Abstract
Efflux pump genes and proteins are present in both antibiotic-susceptible and antibiotic-resistant bacteria. Pumps may be specific for one substrate or may transport a range of structurally dissimilar compounds (including antibiotics of multiple classes); such pumps can be associated with multiple drug (antibiotic) resistance (MDR). However, the clinical relevance of efflux-mediated resistance is species, drug, and infection dependent. This review focuses on chromosomally encoded pumps in bacteria that cause infections in humans. Recent structural data provide valuable insights into the mechanisms of drug transport. MDR efflux pumps contribute to antibiotic resistance in bacteria in several ways: (i) inherent resistance to an entire class of agents, (ii) inherent resistance to specific agents, and (iii) resistance conferred by overexpression of an efflux pump. Enhanced efflux can be mediated by mutations in (i) the local repressor gene, (ii) a global regulatory gene, (iii) the promoter region of the transporter gene, or (iv) insertion elements upstream of the transporter gene. Some data suggest that resistance nodulation division systems are important in pathogenicity and/or survival in a particular ecological niche. Inhibitors of various efflux pump systems have been described; typically these are plant alkaloids, but as yet no product has been marketed.
INTRODUCTION
Efflux is the pumping of a solute out of a cell. Efflux pump genes and proteins are present in both antibiotic-susceptible and antibiotic-resistant bacteria. Some systems can be induced by their substrates so that an apparently susceptible strain can overproduce a pump and become resistant. Antimicrobial resistance in an efflux mutant is due to one of two mechanisms: either (i) expression of the efflux pump protein is increased or (ii) the protein contains an amino acid substitution(s) that makes the protein more efficient at export. In either case, the intracellular concentration of the substrate antimicrobial is lowered and the organism becomes less susceptible to that agent. Efflux pumps may be specific for one substrate or may transport a range of structurally dissimilar compounds (including antibiotics of multiple classes); such pumps can be associated with multiple drug (antibiotic) resistance (MDR). Resistance in this context does not necessarily mean resistance to those agents that would be used to treat an infection by a particular species, or even resistance to clinically achievable concentrations of these drugs, and so the clinical relevance of efflux-mediated resistance is species, drug, and infection dependent. Genes encoding efflux pumps can be found on the chromosome or on transmissible elements such as plasmids (e.g., tet and qac genes); this review focuses on chromosomally encoded MDR efflux pumps.
In addition to the role of enhanced efflux in antimicrobial resistance, it has also been suggested that increased expression of efflux pump genes may be the first step in the bacterium becoming fully resistant (144). It is also thought that increased efflux decreases the intracellular concentration of the antimicrobial, thereby allowing bacterial survival for a greater length of time, such that bacteria containing mutations in other genes, such as those that encode target proteins (e.g., fluoroquinolones and topoisomerase genes), can accumulate. More recently, there have been several publications on different species of bacteria, suggesting natural physiological roles of MDR efflux pumps in addition to export of antimicrobials.
There are still many questions that must be addressed, as genomics has revealed that efflux pumps are ubiquitous throughout nature in all types of cells, from eukaryotic to prokaryotic. One important question is for which species MDR efflux pumps confer clinically relevant resistance, i.e., in which the MIC is greater than the recommended breakpoint concentration of a drug for a particular bacterial species, and specifically with reference to antimicrobials that are used in human or veterinary medicine for an infection caused by that bacterium. Which of the clinically relevant agents, then, are typically affected by bacteria that overexpress MDR efflux pumps?
There have been several keynote publications in this field in recent years describing the various classes of efflux pumps and their substrates, structures, and functions (19, 63, 96, 155, 166, 236). This article focuses on those pumps and bacterial species considered to be of current clinical relevance, the putative natural roles of efflux pumps, and inhibitors.
CLASSES OF MDR EFFLUX PUMPS, GENOMICS, AND STRUCTURAL BIOLOGY
Classes and Organization of Efflux Pump Systems
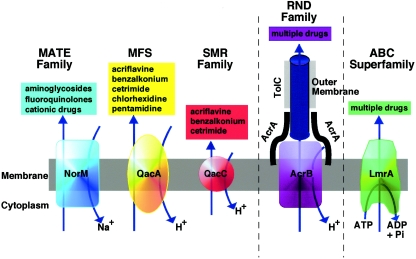
Genomics has revealed that there are many genes that encode putative efflux pumps of all types, of which a subset are thought to confer MDR (196). There is also evidence that the size of the genome is reflected in the number of pump genes present, such that large genomes possess greater numbers of pump genes (156, 182). Often a single organism can possess multiple MDR efflux pumps (e.g., the Mex systems of Pseudomonas aeruginosa or the Acr systems of the Enterobacteriaceae). There are essentially five different families of efflux pump proteins (Fig. 1); to date the important families of chromosomally encoded bacterial efflux pumps, with respect to bacterial MDR efflux, are the resistance nodulation division (RND) family, the major facilitator superfamily (MFS), and the staphylococcal multiresistance (SMR) and multidrug and toxic compound extrusion (MATE) families. A role for ABC (ATP binding cassette) MDR transporters in MDR of clinically relevant bacteria has yet to be established.
FIG. 1.
Diagrammatic comparison of the five families of efflux pumps. (Courtesy of Melissa Brown; reproduced by kind permission.)
