M. leprae is a potent modulator of Mφ effector functions and that its influence is largely restricted to the microenvironment of the granuloma. This work is now being extended to evaluation of M. leprae-infected gene knockout mice.
Recent evidence from our laboratory, using an in vitro system in which M. leprae-infected nu/nu mouse footpad granuloma target Mφ are cocultured with fresh uninfected effector Mφ, suggests that Mφ may play a role in cell toxicity in leprosy lesions (145). When normal effector Mφ were cocultured with target Mφ, the effector Mφ acquired the bacilli from the infected target Mφ. Moreover, if the effector Mφ were activated with IFN-γ, they could kill the target cell-derived M. leprae. Killing of the bacilli in this system was not a rapid process but required 3 to 5 days of coculture for optimal effect. Furthermore, it was dependent on cell-cell contact and production of reactive nitrogen intermediates, but did not require concomitant IFN-γ or TNF-α production. The exact mechanism by which the effector Mφ acquired M. leprae from the target Mφ is not yet known. However, in numerous systems, especially those involving tumor models, Mφ have demonstrated cytotoxic capabilities using a variety of functions, including direct lysis, engulfment, and induction of apoptosis or necrosis. Current studies are aimed at determining which mechanism(s) a new Mφ may employ to effect cellular turnover in a leprosy lesion.
(v) Cytokines in leprosy.
The Th-1/Th-2 paradigm, based on functional discrimination of T-helper cells according to their pattern of cytokine production, asserts that Th-1 and Th-2 cells promote a cellular and humoral immune response, respectively (277). This functional differentiation has offered an attractive hypothesis to explain the differences between tuberculoid and lepromatous responses to M. leprae.
Several major studies of local immune responses in leprosy skin lesions have been published (Table 5). These are difficult to compare because they have used different designs, different methods of quantification, and different conventions to express their results. Overall, however, these studies have generally revealed a predominance of IL-2, TNF-α, and IFN-γ transcripts in tuberculoid lesions and IL-4 and IFN-γ in lepromatous ones, gene expression profiles consistent with Th-1 and Th-2 patterns, respectively (16, 113, 281, 380, 448). CD4+ clones isolated from TT lesions secreted primarily IFN-γ, whereas a CD4+ clone from an LL lesion produced predominantly IL-4 (381), and CD8+ clones isolated from LL patients likewise generated large amounts of IL-4 (328).
TABLE 5.
Cytokine gene expression in nonreactional leprosya
| Gene | Single lesion | T-cell clones
|
Skin lesions
|
PBMC
|
|||
|---|---|---|---|---|---|---|---|
| T | L | T | L | T | L | ||
| IL-1 | + (113, 381, 448) | +/− (113) | |||||
| IL-2 | + (113, 381, 448) | +/− (113) | |||||
| IL-4 | + (381, 448) | +/− (263) | + (263, 285) | +/− (141) | +/− (281) | ||
| IL-5 | + (381, 448) | +/− (141) | |||||
| IL-8 | + (381, 448) | ||||||
| IL-10 | + (391) | +/− (285) | |||||
| IL-12 | + (391) | +/− (285) | |||||
| TNF-α | + (391) | + (381, 448) | + (141, 281) | ||||
| IFN-γ | + (391) | + (381, 448) | + (263) | + (263, 281, 285) | + (141, 281) | + (281) | |
| TGF | + (381, 448) | ||||||
| GM-CSF | + (381, 448) | ||||||
| MIP | + (391) | ||||||
Specimens for reverse transcription-PCR were frozen biopsies (skin lesions), cultures of peripheral blood mononuclear cells stimulated in vitro (PBMC), or T-cell lines and clones established from PBMC of leprosy patients. Positive results were obtained for the cytokines indicated. Evidence from several reports is summarized to present a consensus of the findings, although the studies had different designs and different methods of quantification and used different conventions to express their results. Early, single-lesion leprosy was usually found histologically to be consistent with TT or BT disease in the one study of these lesions. Most studies classified patients only as tuberculoid (T) or lepromatous (L) and did not stratify results within the borderline portion of the leprosy spectrum. +, reported increase in expression (above controls); +/−, minimal or no increase in expression. Numbers in parentheses are references. TGF, transforming growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Further studies have also indicated that IL-12 and IL-18 promote resistance to M. leprae and are highly expressed in tuberculoid lesions (123, 380). Most studies have grouped patients only into tuberculoid or lepromatous groupings, however, and it is not clear whether variations in cytokine production correspond well with the various degrees of cellular immunity represented in the borderline portion of the leprosy spectrum.
The studies noted above have examined biopsies from well-established lesions, usually present for at least 2 years. The immunologic activity within earlier lesions, in patients with only a single lesion, has recently been evaluated by Stefani et al. (391). These early lesions, histologically consistent with TT or BT disease, also displayed a Th-1-like pattern of cytokine gene expression (Table 5).
Circulating leukocytes and T-cell lines from tuberculoid patients stimulated by M. leprae in vitro have also generally been found to produce a Th-1 cytokine pattern (Table 5), while leukocytes and T-cell lines from lepromatous patients generally produce a Th-2 cytokine pattern (263, 285). However, leukocytes from approximately 40% of all patients in one such study produced a mixed Th-0 cytokine profile, i.e., IFN-γ, IL-2, and IL-4 (263). It is possible that some of the patients whose cells produced the Th-0 pattern were in the borderline portion of the leprosy spectrum (BL or BT); alternatively, the human immune response to M. leprae may not correspond entirely with the Th-1/Th-2 model. Fractionated M. leprae antigens have also been found to stimulate IFN-γ in vitro with leukocytes from tuberculoid patients (95).
Experimental immunotherapy with intradermal inoculation of cytokines has provided additional information about their roles in immunological events within leprosy skin lesions (197). Both short- and long-term intradermal administration of IFN-γ resulted in an influx of mononuclear cells and an increase in the CD4/CD8 ratio in the lesions, but did not reverse the specific nonresponsiveness of circulating leukocytes to M. leprae (200). M. leprae exposure in vitro did not elicit IFN-γ in circulating mononuclear cells of lepromatous patients; the addition of IL-2 reversed this in most (but not all) of these patients' peripheral blood mononuclear cells (291). In lepromatous patients, intradermal injections of IL-2 generated apparent increases in cell-mediated immunity within the skin lesions (199) and resulted in increased levels of antibodies to M. leprae antigens (198), but did not enhance systemic cell-mediated immunity to M. leprae.
In summary, studies of cytokine gene expression in leprosy lesions thus far have given us a more detailed description of the immunological parameters of the polar types of leprosy, confirming and supporting the original concept that tuberculoid lesions are manifestations of delayed hypersensitivity and cellular immunity and that lepromatous ones result when immune recognition occurs (as indicated by antibody production) but the host is unable to develop cellular immunity to M. leprae. However, these studies have not yet revealed the mechanisms by which the cellular immune response is so extraordinarily titrated to produce the entire leprosy spectrum. Research in this area continues on the premise that the answer will be found in an understanding of the complex immunoregulatory mechanisms of cytokine production and inhibition. Some of the answers to these questions may also be found in studies of the genetically inherited ability to respond to M. leprae and other pathogens (see Genetic Influences on Leprosy in Humans, above).
LEPROSY REACTIONS
Reactions are acute inflammatory complications often presenting as medical emergencies during the course of treated or untreated Hansen's disease. Two major clinical types of leprosy reactions occur; together they may affect 30 to 50% of all leprosy patients (28, 226, 352). Because M. leprae infects peripheral nerves, the inflammation associated with reactions is a medical emergency, as severe nerve injury may develop rapidly, with subsequent loss of sensation, paralysis, and deformity.
The cause(s), mechanisms, and treatment of these reactions remain highly problematic, for both clinicians and basic scientists. The different types of reactions appear to have different underlying immunologic mechanisms, but these are poorly understood in spite of a substantial body of detailed descriptive information, and the factors that initiate them are unknown.
Clinical Presentation, Diagnosis, and Histopathology
Type 1 reactions occur in patients in the borderline portion of the spectrum (i.e., BL, BB, and BT). They are also known as reversal reactions, because early observations suggested that after the reaction had subsided, clinical and histopathological evidence indicated that the immunity in the lesions had increased (upgrading) or decreased (downgrading) (320). Downgrading is rarely seen in the current era of antimicrobial treatment, and the evidence regarding type 1 reactions reviewed here is based on studies of upgrading reactions.
These reactions present as induration and erythema of existing lesions, frequently with prominent acral edema and often with progressive neuritis, causing sensory and motor neuropathy (Table 6). In severe reactions the lesions may ulcerate. Type 1 reactions usually develop gradually, and their natural course may last for many weeks.
TABLE 6.
Comparison of clinical features of type 1 and type 2 leprosy reactions
| Parameter | Type 1 | Type 2 |
|---|---|---|
| Patients at risk | BL, BB, BT | LL, BL |
| Onset of reaction | Gradual, over a few weeks | Sudden, “overnight” |
| Cutaneous lesions | Increased erythema and induration of previously existing lesions | Numerous erythematous, tender nodules on face, extremities, or trunk, without relationship to prior lesions |
| Neuritis | Frequent, often severe | Frequent, often severe |
| Systemic symptoms | Malaise | Fever, malaise |
| Histopathological features | No specific findings | Polymorphonuclear cell infiltrates in lesions <24 h old |
| Course (untreated) | Weeks or months | Days to weeks |
| Treatment | Corticosteroids | Thalidomide, corticosteroids |
Type 2 reactions, also known as erythema nodosum leprosum, occur in multibacillary patients (LL and BL). These patients experience an abrupt onset of crops of very tender, erythematous nodules that may develop on the face, extremities, or trunk, without predilection for existing lesions (Table 6). Systemically, these patients often also experience fever, malaise, and some degree of neuritis with sensory and motor neuropathy. Iridocyclitis and episcleritis, orchitis, arthritis, and myositis may also accompany this reaction. In severe type 2 reactions, some of the cutaneous lesions may ulcerate. The natural course of type 2 reactions is 1 to 2 weeks, but many patients experience multiple recurrences over several months.
The Lucio phenomenon is an acute, severe, necrotizing vasculitis occurring primarily in patients of Mexican ancestry (96). Fortunately, this complication is rare, because it is associated with high morbidity and mortality. These reactions have been associated with the presence of high levels of cryoglobulins, and M. leprae antigens have been associated with some of these (100, 305), but the role they might play in these reactions is unclear. Characteristically, endothelial cells are unusually heavily infected and may be seen in various stages of degeneration. Lucio reactions may be accompanied by a profound anemia, and severe cases may require intensive wound care and debridement comparable to that used in the management of severe burns. These reactions require intensive inpatient management, and in the United States, consultation with the National Hansen's Disease Program is recommended.
Immunological Features of Reactions
Evidence regarding the mechanisms of leprosy reactions.
All types of leprosy reactions are believed to be immunologically mediated, but the mechanisms responsible for each type of reaction remain poorly understood. Although type 1 and 2 reactions together affect 40 to 50% of all patients at least once in the course of their disease, no clinical or laboratory tests can accurately predict who is most likely to develop a reaction or when it might occur. The factors precipitating reactions and the earliest events in the development of reactions are therefore unknown.
(i) Type 1 (reversal) reactions.
Substantial evidence now indicates that type 1 reactions are the result of spontaneous enhancement of cellular immunity and delayed hypersensitivity to M. leprae antigens, but the causes and mechanisms of this enhancement remain poorly understood. Early functional studies of lymphocytes demonstrated increased lymphocyte proliferation in response to M. leprae antigens in vitro during type 1 reactions (26, 135). Subsequent immunophenotyping studies revealed that the number and percentage of CD4+ T cells are increased in reacting skin lesions (267, 269, 353; reviewed in reference 270). Measurement of soluble IL-2 receptor levels in patient sera found high levels in type 1 reactions when the patients first presented for treatment and found that these levels declined steadily during treatment (416).
During type 1 reactions, increases in expression of the genes for several proinflammatory cytokines, including IL-1, IL-2, IL-12, IFN-γ, and TNF-α, have now been documented in several studies (Table 7). This activation is present both locally, in reacting skin lesions, and systemically, in serum and in circulating leukocytes. The pattern of cytokine expression has suggested to many investigators that type 1 leprosy reactions represent a spontaneously enhanced Th-1 response. However, these studies have not been able to clearly differentiate which changes observed are consequences of reaction and which (if any) reflect initiating events, and several of the reports have not clearly distinguished immunological from inflammatory phenomena.
TABLE 7.
Cytokine gene expression changes during type 1 and type 2 reactionsa
| Category and cytokine | Type 1 reaction compared to BT or BL (reference[s]) | Type 2 reaction compared to BL or LL (reference[s]) |
|---|---|---|
| Circulating | ||
| TNF-α | ↑ (337) | ⇈ (25, 115, 330, 337*) |
| IFN-γ | ↑ (274*, 389*) | ↑ (274*, 330, 389*) |
| IL-1 | ↑ (337) | ↑ (337) |
| IL-2 | ↑ (274) | ↑ (274*) |
| IL-2 receptor | ↑ (416) | ↑ (416) |
| IL-4 | Not increased | ↓ (284, 285*) |
| IL-6 | ↑ (274) | ↑ (274) |
| IL-8 | ↑ (274*) | ↑ (274*) |
| IL-10 | ↑ (389*) | |
| ↓ (284) | ||
| IL-12p40 | ↑ (389*) | ↑ (285*, 389*) |
| Cutaneous | ||
| TNF-α | ↑ (21, 274*, 449) | ↑ (21, 274*, 449) |
| IFN-γ | ↑ (233***, 274*, 389, | ↑ (274*, 389, 449) |
| 423**, 449) | ||
| IL-2 | ↑ (449) | ↑ (449) |
| IL-12p40 | ↑ (233***, 274*) | ↑ (274*) |
| IL-4 | Not increased | ↑ (274*, 449) |
| IL-10 | ↑ (274*) | ↑ (274*, 449) |
| IL-6 | ↑ (274*) | ↑ (274*) |
| IL-8 | ↑ (218***, 274*) | ↑ (274*, 449) |
| MCP-1 | ↑ (218***) | Not done |
| RANTES | ↑ (218***) | Not done |
| iNOS | ↑ (233) | Not done |
| Peripheral nerve | ||
| TNF-α | ↑ (21) | Not done |
This table summarizes the results of many studies that employed a variety of study designs, different methods of cytokine measurement, and different criteria for determination of an increase in gene expression. Most studies of skin lesions assessed mRNA by reverse transcription-PCR; some also measured protein levels of selected cytokines. The observation that expression or level of most of the proinflammatory cytokines is increased in both types of reactions highlights the difficulty in determining whether an increase in any cytokine reflects a causative immunologic event underlying the reaction or is a consequence of the intense inflammation occurring in the reaction. *, mRNA in peripheral blood mononuclear cells; **, mRNA in T-cell clones from lesions; ***, cytokines and chemokines assessed by immunostaining.
Clinical studies have determined that the serum levels of some of these cytokines decline during the course of successful prednisolone treatment of type 1 reactions but show little or no reduction during the treatment of type 2 reactions. Such a decline has been documented, for example, for indicators of inflammation such as neopterin and iNOS (114, 233), as well as for cytokines and receptors more indicative of immunologic function such as soluble IL-2 receptor, IFN-γ, IL-6, IL-10, IL-12, and IL-13 (21, 233, 416). These observations suggest that in addition to the nonspecific anti-inflammatory effects of prednisolone, it may have some inhibitory effect on the underlying immunologic mechanisms of these reactions, although it is not certain that this is the case.
The events or conditions that trigger type 1 leprosy reactions are unknown; notably, only about 15 to 30% of the patients at risk (i.e., BL, BB, and BT) are affected. Type 1 reactions have been observed to follow immunization with other mycobacteria in some circumstances (433, 452), and various environmental factors have been considered but not confirmed to be associated with the onset of these reactions. The possibility that genetic factors might predispose some patients to develop type 1 reactions has not yet been examined (see Genetic Influences, above). Notably, experimental intradermal inoculation of IL-2 or of IFN-γ did not precipitate type 1 reactions (198, 332). In addition, although thalidomide was reported to inhibit TNF-α in several experimental conditions (410), it has no benefit in the treatment of type 1 reactions.
(ii) Type 2 leprosy reaction (erythema nodosum leprosum).
Type 2 reactions occur in patients with poor cellular immunity to M. leprae, abundant bacilli (i.e., antigen) in cutaneous and peripheral nerve lesions, and a strong polyclonal antibody response with high levels of circulating immunoglobulins. The acute lesions are characterized by a neutrophilic infiltrate superimposed upon a chronic lepromatous pattern, observations that have long dominated thinking about this reaction. Based primarily on histological evidence, Wemambu and colleagues proposed that ENL represents an Arthus-like phenomenon mediated by immune complexes (431). Immunoglobulin and complement deposition have been demonstrated in the skin lesions, and serum complement is decreased in these patients, consistent with this hypothesis, and some mycobacterial constituents have been identified in some of these complexes (322). However, neither circulating nor fixed immune complexes have been reproducibly demonstrated in ENL lesions. The demonstration of immune complexes within clinical lesions in other diseases has also been difficult and problematic, however, and the immune complex theory of ENL is thus neither proved nor disproved.
Other studies have identified possible evidence of cellular immune activation in type 2 reactions, including increases in circulating IFN-γ, TNF-α, and IL-12 (Table 7) (449). Increases in mRNA levels for these cytokines have also been observed in biopsies of skin lesions, indicating that cellular immune activation is occurring locally. In contrast, increases in the expression of IL-6, IL-8, and IL-10 mRNAs and sustained expression of IL-4 and IL-5 mRNAs, all cytokines associated with neutrophil chemotaxis, antibody production, and reduced cell-mediated immunity, were observed in ENL lesions.
The factors that trigger type 2 reactions are even more poorly understood than the immunologic mechanisms of the reaction itself. Other infections or viral illness, fever, immunization, and psychological stress have all been invoked, but no convincing evidence has supported any of these. Pregnancy appears to have an inhibitory effect on type 2 reactions, whereas the reaction may recur severely in the postpartum period in women with lepromatous leprosy (101, 236). Anecdotal reports suggest that in some women, the severity of a type 2 reaction fluctuates during the menstrual cycle, and still other anecdotal evidence has suggested that the type 2 reaction is more frequent and severe among children who develop leprosy at the onset of puberty (81).
The only stimulus known to initiate a type 2 reaction is the intralesional injection of IFN-γ (332). In experimental immunotherapeutic trials, multiple intradermal injections of IFN-γ over 6 to 12 months elicited this reaction in 6 of 10 lepromatous patients studied; all 6 of these had polar LL disease. The four patients who did not develop the reaction had BL or subpolar LL types of leprosy, in which the ability to develop cell-mediated immunity to M. leprae is greatly reduced but is not altogether absent. The observation that type 2 reactions developed in patients who had the most profound inability to develop cell-mediated immunity to M. leprae may suggest that, in such patients, the effect of IFN is to activate immunologic mechanisms that, because they cannot generate cell-mediated immunity, are instead channeled into pathways that lead to enhanced humoral immune activity, i.e., Th-2 cytokine responses. Notably, intradermal injections of IL-2 in lepromatous patients did not elicit a type 2 reaction (198).
Evidence from therapeutic trials regarding mechanisms of reactions.
Reactions are poorly understood, and their management is often difficult and perplexing. Corticosteroids are the mainstay of the treatment of type 1 reactions; high doses are often required, sometimes for prolonged periods of time (40), with the attendant risk of serious side effects (397). For type 2 reactions, thalidomide is the treatment of choice (405). (For decades, the type 2 reaction was the only clear indication for the use of thalidomide, and the drug might well have been forgotten entirely had it not been so valuable for the treatment of these reactions.) The anti-inflammatory properties of clofazimine are useful in treating type 2 reactions also. Corticosteroids are widely used for the treatment of these reactions (235, 349), sometimes because they do not respond well to thalidomide, and more often because the drug is highly restricted or unavailable. Antimicrobial therapy should be maintained throughout treatment for both types of reaction.
The great difficulties encountered in treating reactions provide an illuminating example of the importance of understanding the mechanisms of disease: i.e., since the cause(s) and mechanisms of reactions are poorly understood, treatment is largely empirical and is often suboptimal. A number of other immunosuppressive agents have therefore been tested for their effect on reactions in the search for additional regimens that might be effective individually or at least offer a steroid-sparing effect. Since these agents act at different points in the development of an immunological response and some information is available concerning their mechanisms of action, a review of these trials is of interest in trying to understand the immunological mechanisms causing reactions (Table 8).
TABLE 8.
Effects of immunosuppressive agents on leprosy reactionsc
| Agent | Primary mechanism(s) of action (reference[s]) | Effect on type 1 reactiona (reference[s]) | Effect on type 2 reaction (reference[s]) |
|---|---|---|---|
| Thalidomide | Multiple dose-related mechanisms: inhibits TNF-α, stimulates IL-2, inhibits IgM response (333, 362) | 0 (166) | ++++ (275, 276, 367, 404) |
| Methotrexate | Antimetabolite inhibiting lymphoid and myeloid proliferation (56) | ND | ++ (202) |
| Cyclosporine | Inhibits IL-2 and other cytokines (27, 147, 211, 250) | ++ (64, 116, 421) | +/− (225, 261, 283, 418, 421) |
| Azathioprine | Purine antagonist; inhibits lymphocyte proliferation; exact mechanism unknown (27, 147) | +b (246) | +b (243) |
| Pentoxifylline | Inhibits TNF-α and other cytokines (321, 395) | +/− (82, 275) | +/− (82, 276, 287, 331) |
| Mycophenolate mofetil | Blocks guanosine nucleotides, inhibiting proliferation of T and B cells (27, 147) | ND | 0 (47) |
| Corticosteroids | Block transcription factors AP-1 and NF-κB; inhibit synthesis of many proinflammatory cytokines (203, 341) | ++++ (283) | +++ (276) |
ND, no data available.
Effect observed only in combination with prednisolone.
0, not beneficial; +/−, conflicting results; +, beneficial; ++, better results; +++, highly beneficial; ++++, treatment of choice.
Corticosteroids, due to their general anti-inflammatory effects, are highly effective clinically for both type 1 and type 2 reactions. It is not clear, however, that this treatment has a significant effect upon the underlying mechanisms of either type of reaction (282, 283). Reduced levels of proinflammatory cytokines have been observed in peripheral blood monocytes from patients treated with corticosteroids for type 1 reactions (245), but other studies have suggested that alterations in cytokine levels are not always observed in patients who do receive good clinical benefit from anti-inflammatory treatment (12, 275, 404).
Thalidomide was determined to be extraordinarily effective in the treatment of type 2 reactions before its teratogenic properties were recognized (367). The mechanism by which thalidomide exerts this strong anti-inflammatory effect in type 2 reactions is not clearly understood even today, however, in spite of the flurry of interest in the apparent inhibitory action of thalidomide on TNF-α (410). The effects of thalidomide are strikingly different over a wide range of concentrations, and at the concentrations used clinically it has also been observed to enhance the production of IL-2 (360). Early studies noted that thalidomide inhibited the IgM response (361), and it is also known to promote apoptosis in neutrophils (19), both of which are potentially significant effects in the context of type 2 reactions.
Cyclosporine has been used to treat type 2 reactions, with mixed results (260, 421, 418). Cyclosporine is a potent suppressor of cellular immunity, blocking the transcription of IL-2 and several other cytokines by interfering with the calcineurin-dependent translocation of the nuclear factor of T-cell activation (NFAT) to the nucleus (27, 211, 250). The evidence that cyclosporine is beneficial in some severe cases of type 2 reaction may indicate that the mechanisms underlying these reactions are not homogeneous. T-cell-related mechanisms may be involved in the pathogenesis of severe or recurrent ENL, but the broader experience, in which cyclosporine was of little benefit, suggests that such T-cell functions may not be a major feature of most type 2 reactions.
Azathioprine is a purine antagonist and is well documented to inhibit lymphocyte proliferation, although its precise mechanism of immunosuppression is unknown (27). When followed by prednisolone, it has been found to provide results comparable to those with prednisolone alone in the treatment of type 1 reactions (246), thus possibly providing a steroid-sparing regimen in the treatment of this reaction. Azathioprine alone did not provide superior results in this study, however, suggesting that the broad immunosuppression associated with this agent did not interfere with some of the basic mechanisms underlying the reaction. Azathioprine alone has not been assessed in the treatment of type 2 reactions but has also been reported to be useful in combination with prednisolone in the management of intractable type 2 reactions that do not respond well to prednisolone alone (243). This has not been evaluated in a controlled study, however.
Methotrexate has recently been reported to be effective when used in combination with corticosteroids in patients whose type 2 reaction could not be controlled with corticosteroids alone (202). This remains to be confirmed in controlled studies.
Pentoxifylline, like thalidomide, has been observed to produce a reduction in circulating levels of TNF-α in patients with type 2 reactions as well as inhibit TNF-α mRNA in skin lesions (275, 331, 304). However, pentoxifylline has not provided a clinical benefit comparable to that of thalidomide (82, 276), and some evidence has suggested that the inhibition of TNF-α may not be the major beneficial effect of thalidomide in ENL (276).
Mycophenolate mofetil, an inhibitor of B- and T-cell proliferation that blocks the production of the guanosine nucleotides required for DNA synthesis (27), has shown no benefit in type 2 reactions in one small study (47), although the effect of higher doses has not yet been studied. This agent has not been tested in type 1 reactions.
In summary, inhibition of lymphocyte proliferation by several potent antimetabolites has had little or no consistent effect in the treatment of either type of leprosy reaction. Similarly, clinical inhibitors of TNF-α, IL-2, and other cytokines have had minimal effects on reactions in most cases. The mechanism of the remarkably beneficial effect of thalidomide on type 2 reactions remains unexplained. There is no convincing evidence to date that the anti-inflammatory treatments used actually interrupt the underlying immunological processes. The overall beneficial effects of corticosteroids on both types of reaction are probably due largely to their anti-inflammatory mechanisms. Although corticosteroid and thalidomide treatments greatly alleviate suffering and mitigate nerve injury, it is quite possible that the immunological events that fuel reactions simply run their course and abate independently of the anti-inflammatory treatment itself.
MECHANISMS OF NERVE INJURY
Among bacterial pathogens, infection of peripheral nerves is a unique property of M. leprae. Infection of peripheral nerves is the sine qua non of leprosy, but many clinical details regarding the frequency and extent of nerve injury have only recently been described, and the mechanism(s) underlying nerve injury in leprosy is very poorly understood (354).
Neuropathy in leprosy arises not only from the infection of peripheral nerves by M. leprae but also from the inflammatory and immunologic responses to this pathogen. This neuropathy is often devastating to the patient's health and well-being, through the development of anesthesia, paralysis, and potential crippling deformities of fingers and toes due to ulnar, median radial, peroneal, or posterior tibial neuritis or ocular damage in the case of facial nerve involvement. Studies of the pathogenesis of neuropathy in leprosy have been severely hampered by the lack of good experimental models and because biopsy of the most actively inflamed sites of affected peripheral nerve trunks is not possible.
Although localized anesthesia is a serious and well-known consequence of leprosy, recent evidence also indicates that a large percentage of patients experience neuropathic pain (142, 396), sometimes long after they appear to be otherwise cured of infection. Little study has been done concerning the mechanisms of neuropathic pain in leprosy, and the remainder of this discussion of nerve injury will concentrate on mechanisms related primarily to injury resulting in anesthesia and, ultimately, paralysis.
Recent advances in the study of nerve injury in leprosy have been most prominent in five areas: improvements in clinical sensory testing with monofilaments, recognition that the mechanisms of localization of M. leprae to nerves may involve the vascular endothelium, direct examination of immunological parameters in biopsy samples of affected nerves in leprosy patients, identification of the molecular mechanisms of M. leprae binding to Schwann cells, and development of greatly improved Schwann cell culture models for in vitro studies of the consequences of M. leprae infection.
Accurate, reproducible measurement of sensory function using calibrated Semmes-Weinstein monofilaments has been a major advance in the study of nerve injury in leprosy (231). Such testing can clearly identify loss of protective sensation before it results in ulceration and other injury and can identify early neuropathy, with subtle functional impairment, that is otherwise often overlooked. This has contributed to advances in the prevention of disability in leprosy (419). Using this method, a 5-year follow-up study of nerve function impairment in over 200 patients has demonstrated that nerve injury continues to be a problem even after the infection is treated and cured (316).
Although neuritis and neuropathy have often been studied and discussed in the context of leprosy reactions, which may exacerbate neuritis, the more sensitive assessments of sensory loss have also demonstrated that nerve function impairment occurs independently of reactions (419). With these sensitive methods, it is now evident that early nerve function impairment occurs earlier in lepromatous than in tuberculoid patients (258, 316, 339). In addition, a study of prophylactic treatment with prednisolone has shown that it reduces nerve function impairment that is measurable at 4 months, but this improvement was not evident after 1 year (385). Thus, the ability to clinically evaluate different degrees of neuropathy and correlate this with responses to intervention has very important implications for both clinical and basic research on the mechanisms of nerve injury in leprosy.
Overt sensory and motor neuropathies that prompt patients to seek medical attention often occur earlier and more intensely in those patients whose lesions contain few bacilli (BT and TT types), most probably because the granulomatous inflammatory response to M. leprae in these patients leads to injury to adjacent nerves. In contrast, lepromatous patients often develop overt neuropathy more slowly, even though the Schwann cells and macrophages of their peripheral nerves are more heavily infected with M. leprae. After prolonged, untreated infection, however, the nerves of all types of patients are at risk of chronic inflammation and fibrosis that becomes the final common pathway of injury, potentially resulting in anesthesia with paralysis of intrinsic and extensor muscles of the hands and feet. The affected limbs are then at high risk of injury or mutilation, processes that accelerate the physiologic resorption of bone and result in loss of digits. Involvement of the facial nerve leaves patients at risk of corneal anesthesia, abrasion, and blindness.
Four related aspects of nerve injury in leprosy must be considered in understanding the pathogenesis of neuritis in leprosy: localization of M. leprae to peripheral nerves, infection of Schwann cells, immunologic responses, and inflammation. Of these, the infection of Schwann cells is the most obviously remarkable and has received by far the greatest experimental attention, and the other aspects have suffered especially from the inherent difficulties in obtaining material for investigation, since biopsy of affected nerves is seldom clinically indicated and is otherwise unethical. The armadillo appears to provide a model for some aspects of leprosy neuropathy in humans, as noted below, but also has posed major limitations as an experimental animal.
Localization of M. leprae to Peripheral Nerves
The first essential step in leprosy neuritis is the localization of M. leprae to peripheral nerves. Ever since autopsy dissections in the 1890s (85, 130), which followed affected peripheral nerves from the skin lesion to the spinal cord, the infection of peripheral nerves has been understood to be an ascending neuropathy originating in sensory cutaneous nerves and traveling proximally to involve larger nerve trunks carrying mixed sensory and motor fibers (327). This has been extrapolated to imply that M. leprae initially binds to exposed Schwann cells in the dermis and then moves proximally within the nerve, “swimming like fish up a stream” (209).
However, recent studies of peripheral nerves in experimentally infected armadillos have suggested that M. leprae infects nerves from the outside in, first aggregating in epineurial lymphatics and blood vessels and then entering the endoneurial compartment through its blood supply (347, 351).
A model illustrating this hypothesis of localization of M. leprae to peripheral nerves is presented in Fig. 6 (347).This view of the pathogenesis of infection of peripheral nerves raises significant implications with respect to both understanding the process and possible points of preventive or therapeutic intervention. If several steps are required for the ultimate entry of M. leprae into Schwann cells, then there are several potential sites of intervention, e.g., binding to endothelial cells, entry into the endothelium, exit from endothelial cells into the endoneurium, and binding to Schwann cells. If, on the other hand, M. leprae enters nerves exclusively via the single step of direct binding to exposed Schwann cells in the dermis, then this is the only opportunity for preventive or therapeutic intervention, and the likelihood of developing such interventions is correspondingly decreased.
FIG. 6.
Proposed model of infection of peripheral nerve by M. leprae via blood vessels. A cutaneous nerve with three fascicles is represented here to illustrate the proposed steps in the pathogenesis of infection of peripheral nerves by M. leprae. (A) Initially, colonization of the epineurium (e) occurs when bacilli (red) localize in cells in and around blood vessels (blue). It is possible that this is enhanced by drainage of bacilli through the lymphatics (green) that are intertwined with the blood vessels of the epineurium (lymphatics are here illustrated only at the lower end of the drawing). The resulting accumulation of bacilli within and around endothelial cells greatly increases the likelihood that bacilli will be available for circulation through the endoneurial vessels which branch off the epineurial ones. (B) Entry of M. leprae into the endoneurial compartment proceeds along blood vessels from foci on and within the perineurium (p), extending through it into the interior of the nerve. The mechanisms responsible for entry into the interstitial space of the endoneurium remain to be determined. Once inside, however, bacilli are available for phagocytosis by Schwann cells (SC), represented here with concentric layers of myelin surrounding axons. Although these initial events in the localization and entry of M. leprae into peripheral nerves are postulated to be unrelated to specific immune function, the subsequent pathogenesis of neuritis in leprosy probably depends in large part on the patient's immune response to M. leprae. (C) If no effective immune respose develops (e.g., lepromatous leprosy), bacilli proliferate within macrophages and Schwann cells. This results in perineurial inflammation and thickening (proliferation) and an increasing bacterial load both in the epineurium and in the endoneurium. Since M. leprae is an indolent, well-adapted intracellular pathogen, however, axons are not badly damaged for a long time, and a variable degree of nerve function is preserved until late in the course of the disease. (D) If effective cellular immunity and delayed hypersensitivity do develop (e.g., tuberculoid leprosy), a granulomatous response follows at sites of infection near epineurial and endoneurial vessels and Schwann cells. This immunologically elicited inflammation eliminates nearly all of the bacilli in the epi- and perineurium and also stimulates perineurial fibrosis and thickening. However, M. leprae organisms that have already been ingested by Schwann cells may be relatively protected from immunologically mediated destruction and able to maintain a persistent infection in these cells for a longer time. This is where most bacilli are found in diagnostic biopsies of tuberculoid lesions. Granulomatous inflammation is also potentially injurious to adjacent tissue. In M. leprae-infected nerves, this includes injury to axons in the vicinity of the granulomas, resulting in impaired nerve function. (Reprinted from reference 347.)
The Schwann cell, the principal support cell in the peripheral nervous system, appears to be the major target of M. leprae in peripheral nerves. In patients with advanced leprosy, both myelinated and nonmyelinated Schwann cells are infected by M. leprae (163, 179, 180), although some reports have suggested some preference for nonmyelinating Schwann cells (311). In vitro, we have observed a similarly brisk and heavy infection of both cell types (144). Some investigators, however, have reported exclusive infection of nonmyelinating cells in vitro (309).
M. leprae Interactions with Schwann Cells
Adhesion to Schwann cells.
Several potential mechanisms of binding of M. leprae to the Schwann cell (SC) have been elucidated (310, 308, 398). Antibodies directed against the polysaccharide and lipid components of M. leprae inhibited adhesion to SCs, while those directed against both surface and cytoplasmic protein epitopes did not show any such effect (67), indicating that the association of M. leprae with SCs may be mediated by more than one of its cell surface molecules. Recent studies have demonstrated that M. leprae specifically binds to α-dystroglycan in the presence of the G domain of the α2 chain of laminin-2 (310). Using α2 laminins as a probe, a major protein in the M. leprae cell wall fraction (ML-LBP21) that binds α2 laminins on the surface of SCs has been identified (370).
Phenolic glycolipid 1 of M. leprae has also been demonstrated to bind specifically to laminin-2 in the basal lamina of SC-axon units (288). PGL-1, therefore, appears to be involved in the invasion of SCs by M. leprae in a laminin-2-dependent pathway. Importantly, however, evidence clearly indicates that this mechanism of binding to the SC surface via α2-laminins is not unique to M. leprae. Other mycobacterial species, including M. tuberculosis, M. chelonae, and M. smegmatis, have been shown to express an α2-laminin-binding capacity (247) and these species readily interact with the ST88-14 Schwannoma cell line. This suggests that the ability to bind α2-laminins is conserved within the genus Mycobacterium. Other studies have also demonstrated the ability of myelin P0 to bind M. leprae (398).
Ingestion by SCs.
After M. leprae adheres to the SC surface, it is slowly ingested, as described in recent studies using primary denervated rat SC cultures and SC-neuron cocultures (144) (Fig. 7). After ingestion the SC appeared to be incapable of destroying this intracellular parasite when cultures were maintained at 33°C (optimal conditions for M. leprae viability and temperature of peripheral nerves) (414). In vitro studies of ingestion of M. leprae by a human Schwannoma cell line (ST88-14) found that several protein kinases were essential for ingestion but that cyclic AMP-dependent kinases were not (11). In these studies, acidification of vesicles containing irradiated M. leprae proceeded normally but was minimal when live M. leprae was used, suggesting that viable M. leprae interferes with normal endocytic maturation.
FIG. 7.
Transmission electron micrographs of Mycobacterium leprae-infected rat Schwann cell (SC)-neuron cocultures. Infected cultures were obtained by exposure of primary Schwann cells to M. leprae for 48 h. After cultivation for 12 days at 33°C, they were seeded onto embryonic rat neurons. The infected Schwann cell cultures were induced to myelinate and cultured for 30 days at 33°C. A. Myelinating Schwann cells. B. Nonmyelinating Schwann cells. (Reprinted from reference 144 with permission. © 2002 by the Infectious Diseases Society of America. All rights reserved.)
Effects of SCs on M. leprae.
SCs apparently provide an environment suitable for the preservation and proliferation of M. leprae. Studies using highly viable suspensions of nude-mouse-derived M. leprae have demonstrated that the viability of the bacilli in rat SCs is comparable to that previously described for bacilli within macrophages in vitro and that survival of this organism within SCs is greater at 33°C than at 37°C (144). This survival within SCs in vitro is consistent with the long-standing histopathological observations that M. leprae appears to persist and grow within SCs in human nerves.
Effects of M. leprae on SCs.
The effect of M. leprae on the SC has been the subject of many studies in vitro. Notably, however, optimal conditions (highly viable bacilli and cooler cultivation temperatures) have not been used in most studies of this interaction, possibly contributing to the variety of conflicting reports in the literature (264, 279, 382, 392). Infection of SCs with whole, viable M. leprae has not been observed to cause SC loss (144), and even appeared to favor SC survival rather than apoptosis (311). However, human SCs express Toll-like receptor 2 both in vitro and in vivo, and binding of an M. leprae-derived lipoprotein to TLR2 on SCs has been reported to result in apoptosis (294). These investigators also identified SCs that had undergone apoptosis in biopsies of human lesions. The significance of these observations with respect to clinical nerve injury remains uncertain.
M. leprae also appears to have no effect on intact, mature SC-axon units, but did alter SC expression of a small number of genes examined (those for glial fibrillary acidic protein, transforming growth factor β1, NCAM, ICAM, N-cadherin, and L1)(144). However, transcript levels for all but one of these genes, that encoding N-cadherin, varied less than twofold. Therefore, the functional significance of these alterations remains to be determined.
In contrast to these observations, Rambukkana and colleagues, also using a rat SC-axon coculture system, have described rapid demyelination following adherence of M. leprae to SCs in the absence of immune cells, interpreted to be a contact-dependent mechanism dependent on PGL-1, a component of the M. leprae cell wall (311). Similar findings in T- and B-cell-deficient (Rag1−/−) mice led these authors to conclude that attachment of M. leprae to the myelinated SC surface is sufficient to induce rapid demyelination of these cells, thus suggesting a mechanism for demyelination of nerves in leprosy (for a review, see reference 309). These conclusions, however, are at considerable odds with well-documented clinical and histopathological observations. First, patients with untreated lepromatous leprosy may have billions of bacilli in their bodies but show little or no demyelination. Secondly, rapid demyelination (if it occurs in clinical leprosy) is a late manifestation of the disease rather than an early one (295).
Immune responses and SCs.
Finally, the immune response may also be directed at M. leprae-infected SCs. Isolated human SC cultures appear to be able to process and present M. leprae antigens to CD4+ T cells (387). The infected SCs were highly susceptible to killing by CD4+ cytotoxic T-cell clones derived from leprosy patients. Long-term cultures of human SCs also express MHC class I and II, ICAM-1, and CD80 surface molecules involved in antigen presentation. These cells process and present M. leprae and some of its protein and peptide antigens to MHC class II-restricted CD4+ T cells and are efficiently killed by these activated T cells.
As a long-term consequence of these and other, unknown mechanisms, SCs are ultimately functionally impaired or destroyed in infected nerves. The end result is a demyelination neuropathy (164, 399). Segmental demyelination is seen adjacent to areas of infection, as discussed above. Axonal atrophy and accompanying demyelination have also been described in leprous nerves, and this has been associated with abnormal phosphorylation of high- and medium-weight neurofilaments (340).
In summary, M. leprae has a unique ability to infect peripheral nerves, probably entering via their vascular endothelium by mechanisms not yet determined. Once they have gained access to the endoneurial compartment, M. leprae organisms bind to SCs via several binding molecules on the surface membrane. The bacilli are ingested by SCs, and viable organisms appear to be able to interfere with normal endocytic maturation and thus, probably, with potential killing mechanisms. Within SCs in vitro, M. leprae is able to survive for a limited time at 33°C. M. leprae does not appear to induce SC death, by apoptosis or otherwise, and infected SCs are able to associate normally with axons in vitro. Infection does, however, produce measurable changes in transcription of some of the genes that have been examined thus far.
Infected SCs are also able to process and present antigen to T cells, and thus may become targets of immune responses. Immunologically driven inflammation is probably responsible for much of the clinically apparent nerve injury, because nerve function impairment occurs more rapidly and more severely in patients with a strong cellular immune response (i.e., tuberculoid disease). The limited evidence available indicates that the immunological mechanisms operating within nerves are similar to those which have been described in much more detail in the skin. In lepromatous patients, with minimal immunological response to M. leprae, nerves may be heavily infected with only mild to moderate impairment of function. Ultimately, however, selected peripheral nerves in all forms of leprosy undergo demyelination. Without effective treatment, many such nerves will become completely nonfunctional, leaving the patient with an insensate, paralyzed hand or foot. Understanding the many undeciphered mechanisms underlying nerve injury and applying available clinical and rehabilitation tools to prevent and minimize nerve injury are among the greatest challenges and highest priorities in leprosy research today.
CHEMOTHERAPY
Current Therapies and Drug Resistance
In the 1950s, dapsone (diaminodimethyl sulfone) was introduced as standard chemotherapy for leprosy and was used worldwide for treatment of both multibacillary and paucibacillary forms of the disease. Long-term monotherapy with dapsone resulted in poor compliance in many areas, ultimately leading to the emergence of dapsone-resistant leprosy, resulting in treatment failures and resistance levels reported to be as high as 40% in some areas of the world (446, 365).
Fortunately, additional antimicrobial agents such as rifampin and clofazimine were developed and introduced for the treatment of leprosy (44, 232). Although rifampin proved to be a powerful antileprosy drug, use of rifampin alone or in combination with dapsone for the treatment of dapsone-resistant leprosy led to the rapid development of rifampin-resistant organisms (137, 177). Other drugs with antileprosy activity were also evaluated. Clofazimine proved to be only weakly bactericidal against M. leprae and therefore was not suitable as monotherapy for leprosy (169, 177).
To overcome the problem of drug-resistant M. leprae and to improve treatment efficacy, the World Health Organization recommended multidrug therapy for leprosy in 1981. The initial recommendation for patients with multibacillary leprosy was to give daily dapsone and clofazimine with monthly rifampin and clofazimine for 2 years or until the skin smear was negative. These recommendations, as well as diagnostic criteria, have been modified several times since 1981. Currently the World Health Organization recommends counting lesions to distinguish paucibacillary from multibacillary disease, less than five lesions being classified as paucibacillary and five or more lesions as multibacillary. Since 1998 they have also recommended treating multibacillary patients for only 12 months and paucibacillary patients for only 6 months (reviewed in reference 349) (Table 9). In addition, a World Health Organization committee recommended that patients with a single lesion be treated with a single combination dose of rifampin (600 mg), ofloxacin (400 mg), and minocycline (100 mg) (312; www.who.int/lep/romfaq3.htm), but this regimen remains very controversial. These recommendations arise from efforts to reduce the resources allocated to leprosy in developing countries and are the subject of considerable debate. Optimal diagnostic evaluation employing skin smears or biopsies, classifying the lesions on the Ridley-Jopling scale, and conservative, longer duration of treatment with multiple antimicrobials are recommended in the United States and most developed countries (349).
TABLE 9.
Antimicrobial agents for leprosya
| Agent | Routine dose (per day) | Antibacterial mechanismb | References |
|---|---|---|---|
| Dapsone | 100 mg | Weakly bactericidal; competitive PABA antagonist | 10, 225, 317, 359 |
| Clofazimine | 50 mg | Weakly bactericidal; unclear but binds DNA of mycobacteria | 44, 169, 171, 175, 314 |
| Rifampin | 600 mg | Bactericidal; inhibits DNA-dependent RNA polymerase | 156, 178, 232, 280, 403, 441 |
| Minocycline | 100 mg | Bactericidal; inhibits ribosomal protein synthesis | 126, 171, 176, 402 |
| Ofloxacin | 400 g | Bactericidal; inhibits DNA gyrase | 99, 120, 171, 172, 175, 176, 241, 401 |
| Perfloxacin | 800 mg | Bactericidal; inhibits DNA gyrase | 106, 118, 129, 138 |
| Clarithromycin | 500 mg twice | Bactericidal; inhibits ribosomal protein synthesis | 57, 174 |
Modified from reference 349 with permission from Elsevier. Only dapsone is approved specifically for the treatment of leprosy by the Food and Drug Administration.
PABA, p-aminobenzoate.
Multidrug therapy has been very practical and successful for treatment of both multibacillary and paucibacillary leprosy (171, 444, 447), and the overall number of registered cases worldwide has fallen dramatically (171, 445). However, even with these powerful drug combinations, the number of newly registered cases has not fallen consistently, and drug resistance still occurs.
A recent report demonstrated that 19% of 265 M. leprae isolates from biopsied samples of leprosy patients were resistant to various concentrations of dapsone, rifampin, or clofazimine and 6.23% were resistant to more than one drug in the mouse footpad susceptibility assay (102). In addition, several investigators have identified multidrug-resistant strains of M. leprae (reviewed in reference 434). Ofloxacin and minocycline have been added to the drug arsenal for the treatment of leprosy (126, 172, 175, 176; see also http://www.who.int/lep/romfaq3.htm). Current treatment recommendations in the United States have been summarized elsewhere (276a, 349), and Lockwood has provided a rigorous evidence-based discussion of the treatment of leprosy (234).
With or without the development of drug resistance, relapse occurs in some cases even after multidrug therapy. The reported extent of relapse varies greatly, depending on several operational factors and on the duration of follow-up. Because of the very slow growth of this organism, follow-up of at least 10 years is necessary to obtain a reasonable assessment of relapse, and during the last 20 years recommendations concerning treatment duration and drug combinations have changed several times, further complicating this assessment. The largest study, although shorter than 10 years in duration, is a 6-year follow up of 47,276 patients in the Chinese national program (63), which revealed an overall relapse rate of 0.73/1,000 person-years, significantly greater for paucibacillary patients (1.04/1,000 person-years) than for multibacillary patients (0.61/1,000 person-years).
In southern India, a relapse rate equivalent to 20/1,000 person-years was observed among multibacillary patients given fixed-duration (2-year) multidrug therapy, reduced to 10/1,000 person-years in patients treated until smear negative (134). A 10-year prospective study in the Philippines (55) observed an overall relapse rate equivalent to 2.8/1,000 person-years. Significant differences were noted in the rates of relapse in multibacillary patients followed at a referral center (9%) versus field clinics (3%). Importantly, in both the southern India and Philippine studies, higher rates of relapse have been observed in patients with a high bacterial index (BI) (≥4) at the time of diagnosis, underscoring the advice that such patients require longer treatment (128). In the longest study to date, a 16-year follow-up of patients in Karigiri, India (292), a relapse rate equivalent to 0.7/1,000 person-years was observed among multibacillary patients who had received multidrug therapy. Relapses occurred 14 to 15 years after release from treatment and again were more frequent in persons with a high initial BI.
Molecular Mechanisms of Drug Resistance
Dapsone (4,4-diaminodiphenyl sulfone) is a synthetic sulfone, is structurally and functionally related to the sulfonamide drugs, and targets dihydropteroate synthase, a key enzyme in the folate biosynthesis pathway in bacteria, by acting as a competitive inhibitor of p-aminobenzoic acid (317, 359). Dapsone has also been shown to target the folate biosynthetic pathway of M. leprae (225).