The efflux pump systems of the RND family are organized as tripartite efflux pumps. The pump in Escherichia coli and other gram-negative bacteria has three components: a transporter (efflux) protein in the inner membrane (e.g., AcrB), a periplasmic accessory protein (e.g., AcrA), and an outer membrane protein channel (e.g., TolC) (85), either termed an outer membrane protein (OMP) or outer membrane factor. AcrB captures its substrates within either the phospholipid bilayer of the inner membrane of the bacterial cell envelope or the cytoplasm (2) and transports them into the external medium via TolC (42). The cooperation between AcrB and TolC is mediated by the periplasmic protein AcrA. Comparative genomics have revealed high degrees of homology between pump genes (>70% identity) and amino acid sequences (>80% similarity) of pump proteins of the RND family both within a species and across different bacterial species, e.g., E. coli acrB/AcrB, P. aeruginosa mexB/MexB, Campylobacter jejuni cmeB/CmeB, and Neisseria gonorrhoeae mtrD/MtrD (Fig. 2). The genetic organizations of the genes encoding these tripartite efflux systems are also similar among different species. Typically, the genes are organized as an operon: the regulator gene is located adjacent to the gene encoding the periplasmic accessory protein, which is located adjacent to the gene encoding the efflux pump protein, which is located next to that for the OMP. The membrane fusion protein and the pump protein are usually cotranscribed. For some systems and/or species, the OMP is not colocated with the other genes, e.g., E. coli acrAB and tolC (110) and P. aeruginosa mexXY and oprM (1). The RND pumps are proton antiporters, using the proton gradient across the membrane to power efflux, exchanging one H+ ion for one drug molecule (155).
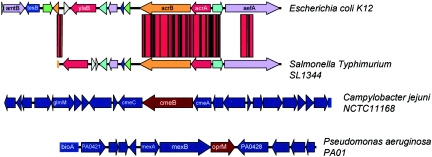
FIG. 2.
Comparison of the genetic organization of E. coli acrRAB with S. enterica serovar Typhimurium acrRAB, C. jejuni cmeABC, and P. aeruginosa mexAB-OprM. This diagram shows the similarities between the RND MDR efflux pump genes of different bacterial species.
In clinically important gram-positive bacteria, the two efflux pumps that have been examined in the most detail to date are members of the MFS: NorA of Staphylococcus aureus and PmrA of Streptococcus pneumoniae. As with the efflux pump (e.g., AcrB) of tripartite efflux pump systems, PmrA and NorA both possess 12 transmembrane-spanning regions (54, 232). Efflux is also driven by the proton motive force (PMF).
MATE MDR efflux pumps have been described for various bacteria, including Vibrio parahaemolyticus (NorM), Vibrio cholerae (VcrM; VcmA), Bacteroides thetaiotaomicron (BexA), Haemophilus influenzae (HmrM), P. aeruginosa (PmpM), Clostridium difficile (CdeA), and S. aureus (MepA). Two energy sources have been identified for MATE efflux pumps: the PMF and the sodium ion gradient. MATE pumps transport some of those agents also transported by RND pumps. However, a key distinguishing feature is that while RND pumps are tripartite, MATE pumps are not (Fig. 1).
Although no ABC transporters that give rise to clinically relevant MDR in human or animal pathogens have so far been described, ABC transporters are present in the genomes of pathogenic bacteria, and it may be predicted that at least one will confer antimicrobial drug resistance in the same way that P glycoprotein (PgP) confers resistance to anticancer agents. It has already been shown that Lactococcus lactis LmrA, an ABC transporter, confers MDR in this organism (223). ABC transporters have structural characteristics that differ from RND and MFS pump proteins, in that there are typically only six transmembrane-spanning regions (178). There are also three signature motifs within ABC transporter proteins; these include the Walker A, Walker B, and ABC signature motifs. In contrast to the RND, MSF, and MATE families of transporters, efflux by ABC transporters is driven by ATP hydrolysis.
To date, DNA sequencing data have not revealed significant variation between the sequences of the efflux pump genes of strains of the same species; however, recent work with C. jejuni cmeB has indicated some sequence diversity (27; B. Guo, J. Lin, D. Reynolds, and Q. Zhang, Abstr. 105th Gen. Meet. Am. Soc. Microbiol., abstr. A-001, 2005). Sequence variations occur throughout cmeB, but no data are available to indicate what effect, if any, these may have on efflux. Kim et al. (82) explored genomic data for similarities between LmrA and MexB (examples of the ABC and RND superfamilies, respectively). They observed that there are many hydrophobic, hydrophilic, and semipolar residues conserved in both proteins and that these are important for structure and/or function in both families.
An important structural feature of efflux pump proteins is that hydropathy plots typically reveal 12 transmembrane-spanning regions. Such predicted structural information from genomic data has been useful in identifying proteins involved in multidrug efflux.
Structural Biology
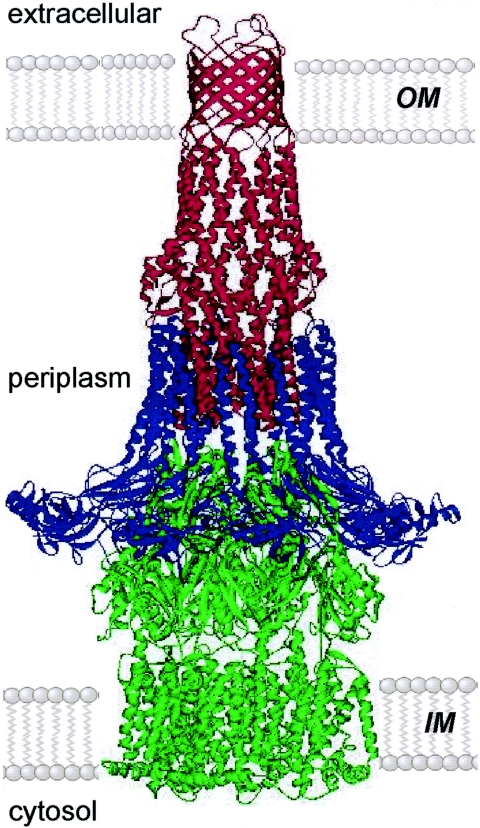
Several publications have described the crystal structures of components of the tripartite RND multidrug efflux pumps of gram-negative bacteria, including E. coli AcrB and TolC and P. aeruginosa MexA, MexB, and OprM (3, 4, 59, 86, 133) (Fig. 3).