Specific mutations within the highly conserved p-aminobenzoic acid binding site of E. coli dihydropteroate synthase, encoded by folP, result in the development of dapsone resistance (80). New evidence from the M. leprae genome sequencing project indicated that M. leprae possesses two folP homologues (folP1 and folP2) (74). Through surrogate genetic studies with M. smegmatis, the relationship between dapsone resistance and the dihydropteroate synthase of M. leprae has been established (439). Missense mutations within codons 53 and 55 of the sulfone resistance-determining region of folP1 result in the development of high-level dapsone resistance in M. leprae (Table 10).
TABLE 10.
Mutations within drug target genes associated with drug resistance in Mycobacterium leprae
| Drug/target gene | Drug susceptibilitya | Mutation(s) | No. of isolates (%)b | Reference(s) |
|---|---|---|---|---|
| Rifampin/rpoB | R | Gly401Ser; His420Asp | 1 (2) | 51 |
| R | Gln407Val | 1 (2) | 51 | |
| R | Phe408/Met409; LysPhe insertion | 1 (2) | 156 | |
| NC | Asp410Asn | 1 (2) | 241 | |
| NC | Asp410Asn; Leu427Pro | 1 (2) | 241 | |
| R | Ser416Cys | 1 (2) | 158 | |
| R | His420Asp | 1 (2) | 156 | |
| R | His420Tyr | 11 (20) | 241 | |
| R | Ser425Leu | 33 (60) | 51, 52, 156, 241, 251, 441 | |
| R | Ser425Met | 1 (2) | 156 | |
| R | Ser425Met; Leu427Val | 1 (2) | 51 | |
| R | Ser425Phe | 1 (2) | 156 | |
| NC | Ser425Trp | 1 (2) | 241 | |
| Dapsone/folP1 | R | Thr53Ala | 12 (40) | 191, 241, 437 |
| NC | Thr53Ala; Pro55Leu | 1 (3) | 241 | |
| R | Thr53Arg | 2 (7) | 437 | |
| R | Thr53Ile | 4 (13) | 241, 439 | |
| R | Pro55Arg | 3 (10) | 437, 439 | |
| R | Pro55Leu | 8 (27) | 191, 241, 437 | |
| Ofloxacin/gyrA | NC | Gly89Cys | 1 (14) | 241 |
| R | Ala91Val | 6 (86) | 51, 241 |
R, resistant phenotype, as determined by mouse footpad or radiorespirometry (Buddemeyer) drug susceptibility analysis; NC, not confirmed by either assay.
Percentage (rounded to the nearest whole number) of isolates in each drug-resistant group that contain a specific mutation.
Rifampin (3-{[(4-methyl-1-piperazinyl)-imino]-methyl}rifamycin) is the key bactericidal component of all recommended antileprosy chemotherapeutic regimens. A single dose of 1,200 mg can reduce the number of viable bacilli in a patient's skin to undetectable levels within a few days (232). The target for rifampin in mycobacteria and E. coli is the β-subunit of the RNA polymerase encoded by rpoB (156, 178, 403, 441). Comparison of the deduced primary structures of β-subunit proteins from several bacteria to that of M. leprae demonstrated that M. leprae shares six highly conserved functional regions common to this enzyme in bacteria.
Mycobacterial resistance to rifampin correlates with changes in the structure of the β-subunit of the DNA-dependent RNA polymerase, primarily due to missense mutations within codons of a highly conserved region of the rpoB gene referred to as the rifampin resistance-determining region (280, 403, 441). Rifampin resistance in M. leprae also correlates with missense mutations within this region of rpoB (156). Substitutions within codon Ser425 have been shown to be the most frequent mutations associated with the development of the rifampin-resistant phenotype in M. leprae (Table 10).
Clofazimine [3-(p-chloroanilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)phenazine] is a substituted iminophenazine with antimycobacterial activity for which the mechanism has not been fully elucidated (175). Clofazimine attains high intracellular levels in mononuclear phagocytic cells, its metabolic elimination is slow, it has an anti-inflammatory effect, and the incidence of resistance to it in M. leprae is low. It is highly lipophilic and appears to bind preferentially to mycobacterial DNA (171). Binding of the drug to DNA appears to occur principally at base sequences containing guanine, explaining clofazimine's preference for the G+C-rich genomes of mycobacteria over human DNA.
Lysophospholipids appear to mediate the activity of clofazimine in some gram-positive bacteria (83). However, it is unclear whether this mechanism of action is operational in M. leprae. Since clofazimine may act through several different mechanisms, it is not difficult to understand why only a few cases of clofazimine-resistant leprosy have been reported over the years (84, 256, 368).
Clarithromycin is a semisynthetic macrolide that differs from erythromycin in its methyl substitution at the number 6 position of the macrolide ring (reviewed in reference 424). It displays significant bactericidal activity against M. leprae in humans (127, 173). In patients with lepromatous leprosy, daily administration of 500 mg of clarithromycin kills 99% of viable M. leprae within 28 days and >99.9% by 56 days. Although the mechanism of action of this antibiotic against M. leprae is unknown, it is thought to be similar to that of erythromycin, which acts by inhibiting protein synthesis by binding to the ribosome.
Clarithromycin resistance in bacteria and mycobacteria appears to be due to a decrease in binding of the drug to ribosomes and is associated with missense mutations within the 23S rRNA gene (257, 424, 428). This has not yet been established to be the case with M. leprae, however, and in a recent study no mutations were observed within the 23S rRNA gene in clarithromycin-resistant M. leprae strains (450).
Minocycline (7-dimethylamino-6-demethyl-6-deoxytetracycline) is the only member of the tetracycline group of antibiotics to demonstrate significant activity against M. leprae, presumably due to its lipophilic property, which may enhance cell wall penetration (126, 176). Minocycline is bactericidal for M. leprae, and its activity is additive when it is combined with dapsone and rifampin. The mechanism of action of minocycline against M. leprae is unknown but is thought to be similar to that of all tetracyclines, which act by inhibiting protein synthesis. Tetracyclines bind reversibly to the 30S ribosomal subunit, blocking the binding of aminoacyl-tRNA to the mRNA-ribosome complex (402).
Resistance to tetracycline may be mediated by three different mechanisms: an energy-dependent efflux of tetracycline brought about by an integral membrane protein; ribosomal protection by a soluble protein; or enzymatic inactivation of tetracycline. The molecular mechanism of minocycline resistance has not been studied in M. leprae primarily because this drug has only recently been used widely (in the treatment of single-lesion, paucibacillary leprosy), and resistant strains have not been identified.
Ofloxacin (4-fluoroquinolone) is a fluorinated carboxyquinolone that has moderate anti-M. leprae activity (175, 176). The mechanism of action of ofloxacin on M. leprae is unknown, but in other bacteria it appears to inhibit DNA replication by inhibiting the DNA gyrase, a tetramer containing two A-subunits (GyrA) and two B-subunits (GyrB) (99).
Mutations within a highly conserved region of gyrA, the quinolone resistance-determining region, are associated with the development of ofloxacin resistance in most resistant strains of mycobacteria (53, 401). The quinolone resistance-determining region of M. leprae gyrA is highly homologous to that of M. tuberculosis, and missense mutations within this region have been found in ofloxacin-resistant strains of M. leprae (Table 10).
Development of Drug Resistance in M. leprae
Lacking direct evidence for the mechanisms of M. leprae's resistance to most of the antileprosy drugs, our current understanding is based on studies carried out in M. tuberculosis (280) and other bacteria and limited studies with M. leprae genes in surrogate hosts. From these studies one can predict that drug resistance in M. leprae is attributable to chromosomal mutations in genes encoding drug targets, these mutations occur spontaneously as a result of errors in DNA replication, and these mutants are further enriched in a population by inappropriate or inadequate drug therapy.
Because M. leprae cannot be cultivated in vitro, the frequency of drug-resistant mutants in a population is primarily inferred from studies with M. tuberculosis. For example, the frequency of dapsone-resistant mutants in a population of M. leprae is estimated to be 10−6 and the frequency for rifampin or ofloxacin resistance is estimated at 10−7 to 10−8 (158, 280). Rates of clofazimine resistance in M. leprae are unknown but appear to be relatively low. Since untreated multibacillary patients can harbor large bacterial loads (>1011 M. leprae), it is feasible that a patient could contain up to 105 dapsone-resistant organisms and thousands of rifampin- or ofloxacin-resistant organisms. Inappropriate therapy (noncompliance or inadequate therapy) for these patients has the potential to enrich the subpopulations of drug-resistant M. leprae, leading to the spread of one or more resistant phenotypes. Indeed, drug-resistant isolates of M. leprae have been found in many parts of the world (49, 51, 52, 84, 86, 102).
Detection of Drug-Resistant Leprosy
Leprosy presents a very special problem for the detection of resistance because of the inability to culture M. leprae axenically. Conventional drug susceptibility testing of M. leprae from clinical specimens relies on the ability to cultivate M. leprae in the hind footpads of mice by the method described by Shepard and Chang (364). This method requires the recovery of a sufficient number of viable organisms from a patient to inoculate the footpads of 20 to 40 mice (depending on the number of drugs to be tested), with each footpad receiving 5 × 103 organisms. Results are available after 6 months to 1 year. While this assay gives definitive information pertaining to the susceptibility of an M. leprae isolate to standard antileprosy drugs, it is cumbersome, very expensive, and very slow.
The first rapid drug-screening assays for M. leprae were developed based on radiorespirometry techniques (BACTEC and Buddemeyer) and have been used successfully to identify new antileprosy drugs (117). However, the use of these techniques for drug susceptibility testing in leprosy is limited by a stringent requirement for very large numbers (≥107) of viable organisms from each patient.
Molecular assays for resistance would simplify susceptibility testing and provide a means for monitoring resistance globally. To reduce the number of organisms needed and to minimize the time required for drug susceptibility testing of M. leprae, several protocols based on genotypic identification of drug-resistant mutants have been developed. These techniques are based on the amplification of specific DNA fragments from crude biological specimens (e.g., skin biopsy specimens from leprosy patients) using PCR amplification and detection of mutations associated with drug resistance within these DNA fragments by direct DNA sequencing, single-strand conformation polymorphism analysis, heteroduplex analysis, and solid-phase reverse hybridization analysis, similar to the line probe assay (Table 11).
TABLE 11.
Target genes for M. leprae drug resistance PCR-based assays
PCR-direct DNA sequencing.
Sequencing of the PCR amplicon is the most definitive of all of the nucleic acid-based mutation detection protocols because it detects the actual nucleotide changes in the target gene in which mutations associated with antibiotic resistance are found. In addition, the assay can be designed to be species specific, providing direct evidence for the presence of a particular pathogen in the specimen being tested. PCR-direct DNA sequencing has been used to identify rifampin-, dapsone-, and ofloxacin-resistant mutants of M. leprae (Table 11). These assays are based on PCR amplification of the appropriate target DNA directly from skin biopsy specimens using oligonucleotide primers that are specific for the rifampin, sulfone, or quinolone resistance-determining region of M. leprae. The DNA sequence of these PCR products is then determined and examined for the presence of mutations previously associated with drug resistance. The Ser425Leu mutation in the B-subunit of the RNA polymerase is the most frequently detected mutation associated with rifampin resistance in M. leprae (Table 10). PCR-direct DNA sequencing can be performed in a well-equipped diagnostic laboratory with either manual or automated DNA sequencing systems and requires approximately 1 to 2 days to obtain drug susceptibility results directly from clinical specimens.
PCR-SSCP.
A PCR-single-strand conformation polymorphism (SSCP) assay has been developed to detect rifampin-resistant M. leprae in human specimens (156, 157). To accomplish this, the rifampin resistance-determining region target was amplified by PCR and the double-stranded PCR products were heated to dissociate them into single strands and then separated by denaturing gel electrophoresis under stringently controlled temperature conditions. Gels were then stained to observe DNA fragment mobility patterns, called SSCP profiles. DNA strands from rifampin-susceptible organisms migrate at a rate proportional to their molecular weight and conformation and give a reproducible SSCP profile. The DNA fragment patterns observed with SSCP are highly reproducible and yield profiles unique to specific mutations.
PCR-solid-phase hybridization.
A PCR-solid-phase hybridization assay has recently been developed for the detection of rifampin-resistant M. leprae (158). An initial PCR step with a biotinylated and an unlabeled primer produces an 83-bp, biotinylated fragment of the M. leprae rifampin resistance-determining region. The amplified PCR product is then hybridized to a set of DNA capture probes which have been immobilized at specific sites on a Biodyne C membrane. The immobilized capture probes are small DNA fragments that are homologous to short segments of the rifampin resistance-determining region of the rpoB gene from a rifampin-susceptible strain of M. leprae or specific mutant strains. The stringency of the hybridization reaction is designed so that the PCR product will only bind to probes with 100% sequence homology. The resultant hybrids are detected by chemiluminescence using a streptavidin-peroxidase conjugate. The genotype of the test organism is determined by the capture probes which hybridize.
PCR-heteroduplex analysis.
A PCR heteroduplex-based assay was initially developed to detect the presence of drug-resistant M. tuberculosis from sputum specimens using a universal heteroduplex generator (UHG) (438). A similar approach has been used to develop a PCR-UHG assay to detect the presence of dapsone-resistant M. leprae in skin biopsy homogenates of lepromatous leprosy patients (437). The assay requires PCR amplification of the sulfone resistance-determining region of folP1 and the mixing of these PCR products with a universal heteroduplex generator (UHG-DDS-141), a synthetic 141-bp sulfone resistance-determining region DNA fragment that contains several base pair mismatches flanking codons that are associated with dapsone resistance. When UHG-DDS-141 is denatured by heat and slowly annealed to denatured sulfone resistance-determining region PCR products from M. leprae, the resultant heteroduplexes form unique structures which, when analyzed by electrophoresis, provide enhanced mutation detection over standard heteroduplex detection. Enhanced mutation detection occurs because large areas of unmatched nucleotides (bubbles) in the newly formed duplexes greatly affect the mobility of the resultant DNA fragments. When the heteroduplexes are separated by electrophoresis on polyacrylamide minigels, unique heteroduplex profiles are observed for susceptible and resistant genotypes. PCR-UHG requires approximately 6 h to complete and uses 6% precast nondenaturing Tris-borate-EDTA minigels and a nonradioactive detection format.
PREVENTION: THE QUEST FOR A LEPROSY VACCINE
Vaccinology has grown from an empirical science with little in the way of biological understanding of events to a highly structured science drawing on detailed immunological studies at the cellular and molecular levels of both the host and the infectious agent. The cells, cytokines, and regulatory pathways active in the host's immune response to M. leprae and other mycobacterial pathogens continue to be elucidated, building a foundation for our understanding of the causes of immunopathogenesis and protective immunity. Annotation of the M. leprae genome and bioinformatic processing of the data have changed the way we investigate potential antigens for new vaccines. Given the potential for developing an effective vaccine for leprosy based on these new tools, the debate continues as to whether there is a need for a vaccine in the overall strategy to control or eradicate leprosy.
As discussed above, the current strategy for controlling leprosy is based on the implementation of effective drug regimens set forth by the World Health Organization. Unfortunately, recent epidemiological data suggest that this strategy appears to have had little effect on reducing the annual incidence of new cases of leprosy. Additionally, recent reports have shown that relapse rates of 16 to 39% among multiibacillary patients with high BIs are appearing 4 to 10 years after completion of 2-year multidrug therapy (128, 134, 168). Acknowledging these clinical realities requires an objective assessment of current control strategies and reminds us that other intervention strategies may be necessary to eradicate leprosy. Improved strategies may require new application of old tools, such as special programs designed to improve drug distribution and treatment compliance, but should also include basic research designed to develop tests for early diagnosis as well as pre- and postexposure vaccines for leprosy.
From the standpoint of disease control, vaccines are similar to drugs in that they may be applied as prophylactic (preexposure) or therapeutic (postexposure) measures. Vaccines, however, have a potential added advantage by producing a relatively long-lived immunological memory component. Accordingly, an effective prophylactic vaccine for leprosy could break transmission by conferring upon recipients immediate as well as extended protection from infection with M. leprae. A prophylactic vaccine should also protect against both drug-susceptible and drug-resistant strains, helping curb the emergence of drug resistance. Used as a therapeutic measure for leprosy control, a postexposure vaccine could improve a patient's response to multidrug therapy by hastening a cure and potentially reducing the incidence of relapse cases. While most of these concepts remain hypothetical, evidence is available that antileprosy vaccines can provide various levels of protection as well as limited beneficial therapeutic effects.
The vaccine studied most in leprosy is M. bovis BCG. Experience with BCG vaccination for leprosy remains enigmatic in that levels of protection vary from 20 to 80% (Table 12). These results are not unexpected considering BCG's variable efficacy against tuberculosis (71). Fine (111) has reviewed many of the issues surrounding the curious variability seen with BCG vaccination for tuberculosis. He speculates that factors such as biological differences in BCG strains, exposure to environmental mycobacteria, and ineffective boosting against reinfection with or reactivation of tuberculosis may give rise to the observed variability in protection seen with BCG vaccination. It is likely that some or all of these factors may play a role in the variability seen in BCG vaccine efficacy against leprosy.
TABLE 12.
Summary of estimates of efficacy of BCG and other vaccines against leprosya
| Country | Study population (incidence per 1,000 person-yr) | Placebo | Vaccinationb | Randomized | % Efficacy (95% CI)c | Reference |
|---|---|---|---|---|---|---|
| Uganda | Child contact (3.1) | Unvaccinated | BCG Glaxo | Yes | 80 (72-85) | 390 |
| Venezuela | Close contacts (3.1) | BCG without prior scar | BCG with prior scar(s) | No | 56 (27-74) | 76 |
| Malawi | Total population (1.3) | No scar | BCG Glaxo, scar | No | 54 (35-68) | 302 |
| Same cohort with restrictions of no early lesion at start | 65 (50-75) | |||||
| Malawi | Total population (0.85) | Placebo with BCG scar | BCG Glaxo with prior BCG scar, with or without HK M. leprae | Yes | 49 (1-74) | 204 |
| Papua New Guinea | Total population (5.4) | Saline | BCG Japan, 4 batches | Yes? | 48 (34-59) | 23 |
| India | HHCd of active BB, BL, and LL patients (16.8) | Unvaccinated | HK M. leprae/BCG Japan/Mix BCG | No | 42 (1-66) | 60 |
| India | General population (unknown) | Saline | BCG | Yes | 34 (14-50) | 140 |
| BCG + HK M. leprae | Yes | 71 (54-81) | ||||
| M. w. | Yes | 31 (3-51) | ||||
| ICRC bacillus | Yes | 65 (47-78) | ||||
| India | Total population (unknown) | Placebo | 2 BCG strains, French-Danish | Yes | 30 (10-38) | 411 |
| Burma | Children ages 0-14 (5.5) | Unvaccinated | BCG Glaxo, 2 batches | Yes? | 20 (12-28) | 240 |
Modified from reference 23a with permission of the publisher.
HK, heat-killed.
CI, confidence interval.
HHC, household contacts.
Because leprosy is a relatively uncommon disease entity, even in countries with the highest prevalence, case-control studies have been particularly useful in obtaining information concerning vaccine efficacy. Two recent case-control studies, in India (455) and in Brazil (78), provide clear evidence that BCG protects against leprosy. In the Brazilian study, the investigators argue that their results suggest that neonatal BCG vaccination may have an important impact on transmission of leprosy and that environmental mycobacteria may not impact vaccine efficacy, at least in the Amazon region of Brazil. Zodpey and coworkers showed an overall vaccine efficacy of 54%, with the greatest protective effect seen for multibacillary leprosy (68%), suggesting that an effective vaccine for leprosy, like BCG, appears to have its greatest effect on the disease form (lepromatous) most likely to transmit M. leprae within the community.
Three relatively recent vaccine trials have studied the hypothesis that combining BCG with killed M. leprae improves vaccine efficacy. The primary reason for testing this hypothesis is to determine whether M. leprae-specific antigens are able to improve the efficacy of BCG. The earliest of the three studies (76) was conducted in Venezuela and concluded that the protective efficacy of BCG was proportional to the number of doses (i.e., the number of BCG scars) and that protection against leprosy was not improved when heat-killed M. leprae was combined with BCG. Similarly, in the double-blind, controlled trial done in Malawi (204), no advantage was observed by including heat-killed M. leprae (HKML) along with BCG. However, among scar-positive individuals, a second BCG vaccination gave further protection against leprosy (about 50%) over a first BCG vaccination.
In contrast, the vaccine trial done in southern India showed no positive effect of vaccinating BCG scar-positive individuals, but enhanced protection over that with BCG alone was seen in individuals receiving BCG plus HKML (140). Additional arms in the southern India vaccine trial included two atypical mycobacteria, Mycobacterium w (207) and ICRC bacillus (313). Both vaccines are nonliving preparations, and both were superior to BCG alone with respect to percent protection at the second and third resurveys of the trial. Interestingly, ICRC alone and BCG plus HKML gave approximately the same level of protection (64 and 65%, respectively), suggesting that a killed mycobacterial vaccine containing M. leprae cross-reactive antigens is as effective as a live, attenuated BCG vaccine in this population and setting. So, while the controversy continues over what elements of a leprosy vaccine are superior in a particular setting, there is little or no controversy over the positive effects of vaccination to reduce leprosy incidence.
Less is known about the application of leprosy vaccines for therapeutic purposes. A number of reports using Mycobacterium w as immunotherapy suggest that when given as adjunct therapy to multidrug therapy, significant clinical improvement does result (88, 206, 453). For example, De Sarkar et al. (88) observed significant improvement in both clinical and histopathological assessments of lesions in patients receiving Mycobacterium w plus multidrug therapy. In addition, patients vaccinated with Mycobacterium w demonstrated reduced bacillary indices over time compared to patients receiving only multidrug therapy for 12 months. An important finding in this study, corroborating findings from an earlier study (453), was that patients in the vaccine group experienced a higher percentage (30%) of type 1 reactions compared to the control group (10%). Fortunately, neuritis was not increased with vaccination in patients with or without reactions, a finding also reported in the earlier study (453). In contrast to the increased incidence of type 1 reactions following vaccination with Mycobacterium w, the incidence of type 2 reactions was similar in controls and vaccinated patients. This contrasts with the experience, noted above, that cytokine treatments using IFN-γ led to upgrading of LL and BL lesions and were accompanied by a high incidence of ENL (332). These findings underscore the challenge of producing an effective postexposure leprosy vaccine with the purpose of increasing cell-mediated immunity without inducing harmful sequelae.
Another important piece of the puzzle that is propelling investigators to search for new molecules with vaccine potential is the completion of the genome sequences of M. leprae (74) and M. tuberculosis (73). Prior to the complete sequencing of the M. leprae genome, the only reliable source of M. leprae antigens was highly purified bacilli originating from infected armadillo tissues. While many major compounds of M. leprae have been discovered and studied in detail using this material, purified proteins have been available in very limited quantities and of poor quality by contemporary standards, making them difficult to use for vaccine development. In addition, certain secreted proteins are almost surely lost upon purification of the bacilli from infected armadillo tissues.
New approaches to identifying genes from completed M. leprae genome sequences are being applied using standardized bioinformatics tools (253). These tools can identify proteins with special features, such as unique or shared amino acid sequence homologies with proteins of M. tuberculosis or other mycobacterial species, and the presence of specialized peptide signatures suggesting their cellular location and possible secretion across the cell membrane. Proteins of interest can be prioritized based on potential B-cell or T-cell epitopes, although strict associations between bioinformatics tools and antigenic epitopes remain underdeveloped. Finally, the proteins selected for further study can be purified as recombinant proteins for an endless supply of the protein for immunologic and vaccine studies (255, 289, 386, 442).
In addition to newly available search algorithms for genes of interest, new vehicles for delivery of protein antigens have been identified from research on recombinant DNA over the last 20 years. Much of this technology is being used to create vaccines to be administered in concert with BCG, either as recombinant BCG overexpressing one or more antigenic proteins or in a prime-boost scenario where antigen is given first in an adjuvant (priming) and then followed by BCG vaccination to boost the initial response. Since BCG is given at birth in many countries, the standard prime-boost schedule for leprosy and tuberculosis vaccines would likely be reversed. Supporting this reversal of priming and boosting with BCG are three studies using different protein vaccines for tuberculosis that have shown favorable protective responses in animal models (36, 254, 271). Application of this strategy for designing leprosy vaccines should not present any unique hurdles now that M. leprae antigens of interest can be cloned and expressed in large quantities.