FIG. 3.
Model of the assembled tripartite drug efflux pump. This possible model of an RND-class drug efflux pump is based on the open-state model of TolC (red) forming a minimal contact interface with the six hairpins at the apex of AcrB (green). A ring of nine MexA molecules (blue) is modeled to form a sheath around AcrB and the α-barrel of TolC (MexA is a close homologue of AcrA, the natural partner of AcrB/TolC). Variants of the model might include a lower-order oligomer of MexA (4) and more extensive interaction between AcrB and TolC. IM, inner membrane; OM, outer membrane. (Reprinted from reference 42 with permission from Elsevier and V. Koronakis.)
The crystal structure of TolC was resolved at 3.5-Å resolution (86). The functional unit of this protein is a homotrimer with a long channel that spans both the outer membrane and the periplasmic space. A set of coiled helices at the end of the tunnel were proposed to untwist and thereby open the channel.
The crystal structure of AcrB was first resolved at 3.5-Å resolution (133). The functional unit of AcrB is a homotrimer, with 12 membrane-spanning α helices and a large periplasmic domain. The structure also revealed that AcrB has a “headpiece” that opens like a funnel, thereby facilitating contact with TolC. Three α helices form a pore to the funnel, with a central cavity located at the bottom of the headpiece. The cavity has three “vestibules” (small pockets made from gaps around the three protomers of AcrB) at the side of the headpiece that lead into the periplasm. In the transmembrane region, each protomer has 12 transmembrane α helices. The structure suggested that substrates are transported from the cytoplasm via the transmembrane region and also from the periplasm via the vestibules. Substrates are then actively transported through the pore into TolC. Yu et al. (235) further suggested that the binding of substrates in AcrB is due to the partial binding of the compound to the phospholipid bilayer of the cytoplasmic membrane, depending on the lipophilicity and charge of the molecule. From there, the substrate must diffuse through the membrane toward the AcrB vestibules. It is then bound to the wall of the central cavity and from there is pumped out with the proton gradient (235). The structure of AcrB has since been further resolved to 2.7 Å (170). Two transmembrane domains are thought to be critical in proton translocation: TM4, which contains Asp401 and Asp 402, and TM10, which contains Lys940. Interestingly, there seems to be only one attachment between monomers, a long loop that protrudes into the center of the monomer in a counter-clockwise direction. Recent evidence with AcrD suggests that RND pumps act as a “periplasmic vacuum cleaner” and capture their substrates from the periplasm and export them into the external medium (2).
Gerken and Misra (51) showed that it is possible to cross-link AcrA and TolC without the presence of AcrB and so proposed that AcrB does not mediate or influence binding in vivo of TolC-AcrA interactions. In experiments with mutants lacking AcrAB, protein levels of TolC were unaffected and receptor functions were still present. Therefore, the authors proposed that TolC can stably insert into the outer membrane without AcrA or AcrB and still perform receptor functions.
MexA is found in Pseudomonas aeruginosa and is homologous to AcrA in E. coli. However, AcrA functions in complex with AcrB, whereas MexA and MexB form a homologous system. MexA and AcrA are anchored to the inner membrane by a single transmembrane helix, or in some cases by a lipid modification on the N terminus of the protein. Higgins et al. (59) have resolved the crystal structure of MexA to 3.0-Å resolution and have resolved amino acids 29 to 259 (a total of 230). MexA is 360 residues long in vivo, so part of this protein was absent. The MexA monomer was shown to be 89 Å long and 35 Å wide and to take the form of a β-barrel/lipoyl domain/α-helical structure, which is normally associated in other structures with ligand binding. It was proposed that, in vivo, the monomers form a homomonomer and make a “sheath” around both TolC (used because at that time the crystal structure of OprM had not been solved) and AcrB. Akama et al. (4) also proposed a similar structure for MexA, although they resolved more of the protein, 241 residues in all, from residues 23 to 274. They also proposed the same β-barrel/lipoyl domain/α-helical hairpin structure as did Higgins et al. (59). However, Akama et al. (3) proposed two in vivo structures, one based upon the sheath, or “seal,” model proposed by Higgins et al. (59) and one based upon a threefold dimer model. As MexA was found in the inner membrane fraction and the N terminus is fatty acid modified, Akama et al. (4) concluded that MexA is highly likely to be anchored to the inner membrane at least once. They also concluded that the α-helical hairpin is directed toward OprM/TolC and the β-barrel toward MexB/AcrB. A domain swapping experiment was also carried out, and they concluded that the C-terminal region of MexA/AcrA is very important in the interaction with MexB/AcrB.