Recalling the results of earlier vaccine trials with BCG reminds us that it is a fairly potent vaccine for leprosy in many settings. Newly designed vaccines for tuberculosis that may utilize BCG altered through genetic engineering or through a prime-boost strategy may or may not provide better protection against leprosy than is afforded by current BCG vaccines. Accordingly, newly configured vaccines for tuberculosis should be tested for efficacy against M. leprae challenge to show that benefits for leprosy control through vaccination for tuberculosis are not lost in the process.
While the immunogenicity of various candidate proteins can be tested in vitro using human cells, animal models in which to test the efficacy of a new vaccine are limited to the mouse and armadillo. Shepard's mouse footpad model is the gold standard for leprosy vaccine studies (366). The infection that develops in the mouse footpad may best be described as limited multiplication at the site of infection and may be somewhat analogous to indeterminate or tuberculoid leprosy in humans. Since many indeterminate cases self-heal and tuberculoid leprosy in humans involves nerve damage (not seen in mice), this model for defining vaccine efficacy has important limitations. The model does, however, enable screening out of vaccines with little or no protective efficacy against a challenge with live M. leprae. Vaccines that are effective in the mouse model could then be tested in the armadillo, an animal that manifests most of the characteristics of human leprosy.
Finally, vaccine efficacy must be tested in humans following appropriate safety and potency trials. These trials must be large due to the relatively low incidence of leprosy in most settings, which continues to be an obstacle for assessing vaccines as disease management tools in leprosy. Therapeutic vaccine trials could be more focused and evaluated in smaller, defined groups of patients. With the advent of a better understanding of the molecular nature of M. leprae and the human response to infection, new vaccines will become a reality. The challenge for the research and public health systems is to unite the political will and financial resources to test one or more of the new vaccines for leprosy.
SUMMARY AND CONCLUSIONS
Based on operational data, with its inherent biases, the number of new cases of leprosy identified annually worldwide has probably not changed over the last two decades, although the number of registered cases has declined as a result of treatment and removal from registries. This disease poses major challenges to our understanding in microbiology, immunology, pathology, treatment, and prevention, requiring continued emphasis on basic research and clinical management in the field. Reviewed above are major advances that have been made in the basic understanding of leprosy since 1990, including the following.
The genome of M. leprae has been sequenced, and this organism has been shown to be able to synthesize far fewer proteins than the other major human-pathogenic mycobacterium, M. tuberculosis. Thus, although M. leprae still cannot be cultivated axenically, the new molecular ability to assess its ability to transcribe and synthesize various proteins in response to different environments and stresses will likely provide valuable information about its mechanisms of pathogenicity in the near future.
PCR analysis of tissues for M. leprae DNA now provides a valuable means for identifying this organism. Mutations in the M. leprae genome that are associated with resistance to several of the drugs used against this pathogen have been identified, and DNA analysis to detect these mutations is likely to replace the mouse footpad technique.
Major advances have been made in experimental models of leprosy. The availability of knockout mice deficient in selected immunologic abilities will enable dissection of the roles of different cytokines and T-cell subsets in the response to this infection. The armadillo genome has been partially sequenced and is being annotated; this will soon enable investigators to identify and synthesize immunologically relevant cytokines and probes for use in this animal model.
Within macrophages, M. leprae is now known to be killed by reactive nitrogen compounds. A variety of mechanisms of innate and adaptive immunity have been identified and postulated to play a role in the development of cellular immunity in leprosy. None of these can yet explain the remarkable spectrum of cellular immune responses to this organism in human subjects, however.
Genetic influences on immunity to M. leprae in humans appear to operate at two levels: some mechanisms act at the level of overall susceptibility, and others function at the level of acquired immunity. One leprosy susceptibility gene has been identified, and several genes possibly influencing adaptive immunity have also been described.
Reactions in leprosy remain poorly understood. Immunopathological studies have generally found that type 1 (reversal) reactions correspond to an up-regulation of Th-1-type immune responses, and that type 2 reactions (ENL) correspond to an enhancement of the Th-2 type of response. These findings are not yet very satisfying, however, since there are several unresolved discrepancies in these associations, and no information thus far indicates what triggers reactions or why they affect some patients but not others.
The mechanisms of nerve injury in leprosy remain poorly understood, although recent studies have shed light on the mechanisms of localization of M. leprae to nerves and on the molecular mechanisms of binding and ingestion of M. leprae by Schwann cells. A major frontier in clinical leprosy is the prevention of disability by active and persistent attention to nerve function impairment, since nerve injury may progress even after completion of chemotherapy.
Several effective antimicrobial agents are now available to treat leprosy, and this infection is curable. Molecular methods can now identify several mutations in M. leprae associated with antimicrobial resistance. The medically conservative approach to treatment recommends using multiple agents (multidrug therapy) for several months or years, depending on the classification of the disease. The World Health Organization has recommended much shorter treatment protocols, and these have been the subject of much controversy among physicians treating patients with leprosy.
BCG provides a low but measurable degree of protection against M. leprae, but no highly effective, specific vaccine has yet been developed. No infectious disease has been eliminated using drug treatment alone, without an effective vaccine, but the difficulties of implementation of a leprosy vaccine also pose profound challenges to the use of such a vaccine if one is developed.
Future of Leprosy Treatment and Research
Global elimination of leprosy has been the overarching goal of laboratory research and health policy for nearly 20 years. This was not accomplished by 2000 or 2005 and does not appear likely, probably due to a complex mixture of social, economic, and biological factors that cannot be resolved in the laboratory alone. Elimination of an infectious disease requires a highly effective vaccine; developing one was the central focus of leprosy research during the 1980s and early 1990s. This has not been successful thus far, although vaccine efforts continue.
With elimination still in mind, the current primary goal is early diagnosis, in order to try to interrupt transmission with treatment as early as possible. This is the major focus of the most concerted laboratory research efforts today, employing advanced molecular tools. Even if this should become technically feasible in the near future, this concept faces enormous challenges to verification before treatment of asymptomatic individuals can be recommended. Implementing treatment of asymptomatic persons would be even more difficult; at this writing, in many areas with endemic leprosy, even patients with overt disease are finding that resources for diagnosis and treatment are being systematically reduced.
An alternative paradigm to elimination of leprosy is living with leprosy but rendering it harmless, an idea advanced by Yo Yuasa of the Sasakawa Memorial Health Foundation (451). Recognizing the high cost and apparent futility of elimination campaigns in the most highly leprosy-endemic regions of the world, this approach calls for improved tools for management of the infection and its complications and better methods for the prevention and treatment of nerve injury. Both of these paradigms, as well as the tension between them, reflect the continuing challenges of leprosy.
Acknowledgments
The photographs in Fig. 2 were taken by G. McCormick, NHDP. Figure 6 was created by T. Thomassie, NHDP.
This work was supported in part by NIH grants AI050027 and AI045725 from the National Institute of Allergy and Infectious Diseases, NIAID contract Y1-AI-2646-01, and grants from the Heiser Foundation and from American Leprosy Missions.
REFERENCES
- 1.Abel, L., and F. Demenais. 1988. Detection of major genes for susceptibility to leprosy and its subtypes in a Caribbean island: Desirade Island. Am. J. Hum. Genet. 42:256-266. [PMC free article] [PubMed] [Google Scholar]
- 2.Abel, L., F. O. Sanchez, J. Oberti, N. V. Thuc, L. V. Hoa, V. D. Lap, E. Skamene, P. H. Lagrange, and E. Schurr. 1998. Susceptibility to leprosy is linked to the human NRAMP1 gene. J. Infect. Dis. 177:133-145. [DOI] [PubMed] [Google Scholar]
- 3.Adams, L. B., M. C. Dinauer, D. E. Morgenstern, and J. L. Krahenbuhl. 1997. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tubercle Lung Dis. 7 8:237-246. [DOI] [PubMed] [Google Scholar]
- 4.Adams, L. B., S. G. Franzblau, Z. Vavrin, J. B. Hibbs, Jr., and J. L. Krahenbuhl. 1991. l-Arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J. Immunol. 147:1642-1646. [PubMed] [Google Scholar]
- 5.Adams, L. B., Y. Fukutomi, and J. L. Krahenbuhl. 1993. Regulation of murine macrophage effector functions by lipoarabinomannan from mycobacterial strains with different degrees of virulence. Infect. Immun. 61:4173-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams, L. B., C. K. Job, and J. L. Krahenbuhl. 2000. Role of inducible nitric oxide synthase in resistance to Mycobacterium leprae infection in mice. Infect. Immun. 68:5462-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams, L. B., D. M. Scollard, N. A. Ray, A. M. Cooper, A. A. Frank, I. M. Orme, and J. L. Krahenbuhl. 2002. The study of Mycobacterium leprae infection in interferon-γ gene-disrupted mice as a model to explore the immunopathologic spectrum of leprosy. J. Infect. Dis. 185:S1-S8. [DOI] [PubMed] [Google Scholar]
- 8.Alcais, A., F. O. Sanchez, N. V. Thuc, V. D. Lap, J. Oberti, P. H. Lagrange, E. Schurr, and L. Abel. 2000. Granulomatous reaction to intradermal injection of lepromin (Mitsuda reaction) is linked to the human NRAMP1 gene in Vietnamese leprosy sibships. J. Infect. Dis. 181:302-308. [DOI] [PubMed] [Google Scholar]
- 9.Allavena, P., M. Chieppa, P. Monti, and L. Piemonti. 2004. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit. Rev. Immunol. 24:179-192. [DOI] [PubMed] [Google Scholar]
- 10.Allegra, C. J., D. Boarman, J. A. Kovacs, P. Morrison, J. Beaver, B. A. Chabner, and H. Masur. 1990. Interaction of sulfonamide and sulfone compounds with Toxoplasma gondii dihydropteroate synthase. J. Clin. Investig. 85:371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves, L., L. de Mendonca Lima, E. da Silva Maeda, L. Carvalho, J. Holy, E. N. Sarno, M. C. Pessolani, and L. P. Barker. 2004. Mycobacterium leprae infection of human Schwann cells depends on selective host kinases and pathogen-modulated endocytic pathways. FEMS Microbiol. Lett. 238:429-437. [DOI] [PubMed] [Google Scholar]
- 12.Andersson, A. K., M. Chaduvula, S. E. Atkinson, S. Khanolkar-Young, S. Jain, L. Suneetha, S. Suneetha, and D. N. Lockwood. 2005. Effects of prednisolone treatment on cytokine expression in patients with leprosy type 1 reactions. Infect. Immun. 73:3725-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade, V. L., J. C. Avelleira, A. Marques, and F. R. Vianna. 1991. Leprosy as cause of false positive results in serological assays for the detection of antibodies to HIV-1. Int. J. Lepr. Other Mycobact. Dis. 59:125-126. [PubMed] [Google Scholar]
- 14.Anthony, L. S. D., D. Chatterjee, P. Brennan, and F. E. Nano. 1994. Lipoarabinomannan from Mycobacterium tuberculosis modulates the generation of reactive nitrogen intermediates by gamma interferon-activated macrophages. FEMS Immunol. Med. Microbiol. 8:299-306. [DOI] [PubMed] [Google Scholar]
- 15.Antunes, A., J. Nina, and S. David. 2002. Serological screening for tuberculosis in the community: an evaluation of the Mycodot procedure in an African population with high HIV-2 prevalence (Republic of Guinea-Bissau). Res. Microbiol. 153:301-305. [DOI] [PubMed] [Google Scholar]
- 16.Arnoldi, J., J. Gerdes, and H.-D. Flad. 1990. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am. J. Pathol. 137:749-753. [PMC free article] [PubMed] [Google Scholar]
- 17.Arnoldi, J., C. Schluter, M. Duchrow, L. Hubner, M. Ernst, A. Teske, H. D. Flad, J. Gerdes, and E. C. Bottger. 1992. Species-specific assessment of Mycobacterium leprae in skin biopsies by in situ hybridization and polymerase chain reaction. Lab. Investig. 66:618-623. [PubMed] [Google Scholar]
- 18.Arunthathi, S., L. Ebenezer, and C. Kumuda. 1998. Reversal reaction, nerve damage and steroid therapy in three multibacillary HIV positive patients. Lepr. Rev. 69:173-177. [DOI] [PubMed] [Google Scholar]
- 19.Aseffa, A., M. A. Dietrich, and E. J. Shannon. 1997. Effect of thalidomide on apoptosis of lymphocytes and neutrophils. Immunopharmacol. Immunotoxicol. 19:313-326. [DOI] [PubMed] [Google Scholar]
- 20.Astarie-Dequeker, C., E.-N. N'Diaye, V. Le Cabec, M. G. Rittig, J. Prandi, and I. Mariconneau-Parini. 1999. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun. 67:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkinson, S. E., S. Khanolkar-Young, S. Marlowe, S. Jain, R. G. Reddy, S. Suneetha, and D. N. Lockwood. 2004. Detection of IL-13, IL-10, and IL-6 in the leprosy skin lesions of patients during prednisolone treatment for type 1 (T1R) reactions. Int. J. Lepr. Other Mycobact. Dis. 72:27-34. [DOI] [PubMed] [Google Scholar]
- 22.Azouaou, N., R. H. Gelber, K. Abel, D. T. Sasaki, L. P. Murray, R. M. Locksley, and N. Mohagheghpour. 1993. Reconstitution of Mycobacterium leprae immunity in severe combined immunodeficient mice using a T-cell line. Int. J. Lepr. Other Mycobact. Dis. 61:398-405. [PubMed] [Google Scholar]
- 23.Bagshawe, A., G. C. Scott, D. A. Russell, S. C. Wigley, A. Merianos, and G. Berry. 1989. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea, 1963-79. Bull. W. H. O. 67:389-399. [PMC free article] [PubMed] [Google Scholar]
- 23a.Bakker, M. J. 2005. Epidemiology and prevention of leprosy: a cohort study in Indonesia. Ph.D. thesis. KIT Biomedical Research, Amsterdam, The Netherlands.
- 24.Barnes, P. F., D. Chatterjee, J. S. Abrams, S. Lu, E. Wang, M. Yamamura, P. J. Brennan, and R. L. Modlin. 1992. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan: relationship to chemical structure. J. Immunol. 149:541. [PubMed] [Google Scholar]
- 25.Barnes, P. F., D. Chatterjee, P. J. Brennan, T. H. Rea, and R. L. Modlin. 1992. Tumor necrosis factor production in patients with leprosy. Infect. Immun. 60:1441-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnetson, R. S., G. Bjune, J. M. Pearson, and G. Kronvall. 1976. Cell mediated and humoral immunity in “reversal reactions.” Int. J. Lepr. Other Mycobact. Dis. 44:267-274. [PubMed] [Google Scholar]
- 27.Barshes, N. R., S. E. Goodpastor, and J. A. Goss. 2004. Pharmacologic immunosuppression. Front. Biosci. 9:411-420. [DOI] [PubMed] [Google Scholar]
- 28.Becx-Bleumink, M., and D. Berhe. 1992. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy: experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. Other Mycobact. Dis. 60:173-184. [PubMed] [Google Scholar]
- 29.Bedi, B. M., E. B. Harris, E. Narayanan, and W. F. Kirchheimer. 1976. Delayed hypersensitivity tests with Mycobacterium leprae purified protein derivative. Lepr. India 48:8-18. [PubMed] [Google Scholar]
- 30.Beiguelman, B., and R. Quagliato. 1965. Nature and familial character of the lepromin reactions. Int. J. Lepr. Other Mycobact. Dis. 33:800-807. [PubMed] [Google Scholar]
- 31.Belliappa, A. D., R. M. Bhat, and J. Martis. 2002. Leprosy in type I reaction and diabetes mellitus in a patient with HIV infection. Int. J. Dermatol. 41:694-695. [DOI] [PubMed] [Google Scholar]
- 32.Beyene, D., A. Aseffa, M. Harboe, D. Kidane, M. Macdonald, P. R. Klatser, G. A. Bjune, and W. C. Smith. 2003. Nasal carriage of Mycobacterium leprae DNA in healthy individuals in Lega Robi village, Ethiopia. Epidemiol. Infect. 131:841-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackwell, J. M., S. Searle, T. Goswami, and E. N. Miller. 2000. Understanding the multiple functions of Nramp1. Microbes Infect. 2:317-321. [DOI] [PubMed] [Google Scholar]
- 34.Blum, L., B. Flageul, S. Sow, P. Launois, M. D. Vignon-Pennamen, A. Coll, and J. Millan. 1993. Leprosy reversal reaction in HIV-positive patients. Int. J. Lepr. Other Mycobact. Dis. 61:214-217. [PubMed] [Google Scholar]
- 35.Bochud, P. Y., T. R. Hawn, and A. Aderem. 2003. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 170:3451-3454. [DOI] [PubMed] [Google Scholar]
- 36.Brandt, L., Y. A. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 72:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brennan, P. J. 2000. Skin test development in leprosy: progress with first-generation skin test antigens, and an approach to the second generation. Lepr. Rev. 71(Suppl.):S50-S54. [DOI] [PubMed] [Google Scholar]
- 38.Brennan, P. J., and V. D. Vissa. 2001. Genomic evidence for the retention of the essential mycobacterial cell wall in the otherwise defective Mycobacterium leprae. Lepr. Rev. 72:415-428. [DOI] [PubMed] [Google Scholar]
- 39.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 40.Britton, W. J. 1998. The management of leprosy reversal reactions. Lepr. Rev. 69:225-234. [DOI] [PubMed] [Google Scholar]
- 41.Britton, W. J., L. Hellqvist, A. Basten, and R. L. Raison. 1985. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135:4171-4177. [PubMed] [Google Scholar]
- 42.Britton, W. J., and D. N. Lockwood. 2004. Leprosy. Lancet 363:1209-1219. [DOI] [PubMed] [Google Scholar]
- 43.Brosch, R., S. V. Gordon, K. Eiglmeier, T. Garnier, and S. T. Cole. 2000. Comparative genomics of the leprosy and tubercle bacilli. Res. Microbiol. 151:135-142. [DOI] [PubMed] [Google Scholar]
- 44.Browne, S. G., and L. M. Hogerzeil. 1962. “B 663” in the treatment of leprosy. Preliminary report of a pilot trial. Lepr. Rev. 33:6-10. [PubMed] [Google Scholar]
- 45.Bruce, S., T. L. Schroeder, K. Ellner, H. Rubin, T. Williams, and J. E. Wolf, Jr. 2000. Armadillo exposure and Hansen's disease: an epidemiologic survey in southern Texas. J. Am. Acad. Dermatol. 43:223-228. [DOI] [PubMed] [Google Scholar]
- 46.Buhrer, S. S., H. L. Smits, G. C. Gussenhoven, C. W. van Ingen, and P. R. Klatser. 1998. A simple dipstick assay for the detection of antibodies to phenolic glycolipid-I of Mycobacterium leprae. Am. J. Trop. Med. Hyg. 58:133-136. [DOI] [PubMed] [Google Scholar]
- 47.Burdick, A. A., and C. C. Ramirez. 2005. The role of mycophenolate mofetil in the treatment of leprosy reactions. Int. J. Lepr. Other Mycobact. Infect. 73:127-128. [PubMed] [Google Scholar]
- 48.Buschman, E., and E. Skamene. 2004. Linkage of leprosy susceptibility to Parkinson's disease genes. Int. J. Lepr. Other Mycobact. Dis. 72:169-170. [DOI] [PubMed] [Google Scholar]
- 49.Butlin, C. R., K. D. Neupane, S. S. Failbus, A. Morgan, and W. J. Britton. 1996. Drug resistance in Nepali leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 64:136-141. [PubMed] [Google Scholar]
- 50.Bwire, R., and H. J. Kawuma. 1994. Type 1 reactions in leprosy, neuritis and steroid therapy: the impact of the human immunodeficiency virus. Trans. R Soc. Trop. Med. Hyg. 88:315-316. [DOI] [PubMed] [Google Scholar]
- 51.Cambau, E., P. Bonnafous, E. Perani, W. Sougakoff, B. Ji, and V. Jarlier. 2002. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin. Infect. Dis. 34:39-45. [DOI] [PubMed] [Google Scholar]
- 52.Cambau, E., E. Perani, I. Guillemin, P. Jamet, and B. Ji. 1997. Multidrug-resistance to dapsone, rifampicin, and ofloxacin in Mycobacterium leprae. Lancet 349:103-104. [DOI] [PubMed] [Google Scholar]
- 53.Cambau, E., W. Sougakoff, and V. Jarlier. 1994. Amplification and nucleotide sequence of the quinolone resistance-determining region in the gyrA gene of mycobacteria. FEMS Microbiol. Lett. 116:49-54. [DOI] [PubMed] [Google Scholar]
- 54.Carus, N. H., M. B. Raizman, D. L. Williams, and A. S. Baker. 1995. Relapse of Mycobacterium leprae infection with ocular manifestations. Clin. Infect. Dis. 20:776-780. [DOI] [PubMed] [Google Scholar]
- 55.Cellona, R. V., M. F. Balagon, E. C. dela Cruz, J. A. Burgos, R. M. Abalos, G. P. Walsh, R. Topolski, R. H. Gelber, and D. S. Walsh. 2003. Long-term efficacy of 2 year WHO multiple drug therapy (MDT) in multibacillary (MB) leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 71:308-319. [DOI] [PubMed] [Google Scholar]
- 56.Chan, E. S., and B. N. Cronstein. 2002. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 4:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan, G. P., B. Y. Garcia-Ignacio, V. E. Chavez, J. B. Livelo, C. L. Jimenez, M. L. Parrilla, and S. G. Franzblau. 1994. Clinical trial of clarithromycin for lepromatous leprosy. Antimicrob. Agents Chemother. 38:515-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan, J., T. Fujiwara, P. Brennan, M. McNeil, S. J. Turco, J.-C. Sibille, M. Snapper, P. Aisen, and B. R. Bloom. 1989. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc. Natl. Acad. Sci. USA 86:2453-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatterjee, D., A. D. Roberts, K. Lowell, P. J. Brennan, and I. M. Orme. 1992. Structural basis for the capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect. Immun. 60:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhury, S., S. K. Hazra, B. Saha, B. Mazumder, P. C. Biswas, D. Chattopadhya, and K. Saha. 1994. An eight-year field trial on antileprosy vaccines among high-risk household contacts in the Calcutta metropolis. Int. J. Lepr. Other Mycobact. Dis. 62:389-394. [PubMed] [Google Scholar]
- 61.Chehl, S., C. K. Job, and R. C. Hastings. 1985. Transmission of leprosy in nude mice. Am. J. Trop. Med. Hyg. 34:1161-1166. [DOI] [PubMed] [Google Scholar]
- 62.Chemouilli, P., S. Woods, G. Said, and S. T. Cole. 1996. Detection of Mycobacterium leprae in nerve lesions by the polymerase chain reaction. Int. J. Lepr. Other Mycobact. Dis. 64:1-5. [PubMed] [Google Scholar]
- 63.Chen, X. S., W. Z. Li, C. Jiang, and G. Y. Ye. 1999. Studies on risk of leprosy relapses in China: relapses after treatment with multidrug therapy. Int. J. Lepr. Other Mycobact. Dis. 67:379-387. [PubMed] [Google Scholar]
- 64.Chin-A-Lien, R. A., W. R. Faber, and B. Naafs. 1994. Cyclosporin A treatment in reversal reaction. Trop. Geogr. Med. 46:123-124. [PubMed] [Google Scholar]
- 65.Chiplunkar, S., G. De Libero, and S. H. Kaufmann. 1986. Mycobacterium leprae-specific Lyt-2+ T lymphocytes with cytolytic activity. Infect. Immun. 54:793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiplunkar, S. V., M. V. Deshmukh, P. D. Samson, R. Butlin, W. S. Bhatki, R. G. Chulawalla, M. G. Deo, and S. G. Gangal. 1990. Natural killer-cell-mediated and antibody-dependent cellular cytotoxicity in leprosy. Int. J. Lepr. Other Mycobact. Dis. 58:334-341. [PubMed] [Google Scholar]
- 67.Choudhury, A., N. F. Mistry, and N. H. Antia. 1989. Blocking of Mycobacterium leprae adherence to dissociated Schwann cells by anti-mycobacterial antibodies. Scand. J. Immunol. 30:505-509. [DOI] [PubMed] [Google Scholar]
- 68.Clark-Curtiss, J. E., and M. A. Docherty. 1989. A species-specific repetitive sequence in Mycobacterium leprae DNA. J. Infect. Dis. 159:7-15. [DOI] [PubMed] [Google Scholar]
- 69.Clark-Curtiss, J. E., and G. P. Walsh. 1989. Conservation of genomic sequences among isolates of Mycobacterium leprae. J. Bacteriol. 171:4844-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clements, B., and D. M. Scollard. 1996. Leprosy, p. 9.2-9.28. In G. L. Mandell and R. Fekety (ed.), Atlas of infectious diseases. Current Medicine, Philadelphia, Pa.
- 71.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 72.Cole, S. T. 1998. Comparative mycobacterial genomics. Curr. Opin. Microbiol. 1:567-571. [DOI] [PubMed] [Google Scholar]
- 73.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 74.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, J. R. Thomson, R. P. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Color, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, A. Fraser, N. Hamlin, S. Holroyd, T. Hornby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 75.Colston, M. J., and G. R. Hilson. 1976. Growth of Mycobacterium leprae and M. marinum in congenitally athymic (nude) mice. Nature 262:736-741. [DOI] [PubMed] [Google Scholar]
- 76.Convit, J., C. Sampson, M. Zuniga, P. G. Smith, J. Plata, J. Silva, J. Molina, M. E. Pinardi, B. R. Bloom, and A. Salgado. 1992. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet 339:446-450. [DOI] [PubMed] [Google Scholar]
- 77.Couppie, P., S. Abel, H. Voinchet, M. Roussel, R. Helenon, M. Huerre, D. Sainte-Marie, and A. Cabie. 2004. Immune reconstitution inflammatory syndrome associated with HIV and leprosy. Arch. Dermatol. 140:997-1000. [DOI] [PubMed] [Google Scholar]
- 78.Cunha, S. S., L. C. Rodrigues, V. Pedrosa, I. M. Dourado, M. L. Barreto, and S. M. Pereira. 2004. Neonatal BCG protection against leprosy: a study in Manaus, Brazilian Amazon. Lepr. Rev. 75:357-366. [PubMed] [Google Scholar]
- 79.Daffe, M., M. McNeil, and P. J. Brennan. 1993. Major structural features of the cell wall arabinogalactans of Mycobacterium, Rhodococcus, and Nocardia spp. Carbohydr Res. 249:383-398. [DOI] [PubMed] [Google Scholar]
- 80.Dallas, W. S., J. E. Gowen, P. H. Ray, M. J. Cox, and I. K. Dev. 1992. Cloning, sequencing, and enhanced expression of the dihydropteroate synthase gene of Escherichia coli MC4100. J. Bacteriol. 174:5961-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davey, T. F., and R. R. Schenck. 1964. The endocrines in leprosy, p. 198. In R. G. Cochrane and T. F. Davey (ed.), Leprosy in theory and practice. John Wright and Sons, Ltd., Bristol, United Kingdom.
- 82.Dawlah, Z. M., A. Cabrera, K. Ahern, and W. R. Levis. 2002. A phase 2 open trial of pentoxifylline for the treatment of leprosy reactions. Int. J. Lepr. Other Mycobact. Dis. 70:38-43. [PubMed] [Google Scholar]
- 83.De Bruyn, E. E., H. C. Steel, E. J. Van Rensburg, and R. Anderson. 1996. The riminophenazines, clofazimine and B669, inhibit potassium transport in gram-positive bacteria by a lysophospholipid-dependent mechanism. J. Antimicrob. Chemother. 38:349-362. [DOI] [PubMed] [Google Scholar]
- 84.de Carsalade, G. Y., D. Wallach, E. Spindler, J. Pennec, F. Cottenot, and B. Flageul. 1997. Daily multidrug therapy for leprosy: results of a fourteen-year experience. Int. J. Lepr. Other Mycobact. Dis. 65:37-44. [PubMed] [Google Scholar]
- 85.Dehio, K. 1952. On the Lepra Anesthetica and the pathogenetic relation of its disease-appearances. Proceedings of the International Scientific Leprosy Conference in Berlin, October 1897. Lepr. India 24:78-83. [Google Scholar]
- 86.dela Cruz, E., R. V. Cellona, M. V. Balagon, L. G. Villahermosa, T. T. Fajardo, Jr., R. M. Abalos, E. V. Tan, and G. P. Walsh. 1996. Primary dapsone resistance in Cebu, The Philippines; cause for concern. Int. J. Lepr. Other Mycobact. Dis. 64:253-256. [PubMed] [Google Scholar]
- 87.Demangel, C., and W. J. Britton. 2000. Interaction of dendritic cells with mycobacteria: where the action starts. Immunol. Cell Biol. 78:318-324. [DOI] [PubMed] [Google Scholar]
- 88.De Sarkar, A., I. Kaur, B. D. Radotra, and B. Kumar. 2001. Impact of combined Mycobacterium w vaccine and 1 year of MDT on multibacillary leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 69:187-194. [PubMed] [Google Scholar]
- 89.de Vries, R. R., R. F. Fat, L. E. Nijenhuis, and J. J. van Rood. 1976. HLA-linked genetic control of host response to Mycobacterium leprae. Lancet ii:1328-1330. [DOI] [PubMed] [Google Scholar]
- 90.de Wit, M. Y., J. T. Douglas, J. McFadden, and P. R. Klatser. 1993. Polymerase chain reaction for detection of Mycobacterium leprae in nasal swab specimens. J. Clin. Microbiol. 31:502-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Wit, M. Y., W. R. Faber, S. R. Krieg, J. T. Douglas, S. B. Lucas, N. Montreewasuwat, S. R. Pattyn, R. Hussain, J. M. Ponnighaus, R. A. Hartskeerl, et al. 1991. Application of a polymerase chain reaction for the detection of Mycobacterium leprae in skin tissues. J. Clin. Microbiol. 29:906-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Wit, M. Y., and P. R. Klatser. 1994. Mycobacterium leprae isolates from different sources have identical sequences of the spacer region between the 16S and 23S ribosomal RNA genes. Microbiology 140:1983-1987. [DOI] [PubMed] [Google Scholar]
- 93.Dinauer, M. C., M. B. Deck, and E. R. Unanue. 1997. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J. Immunol. 158:5581-5583. [PubMed] [Google Scholar]
- 94.Dockrell, H. M., S. Brahmbhatt, B. D. Robertson, S. Britton, U. Fruth, N. Gebre, M. Hunegnaw, R. Hussain, R. Manadhar, L. Murrillo, M. C. Pessolani, P. Roche, J. L. Salgado, E. Sampaio, F. Shahid, J. E. Thole, and D. B. Young. 2000. Diagnostic assays for leprosy based on T-cell epitopes. Lepr. Rev. 71(Suppl.):S55-59. [DOI] [PubMed]
- 95.Dockrell, H. M., S. K. Young, K. Britton, P. J. Brennan, B. Rivoire, M. F. Waters, S. B. Lucas, F. Shahid, M. Dojki, T. J. Chiang, Q. Ehsan, K. P. McAdam, and R. Hussain. 1996. Induction of Th1 cytokine responses by mycobacterial antigens in leprosy. Infect. Immun. 64:4385-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donner, R. S., and J. A. Shively. 1967. The “Lucio phenomenon” in diffuse leprosy. Ann. Intern. Med. 67:831-836. [DOI] [PubMed] [Google Scholar]
- 97.Douglas, J. T., R. V. Celona, R. M. Abalos, M. G. Madarang, and T. Fajardo. 1987. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Cebu, the Philippines. Int. J. Lepr. Other Mycobact. Dis. 55:718-721. [PubMed] [Google Scholar]
- 98.Draper, P., O. Kandler, and A. Darbre. 1987. Peptidoglycan and arabinogalactan of Mycobacterium leprae. J. Gen. Microbiol. 133:1187-1194. [DOI] [PubMed] [Google Scholar]
- 99.Drlica, K., C. Xu, J. Y. Wang, R. M. Burger, and M. Malik. 1996. Fluoroquinolone action in mycobacteria: similarity with effects in Escherichia coli and detection by cell lysate viscosity. Antimicrob. Agents Chemother. 40:1594-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drosos, A. A., P. J. Brennan, M. S. Elisaf, S. G. Stefanou, C. S. Papadimitriou, and H. M. Moutsopoulos. 1986. Specific antigen and antibody to Mycobacterium leprae in the cryoprecipitate of a patient with Lucio phenomenon. Rheumatol. Int. 6:93-94. [DOI] [PubMed] [Google Scholar]
- 101.Duncan, M. E., J. M. Pearson, D. S. Ridley, R. Melsom, and G. Bjune. 1982. Pregnancy and leprosy: the consequences of alterations of cell-mediated and humoral immunity during pregnancy and lactation. Int. J. Lepr. Other Mycobact. Dis. 50:425-435. [PubMed] [Google Scholar]
- 102.Ebenezer, G. J., G. Norman, G. A. Joseph, S. Daniel, and C. K. Job. 2002. Drug resistant Mycobacterium leprae—results of mouse footpad studies from a laboratory in south India. Indian J. Lepr. 74:301-312. [PubMed] [Google Scholar]
- 103.Eiglmeier, K., N. Honore, S. A. Woods, B. Caudron, and S. T. Cole. 1993. Use of an ordered cosmid library to deduce the genomic organization of Mycobacterium leprae. Mol. Microbiol. 7:197-206. [DOI] [PubMed] [Google Scholar]
- 104.Eiglmeier, K., J. Parkhill, N. Honore, T. Garnier, F. Tekaia, A. Telenti, P. Klatser, K. D. James, N. R. Thomson, P. R. Wheeler, C. Churcher, D. Harris, K. Mungall, B. G. Barrell, and S. T. Cole. 2001. The decaying genome of Mycobacterium leprae. Lepr. Rev. 72:387-398. [PubMed] [Google Scholar]
- 105.Eiglmeier, K., S. Simon, T. Garnier, and S. T. Cole. 2001. The integrated genome map of Mycobacterium leprae. Lepr. Rev. 72:462-469. [PubMed] [Google Scholar]
- 106.Fajardo, T. T., Jr., L. G. Villahermosa, E. C. Cruz, R. V. Cellona, M. V. Balagon, R. M. Abalos, and R. H. Gelber. 2004. A clinical trial of pefloxacin and ofloxacin in lepromatous leprosy. Lepr. Rev. 75:389-397. [PubMed] [Google Scholar]
- 107.Feitosa, M., H. Krieger, I. Borecki, B. Beiguelman, and D. C. Rao. 1996. Genetic epidemiology of the Mitsuda reaction in leprosy. Hum. Hered. 46:32-35. [DOI] [PubMed] [Google Scholar]
- 108.Ferreira, F. R., L. Z. Goulart, H. D. Silva, and I. M. B. Goulart. 2004. Susceptibility to leprosy may be conditioned by an interaction between NRAMP1 promoter polymorphisms and the lepromin response. Int. J. Lepr. Other Mycobact. Dis. 72:457-467. [DOI] [PubMed] [Google Scholar]
- 109.Fiallo, P., D. L. Williams, G. P. Chan, and T. P. Gillis. 1992. Effects of fixation on polymerase chain reaction detection of Mycobacterium leprae. J. Clin. Microbiol. 30:3095-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Filice, G. A., R. N. Greenberg, and D. W. Fraser. 1977. Lack of observed association between armadillo contact and leprosy in humans. Am. J. Trop. Med. Hyg. 26:137-139. [DOI] [PubMed] [Google Scholar]
- 111.Fine, P. E. 2001. BCG: the challenge continues. Scand. J. Infect. Dis. 33:243-245. [DOI] [PubMed] [Google Scholar]
- 112.Fine, P. E. M., and R. W. Truman. 1998. Report of the workshop on epidemiology, transmission and vaccines in leprosy. Int. J. Lepr. Other Mycobact. Dis. 66:596-597. [PubMed] [Google Scholar]
- 113.Flad, H. D., J. Arnoldi, A. Ohlert, J. Kazda, and J. Gerdes. 1990. Cytokine production and proliferative capacity of infiltrating cells in various forms of leprosy. Trop. Med. Parasitol. 41:307-309. [PubMed] [Google Scholar]
- 114.Fleischmann, R. D., D. Alland, J. A. Eisen, L. Carpenter, O. White, J. Peterson, R. DeBoy, R. Dodson, M. Gwinn, D. Haft, E. Hickey, J. F. Kolonay, W. C. Nelson, L. A. Umayam, M. Ermolaeva, S. L. Salzberg, A. Delcher, T. Utterback, J. Weidman, H. Khouri, J. Gill, A. Mikula, W. Bishai, W. R. Jacobs, Jr., J. C. Venter, and C. M. Fraser. 2002. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J. Bacteriol. 184:5479-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Foss, N. T., E. B. de Oliveira, and C. L. Silva. 1993. Correlation between TNF production, increase of plasma C-reactive protein level and suppression of T lymphocyte response to concanavalin A during erythema nodosum leprosum. Int. J. Lepr. Other Mycobact. Dis. 61:218-226. [PubMed] [Google Scholar]
- 116.Frankel, R. I., R. T. Mita, R. Kim, and F. J. Dann. 1992. Resolution of type 1 reaction in multibacillary Hansen's disease as a result of treatment with cyclosporine. Int. J. Lepr. Other Mycobact. Dis. 60:8-12. [PubMed] [Google Scholar]
- 117.Franzblau, S. G. 1989. Drug susceptibility testing of Mycobacterium leprae in the BACTEC 460 system. Antimicrob. Agents Chemother. 33:2115-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franzblau, S. G., and K. E. White. 1990. Comparative in vitro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob. Agents Chemother. 34:229-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fsihi, H., and S. T. Cole. 1995. The Mycobacterium leprae genome: systematic sequence analysis identifies key catabolic enzymes, ATP-dependent transport systems and a novel polA locus associated with genomic variability. Mol. Microbiol. 16:909-919. [DOI] [PubMed] [Google Scholar]
- 120.Fsihi, H., V. Vincent, and S. T. Cole. 1996. Homing events in the gyrA gene of some mycobacteria. Proc. Natl. Acad. Sci. USA 93:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fujita, M., Y. Miyachi, S. Izumi, H. Ishida, A. Obara, and S. Imamura. 1991. Immunohistochemical study of lepromin reaction in healthy adults. J. Dermatol Sci. 2:179-182. [DOI] [PubMed] [Google Scholar]
- 122.Fukutomi, Y. 2004. Functional changes of macrophages in Hansen's disease. Nihon Hansenbyo Gakkai Zasshi 73:253-261. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 123.Garcia, V. E., K. Uyemura, P. A. Sieling, M. T. Ochoa, C. T. Morita, H. Okamura, M. Kurimoto, T. H. Rea, and R. L. Modlin. 1999. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 162:6114-6121. [PubMed] [Google Scholar]
- 124.Gebre, S., P. Saunderson, T. Messele, and P. Byass. 2000. The effect of HIV status on the clinical picture of leprosy: a prospective study in Ethiopia. Lepr. Rev. 71:338-343. [DOI] [PubMed] [Google Scholar]
- 125.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gelber, R. H. 1987. Activity of minocycline in Mycobacterium leprae-infected mice. J. Infect. Dis. 156:236-239. [DOI] [PubMed] [Google Scholar]
- 127.Gelber, R. H. 1995. Successful treatment of a lepromatous patient with clarithromycin. Int. J. Lepr. Other Mycobact. Dis. 63:113-115. [PubMed] [Google Scholar]
- 128.Gelber, R. H., V. F. Balagon, and R. V. Cellona. 2004. The relapse rate in MB leprosy patients treated with 2-years of WHO-MDT is not low. Int. J. Lepr. Other Mycobact. Dis. 72:493-500. [DOI] [PubMed] [Google Scholar]
- 129.Gelber, R. H., A. Iranmanesh, L. Murray, P. Siu, and M. Tsang. 1992. Activities of various quinolone antibiotics against Mycobacterium leprae in infected mice. Antimicrob. Agents Chemother. 36:2544-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gerlach, W. 1891. Investigations on anaesthetic skin patches. Virchows Arch. 125:126-145. [Google Scholar]
- 131.Gillis, T. P., and T. M. Buchanan. 1982. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect. Immun. 37:172-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gillis, T. P., and D. L. Williams. 1991. Polymerase chain reaction and leprosy. Int. J. Lepr. Other Mycobact. Dis. 59:311-316. [PubMed] [Google Scholar]
- 133.Gimenez, M. F., I. Gigli, and F. A. Tausk. 1989. Differential expression of Langerhans cells in the epidermis of patients with leprosy. Br. J. Dermatol. 121:19-26. [DOI] [PubMed] [Google Scholar]
- 134.Girdhar, B. K., A. Girdhar, and A. Kumar. 2000. Relapses in multibacillary leprosy patients: effect of length of therapy. Lepr. Rev. 71:144-153. [DOI] [PubMed] [Google Scholar]
- 135.Godal, T., B. Myrvang, D. R. Samuel, W. F. Ross, and M. Lofgren. 1973. Mechanism of “reactions” in borderline tuberculoid (BT) leprosy. A preliminary report. Acta Pathol. Microbiol. Scand. A 236:45-53. [PubMed] [Google Scholar]
- 136.Goodless, D. R., A. L. Viciana, R. J. Pardo, and P. Ruiz. 1994. Borderline tuberculoid Hansen's disease in AIDS. J. Am. Acad. Dermatol. 30:866-869. [DOI] [PubMed] [Google Scholar]
- 137.Grosset, J. H., C. C. Guelpa-Lauras, P. Bobin, G. Brucker, J. L. Cartel, M. Constant-Desportes, B. Flageul, M. Frederic, J. C. Guillaume, and J. Millan. 1989. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int. J. Lepr. Other Mycobact. Dis. 57:607-614. [PubMed] [Google Scholar]
- 138.Grosset, J. H., B. H. Ji, C. C. Guelpa-Lauras, E. G. Perani, and L. N. N'Deli. 1990. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. Other Mycobact. Dis. 58:281-295. [PubMed] [Google Scholar]
- 139.Gruenheid, S., and P. Gros. 2000. Genetic susceptibility to intracellular infections: Nramp1, macrophage function and divalent cations transport. Curr. Opin. Microbiol. 3:43-48. [DOI] [PubMed] [Google Scholar]
- 140.Gupte, M. D., R. S. Vallishayee, D. S. Anantharaman, B. Nagaraju, Sreevatsa, S. Balasubramanyam, R. L. de Britto, N. Elango, N. Uthayakumaran, V. N. Mahalingam, G. Lourdusamy, A. Ramalingam, S. Kannan, and J. Arokiasamy. 1998. Comparative leprosy vaccine trial in south India. Indian J. Lepr. 70:369-388. [PubMed] [Google Scholar]
- 141.Haanen, J. B., R. de Waal Malefijt, P. C. Res, E. M. Kraakman, T. H. Ottenhoff, R. R. de Vries, and H. Spits. 1991. Selection of a human T helper type 1-like T cell subset by mycobacteria. J. Exp. Med. 174:583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haanpaa, M., D. N. Lockwood, and A. Hietaharju. 2004. Neuropathic pain in leprosy. Lepr. Rev. 75:7-18. [PubMed] [Google Scholar]
- 143.Hackel, C., S. Houard, F. Portaels, A. van Elsen, A. Herzog, and A. Bollen. 1990. Specific identification of Mycobacterium leprae by the polymerase chain reaction. Mol. Cell. Probes 4:205-210. [DOI] [PubMed] [Google Scholar]
- 144.Hagge, D., S. O. Robinson, D. Scollard, G. McCormick, and D. L. Williams. 2002. A new model for studying the effects of Mycobacterium leprae on Schwann cell and neuron interactions. J. Infect. Dis. 186:1283-1296. [DOI] [PubMed] [Google Scholar]
- 145.Hagge, D. A., N. A. Ray, J. L. Krahenbuhl, and L. B. Adams. 2004. An in vitro model for the lepromatous leprosy granuloma: fate of Mycobacterium leprae from target macrophages after interaction with normal and activated effector macrophages. J. Immunol. 172:7771-7779. [DOI] [PubMed] [Google Scholar]
- 146.Haile, Y., and J. J. Ryon. 2004. Colorimetric microtitre plate hybridization assay for the detection of Mycobacterium leprae 16S rRNA in clinical specimens. Lepr. Rev. 75:40-49. [PubMed] [Google Scholar]
- 147.Halloran, P. F. 2004. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 351:2715-2729. [DOI] [PubMed] [Google Scholar]
- 148.Hamerlinck, F. F., P. R. Klatser, D. S. Walsh, J. D. Bos, G. P. Walsh, and W. R. Faber. 1999. Serum neopterin as a marker for reactional states in leprosy. FEMS Immunol. Med. Microbiol. 24:405-409. [DOI] [PubMed] [Google Scholar]
- 149.Hancock, G. E., A. Molloy, B. K. Ab, R. Kiessling, M. Becx-Bleumink, Z. A. Cohn, and G. Kaplan. 1991. In vivo administration of low-dose human interleukin-2 induces lymphokine-activated killer cells for enhanced cytolysis in vitro. Cell. Immunol. 132:277-284. [DOI] [PubMed] [Google Scholar]
- 150.Hartskeerl, R. A., M. Y. de Wit, and P. R. Klatser. 1989. Polymerase chain reaction for the detection of Mycobacterium leprae. J. Gen. Microbiol. 135:2357-2364. [DOI] [PubMed] [Google Scholar]
- 151.Hashimoto, K., Y. Maeda, H. Kimura, K. Suzuki, A. Masuda, M. Matsuoka, and M. Makino. 2002. Mycobacterium leprae infection in monocyte-derived dendritic cells and its influence on antigen-presenting function. Infect. Immun. 70:5167-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hatagima, A., D. V. Opromolla, S. Ura, M. F. Feitosa, B. Beiguelman, and H. Krieger. 2001. No evidence of linkage between Mitsuda reaction and the NRAMP1 locus. Int. J. Lepr. Other Mycobact. Dis. 69:99-103. [PubMed] [Google Scholar]
- 153.Hayes, C. E., F. E. Nashold, K. M. Spach, and L. B. Pedersen. 2003. The immunological functions of the vitamin D endocrine system. Cell. Mol. Biol (Noisy-Le-Grand) 49:277-300. [PubMed] [Google Scholar]
- 154.Heine, H., and E. Lien. 2003. Toll-like receptors and their function in innate and adaptive immunity. Int. Arch. Allergy Immunol. 130:180-192. [DOI] [PubMed] [Google Scholar]
- 155.Holzer, T. J., K. E. Nelson, R. G. Crispen, and B. R. Anderson. 1986. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Honore, N., and S. T. Cole. 1993. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob. Agents Chemother. 37:414-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Honore, N., E. Perrani, A. Telenti, J. Grosset, and S. T. Cole. 1993. A simple and rapid technique for the detection of rifampin resistance in Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 61:600-604. [PubMed] [Google Scholar]
- 158.Honore, N., P. W. Roche, J. H. Grosset, and S. T. Cole. 2001. A method for rapid detection of rifampicin-resistant isolates of Mycobacterium leprae. Lepr. Rev. 72:441-448. [DOI] [PubMed] [Google Scholar]
- 159.Hori, S., and S. Sakaguchi. 2004. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 6:745-751. [DOI] [PubMed] [Google Scholar]
- 160.Humphres, R. C., R. H. Gelber, and J. L. Krahenbuhl. 1982. Suppressed natural killer cell activity during episodes of erythema nodosum leprosum in lepromatous leprosy. Clin. Exp. Immunol. 49:500-508. [PMC free article] [PubMed] [Google Scholar]
- 161.Hunger, R. E., P. A. Sieling, M. T. Ochoa, M. Sugaya, A. E. Burdick, T. H. Rea, P. J. Brennan, J. T. Belisle, A. Blauvelt, S. A. Porcelli, and R. L. Modlin. 2004. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J. Clin. Investig. 113:701-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hussain, T., S. Sinha, K. Kulshreshtha, K. Katoch, V. Yadev, U. Sengupta, and V. M. Katoch. 2005. Seroprevalence of HIV infection among leprosy patients in Agra, India: trends and perspective. Int. J. Lepr. Other Mycobact. Infect. 73:93-99. [PubMed] [Google Scholar]
- 163.Jacobs, J. M., V. P. Shetty, and N. H. Antia. 1987. Myelin changes in leprous neuropathy. Acta Neuropathol. 74:75-80. [DOI] [PubMed] [Google Scholar]
- 164.Jacobs, J. M., V. P. Shetty, and N. H. Antia. 1987. Teased fibre studies in leprous neuropathy. J. Neurol. Sci. 79:301-313. [DOI] [PubMed] [Google Scholar]
- 165.Jacobs, W. R., M. A. Docherty, R. Curtiss III, and J. E. Clark-Curtiss. 1986. Expression of Mycobacterium leprae genes from a Streptococcus mutans promoter in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 83:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jacobson, R. R. 1997. Treatment of leprosy, p. 470. In R. C. Hastings (ed.), Leprosy. Chruchill Livingstone, Edinburgh, Scotland.