SUBSTRATES OF MDR EFFLUX PUMPS
The phenotype of an “efflux mutant” (those that phenotypically resemble mutants that overexpress an efflux pump) is that the strain is typically MDR, i.e., resistant (less susceptible) to antimicrobials from at least three different classes of antibiotics, disinfectants (biocides), dyes, and detergents. The agents typically include a quinolone (nalidixic acid, ciprofloxacin, norfloxacin), tetracycline, chloramphenicol, ethidium bromide, acriflavine, sodium dodecyl sulfate (SDS), Triton X-100, and triclosan, but the agents considered to be substrates of each pump are slightly different depending on the pump and bacterial species. Poole (166, 167) lists details available for specific bacterial efflux pumps. The MICs of these substrates for strains overexpressing an efflux pump are typically two- to eightfold higher than those for a typical representative susceptible strain of that species or an isogenic parent strain (see Tables 1 to 8). Likewise, in those mutants in which an efflux pump gene has been disrupted or deleted, the MICs of substrates of that pump decrease, giving rise to a hypersusceptible strain. In most cases, the increases in MICs due to overexpression of an MDR efflux pump do not confer the same magnitude of increase as other mechanisms of resistance, such as β-lactamases, aminoglycoside modifying enzymes, or topoisomerase substitutions.
TABLE 1.
Susceptibility to antibiotics of P. aeruginosa lacking or overexpressing an efflux pump
| P. aeruginosa strain or characteristica | MIC (μg/ml)b
|
|||
|---|---|---|---|---|
| CAR | CIP | CHL | TET | |
| CLSI recommended breakpoint concn | 512 | 4 | 32 | 16 |
| BSAC recommended breakpoint concn | 256 | 8 | 8 | 2 |
| PAO1 (wild type) | 64 | 0.12 | 64 | 16 |
| mexAB-M::kan | 0.5 | 0.03 | 2 | 1 |
| MexAB-M+++ | 256 | 0.25 | 256 | 64 |
| MexAB-M+++ (strain 128)c | 128d | 1 | 256 | |
| ΔmexCD-oprJ | 0.002 | 1 | 0.12 | |
| ΔmexXY-oprM | 0.25 | 512 | 64 | |
| mexXY-oprM+++ | 0.5 | 8 | 32 | |
TABLE 8.
Susceptibility to antimicrobial agents of S. pneumoniae lacking or overexpressing pmrA
CLINICAL RELEVANCE OF MDR EFFLUX PUMPS
MDR efflux pumps contribute to antibiotic resistance in bacteria in several ways: (i) inherent resistance of gram-negative bacteria to an entire class of agents, (ii) inherent resistance of some species of gram-negative bacteria to specific agents, and (iii) resistance in clinically relevant bacteria conferred by overexpression of an efflux pump.
Inherent Resistance of Gram-Negative Bacteria to an Entire Class of Agents
Some agents have narrow spectra of activity, typically including gram-positive bacteria only, such as the oxalidinone class, e.g., linezolid, and more recently the deformylase inhibitors (69, 103). However, it has been shown that gram-negative bacteria in which specific efflux pump genes have been deleted are susceptible, indicating that these agents are substrates of the RND family of MDR efflux pumps (J. M. Buysse, W. F. Demyan, D. S. Dunyak, D. Stapert, J. C. Hamel, and C. W. Ford, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother, abstr. C-42, p. 41, 1996; J. Clements, personal communication). Chollet et al. (30) also showed that mutants lacking components of the E. coli AcrAB-TolC system were more susceptible to clarithromycin and erythromycin, but that the ketolide telithromycin was unaffected. The modest in vitro activity of the macrolides for Haemophilus influenzae has also been attributed to efflux via AcrAB in this species (159, 197). In addition, the new glycycline, tigecycline, has been shown to be less active against P. aeruginosa and Proteus mirabilis due to the MexXY-OprM and AcrAB-TolC efflux pumps, respectively (37, 225). As there are now increasing numbers of MDR gram-negative bacteria, the focus on drug development will inevitably turn to extending the spectrum of activity of these agents, with efforts to identify agents that are not substrates of efflux pumps.
Inherent Resistance of Some Species of Gram-Negative Bacteria to Specific Agents
P. aeruginosa is often considered to be resistant to many antibiotics, and historically this was attributed to the low “permeability” of the outer membrane. However, Livermore and Davy (104) provided evidence to refute this hypothesis and, in 1994, Li et al. (97) provided data to indicate that the resistance to tetracycline, chloramphenicol, and some fluoroquinolones was mediated by efflux. The previous year, Poole et al. (168) had described an efflux operon, MexAB-OprM, in wild-type P. aeruginosa; deletion of components of this system conferred hypersusceptibility to a variety of antimicrobial agents (Table 1).
The Enterobacteriaceae and other gram-negative bacteria are also generally considered to be less susceptible to many antimicrobials than gram-positive bacteria. Historically, this too has been attributed to the bacterial cell envelope conferring a “permeability barrier” (i.e., preventing uptake into the cell); however, it is increasingly recognized that this “barrier” is often due to efflux pumps acting alone or in concert with decreased expression of porins. Nonetheless, decreased transport of drugs into the bacterial cell is still a factor to be considered.
Efflux Pumps of Clinically Relevant Bacteria That Confer MDR via Overexpression
Gram-negative bacteria. (i) Pseudomonas aeruginosa.