- 167.Jacobson, R. R., and J. L. Krahenbuhl. 1999. Leprosy. Lancet 353:655-660. [DOI] [PubMed] [Google Scholar]
- 168.Jamet, P., B. Ji, et al. 1995. Relapse after long-term follow up of multibacillary patients treated by WHO multidrug regimen. Int. J. Lepr. Other Mycobact. Dis. 63:195-201. [PubMed] [Google Scholar]
- 169.Jamet, P., I. Traore, J. A. Husser, and B. Ji. 1992. Short-term trial of clofazimine in previously untreated lepromatous leprosy. Int. J. Lepr. Other Mycobact. Dis. 60:542-548. [PubMed] [Google Scholar]
- 170.Jamil, S., S. M. Wilson, M. Hacket, R. Hussain, and N. G. Stoker. 1994. A colorimetric PCR method for the detection of M. leprae in skin biopsies from leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 62:512-520. [PubMed] [Google Scholar]
- 171.Ji, B. 1998. Treatment of leprosy, p. 398-424. In P. R. Gangadharam and P. A. Jenkins (ed.), Mycobacteria, vol. II: chemotherapy. International Thomson Publishing Co., New York, N.Y. [Google Scholar]
- 172.Ji, B., and J. Grosset. 1991. Ofloxacin for the treatment of leprosy. Acta Leprol. 7:321-326. [PubMed] [Google Scholar]
- 173.Ji, B., P. Jamet, E. G. Perani, P. Bobin, and J. H. Grosset. 1993. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168:188-190. [DOI] [PubMed] [Google Scholar]
- 174.Ji, B., P. Jamet, E. G. Perani, S. Sow, C. Lienhardt, C. Petinon, and J. H. Grosset. 1996. Bactericidal activity of single dose of clarithromycin plus minocycline, with or without ofloxacin, against Mycobacterium leprae in patients. Antimicrob. Agents Chemother. 40:2137-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ji, B., E. G. Perani, C. Petinom, L. N′Deli, and J. H. Grosset. 1994. Clinical trial of ofloxacin alone and in combination with dapsone plus clofazimine for treatment of lepromatous leprosy. Antimicrob. Agents Chemother. 38:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ji, B., S. Sow, E. Perani, C. Lienhardt, V. Diderot, and J. Grosset. 1998. Bactericidal activity of a single-dose combination of ofloxacin plus minocycline, with or without rifampin, against Mycobacterium leprae in mice and in lepromatous patients. Antimicrob. Agents Chemother. 42:1115-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ji, B. H. 1985. Drug resistance in leprosy—a review. Lepr. Rev. 56:265-278. [PubMed] [Google Scholar]
- 178.Jin, D. J., and C. A. Gross. 1988. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 202:45-58. [DOI] [PubMed] [Google Scholar]
- 179.Job, C. K. 1971. Pathology of peripheral nerve lesions in lepromatous leprosy—a light and electron microscopic study. Int. J. Lepr. Other Mycobact. Dis. 39:251-268. [PubMed] [Google Scholar]
- 180.Job, C. K. 1989. Nerve damage in leprosy. Int. J. Lepr. Other Mycobact. Dis. 57:532-539. [PubMed] [Google Scholar]
- 181.Job, C. K., V. Drain, R. Truman, A. T. Deming, R. M. Sanchez, and R. C. Hastings. 1992. The pathogenesis of leprosy in the nine-banded armadillo and the significance of IgM antibodies to PGL-1. Indian J. Lepr. 64:137-151. [PubMed] [Google Scholar]
- 182.Job, C. K., W. F. Kirchheimer, and R. M. Sanchez. 1982. Tissue response to lepromin, an index of susceptibility of the armadillo to M. leprae infection—a preliminary report. Int. J. Lepr. Other Mycobact. Dis. 50:177-182. [PubMed] [Google Scholar]
- 183.Job, C. K., W. F. Kirchheimer, and R. M. Sanchez. 1982. Borderline leprosy in an experimentally infected armadillo. Int. J. Lepr. Other Mycobact. Dis. 50:488-493. [PubMed] [Google Scholar]
- 184.Job, C. K., W. F. Kirchheimer, and R. M. Sanchez. 1983. Variable lepromin response to Mycobacterium leprae in resistant armadillos. Int. J. Lepr. Other Mycobact. Dis. 51:347-353. [PubMed] [Google Scholar]
- 185.Job, C. K., R. M. Sanchez, and R. C. Hastings. 1985. Manifestations of experimental leprosy in the armadillo. Am. J. Trop. Med. Hyg. 34:151-161. [DOI] [PubMed] [Google Scholar]
- 186.Job, C. K., R. M. Sanchez, and R. C. Hastings. 1991. An attempt to produce experimental tuberculoid leprosy in the nine-banded armadillo. Indian J. Lepr. 63:159-165. [PubMed] [Google Scholar]
- 187.Job, C. K., R. M. Sanchez, R. Hunt, and R. C. Hastings. 1987. Prevalence and significance of Mitsuda reaction in the nine-banded armadillo. Int. J. Lepr. Other Mycobact. Dis. 55:685-688. [PubMed] [Google Scholar]
- 188.Job, C. K., R. M. Sanchez, R. Hunt, R. W. Truman, and R. C. Hastings. 1993. Armadillos (Dasypus novemcinctus) as a model to test antileprosy vaccines: a preliminary report. Int. J. Lepr. Other Mycobact. Dis. 61:394-397. [PubMed] [Google Scholar]
- 189.Job, C. K., and R. W. Truman. 1999. Mitsuda-negative, resistant nine-banded armadillos and enhanced Mitsuda response to live M. leprae [letter]. Int. J. Lepr. Other Mycobact. Dis. 67:475-477. [PubMed] [Google Scholar]
- 190.Johnstone, P. A. 1987. The search for animal models of leprosy. Int. J. Lepr. Other Mycobact. Dis. 55:535-547. [PubMed] [Google Scholar]
- 191.Kai, M., M. Matsuoka, N. Nakata, S. Maeda, M. Gidoh, Y. Maeda, K. Hashimoto, K. Kobayashi, and Y. Kashiwabara. 1999. Diaminodiphenylsulfone resistance of Mycobacterium leprae due to mutations in the dihydropteroate synthase gene. FEMS Microbiol. Lett. 177:231-235. [DOI] [PubMed] [Google Scholar]
- 192.Kaleab, B., T. Ottenoff, P. Converse, E. Halapi, G. Tadesse, M. Rottenberg, and R. Kiessling. 1990. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur. J. Immunol. 20:2651-2659. [DOI] [PubMed] [Google Scholar]
- 193.Kang, T. J., and G. T. Chae. 2001. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol. Med. Microbiol. 31:53-58. [DOI] [PubMed] [Google Scholar]
- 194.Kang, T. J., S. K. Kim, S. B. Lee, G. T. Chae, and J. P. Kim. 2003. Comparison of two different PCR amplification products (the 18-kDa protein gene vs. RLEP repetitive sequence) in the diagnosis of Mycobacterium leprae. Clin. Exp. Dermatol. 28:420-424. [DOI] [PubMed] [Google Scholar]
- 195.Kang, T. J., S. B. Lee, and G. T. Chae. 2002. A polymorphism in the toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine 20:56-62. [DOI] [PubMed] [Google Scholar]
- 196.Kang, T. J., C. E. Yeum, B. C. Kim, E. Y. You, and G. T. Chae. 2004. Differential production of interleukin-10 and interleukin-12 in mononuclear cells from leprosy patients with a Toll-like receptor 2 mutation. Immunol. 112:674-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaplan, G. 1993. Recent advances in cytokine therapy in leprosy. J. Infect. Dis. 167:S18-22. [DOI] [PubMed] [Google Scholar]
- 198.Kaplan, G., W. J. Britton, G. E. Hancock, W. J. Theuvenet, K. A. Smith, C. K. Job, P. W. Roche, A. Molloy, R. Burkhardt, J. Barker, et al. 1991. The systemic influence of recombinant interleukin 2 on the manifestations of lepromatous leprosy. J. Exp. Med. 173:993-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kaplan, G., R. Kiessling, S. Teklemariam, G. Hancock, G. Sheftel, C. K. Job, P. Converse, T. H. Ottenhoff, M. Becx-Bleumink, M. Dietz, and Z. A. Cohn. 1989. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J. Exp. Med. 169:893-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kaplan, G., N. K. Mathur, C. K. Job, I. Nath, and Z. A. Cohn. 1989. Effect of multiple interferon gamma injections on the disposal of Mycobacterium leprae. Proc. Natl. Acad. Sci. USA 86:8073-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kaplan, G., G. Walsh, L. S. Guido, P. Meyn, R. A. Burkhardt, R. M. Abalos, J. Barker, P. A. Frindt, T. T. Fajardo, R. Celona, and Z. A. Cohn. 1992. Novel responses of human skin to intradermal recombinant granulocyte/macrophage-colony-stimulating factor: Langerhans cell recruitment, keratinocyte growth, and enhanced wound healing. J. Exp. Med. 175:1717-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kar, B. R., and R. Babu. 2004. Methotrexate in resistant ENL. Int. J. Lepr. Other Mycobact. Dis. 72:480-482. [DOI] [PubMed] [Google Scholar]
- 203.Karin, M. 1998. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell 93:487-490. [DOI] [PubMed] [Google Scholar]
- 204.Karonga Prevention Trial Group. 1996. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 205.Kashala, O., R. Marlink, M. Ilunga, M. Diese, B. Gormus, K. Xu, P. Mukeba, K. Kasongo, and M. Essex. 1994. Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross-reactivity and antibodies to lipoarabinomannan. J. Infect. Dis. 169:296-304. [DOI] [PubMed] [Google Scholar]
- 206.Katoch, K., V. M. Katoch, M. Natrajan, A. S. Bhatia, Sreevatsa, U. D. Gupta, V. D. Sharma, C. T. Shivannavar, M. A. Patil, and V. P. Bharadwaj. 1995. Treatment of bacilliferous BL/LL cases with combined chemotherapy and immunotherapy. Int. J. Lepr. Other Mycobact. Dis. 63:202-212. [PubMed] [Google Scholar]
- 207.Katoch, V. M. 1981. A report on the biochemical analysis of Mycobacterium w. Lepr. India 53:385-389. [PubMed] [Google Scholar]
- 208.Katoch, V. M. 1999. Molecular techniques for leprosy: present applications and future perspectives. Indian J. Lepr. 71:45-59. [PubMed] [Google Scholar]
- 209.Khanolkar, V. R. 1964. Pathology of leprosy, p. 125-151. In R. G. Cochrane and T. F. Davey (ed.), Leprosy in theory and practice. John Wright and Sons, Ltd., Bristol, United Kingdom.
- 210.Khanolkar-Young, S., D. Snowdon, and D. N. J. Lockwoood. 1998. Immunocytochemical localization of inducible nitric oxide synthase and transforming growth factor-beta (TGF-B) in leprosy lesions. Clin. Exp. Immunol.. 113:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kiani, A., A. Rao, and J. Aramburu. 2000. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity 12:359-372. [DOI] [PubMed] [Google Scholar]
- 212.Kimura, H., Y. Maeda, F. Takeshita, L. E. Takaoka, M. Matsuoka, and M. Makino. 2004. Upregulation of T-cell-stimulating activity of mycobacteria-infected macrophages. Scand. J. Immunol. 60:278-286. [DOI] [PubMed] [Google Scholar]
- 213.Kirchheimer, W. F., and R. M. Sanchez. 1973. Leprosy-susceptibility testing of armadillos. 2. Late cell and bacterial responses at inoculation site of living leprosy bacilli in ‘resistant’ armadillos. Microbios 8:241-246. [PubMed] [Google Scholar]
- 214.Kirchheimer, W. F., and R. M. Sanchez. 1981. Intraspecies differences of resistance against leprosy in nine-banded armadillos. Lepr. India 53:525-530. [PubMed] [Google Scholar]
- 215.Kirchheimer, W. F., R. M. Sanchez, and E. J. Shannon. 1978. Effect of specific vaccine on cell-mediated immunity of armadillos against M. leprae. Int. J. Lepr. Other Mycobact. Dis. 46:353-357. [PubMed] [Google Scholar]
- 216.Kirchheimer, W. F., and E. E. Storrs. 1971. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int. J. Lepr. Other Mycobact. Dis. 39:693-702. [PubMed] [Google Scholar]
- 217.Kirchheimer, W. F., E. E. Storrs, and C. H. Binford. 1972. Attempts to establish the armadillo (Dasypus novemcinctus linn.) as a model for the study of leprosy. II. Histopathologic and bacteriologic post-mortem findings in lepromatoid leprosy in the armadillo. Int. J. Lepr. Other Mycobact. Dis. 40:229-242. [PubMed] [Google Scholar]
- 218.Kirkaldy, A. A., A. C. Musonda, S. Khanolkhar-Young, S. Suneetha, and D. N. Lockwood. 2003. Expression of CC and CXC chemokines and chemokine receptors in human leprosy skin lesions. Clin. Exp. Immunol. 134:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kissenpfennig, A., S. Ait-Yahia, V. Clair-Moninot, H. Stossel, E. Badell, Y. Bordat, J. L. Pooley, T. Lang, E. Prina, I. Coste, O. Gresser, T. Renno, N. Winter, G. Milon, K. Shortman, N. Romani, S. Lebecque, B. Malissen, S. Saeland, and P. Douillard. 2005. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol. Cell. Biol. 25:88-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Koppel, E. A., I. S. Ludwig, M. S. Hernandez, T. L. Lowary, R. R. Gadikota, A. B. Tuzikov, C. M. Vandenbroucke-Grauls, Y. van Kooyk, B. J. Appelmelk, and T. B. Geijtenbeek. 2004. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology 209:117-127. [DOI] [PubMed] [Google Scholar]
- 221.Krahenbuhl, J. L., and L. B. Adams. 1999. Mycobacterium leprae as an opportunistic pathogen, p. 75-90. In L. J. Paradise (ed.), Opportunistic intracellular bacteria and immunity. Plenum Press, New York, N.Y.
- 222.Krahenbuhl, J. L., and L. B. Adams. 2000. Exploitation of gene knockout mice models to study the pathogenesis of leprosy. Lepr. Rev. 71:S170-S175. [DOI] [PubMed] [Google Scholar]
- 223.Krutzik, S. R., M. T. Ochoa, P. A. Sieling, S. Uematsu, Y. W. Ng, A. Legaspi, P. T. Liu, S. T. Cole, P. J. Godowski, Y. Maeda, E. N. Sarno, M. V. Norgard, P. J. Brennan, S. Akira, T. H. Rea, and R. L. Modlin. 2003. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 9:525-532. [DOI] [PubMed] [Google Scholar]
- 224.Krutzik, S. R., B. Tan, H. Li, M. T. Ochoa, P. T. Liu, S. E. Sharfstein, T. G. Graeber, P. A. Sieling, Y. J. Liu, T. H. Rea, B. R. Bloom, and R. L. Modlin. 2005. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kulkarni, V. M., and J. K. Seydel. 1983. Inhibitory activity and mode of action of diaminodiphenylsulfone in cell-free folate-synthesizing systems prepared from Mycobacterium lufu and Mycobacterium leprae. A comparison. Chemotherapy 29:58-67. [DOI] [PubMed] [Google Scholar]
- 226.Kumar, B., S. Dogra, and I. Kaur. 2004. Epidemiological characteristics of leprosy reactions: 15 years experience from north India. Int. J. Lepr. Other Mycobact. Dis. 72:125-133. [DOI] [PubMed] [Google Scholar]
- 227.Kuper, S. W. A. 1964. The lepromin reaction, p. 183-189. In R. G. Cochrane and T. F. Davey (ed.), Leprosy in theory and practice. John Wright and Sons, Ltd., Bristol, United Kingdom.
- 228.Kurabachew, M., A. Wondimu, and J. J. Ryon. 1998. Reverse transcription-PCR detection of Mycobacterium leprae in clinical specimens. J. Clin. Microbiol. 36:1352-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lahiri, R., B. Randhawa, and J. Krahenbuhl. 2005. Application of a viability-staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J. Med. Microbiol. 54:235-242. [DOI] [PubMed] [Google Scholar]
- 230.Lawn, S. D., C. Wood, and D. N. Lockwood. 2003. Borderline tuberculoid leprosy: an immune reconstitution phenomenon in a human immunodeficiency virus-infected person. Clin. Infect. Dis. 36:e5-6. [DOI] [PubMed] [Google Scholar]
- 231.Lehman, L. F., M. B. Orsini, and A. R. Nicholl. 1993. The development and adaptation of the Semmes-Weinstein monofilaments in Brazil. J. Hand Ther. 6:290-297. [DOI] [PubMed] [Google Scholar]
- 232.Levy, L., C. C. Shepard, and P. Fasal. 1976. The bactericidal effect of rifampicin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int. J. Lepr. Other Mycobact. Dis. 44:183-187. [PubMed] [Google Scholar]
- 233.Little, D., S. Khanolkar-Young, Coulthart.A, S. Suneetha, and D. N. J. Lockwood. 2001. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect. Immun. 69:3413-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lockwood, D. 2004. Leprosy. Clin. Evid. 10:1103-1114. [PubMed]
- 235.Lockwood, D. N. 1996. The management of erythema nodosum leprosum: current and future options. Lepr. Rev. 67:253-259. [DOI] [PubMed] [Google Scholar]
- 236.Lockwood, D. N., and H. H. Sinha. 1999. Pregnancy and leprosy: a comprehensive literature review. Int. J. Lepr. Other Mycobact. Dis. 67:6-12. [PubMed] [Google Scholar]
- 237.Lockwood, D. N., and S. Suneetha. 2005. Leprosy: too complex a disease for a simple elimination paradigm. Bull. W. H. O. 83:230-235. [PMC free article] [PubMed] [Google Scholar]
- 238.Lucas, S. 1993. Human immunodeficiency virus and leprosy. Lepr. Rev. 64:97-103. [DOI] [PubMed] [Google Scholar]
- 239.Lumpkin, L. R., III, G. F. Cox, and J. E. Wolf, Jr. 1983. Leprosy in five armadillo handlers. J. Am. Acad. Dermatol. 9:899-903. [DOI] [PubMed] [Google Scholar]
- 240.Lwin, K., T. Sundaresan, M. M. Gyi, L. M. Bechelli, C. Tamondong, P. G. Garbajosa, H. Sansarricq, and S. K. Noordeen. 1985. BCG vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull. W. H. O. 63:1069-1078. [PMC free article] [PubMed] [Google Scholar]
- 241.Maeda, S., M. Matsuoka, N. Nakata, M. Kai, Y. Maeda, K. Hashimoto, H. Kimura, K. Kobayashi, and Y. Kashiwabara. 2001. Multidrug-resistant Mycobacterium leprae from patients with leprosy. Antimicrob. Agents Chemother. 45:3635-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Maeda, Y., M. Gidoh, N. Ishii, C. Mukai, and M. Makino. 2003. Assessment of cell mediated immunogenicity of Mycobacterium leprae-derived antigens. Cell. Immunol. 222:69-77. [DOI] [PubMed] [Google Scholar]
- 243.Mahajan, V. K., N. L. Sharma, R. C. Sharma, and A. Sharma. 2003. Pulse dexamethasone, oral steroids and azathioprine in the management of erythema nodosum leprosum. Lepr. Rev. 74:171-174. [PubMed] [Google Scholar]
- 244.Mak, T. W., J. M. Penninger, and P. S. Ohashi. 2001. Knockout mice: a paradigm shift in modern immunology. Nat. Rev. Immunol. 1:11-19. [DOI] [PubMed] [Google Scholar]
- 245.Manandhar, R., N. Shrestha, C. R. Butlin, and P. W. Roche. 2002. High levels of inflammatory cytokines are associated with poor clinical response to steroid treatment and recurrent episodes of type 1 reactions in leprosy. Clin. Exp. Immunol. 128:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Marlowe, S. N., R. A. Hawksworth, C. R. Butlin, P. G. Nicholls, and D. N. Lockwood. 2004. Clinical outcomes in a randomized controlled study comparing azathioprine and prednisolone versus prednisolone alone in the treatment of severe leprosy type 1 reactions in Nepal. Trans. R. Soc. Trop. Med. Hyg. 98:602-609. [DOI] [PubMed] [Google Scholar]
- 247.Marques, M. A., V. L. Antonio, E. N. Sarno, P. J. Brennan, and M. C. Pessolani. 2001. Binding of alpha2-laminins by pathogenic and non-pathogenic mycobacteria and adherence to Schwann cells. J. Med. Microbiol. 50:23-28. [DOI] [PubMed] [Google Scholar]
- 248.Marques, M. A., B. J. Espinosa, E. K. Xavier da Silveira, M. C. Pessolani, A. Chapeaurouge, J. Perales, K. M. Dobos, J. T. Belisle, J. S. Spencer, and P. J. Brennan. 2004. Continued proteomic analysis of Mycobacterium leprae subcellular fractions. Proteomics 4:2942-2953. [DOI] [PubMed] [Google Scholar]
- 249.Marquet, S., and E. Schurr. 2001. Genetics of susceptibility to infectious diseases: tuberculosis and leprosy as examples. Drug Metab. Dispos. 29:479-483. [PubMed] [Google Scholar]
- 250.Mascarell, L., and P. Truffa-Bachi. 2003. New aspects of cyclosporin a mode of action: from gene silencing to gene up-regulation. Mini Rev. Med. Chem. 3:205-214. [DOI] [PubMed] [Google Scholar]
- 251.Matsuoka, M., Y. Kashiwabara, and M. Namisato. 2000. A Mycobacterium leprae isolate resistant to dapsone, rifampin, ofloxacin and sparfloxacin. Int. J. Lepr. Other Mycobact. Dis. 68:452-455. [PubMed] [Google Scholar]
- 252.Matsuoka, M., S. Maeda, M. Kai, N. Nakata, G. T. Chae, T. P. Gillis, K. Kobayashi, S. Izumi, and Y. Kashiwabara. 2000. Mycobacterium leprae typing by genomic diversity and global distribution of genotypes. Int. J. Lepr. Other Mycobact. Dis. 68:121-128. [PubMed] [Google Scholar]
- 253.McMurry, J., H. Sbai, M. L. Gennaro, E. J. Carter, W. Martin, and A. S. De Groot. 2005. Analyzing Mycobacterium tuberculosis proteomes for candidate vaccine epitopes. Tuberculosis (Edinburgh) 85:95-105. [DOI] [PubMed] [Google Scholar]
- 254.McShane, H., R. Brookes, S. C. Gilbert, and A. V. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mehra, V., B. R. Bloom, A. C. Bajardi, C. L. Grisso, P. A. Sieling, D. Alland, J. Convit, X. D. Fan, S. W. Hunter, P. J. Brennan, et al. 1992. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J. Exp. Med. 175:275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mehta, V. R. 1996. Leprosy resistant to multi-drug-therapy (MDT) successfully treated with ampicillin-sulbactam combination—a case report. Indian J. Med. Sci. 50:305-307. [PubMed] [Google Scholar]
- 257.Meier, A., L. Heifets, R. J. Wallace, Jr., Y. Zhang, B. A. Brown, P. Sander, and E. C. Bottger. 1996. Molecular mechanisms of clarithromycin resistance in Mycobacterium avium: observation of multiple 23S rDNA mutations in a clonal population. J. Infect. Dis. 174:354-360. [DOI] [PubMed] [Google Scholar]
- 258.Meima, A., P. R. Saunderson, S. Gebre, K. Desta, and J. D. Habbema. 2001. Dynamics of impairment during and after treatment: the AMFES cohort. Lepr. Rev. 72:158-170. [DOI] [PubMed] [Google Scholar]
- 259.Meima, A., W. C. Smith, G. J. van Oortmarssen, J. H. Richardus, and J. D. Habbema. 2004. The future incidence of leprosy: a scenario analysis. Bull. W. H. O. 82:373-380. [PMC free article] [PubMed] [Google Scholar]
- 260.Miller, R. A., J. Y. Shen, T. H. Rea, and J. P. Harnisch. 1987. Treatment of chronic erythema nodosum leprosum with cyclosporine A produces clinical and immunohistologic remission. Int. J. Lepr. Other Mycobact. Dis. 55:441-449. [PubMed] [Google Scholar]
- 261.Reference deleted.