In addition to the MexAB-OprM system, three further RND efflux pumps have also been characterized: MexXY-OprM, MexCD-OprJ, and MexEF-OprN (169, 208). Like the MexAB-OprM system, MexXY-OprM is constitutively expressed in wild-type cells and confers intrinsic MDR. However, MexCD-OprJ and MexEF-OprN are inducible by some of their substrates. In addition to exporting fluoroquinolones, tetracyline, chloramphenicol, and some β-lactams, these pumps also export ethidium bromide, acriflavine, SDS, triclosan, organic solvents, and acylated homoserine lactones involved in quorum sensing. Of these four RND pumps, the MexAB-OprM system is most similar to the AcrAB-TolC efflux pump of E. coli. MexA has 71% similarity with AcrA, and MexB has 89% similarity with AcrB. OprM has 35% similarity with TolC. Comparison of the Mex pumps reveals that MexC is more similar to MexA than to MexE (60% and 49%, respectively); likewise MexD is more similar to MexB than to MexF (69% and 61%, respectively). OprN has 48% similarity with OprM. While there are some substrates exported by all four systems, the MexXY system exports aminoglycosides as well, whereas the MexAB-OprM system also exports certain β-lactams, including carbenicillin (122) (Table 1). Both MexAB-OprM and MexCD-OprJ export cefsulodin and novobiocin. Infections by P. aeruginosa are usually treated with ceftazidime, ciprofloxacin, imipenem, gentamicin, tobramycin, ticarcillin-clavulanate, or piperacillin-tazobactam in combination or alone. Some of these agents are substrates of the Mex efflux pumps. However, despite the increase in MICs when these pumps are overexpressed, for agents such as ciprofloxacin the increase may not take the MIC above the recommended breakpoint concentration (Table 1).
Recently, a MATE transporter, PmpM, in P. aeruginosa has been described (58). PmpM transports fluoroquinolones, benzalkonium chloride, ethidium bromide, acriflavine, and tetraphenylphosphonium chloride. This system uses utilizes hydrogen ions, but not sodium ions, as an energy source.
(ii) Escherichia coli.
In E. coli, the AcrAB-TolC system is highly homologous to the MexAB-OprM RND system in P. aeruginosa (138). AcrA is a 397-amino-acid protein that interacts with AcrB, a much larger protein, 1,048 amino acids. TolC, a 506-amino-acid protein, is also associated with AcrA (234). The substrate profile of the AcrAB-TolC pump includes chloramphenicol, lipophilic β-lactams, fluoroquinolones, tetracycline, rifampin, novobiocin, fusidic acid, nalidixic acid, ethidium bromide, acriflavine, bile salts, short-chain fatty acids, SDS, Triton X-100, and triclosan (46, 139, 149, 216, 230). In E. coli, acrD and the acrEF operon also encode efflux pumps (188, 236), and AcrD has been shown to efflux aminoglycosides (140, 188). AcrE and AcrF are 80 and 88% similar to AcrA and AcrB, respectively (110). While recognized as a commensal organism, E. coli is also the most common cause of urinary tract infections, and treatment is usually with a fluoroquinolone, trimoxazole or nitrofurantoin. Enteropathogenic E. coli and enterotoxigenic E. coli are a common cause of diarrhea in developing countries and for travelers to these locations, and if antimicrobial therapy is indicated, the same agents are often used as for the treatment of urinary tract infections. In children and the immunocompromised, E. coli can cause more serious infections, associated with higher morbidity and mortality. For these patient groups, antimicrobial therapy is required; treatment may be with a broad-spectrum cephalosporin (e.g., ceftriaxone) or a fluoroquinolone. While some of these agents are substrates of the AcrAB-TolC system, overexpression alone is unlikely to give rise to clinical levels of resistance (Table 2). For fluoroquinolones, a mutation(s) in a topoisomerase gene is also unlikely to give rise to clinical levels of resistance; however, when combined with enhanced efflux, such isolates are resistant to the breakpoint concentration of ciprofloxacin (e.g., see references 45, 124, 125, and 145). Of current concern is the increasing number of isolates of E. coli expressing an extended-spectrum β-lactamase, in particular a CTM enzyme. Infections with such E. coli isolates are often treated with second- and third-line agents, which are often substrates of efflux pumps; therefore, the selective pressure on this species toward selection of highly MDR strains is increasing.
TABLE 2.
Susceptibility to antibiotics of E. coli lacking an efflux pump
| E. coli strain or characteristic | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| CIP | CHL | TET | CO-TRIM | |
| CLSI recommended breakpoint concn | 4 | 32 | 16 | 4/16 |
| BSAC recommended breakpoint concn | 8 | 16 | 2 | 4 |
| Wild-type strain W3110 | 0.015 | 8 | 1 | >32 |
| tolC::kan | 0.0025 | 1 | 0.12 | 1 |
| acrAB::kan | 0.0025 | 1 | 0.12 | 16 |
| acrD::kan | 0.015 | 8 | 1 | >32 |
| acrEF::kan | 0.0025 | 1 | 1 | >32 |
CIP, ciprofloxacin; CHL, chloramphenicol; TET, tetracycline; CO-TRIM, cotrimoxazole. Data extracted from Sulavik et al. (215). (Note that values have been rounded to typical doubling dilutions for ease of comparison between tables.)
(iii) Salmonella enterica.
Another area in which MDR efflux pumps are thought to play a role is in the antibiotic resistance of food-borne pathogens. It is well known that over the last two decades there has been an increase in the numbers of antibiotic-resistant bacteria isolated, both from humans and from animals. It is also recognized that antimicrobial-resistant organisms can spread from one ecosystem to another. Particular concern has been expressed about antibiotic-resistant food-borne zoonoses such as C. jejuni and various serovars of S. enterica. For both of these species, poultry meat consumption is a significant route of transmission of these bacteria to humans. Bacteria isolated from both animals and humans have been shown to be cross resistant to antibiotics used both in veterinary and human medicine. Agents used in treating infections in poultry not only include fluoroquinolones but also β-lactams, macrolides, and tetracycline. All of these agents are substrates for MDR efflux pumps.
S. enterica serovar Typhimurium AcrA and AcrB are very similar to AcrA (94%) and AcrB (97%), respectively, of E. coli (40) (Fig. 1). Mutants of S. enterica serovar Typhimurium that lack various efflux pump genes have been constructed (15, 25, 40, 185). Mutants lacking AcrB were hypersusceptible to quinolones, tetracycline, chloramphenicol, bile salts, SDS, Triton-X100, acriflavine, ethidium bromide, cetyltrimethylammonium bromide (CTAB), and triclosan. Overexpression of AcrB has also been associated with MDR in human clinical and veterinary isolates (and laboratory mutants) of S. enterica serovar Typhimurium (14, 56, 165). The MICs of nalidixic acid, tetracycline, and chloramphenicol for an AcrB-overexpressing strain were above the recommended breakpoint concentrations (Table 3). The MIC of ciprofloxacin is usually 0.5 μg of ciprofloxacin/ml for an AcrB-overexpressing strain, below the CLSI (Clinical and Laboratory Standards Institute) and BSAC (British Society of Antimicrobial Chemotherapy) recommended breakpoint concentrations for this agent for this organism. However, serovars of S. enterica with mutations in gyrA are inhibited by 0.25 μg of ciprofloxacin/ml, but such strains have been shown to fail therapy with a fluoroquinolone (130, 160, 226). There has been considerable discussion in the literature that the recommended breakpoint concentration of ciprofloxacin should be lowered to 0.25 μg of ciprofloxacin/ml. If this were the recommended value, then the MIC of ciprofloxacin for an AcrB-overexpressing strain would be above this concentration and so would be deemed clinically resistant.
TABLE 3.
Susceptibility to antimicrobial agents of S. enterica serovar Typhimurium lacking or overexpressing an efflux pump
| Serovar Typhimurium strain or characteristica | MIC (μg/ml)b
|
|||||
|---|---|---|---|---|---|---|
| NAL | CIP | TET | CHL | CTB | TRIC | |
| CLSI recommended breakpoint concn | 32 | 4 | 16 | 32 | ||
| BSAC recommended breakpoint concn | 16 | 2 | 2 | 16 | ||
| SL1344 (wild type) | 4 | 0.06 | 4 | 4 | 128 | 0.12 |
| acrB::kan | 1 | 0.015 | 2 | 0.5 | 64 | 0.06 |
| acrB+++ | 32 | 0.5 | 16 | 16 | >256 | 0.5 |
| acrD::kan | 4 | 0.03 | 4 | 4 | >256 | 0.06 |
| acrF::kan | 4 | 0.06 | 4 | 1 | 256 | 0.12 |
| tolC::kan | 1 | 0.015 | 0.5 | 1 | 64 | 0.015 |
Two other RND efflux pumps AcrD and AcrF, are present on the S. enterica genome. Genomic analysis reveals that S. enterica serovar Typhimurium LT2 AcrF is 88% similar to E. coli AcrF. Furthermore, E. coli AcrB is 90% similar to S. enterica AcrF. S. enterica serovar Typhimurium LT2 AcrD is 79 and 78% similar to S. enterica serovar Typhimurium AcrB and AcrF, respectively (40). Deletion of acrD or acrF from S. enterica serovar Typhimurium had little effect on the MICs of clinically relevant antibiotics (40). However, it was shown that when either of these genes was deleted, AcrB expression was increased (40); likewise, when acrB was deleted, expression of acrD or acrF increased (40, 185). It may be that the bacterium can compensate for the lack of AcrD or AcrF, and consequently there is no effect on MICs. However, a double-knockout mutant lacking AcrB and AcrF was no more hypersusceptible than a construct lacking AcrB alone (40). These data suggest that the major efflux pump protein in S. enterica serovar Typhimurium, and probably all serovars of S. enterica, is the AcrAB-TolC pump.
(iv) Campylobacter spp.
In 2003, two teams independently showed that CmeABC mediated efflux in C. jejuni and conferred MDR (100, 175). CmeA and CmeB have some similarity to AcrA (51%) and MexA (49%) and to AcrB (63%) and MexB (62%), respectively, of E. coli and P. aeruginosa. Deletion of cmeB revealed that the substrates of CmeABC include ciprofloxacin and erythromycin, both common first-line agents should antimicrobial treatment be warranted to treat a human campylobacter infection. In addition, overexpression of CmeB confers resistance to ciprofloxacin, ampicillin, tetracycline, and chloramphenicol and decreased susceptibility to triclosan, bile salts, SDS, and Triton X-100 (Table 4) (102, 177, 178). A second efflux pump system, CmeDEF, has also been identified, but this system does not appear to confer resistance to ciprofloxacin or erythromycin (176).
TABLE 4.