- 262.Mira, M. T., A. Alcais, V. T. Nguyen, M. O. Moraes, C. Di Flumeri, H. T. Vu, C. P. Mai, T. H. Nguyen, N. B. Nguyen, X. K. Pham, E. N. Sarno, A. Alter, A. Montpetit, M. E. Moraes, J. R. Moraes, C. Dore, C. J. Gallant, P. Lepage, A. Verner, E. Van De Vosse, T. J. Hudson, L. Abel, and E. Schurr. 2004. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature 427:636-640. [DOI] [PubMed] [Google Scholar]
- 263.Misra, N., A. Murtaza, B. Walker, N. P. Narayan, R. S. Misra, V. Ramesh, S. Singh, M. J. Colston, and I. Nath. 1995. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunology 86:97-103. [PMC free article] [PubMed] [Google Scholar]
- 264.Mistry, Y., D. B. Young, and R. Mukherjee. 1992. hsp70 synthesis in Schwann cells in response to heat shock and infection with Mycobacterium leprae. Infect. Immun. 60:3105-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mittal, A., R. S. Mishra, and I. Nath. 1989. Accessory cell heterogeneity in lepromatous leprosy: dendritic cells and not monocytes support T cell responses. Clin. Exp. Immunol. 76:233-239. [PMC free article] [PubMed] [Google Scholar]
- 266.Modi, K., M. Mancini, and M. P. Joyce. 2003. Lepromatous leprosy in a heart transplant recipient. Am. J. Transplant. 3:1600-1603. [DOI] [PubMed] [Google Scholar]
- 267.Modlin, R. L., J. F. Gebhard, C. R. Taylor, and T. H. Rea. 1983. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin. Exp. Immunol.. 53:17-24. [PMC free article] [PubMed] [Google Scholar]
- 268.Modlin, R. L., F. M. Hofman, C. R. Taylor, and T. H. Rea. 1983. T lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 8:182-189. [DOI] [PubMed] [Google Scholar]
- 269.Modlin, R. L., J. Melancon-Kaplan, S. M. M. Young, C. Pirmez, H. Kino, J. Convit, T. H. Rea, and B. R. Bloom. 1988. Learning from lesions: patterns of tissue inflammation in leprosy. Proc. Natl. Acad. Sci. USA 85:1213-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Modlin, R., and T. Rea. 1994. Immunopathology of leprosy, p. 225-234. In R. C. Hastings and D. V. A. Opromolla (ed.), Leprosy. Churchill Livingstone, New York, N.Y.
- 271.Mollenkopf, H. J., L. Grode, J. Mattow, M. Stein, P. Mann, B. Knapp, J. Ulmer, and S. H. Kaufmann. 2004. Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect. Immun. 72:6471-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Monot, M., N. Honore, T. Garnier, R. Araoz, J. Y. Coppee, C. Lacroix, S. Sow, J. S. Spencer, R. W. Truman, D. L. Williams, R. Gelber, M. Virmond, B. Flageul, S. N. Cho, B. Ji, A. Paniz-Mondolfi, J. Convit, S. Young, P. E. Fine, V. Rasolofo, P. J. Brennan, and S. T. Cole. 2005. On the origin of leprosy. Science 308:1040-1042. [DOI] [PubMed] [Google Scholar]
- 273.Moraes, M. O., N. C. Duppre, P. N. Suffys, A. R. Santos, A. S. Almeida, J. A. Nery, E. P. Sampaio, and E. N. Sarno. 2001. Tumor necrosis factor-alpha promoter polymorphism TNF2 is associated with a stronger delayed-type hypersensitivity reaction in the skin of borderline tuberculoid leprosy patients. Immunogenetics 53:45-47. [DOI] [PubMed] [Google Scholar]
- 274.Moraes, M. O., E. N. Sarno, A. S. Almeida, B. C. Saraiva, J. A. Nery, R. C. Martins, and E. P. Sampaio. 1999. Cytokine mRNA expression in leprosy: a possible role for interferon-gamma and interleukin-12 in reactions (RR and ENL). Scand. J. Immunol. 50:541-549. [DOI] [PubMed] [Google Scholar]
- 275.Moraes, M. O., E. N. Sarno, R. M. Teles, A. S. Almeida, B. C. Saraiva, J. A. Nery, and E. P. Sampaio. 2000. Anti-inflammatory drugs block cytokine mRNA accumulation in the skin and improve the clinical condition of reactional leprosy patients. J. Investig. Dermatol. 115:935-941. [DOI] [PubMed] [Google Scholar]
- 276.Moreira, A. L., G. Kaplan, L. G. Villahermosa, T. J. Fajardo, R. M. Abalos, R. V. Cellona, M. V. Balagon, E. V. Tan, and G. P. Walsh. 1998. Comparison of pentoxifylline, thalidomide and prednisone in the treament of ENL. Int. J. Lepr. Other Mycobact. Dis. 66:61-65. [PubMed] [Google Scholar]
- 276a.Moschella, S. L. 2004. An update on the diagnosis and treatment of leprosy. J. Am. Acad. Dermatol. 51:417-426. [DOI] [PubMed] [Google Scholar]
- 277.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. 1. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 146:2348-2357. [PubMed] [Google Scholar]
- 278.Mueller, D. L. 2004. E3 ubiquitin ligases as T cell anergy factors. Nat. Immunol. 5:883-890. [DOI] [PubMed] [Google Scholar]
- 279.Mukherjee, R., and N. H. Antia. 1986. Host-parasite interrelationship between M. leprae and Schwann cells in vitro. Int. J. Lepr. Other Mycobact. Dis. 54:632-638. [PubMed] [Google Scholar]
- 280.Musser, J. M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8:496-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mutis, T., E. M. Kraakman, Y. E. Cornelisse, J. B. Haanen, H. Spits, R. R. De Vries, and T. H. Ottenhoff. 1993. Analysis of cytokine production by Mycobacterium-reactive T cells. Failure to explain Mycobacterium leprae-specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients. J. Immunol. 150:4641-4651. [PubMed] [Google Scholar]
- 282.Naafs, B. 1996. Bangkok Workshop on Leprosy Research: treatment of reactions and nerve damage. Int. J. Lepr. Other Mycobact. Dis. 64:S21-28. [PubMed] [Google Scholar]
- 283.Naafs, B. 2003. Treatment duration of reversal reaction: a reappraisal. Back to the past. Lepr. Rev. 74:328-336. [PubMed] [Google Scholar]
- 284.Nath, I., N. Vemuri, A. L. Reddi, M. Bharadwaj, P. Brooks, M. J. Colston, R. S. Misra, and V. Ramesh. 2000. Dysregulation of IL-4 expression in lepromatous leprosy patients with and without erythema nodosum leprosum. Lepr. Rev. 71(Suppl.):S130-137. [DOI] [PubMed]
- 285.Nath, I., N. Vemuri, A. L. Reddi, S. Jain, P. Brooks, M. J. Colston, R. S. Misra, and V. Ramesh. 2000. The effect of antigen presenting cells on the cytokine profiles of stable and reactional lepromatous leprosy patients. Immunol. Lett. 75:69-76. [DOI] [PubMed] [Google Scholar]
- 286.Nelson, K. E. 2005. Leprosy and HIV infection (rarely the twain shall meet?). Int. J. Lepr. Other Mycobact. Dis. 73:131-133. [PubMed] [Google Scholar]
- 287.Nery, J. A., A. R. Perisse, A. M. Sales, L. M. Vieira, R. V. Souza, E. P. Sampaio, and E. N. Sarno. 2000. The use of pentoxifylline in the treatment of type 2 reactional episodes in leprosy. Indian J. Lepr. 72:457-467. [PubMed] [Google Scholar]
- 288.Ng, V., G. Zanazzi, R. Timpl, J. Talts, J. L. Salzer, P. J. Brennan, and A. Rambukkana. 2000. Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell 103:511-529. [DOI] [PubMed] [Google Scholar]
- 289.Ngamying, M., P. Sawanpanyalert, R. Butraporn, J. Nikasri, S. N. Cho, L. Levy, and P. J. Brennan. 2003. Effect of vaccination with refined components of the organism on infection of mice with Mycobacterium leprae. Infect. Immun. 71:1596-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477-8485. [DOI] [PubMed] [Google Scholar]
- 291.Nogueira, N., G. Kaplan, E. Levy, E. N. Sarno, P. Kushner, A. Granelli-Piperno, L. Vieira, V. Colomer Gould, W. Levis, R. Steinman, et al. 1983. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J. Exp. Med. 158:2165-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Norman, G., G. Joseph, and J. Richard. 2004. Relapses in multibacillary patients treated with multi-drug therapy until smear negativity: findings after twenty years. Int. J. Lepr. Other Mycobact. Dis. 72:1-7. [DOI] [PubMed] [Google Scholar]
- 293.Ochoa, M. T., S. Stenger, P. A. Sieling, S. Thoma-Uszynski, S. Sabet, S. Cho, A. M. Krensky, M. Rollinghoff, E. Nunes Sarno, A. E. Burdick, T. H. Rea, and R. L. Modlin. 2001. T-cell release of granulysin contributes to host defense in leprosy. Nat. Med. 7:174-179. [DOI] [PubMed] [Google Scholar]
- 294.Oliveira, R. B., M. T. Ochoa, P. A. Sieling, T. H. Rea, A. Rambukkana, E. N. Sarno, and R. L. Modlin. 2003. Expression of Toll-like receptor 2 on human Schwann cells: a mechanism of nerve damage in leprosy. Infect. Immun. 71:1427-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ottenhoff, T. H. 2002. Mycobacterium leprae and demyelination. Science 297:1475-1476. [DOI] [PubMed] [Google Scholar]
- 296.Ottenhoff, T. H., and R. R. de Vries. 1987. HLA class II immune response and suppression genes in leprosy. Int. J. Lepr. Other Mycobact. Dis. 55:521-534. [PubMed] [Google Scholar]
- 297.Pattyn, S. R., D. Ursi, M. Ieven, V. Raes, and P. Jamet. 1992. Polymerase chain reaction amplifying DNA coding for species-specific rRNA of Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 60:234-243. [PubMed] [Google Scholar]
- 298.Pereira, G. A., M. M. Stefani, J. A. Araujo Filho, L. C. Souza, G. P. Stefani, and C. M. Martelli. 2004. Human immunodeficiency virus type 1 (HIV-1) and Mycobacterium leprae co-infection: HIV-1 subtypes and clinical, immunologic, and histopathologic profiles in a Brazilian cohort. Am. J. Trop. Med. Hyg. 71:679-684. [PubMed] [Google Scholar]
- 299.Petri, V., E. V. Mendes, and B. Beiguelman. 1985. Histology of the Mitsuda reaction of healthy adults with no known contacts with leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 53:540-545. [PubMed] [Google Scholar]
- 300.Pignataro, P., S. Rocha Ada, J. A. Nery, A. Miranda, A. M. Sales, H. Ferrreira, V. Valentim, and P. N. Suffys. 2004. Leprosy and AIDS: two cases of increasing inflammatory reactions at the start of highly active antiretroviral therapy. Eur. J. Clin. Microbiol. Infect. Dis. 23:408-411. [DOI] [PubMed] [Google Scholar]
- 301.Plikaytis, B. B., R. H. Gelber, and T. M. Shinnick. 1990. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J. Clin. Microbiol. 28:1913-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ponnighaus, J. M., P. E. Fine, J. A. Sterne, R. J. Wilson, E. Msosa, P. J. Gruer, P. A. Jenkins, S. B. Lucas, N. G. Liomba, and L. Bliss. 1992. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 339:636-639. [DOI] [PubMed] [Google Scholar]
- 303.Ponnighaus, J. M., L. J. Mwanjasi, P. E. M. Fine, M.-A. Shaw, A. C. Turner, S. M. Oxborrow, S. B. Lucas, P. A. Jenkins, J. A. C. Sterne, and L. Bliss. 1991. Is HIV infection a risk factor for leprosy? Int. J. Lepr. 59:221-228. [PubMed] [Google Scholar]
- 304.Prigozy, T. I., P. A. Sieling, D. Clemens, P. L. Stewart, S. M. Behar, S. A. Porcelli, M. B. Brenner, R. L. Modlin, and M. Kronenberg. 1997. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6:187-197. [DOI] [PubMed] [Google Scholar]
- 305.Quismorio, F. P., Jr., T. Rea, S. Chandor, N. Levan, and G. J. Friou. 1978. Lucio's phenomenon: an immune complex deposition syndrome in lepromatous leprosy. Clin. Immunol. Immunopathol. 9:184-193. [DOI] [PubMed] [Google Scholar]
- 306.Rajalingam, R., D. P. Singal, and N. K. Mehra. 1997. Transporter associated with antigen-processing (TAP) genes and susceptibility to tuberculoid leprosy and pulmonary tuberculosis. Tissue Antigens 49:168-172. [DOI] [PubMed] [Google Scholar]
- 307.Ramasesh, N., L. B. Adams, S. G. Franzblau, and J. L. Krahenbuhl. 1991. Effects of activated macrophages on Mycobacterium leprae. Infect. Immun. 59:2864-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rambukkana, A. 2000. How does Mycobacterium leprae target the peripheral nervous system? Trends Microbiol. 8:23-28. [DOI] [PubMed] [Google Scholar]
- 309.Rambukkana, A. 2004. Mycobacterium leprae-induced demyelination: a model for early nerve degeneration. Curr. Opin. Immunol. 16:511-518. [DOI] [PubMed] [Google Scholar]
- 310.Rambukkana, A., J. L. Salzer, P. D. Yurchenco, and E. I. Tuomanen. 1997. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin alpha 2 chain. Cell 88:811-821. [DOI] [PubMed] [Google Scholar]
- 311.Rambukkana, A., G. Zanazzi, N. Tapinos, and J. L. Salzer. 2002. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science 296:927-931. [DOI] [PubMed] [Google Scholar]
- 312.Ramu, G. 1998. Single-dose rifampicin, oflaxicin and minocycline (ROM) therapy for single leprosy lesions. Lepr. Rev. 69:78-82. [PubMed] [Google Scholar]
- 313.Randive, K. J., C. V. Bapat, and V. R. Khanolkar. 1962. Studies of pathogenicity of the ICRC bacillus isolated from human lepromatous leprosy. Int. J. Lepr. 30:442-456. [PubMed] [Google Scholar]
- 314.Reddy, V. M., J. F. O'Sullivan, and P. R. Gangadharam. 1999. Antimycobacterial activities of riminophenazines. J. Antimicrob. Chemother. 43:615-623. [DOI] [PubMed] [Google Scholar]
- 315.Rees, R. J. W., M. F. R. Waters, A. G. M. Weddell, and E. Palmer. 1967. Experimental lepromatous leprosy. Nature 215:599-602. [DOI] [PubMed] [Google Scholar]
- 315a.Rees, R. J. 1973. A century of progress in experimental leprosy. Int. J. Lepr. Other Mycobact. Dis. 41:320-328. [PubMed] [Google Scholar]
- 316.Richardus, J. H., P. G. Nicholls, R. P. Croft, S. G. Withington, and W. C. Smith. 2004. Incidence of acute nerve function impairment and reactions in leprosy: a prospective cohort analysis after 5 years of follow-up. Int. J. Epidemiol. 33:337-343. [DOI] [PubMed] [Google Scholar]
- 317.Richey, D. P., and G. M. Brown. 1969. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J. Biol. Chem. 244:1582-1592. [PubMed] [Google Scholar]
- 318.Richter, E., M. Duchrow, C. Schluter, M. Hahn, H. D. Flad, and J. Gerdes. 1994. Detection of Mycobacterium leprae by three-primer PCR. Immunobiology 191:351-353. [DOI] [PubMed] [Google Scholar]
- 319.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity—a five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 320.Ridley, D. S., and K. B. Radia. 1981. The histological course of reactions in borderline leprosy and their outcome. Int. J. Lepr. Other Mycobact. Dis. 49:383-392. [PubMed] [Google Scholar]
- 321.Rieneck, K., M. Diamant, P. M. Haahr, M. Schonharting, and K. Bendtzen. 1993. In vitro immunomodulatory effects of pentoxifylline. Immunol. Lett. 37:131-138. [DOI] [PubMed] [Google Scholar]
- 322.Rojas, R. E., S. O. Demichelis, E. N. Sarno, and A. Segal-Eiras. 1997. IgM anti-phenolic glycolipid I and IgG anti-10-kDa heat shock protein antibodies in sera and immune complexes isolated from leprosy patients with or without erythema nodosum leprosum and contacts. FEMS Immunol. Med. Microbiol. 19:65-74. [DOI] [PubMed] [Google Scholar]
- 323.Rook, G. A. 1988. The role of vitamin D in tuberculosis. Am. Rev. Respir. Dis. 138:768-770. [DOI] [PubMed] [Google Scholar]
- 324.Rosat, J. P., E. P. Grant, E. M. Beckman, C. C. Dascher, P. A. Sieling, D. Frederique, R. L. Modlin, S. A. Porcelli, S. T. Furlong, and M. B. Brenner. 1999. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ ab T-cell pool. J. Immunol. 162:366-371. [PubMed] [Google Scholar]
- 325.Roy, S., A. Frodsham, B. Saha, S. K. Hazra, C. G. N. Mascie-Taylor, and A. V. S. Hill. 1999. Association of vitamin D receptor genotype with leprosy type. J. Infect. Dis. 179:187-191. [DOI] [PubMed] [Google Scholar]
- 326.Roy, S., W. McGuire, C. G. N. Mascie-Taylor, S. Hazra, A. V. S. Hill, and D. Kwiatkowski. 1997. Tumor necrosis factor promoter polymorphism and susceptibility to lepromatous leprosy. J. Infect. Dis. 176:530-532. [DOI] [PubMed] [Google Scholar]
- 327.Sabin, T. D., T. R. Swift, and R. R. Jacobson. 1993. Leprosy, p. 1354-1379. In P. J. Dyck and P. K. Thomas (ed.), Peripheral neuropathy. W. B. Saunders, Philadelphia, Pa.
- 328.Salgame, P., J. S. Abrams, C. Clayberger, H. Goldstein, J. Convit, R. L. Modlin, and B. R. Bloom. 1991. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254:279-282. [DOI] [PubMed] [Google Scholar]
- 329.Sampaio, E. P., J. R. T. Caneshi, J. A. C. Nery, N. C. Duppre, G. M. B. Pereira, L. M. M. Vieira, A. L. Moreira, G. Kaplan, and E. N. Sarno. 1995. Cellular immune response to Mycobacterium leprae infection in human immunodeficiency virus-infected individuals. Infect. Immun. 63:1848-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sampaio, E. P., G. Kaplan, A. Miranda, J. A. Nery, C. P. Miguel, S. M. Viana, and E. N. Sarno. 1993. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J. Infect. Dis. 168:408-414. [DOI] [PubMed] [Google Scholar]
- 331.Sampaio, E. P., M. O. Moraes, J. A. Nery, A. R. Santos, H. C. Matos, and E. N. Sarno. 1998. Pentoxifylline decreases in vivo and in vitro tumour necrosis factor-alpha (TNF-alpha) production in lepromatous leprosy patients with erythema nodosum leprosum (ENL). Clin. Exp. Immunol. 111:300-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sampaio, E. P., A. L. Moreira, E. N. Sarno, A. M. Malta, and G. Kaplan. 1992. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175:1729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sampaio, E. P., E. N. Sarno, R. Galilly, Z. A. Conh, and G. Kaplan. 1991. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J. Exp. Med. 173:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Santos, A. R., V. Balassiano, M. L. Oliveira, M. A. Pereira, P. B. Santos, W. M. Degrave, and P. N. Suffys. 2001. Detection of Mycobacterium leprae DNA by polymerase chain reaction in the blood of individuals, eight years after completion of anti-leprosy therapy. Mem. Inst. Oswaldo Cruz 96:1129-1133. [PubMed] [Google Scholar]
- 335.Santos, A. R., P. N. Suffys, P. R. Vanderborght, M. O. Moraes, L. M. Vieira, P. H. Cabello, A. M. Bakker, H. J. Matos, T. W. Huizinga, T. H. Ottenhoff, E. P. Sampaio, and E. N. Sarno. 2002. Role of tumor necrosis factor-alpha and interleukin-10 promoter gene polymorphisms in leprosy. J. Infect. Dis. 186:1687-1691. [DOI] [PubMed] [Google Scholar]
- 336.Santos, D. O., S. L. Santos, D. Esquenazi, J. A. Nery, M. Defruyt, K. Lorre, and H. van Heuverswyn. 2001. Evaluation of B7-1 (CD80) and B7-2 (CD86) costimulatory molecules and dendritic cells on the immune response in leprosy. Nihon Hansenbyo Gakkai Zasshi 70:15-24. [DOI] [PubMed] [Google Scholar]
- 337.Sarno, E. N., G. E. Grau, L. M. M. Vieira, and J. A. Nery. 1991. Serum levels of tumour necrosis factor-alpha and interleukin-1B during leprosy reactional states. Clin. Exp. Immunol. 84:103-108. [PMC free article] [PubMed] [Google Scholar]
- 338.Sathish, M., R. E. Esser, J. E. Thole, and J. E. Clark-Curtiss. 1990. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect. Immun. 58:1327-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Saunderson, P., S. Gebre, K. Desta, P. Byass, and D. N. Lockwood. 2000. The pattern of leprosy-related neuropathy in the AMFES patients in Ethiopia: definitions, incidence, risk factors and outcome. Lepr. Rev. 71:285-308. [DOI] [PubMed] [Google Scholar]
- 340.Save, M. P., V. P. Shetty, K. T. Shetty, and N. H. Antia. 2004. Alterations in neurofilament protein(s) in human leprous nerves: morphology, immunohistochemistry and Western immunoblot correlative study. Neuropathol. Appl. Neurobiol. 30:635-650. [DOI] [PubMed] [Google Scholar]
- 341.Schleimer, R. P. 1993. An overview of glucocorticoid anti-inflammatory actions. Eur. J. Clin. Pharmacol. 45(Suppl. 1):S3-S7; discussion, S43-S44. [DOI] [PubMed]
- 342.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 343.Schlesinger, L. S., and M. A. Horwitz. 1991. Phenolic glycolipid-1 of Mycobacterium leprae binds complement component C3 in serum and mediates phagocytosis by human monocytes. J. Exp. Med. 174:1031-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Schon, T., N. Gebre, T. Sundqvist, H. S. Habetmariam, T. Engeda, and S. Britton. 1999. Increased levels of nitric oxide metabolites in urine from leprosy patients in reversal reaction. Lepr. Rev. 70:52-55. [DOI] [PubMed] [Google Scholar]
- 345.Schon, T., R. H. Hernandez-Pando, Y. Negesse, R. Leekassa, T. Sundzvist, and S. Britton. 2001. Expression of inducible nitric oxide synthase and nitrotyrosine in borderline leprosy lesions. Br. J. Dermatol. 145:809-815. [DOI] [PubMed] [Google Scholar]
- 346.Schon, T., R. Leekassa, N. Gebre, T. Sundqvist, E. Bizuneh, and S. Britton. 2000. High dose prednisolone treatment of leprosy patients undergoing reactions is associated with a rapid decrease in urinary nitric oxide metabolites and clinical improvement. Lepr. Rev. 71:355-362. [DOI] [PubMed] [Google Scholar]
- 347.Scollard, D. M. 2000. Endothelial cells and the pathogenesis of lepromatous neuritis: insights from the armadillo model. Microbes Infect. 2:1835-1843. [DOI] [PubMed] [Google Scholar]
- 348.Scollard, D. M., T. P. Gillis, and D. L. Williams. 1998. Polymerase chain reaction assay for the detection and identification of Mycobacterium leprae in patients in the United States. Am. J. Clin. Pathol. 109:642-646. [DOI] [PubMed] [Google Scholar]
- 349.Scollard, D. M., and M. P. Joyce. 2003. Leprosy (Hansen's disease), p. 101-106. In R. E. Rakel and E. T. Bope (ed.), Conn's current therapy. Elsevier Science, St. Louis, Mo.