Susceptibility to antimicrobial agents of C. jejuni 11168 lacking or overexpressing an efflux pump
| C. jejuni strain or characteristica | MIC (μg/ml)b
|
|||||
|---|---|---|---|---|---|---|
| CIP | ERY | AMP | TET | CHL | TRIC | |
| CLSI suggested breakpoint concn | 4 | 8* | 32* | 16* | 32* | |
| BSAC suggested breakpoint concn | 1 | 16* | 2* | 8* | ||
| Wild type | 0.25 | 0.5 | 4 | 0.5 | 1 | 4 |
| cmeB::aph | 0.12 | 0.25 | 2 | 0.25 | 0.5 | 1 |
| cmeB+++ | 8 | 4 | 32 | 16 | 16 | 32 |
| cmeF::aph | 0.25 | 0.5 | 0.5 | 0.25 | 1 | 1 |
+++, overexpressing mutant.
*, recommended breakpoint concentration for Enterobacteriaceae (no recommended breakpoint concentrations for C. jejuni exist); CIP, ciprofloxacin; ERY, erythromycin; AMP, ampicillin; TET, tetracycline; CHL, chloramphenicol; TRIC, triclosan. Data are taken from Pumbwe and Piddock (175) and Pumbwe et al. (176).
(v) Acinetobacter baumannii.
A. baumannii is a multidrug-resistant gram-negative bacillus that is causing increasing problems in the nosocomial setting, particularly intensive care units. This organism is commonly MDR due to the presence of multiple mechanisms of resistance, including chromosomally mediated fluoroquinolone resistance (due to mutations in gyrA) and a species-specific cephalosporinase. It can also possess plasmid- or transposon-encoded genes encoding β-lactamases and aminoglycoside inactivating enzymes. In addition to these mechanisms of resistance, an RND MDR tripartite efflux pump, AdeABC, has been described. AdeA and AdeB have some similarity to AcrA (55%) and MexA (58%) and to AcrB (68%) and MexB (67%), respectively, of E. coli and P. aeruginosa. When adeB was deleted in a clinical isolate, BM4454, the organism became susceptible to gentamicin, ofloxacin, cefotaxime, and tetracycline (112), with MICs below the recommended breakpoint concentration (Table 5). Overexpression of AdeABC confers resistance to aminoglycosides and decreased susceptibility to fluoroquinolones, tetracycline, chloramphenicol, erythromycin, trimethoprim, and ethidium bromide (112), as well as to netilmicin and meropenem (60). Treatment of A. baumannii infection typically includes aminoglycosides, such as gentamicin, in combination with a β-lactamase-stable β-lactam such as piperacillin or imipenem. An alternative therapy would be another β-lactam, a fluoroquinolone, rifampin, or colistin, but these alternative therapies are relatively new to the armory and have not been supported by much clinical data. As can be seen, overexpression of the AdeABC efflux pump reduces the therapeutic options.
TABLE 5.
Susceptibility to antimicrobial agents of A. baumanii lacking or overexpressing an efflux pump
(vi) Neisseria gonorrhoeae.
MtrCDE mediates MDR and resistance to certain antimicrobial peptides produced at host mucosal surfaces. Compared with homologies between other RND pump systems, MtrC has low similarity with E. coli AcrA and P. aeruginosa MexA (47% and 49%, respectively), whereas similarity of MtrD with E. coli AcrB and MexB is higher (67% and 68%, respectively). MtrE corresponds to TolC. In penicillin-resistant strains, it has been shown that the MtrCDE efflux pump interacts synergistically with other mechanisms of β-lactam resistance in N. gonorrhoeae, including porins (penB) and low-affinity penicillin binding proteins (224). Increased expression of MtrCDE alone does not increase the MICs of antimicrobial agents sufficiently to be resistant to the recommended breakpoint concentration (Table 6). Ciprofloxacin is an alternative agent for the treatment of gonorrhea, and this agent is not a substrate of the Mtr system.
TABLE 6.
Susceptibility to antimicrobial agents of N. gonorrhoeae lacking or overexpressing an efflux pump
| N. gonorrhoeae strain or characteristica | MIC (μg/ml)b
|
|||
|---|---|---|---|---|
| PEN | NAF | CIP | TET | |
| CLSI recommended breakpoint concn | 2 | 1 | 2 | |
| BSAC recommended breakpoint concn | 4 | 4 | 16 | |
| FA19 (wild type) | 0.015 | 0.25 | 0.0025 | 0.25 |
| WV30 mtrR171(+++) | 0.03 | 1 | 0.0025 | 0.25 |
| WV31 mtrR171 mtrD::kan | 0.015 | 0.03 | 0.0025 | 0.25 |
+++, MtrCDE-overexpressing mutant.
PEN, penicillin; NAF, nafcillin; CIP, ciprofloxacin; TET, tetracycline. Data extracted from Veal et al. (224); data for ciprofloxacin provided by W. Shafer (personal communication).
(vii) Other gram-negative bacteria.
Homologues of the RND Mex and Acr efflux systems associated with MDR have also been found in other Enterobacteriaceae, including Enterobacter aerogenes (171), Klebsiella spp. (125), Proteus mirabilis (225), Serratia marcescens (91), Morganella morganii (194), H. influenzae (197), and Helicobacter pylori (221).
MDR pumps of the MATE family have been described for several gram-negative bacteria: V. parahaemolyticus (NorM) (132), B. thetaiotaomicron (BexA) (129), V. cholerae (VcmA, VcrM) (65, 66), Brucella melitensis (NorMI) (21), N. gonorrhoeae (NorM) (189), H. influenzae (HmrM) (231), and P. aeruginosa (PmpM) (58). The substrate profile typically includes a fluoroquinolone (norfloxacin and ciprofloxacin), DNA-intercalating dyes, and detergents. However, the clinical relevance of these systems has not been established.
Gram-positive bacteria.