- 350.Scollard, D. M., G. W. Lathrop, and R. W. Truman. 1996. Infection of distal peripheral nerves by M. leprae in infected armadillos; an experimental model of nerve involvement in leprosy. Int. J. Lepr. Other Mycobact. Dis. 64:146-151. [PubMed] [Google Scholar]
- 351.Scollard, D. M., G. McCormick, and J. Allen. 1999. Localization of Mycobacterium leprae to endothelial cells of epineural and perineural blood vessels and lymphatics. Am. J. Pathol. 154:1611-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Scollard, D. M., T. Smith, L. Bhoopat, C. Theetranont, S. Rangdaeng, and D. M. Morens. 1994. Epidemiologic characteristics of leprosy reactions. Int. J. Lepr. Other Mycobact. Dis. 62:559-567. [PubMed] [Google Scholar]
- 353.Scollard, D. M., V. Suriyanon, L. Bhoopat, D. K. Wagner, T. C. Smith, K. Thamprasert, D. L. Nelson, and C. Theetranont. 1990. Studies of human leprosy lesions in situ using suction-induced blisters. 2. Cell changes and soluble interleukin 2 receptor (Tac peptide) in reversal reactions. Int. J. Lepr. Other Mycobact. Dis. 58:469-479. [PubMed] [Google Scholar]
- 354.Scollard, D. M., and D. L. Williams. 2002. Nerve injury in leprosy. UpToDate. [Online.] http://www.uptodate.com.
- 355.Scollard, D. M. 2005. Leprosy research declines, but most of the basic questions remain unanswered. Int. J. Lepr. Other Mycobact. Dis. 73:25-27. [DOI] [PubMed] [Google Scholar]
- 355a.Scollard, D. M., M. P. Joyce, and T. P. Gillis. Development of leprosy and type 1 leprosy reactions after treatment with Infliximab: a report of two cases. Clin. Infect. Dis., in press. [DOI] [PubMed]
- 356.Sela, S., and J. E. Clark-Curtiss. 1991. Cloning and characterization of the Mycobacterium leprae putative ribosomal RNA promoter in Escherichia coli. Gene 98:123-127. [DOI] [PubMed] [Google Scholar]
- 357.Sengupta, U. 1991. Studies on lepromin and soluble antigens of M. leprae: their classification standardization and use. Indian J. Lepr. 63:457-465. [PubMed] [Google Scholar]
- 358.Sergeantson, S. 1983. HLA and susceptibility to leprosy. Immunol. Rev. 70:89-112. [DOI] [PubMed] [Google Scholar]
- 359.Seydel, J. K., M. Richter, and E. Wempe. 1980. Mechanism of action of the folate blocker diaminodiphenylsulfone (dapsone, DDS) studied in E. coli cell-free enzyme extracts in comparison to sulfonamides (SA). Int. J. Lepr. Other Mycobact. Dis. 48:18-29. [PubMed] [Google Scholar]
- 360.Shannon, E., and F. Sandoval. 1996. Thalidomide can be either agonistic or antagonistic to LPS evoked synthesis of TNF-alpha by mononuclear cells. Immunopharmacol. Immunotoxicol. 18:59-72. [DOI] [PubMed] [Google Scholar]
- 361.Shannon, E. J., R. O. Miranda, M. J. Morales, and R. C. Hastings. 1981. Inhibition of de novo IgM antibody synthesis by thalidomide as a relevant mechanism of action in leprosy. Scand. J. Immunol. 13:553-562. [DOI] [PubMed] [Google Scholar]
- 362.Shannon, E. J., and F. Sandoval. 1995. Thalidomide increases the synthesis of IL-2 in cultures of human mononuclear cells stimulated with concanavalin-A, staphylococcal enterotoxin A, and purified protein derivative. Immunopharmacology 31:109-116. [DOI] [PubMed] [Google Scholar]
- 363.Shepard, C. C. 1960. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Shepard, C. C., and Y. T. Chang. 1962. Effect of several anti-leprosy drugs on multiplication of human leprosy bacilli in footpads of mice. Proc. Soc. Exp. Biol. Med. 109:636-638. [DOI] [PubMed] [Google Scholar]
- 365.Shepard, C. C., R. J. Rees, L. Levy, S. R. Pattyn, J. Baohong, and E. C. Dela Cruz. 1986. Susceptibility of strains of Mycobacterium leprae isolated prior to 1977 from patients with previously untreated lepromatous leprosy. Int. J. Lepr. Other Mycobact. Dis. 54:11-15. [PubMed] [Google Scholar]
- 366.Shepard, C. C., L. L. Walker, and R. Van Landingham. 1978. Heat stability of Mycobacterium leprae immunogenicity. Infect. Immun. 22:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sheskin, J. 1965. Thalidomide in the treatment of lepra reactions. Clin. Pharmacol. Ther. 6:303-306. [DOI] [PubMed] [Google Scholar]
- 368.Shetty, V. P., M. W. Uplekar, and N. H. Antia. 1996. Primary resistance to single and multiple drugs in leprosy—a mouse footpad study. Lepr. Rev. 67:280-286. [DOI] [PubMed] [Google Scholar]
- 369.Shi, L., R. P. Kraut, R. Aebersold, and A. H. Greenberg. 1992. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J. Exp. Med. 175:553-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Shimoji, Y., V. Ng, K. Matsumura, V. A. Fischetti, and A. Rambukkana. 1999. A 21 kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl. Acad. Sci. USA 96:9857-9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Shin, Y. C., H. Lee, H. Lee, G. P. Walsh, J. D. Kim, and S. N. Cho. 2000. Variable numbers of TTC repeats in Mycobacterium leprae DNA from leprosy patients and use in strain differentiation. J. Clin. Microbiol. 38:4535-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.ShivRaj, L., S. A. Patil, A. Girdhar, U. Sengupta, K. V. Desikan, and H. Srinivasan. 1988. Antibodies to HIV-1 in sera from patients with mycobacterial infections. Int. J. Lepr. Other Mycobact. Dis. 56:546-551. [PubMed] [Google Scholar]
- 373.Sibley, L. D., S. G. Franzblau, and J. L. Krahenbuhl. 1987. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect. Immun. 55:680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Sibley, L. D., and J. L. Krahenbuhl. 1987. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect. Immun. 55:446-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sibley, L. D., and J. L. Krahenbuhl. 1988. Defective activation of granuloma macrophages from Mycobacterium leprae-infected nude mice. J. Leukoc. Biol. 43:60-66. [DOI] [PubMed] [Google Scholar]
- 376.Sibley, L. D., and J. L. Krahenbuhl. 1988. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect. Immun. 56:1912-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Siddiqui, M. R., S. Meisner, K. Tosh, K. Balakrishnan, S. Ghei, S. E. Fisher, M. Golding, N. P. Shanker Narayan, T. Sitaraman, U. Sengupta, R. Pitchappan, and A. V. Hill. 2001. A major susceptibility locus for leprosy in India maps to chromosome 10p13. Nat. Genet. 27:439-441. [DOI] [PubMed] [Google Scholar]
- 378.Sieling, P. A., D. Chatterjee, S. A. Porcelli, T. I. Prigozy, R. J. Mazzaccaro, T. Soriano, B. R. Bloom, M. B. Brenner, M. Kronenberg, P. J. Brennan, and R. L. Modlin. 1995. CD-1-restricted T cell recognition of microbial lipoglycan antigens. Science 269:227-230. [DOI] [PubMed] [Google Scholar]
- 379.Sieling, P. A., D. Jullien, M. Dahlem, T. F. Tedder, T. H. Rea, R. L. Modlin, and S. A. Procelli. 1999. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J. Immunol. 162:1851-1858. [PubMed] [Google Scholar]
- 380.Sieling, P. A., and R. L. Modlin. 1994. Cytokine patterns at the site of mycobacterial infection. Immunopharmacology 191:378-387. [DOI] [PubMed] [Google Scholar]
- 381.Sieling, P. A., X. H. Wang, M. K. Gately, J. L. Oliveros, T. McHugh, P. F. Barnes, S. F. Wolf, L. Golkar, M. Yamamura, and Y. Yogi. 1994. IL-12 regulates T helper type 1 cytokine responses in human infectious disease. J. Immunol. 153:3639-3647. [PubMed] [Google Scholar]
- 382.Singh, N., T. J. Birdi, S. Chandrashekar, and N. H. Antia. 1997. Schwann cell extracellular matrix protein production is modulated by Mycobacterium leprae and macrophage secretory products. J. Neurol. Sci. 151:13-22. [DOI] [PubMed] [Google Scholar]
- 383.Skamene, E., P. Gros, A. Forget, P. A. Kongshavn, C. St. Charles, and B. A. Taylor. 1982. Genetic regulation of resistance to intracellular pathogens. Nature 297:506-509. [DOI] [PubMed] [Google Scholar]
- 384.Skinsnes, O. K. 1964. The immunopathologic spectrum of leprosy, p. 152-182. In R. G. Cochrane and T. F. Davey (ed.), Leprosy in theory and practice. John Wright and Sons, Ltd., Bristol, United Kingdom.
- 385.Smith, W. C., A. M. Anderson, S. G. Withington, W. H. van Brakel, R. P. Croft, P. G. Nicholls, and J. H. Richardus. 2004. Steroid prophylaxis for prevention of nerve function impairment in leprosy: randomised placebo controlled trial (TRIPOD 1). Br. Med. J. 328:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Spencer, J. S., M. A. Marques, M. C. Lima, A. P. Junqueira-Kipnis, B. C. Gregory, R. W. Truman, and P. J. Brennan. 2002. Antigenic specificity of the Mycobacterium leprae homologue of ESAT-6. Infect. Immun. 70:1010-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Spierings, E., T. De Boer, L. Zulianello, and T. H. Ottenhoff. 2000. Novel mechanisms in the immunopathogenesis of leprosy nerve damage: the role of Schwann cells, T cells and Mycobacterium leprae. Immunol. Cell Biol. 78:349-355. [DOI] [PubMed] [Google Scholar]
- 388.Spies, T., M. Bresnahan, S. Bahram, D. Arnold, G. Blanck, E. Mellins, D. Pious, and R. DeMars. 1990. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature 348:744-747. [DOI] [PubMed] [Google Scholar]
- 389.Sreenivasan, P., R. S. Misra, D. Wilfred, and I. Nath. 1998. Lepromatous leprosy patients show T helper 1-like cytokine profile with differential expression of interleukin-10 during type 1 and 2 reactions. Immunology 95:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Stanley, S. J., C. Howland, M. M. Stone, and I. Sutherland. 1981. BCG vaccination of children against leprosy in Uganda: final results. J. Hyg. (London) 87:233-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Stefani, M. M., C. M. Martelli, T. P. Gillis, and J. L. Krahenbuhl. 2003. In situ type 1 cytokine gene expression and mechanisms associated with early leprosy progression. J. Infect. Dis. 188:1024-1031. [DOI] [PubMed] [Google Scholar]
- 392.Steinhofff, U., A. Wand-Wurttenberger, A. Bremerich, and S. H. E. Kaufmann. 1991. Mycobacterium leprae renders Schwann cells and mononuclear phagocytes susceptible or resistant to killer cells. Infect. Immun. 59:684-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Stenger, S., D. A. Hanson, R. Teitlebaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 394.Storrs, E. E., and W. E. Greer. 1973. Maintenance and husbandry of armadillo colonies. Lab. Anim. Sci. 23:823-829. [PubMed] [Google Scholar]
- 395.Strieter, R. M., D. G. Remick, P. A. Ward, R. N. Spengler, J. P. Lynch III, J. Larrick, and S. L. Kunkel. 1988. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem. Biophys. Res. Commun. 155:1230-1236. [DOI] [PubMed] [Google Scholar]
- 396.Stump, P. R., R. Baccarelli, L. H. Marciano, J. R. Lauris, M. J. Teixeira, S. Ura, and M. C. Virmond. 2004. Neuropathic pain in leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 72:134-138. [DOI] [PubMed] [Google Scholar]
- 397.Sugumaran, D. S. 1998. Leprosy reactions—complications of steroid therapy. Int. J. Lepr. Other Mycobact. Dis. 66:10-15. [PubMed] [Google Scholar]
- 398.Suneetha, L. M., D. Vardhini, S. Suneetha, A. S. Balasubramanian, C. K. Job, and D. Scollard. 2001. Biochemical aspects of Mycobacterium leprae binding proteins: a review of their role in pathogenesis. Int. J. Lepr. Other Mycobact. Dis. 69:341-348. [PubMed] [Google Scholar]
- 399.Swift, T. R. 1974. Peripheral nerve involvement in leprosy: quantitative histologic aspects. Acta Neuropathol. (Berlin) 29:1-8. [DOI] [PubMed] [Google Scholar]
- 400.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Takiff, H. E., L. Salazar, C. Guerrero, W. Philipp, W. M. Huang, B. Kreiswirth, S. T. Cole, W. R. Jacobs, Jr., and A. Telenti. 1994. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Taylor, D. E., and A. Chau. 1996. Tetracycline resistance mediated by ribosomal protection. Antimicrob. Agents Chemother. 40:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Telenti, A., P. Imboden, F. Marchesi, D. Lowrie, S. Cole, M. J. Colston, L. Matter, K. Schopfer, and T. Bodmer. 1993. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 341:647-650. [DOI] [PubMed] [Google Scholar]
- 404.Teles, R. M., M. O. Moraes, N. T. Geraldo, A. M. Salles, E. N. Sarno, and E. P. Sampaio. 2002. Differential TNFalpha mRNA regulation detected in the epidermis of leprosy patients. Arch. Dermatol. Res. 294:355-362. [DOI] [PubMed] [Google Scholar]
- 405.Teo, S. K., K. E. Resztak, M. A. Scheffler, K. A. Kook, J. B. Zeldis, D. I. Stirling, and S. D. Thomas. 2002. Thalidomide in the treatment of leprosy. Microbes Infect. 4:1193-1202. [DOI] [PubMed] [Google Scholar]
- 406.Thangaraj, H. S., F. I. Lamb, E. O. Davis, P. J. Jenner, L. H. Jeyakumar, and M. J. Colston. 1990. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect. Immun. 58:1937-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Thole, J. E., B. Wieles, J. E. Clark-Curtiss, T. H. Ottenhoff, and T. F. Rinke de Wit. 1995. Immunological and functional characterization of Mycobacterium leprae protein antigens: an overview. Mol. Microbiol. 18:791-800. [DOI] [PubMed] [Google Scholar]
- 408.Thomas, D. A., J. S. Mines, T. M. Mack, D. C. Thomas, and T. H. Rea. 1987. Armadillo exposure among Mexican-born patients with lepromatous leprosy. J. Infect. Dis. 156:990-993. [DOI] [PubMed] [Google Scholar]
- 409.Torres, P., J. J. Camarena, J. R. Gomez, J. M. Nogueira, V. Gimeno, J. C. Navarro, and A. Olmos. 2003. Comparison of PCR mediated amplification of DNA and the classical methods for detection of Mycobacterium leprae in different types of clinical samples in leprosy patients and contacts. Lepr. Rev. 74:18-30. [PubMed] [Google Scholar]
- 410.Tramontana, J. M., U. Utaipat, A. Molloy, P. Akarasewi, M. Burroughs, S. Makonkawkeyoon, B. Johnson, J. D. Klausner, W. Rom, and G. Kaplan. 1995. Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol. Med. 1:384-397. [PMC free article] [PubMed] [Google Scholar]
- 411.Tripathy, S. P. 1983. The case for B.C.G. Ann. Natl. Acad. Med. Sci. (India) 19:11-21. [PubMed] [Google Scholar]
- 412.Truman, R., A. B. Fontes, A. B. De Miranda, P. Suffys, and T. Gillis. 2004. Genotypic variation and stability of four variable-number tandem repeats and their suitability for discriminating strains of Mycobacterium leprae. J. Clin. Microbiol. 42:2558-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Truman, R., and R. Sanchez. 1993. Armadillos: models for leprosy. Lab. Anim. 22:28-32. [Google Scholar]
- 414.Truman, R. W., and J. L. Krahenbuhl. 2001. Viable M. leprae as a research reagent. Int. J. Lepr. Other Mycobact. Dis. 69:1-12. [PubMed] [Google Scholar]
- 415.Truman, R. 2005. Leprosy in wild armadillos. Lepr. Rev. 76:198-208. [PubMed] [Google Scholar]
- 416.Tung, K. S., E. Umland, P. Matzner, K. Nelson, V. Schauf, L. Rubin, D. Wagner, D. Scollard, P. Vithayasai, V. Vithayasai, et al. 1987. Soluble serum interleukin 2 receptor levels in leprosy patients. Clin. Exp. Immunol. 69:10-15. [PMC free article] [PubMed] [Google Scholar]
- 417.Underhill, D. M., A. Ozinsku, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signals in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Uyemura, K., J. F. Dixon, L. Wong, T. H. Rea, and R. L. Modlin. 1986. Effect of cyclosporine A in erythema nodosum leprosum. J. Immunol. 137:3620-3623. [PubMed] [Google Scholar]
- 419.Van Brakel, W. H., A. M. Anderson, S. G. Withington, R. P. Croft, P. G. Nicholls, J. H. Richardus, and W. C. Smith. 2003. The prognostic importance of detecting mild sensory impairment in leprosy: a randomized controlled trial (TRIPOD 2). Lepr. Rev. 74:300-310. [PubMed] [Google Scholar]
- 420.van de Vosse, E., M. A. Hoeve, and T. H. Ottenhoff. 2004. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect. Dis. 4:739-749. [DOI] [PubMed] [Google Scholar]
- 421.van Gompel, A., E. van den Enden, and J. van den Ende. 1994. Cyclosporin A is not very effective in erythema nodosum leprosum (ENL). Trop. Geogr. Med. 46:331. [PubMed] [Google Scholar]
- 422.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 423.Verhagen, C. E., E. A. Wierenga, A. A. Buffing, M. A. Chand, W. R. Faber, and P. K. Das. 1997. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin: a follow-study. J. Immunol. 159:4474-4483. [PubMed] [Google Scholar]
- 424.Vester, B., and S. Douthwaite. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 45:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Vissa, V. D., and P. J. Brennan. 2001. The genome of Mycobacterium leprae: a minimal mycobacterial gene set. Genome Biol. 2:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Reference deleted.
- 427.Wagener, D. K., V. Schauf, K. E. Nelson, D. Scollard, A. Brown, and T. Smith. 1988. Segregation analysis of leprosy in families of northern Thailand. Genet. Epidemiol. 5:95-105. [DOI] [PubMed] [Google Scholar]
- 428.Wallace, R. J., Jr., A. Meier, B. A. Brown, Y. Zhang, P. Sander, G. O. Onyi, and E. C. Bottger. 1996. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob. Agents Chemother. 40:1676-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Watts, C. 2004. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat. Immunol. 5:685-692. [DOI] [PubMed] [Google Scholar]
- 430.Weinberg, J. B. 1998. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol. Med. 4:557-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wemambu, S. N., J. L. Turk, M. F. Waters, and R. J. Rees. 1969. Erythema nodosum leprosum: a clinical manifestation of the arthus phenomenon. Lancet ii:933-935. [DOI] [PubMed] [Google Scholar]
- 432.Wheeler, P. R. 2001. The microbial physiologist's guide to the leprosy genome. Lepr. Rev. 72:399-407. [DOI] [PubMed] [Google Scholar]
- 433.Wilkinson, R. J., and D. N. Lockwood. 2005. Antigenic trigger for type 1 reaction in leprosy. J. Infect. 50:242-243. [DOI] [PubMed] [Google Scholar]
- 434.Williams, D. L., and T. P. Gillis. 2004. Molecular detection of drug resistance in Mycobacterium leprae. Lepr. Rev. 75:118-130. [PubMed] [Google Scholar]
- 435.Williams, D. L., T. P. Gillis, R. J. Booth, D. Looker, and J. D. Watson. 1990. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J. Infect. Dis. 162:193-200. [DOI] [PubMed] [Google Scholar]
- 436.Williams, D. L., T. P. Gillis, and F. Portaels. 1990. Geographically distinct isolates of Mycobacterium leprae exhibit no genotypic diversity by restriction fragment-length polymorphism analysis. Mol. Microbiol. 4:1653-1659. [DOI] [PubMed] [Google Scholar]
- 437.Williams, D. L., T. L. Pittman, T. P. Gillis, M. Matsuoka, and Y. Kashiwabara. 2001. Simultaneous detection of Mycobacterium leprae and its susceptibility to dapsone using DNA heteroduplex analysis. J. Clin. Microbiol. 39:2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Williams, D. L., L. Spring, T. P. Gillis, M. Salfinger, and D. H. Persing. 1998. Evaluation of a polymerase chain reaction-based universal heteroduplex generator assay for direct detection of rifampin susceptibility of Mycobacterium tuberculosis from sputum specimens. Clin. Infect. Dis. 26:446-450. [DOI] [PubMed] [Google Scholar]
- 439.Williams, D. L., L. Spring, E. Harris, P. Roche, and T. P. Gillis. 2000. Dihydropteroate synthase of Mycobacterium leprae and dapsone resistance. Antimicrob. Agents Chemother. 44:1530-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Williams, D. L., M. Torrero, P. R. Wheeler, R. W. Truman, M. Yoder, N. Morrison, W. R. Bishai, and T. P. Gillis. 2004. Biological implications of Mycobacterium leprae gene expression during infection. J. Mol. Microbiol. Biotechnol. 8:58-72. [DOI] [PubMed] [Google Scholar]
- 441.Williams, D. L., C. Waguespack, K. Eisenach, J. T. Crawford, F. Portaels, M. Salfinger, C. M. Nolan, C. Abe, V. Sticht-Groh, and T. P. Gillis. 1994. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob. Agents Chemother. 38:2380-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Winter, N., J. A. Triccas, B. Rivoire, M. C. Pessolani, K. Eiglmeier, E. M. Lim, S. W. Hunter, P. J. Brennan, and W. J. Britton. 1995. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol. Microbiol. 16:865-876. [DOI] [PubMed] [Google Scholar]
- 443.Woods, S. A., and S. T. Cole. 1990. A family of dispersed repeats in Mycobacterium leprae. Mol. Microbiol. 4:1745-1751. [DOI] [PubMed] [Google Scholar]
- 444.World Health Organization. 1998. Leprosy. Wkly. Epidemiol. Rec. 73:153-160.9619084 [Google Scholar]
- 445.World Health Organization. 2004. Leprosy elimination project. Status report 2002-2003. World Health Organization, Geneva, Switzerland.
- 446.World Health Organization Expert Committee on Leprosy. 1977. Fifth report. World Health Organization, Geneva, Switzerland.
- 447.World Health Organization Study Group. 1982. Chemotherapy of leprosy for control programmes. World Health Organization, Geneva, Switzerland. [PubMed]
- 448.Yamamura, M., K. Uyemura, R. J. Deans, K. Weinberg, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277-279. [DOI] [PubMed] [Google Scholar]
- 449.Yamamura, M., X. H. Wang, J. D. Ohmen, K. Uyemura, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1992. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149:1470-1475. [PubMed] [Google Scholar]
- 450.You, E. Y., T. J. Kang, S. K. Kim, S. B. Lee, and G. T. Chae. 2005. Mutations in genes related to drug resistance in Mycobacterium leprae isolates from leprosy patients in Korea. J. Infect. 50:6-11. [DOI] [PubMed] [Google Scholar]
- 451.Yuasa, Y. 2003. Remarks by Damien Dutton Award Recipient Dr. Yo Yuasa. Int. J. Lepr. Other Mycobact. Infect. 71:33-35. [Google Scholar]
- 452.Zaheer, S. A., K. R. Beena, H. K. Kar, A. K. Sharma, R. S. Misra, A. Mukherjee, R. Mukherjee, H. Kaur, R. M. Pandey, R. Walia, et al. 1995. Addition of immunotherapy with Mycobacterium w vaccine to multi-drug therapy benefits multibacillary leprosy patients. Vaccine 13:1102-1110. [DOI] [PubMed] [Google Scholar]
- 453.Zaheer, S. A., R. Mukherjee, B. Ramkumar, R. S. Misra, A. K. Sharma, H. K. Kar, H. Kaur, S. Nair, A. Mukherjee, and G. P. Talwar. 1993. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J. Infect. Dis. 167:401-410. [DOI] [PubMed] [Google Scholar]
- 454.Zhang, Y., J. Gao, K. K. Chung, H. Huang, V. L. Dawson, and T. M. Dawson. 2000. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl. Acad. Sci. USA 97:13354-13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Zodpey, S. P., N. N. Ambadekar, and A. Thakur. 2005. Effectiveness of bacillus Calmette Guerin (BCG) vaccination in the prevention of leprosy: a population-based case-control study in Yavatmal District, India. Public Health 119:209-216. [DOI] [PubMed] [Google Scholar]