It has been known for over a decade that Bacillus subtilis possesses an MDR efflux pump, Bmr, which belongs to the MFS family of efflux pumps (134, 135). While there is little clinical significance of this efflux pump in human and veterinary medicine, it has been shown that the NorA pump of S. aureus and PmrA of S. pneumoniae bear significant similarity and identity at the DNA and amino acid levels (55, 136). Therefore, a considerable number of analogies have been made between the properties of Bmr and of NorA (136) and PmrA (55).
(i) S. aureus.
S. aureus NorA has been shown to have 44% amino acid identity and 67% similarity with Bmr. Bmr and NorA are structurally similar to the plasmid-encoded efflux proteins TetA, TetB, and TetC, with 24 to 25% sequence identity with these proteins (134). Overexpression of both Bmr and NorA confers MDR to fluoroquinolones, chloramphenicol, antiseptics, dyes, and disinfectants (Table 7) (77, 134, 136, 137, 232). NorA is present in both methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA). The agents used to treat infections by MSSA include flucloxacillin, nafcillin, and ciprofloxacin. The agents used to treat MRSA include ciprofloxacin (where the MRSA strain has been shown to be susceptible), vancomycin, and linezolid (8). The MICs of nafcillin and vancomycin are unaffected by overexpression of NorA (G. W. Kaatz, personal communication).
TABLE 7.
Susceptibility to antimicrobial agents of S. aureus overexpressing norA
| S. aureus strain or characteristic | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| NOR | CIP | NAF | VANC | |
| CLSI recommended breakpoint concn | 16 | 4 | 4 | 32 |
| BSAC recommended breakpoint concn | 2 | 8 | ||
| SA-1199 | 0.5 | 0.5 | 0.5 | 0.5 |
| SA-1199Bb | 64 | 16 | 0.5 | 0.5 |
| SA-1199-3c | 16 | 4 | 0.5 | 0.5 |
NOR, norfloxacin; CIP, ciprofloxacin; NAF, nafcillin; VANC, vancomycin. Data are from Kaatz and Seo (73) and Kaatz (personal communication).
Possesses a mutation in grlA and overexpresses norA.
Inducible overexpression of norA.
Other efflux pump genes are also present on the S. aureus genome, three of which have been investigated. Overexpression of NorB confers decreased susceptibility to fluoroquinolones, tetracycline, disinfectants, and dyes (218). Overexpression of Tet38 confers resistance to tetracycline only (218). Overexpression of MepA confers resistance to fluoroquinolones and biocides (74). Little detailed work has been performed on the other putative transporters, and so it remains to be seen whether any further transporters play a role in antimicrobial resistance in S. aureus. In addition, any clinical relevance of these new transporters has yet to be defined.
(ii) S. pneumoniae.
Over the last decade or so, considerable effort has been expended by pharmaceutical companies to develop antipneumococcal agents, so there has been considerable focus on S. pneumoniae and the presence of efflux pump proteins that could confer MDR, including to new agents. In 1999, Gill et al. identified PmrA. This protein has 43% amino acid similarity with NorA and 42% similarity with Bmr. These workers showed that when norfloxacin resistance was transformed from a clinical isolate into strain R6 (widely used by geneticists, as it is highly transformable and nonencapsulated, i.e., nonpathogenic; ∼40 kb of the genome is deleted compared with the parent strain from which it originates, and this removes the capsule locus), the MIC of norfloxacin for R6 increased to 16 μg/ml for the construct, R6N. Gill et al. (55) also introduced the cat gene into R6N to construct strain R6N-cat. Insertion of cat into pmrA gave rise to the same susceptibility to norfloxacin, ciprofloxacin, ethidium bromide, and acriflavine in R6N-cat as in R6 (Table 8). The MIC of ciprofloxacin for strain R6N is within one doubling dilution of the recommended breakpoint concentration for this agent. No data are available as to whether overexpression of pmrA has any effect on the MIC of penicillin or cephalosporins. These workers also investigated the activity of fluoroquinolones for pneumococci in the presence of reserpine, an inhibitor of Bmr (see “Inhibitors of Efflux Pumps,” below). Due to the synergistic effect of reserpine and antimicrobial agents for B. subtilis, this agent has been widely used in MIC studies of a variety of different antimicrobials with S. pneumoniae as a means of identifying those isolates that overexpress an efflux pump (e.g., see references 11, 22-24, and 164). Gill et al. (55) also demonstrated that the MIC of norfloxacin for strain R6N was reduced fourfold in the presence of reserpine; they interpreted these data to indicate that reserpine interacted with PmrA to inhibit its efflux activity, so that the activity of norfloxacin was potentiated. However, to date there is no biochemical evidence showing a direct interaction between reserpine and the PmrA protein. Piddock and Johnson (162) examined the concentration of fluoroquinolones accumulated by strain R6N compared with strain R6. They showed that R6N did indeed accumulate significantly lower concentrations of norfloxacin than did R6, supporting the hypothesis that PmrA transported norfloxacin. However, the addition of reserpine at the same concentration as used in MIC studies did not significantly affect the concentration of norfloxacin accumulated. It may be that reserpine interacts with another protein, hence giving the observed synergy in MIC studies. In addition, the concentrations of 10 other fluoroquinolones accumulated by strain R6N were similar to those accumulated by strain R6, suggesting that PmrA did not transport these other drugs. Recently, Marrer et al. (120) identified an ABC transporter associated with ciprofloxacin resistance. Robertson et al. (186) showed that deletion of this transporter conferred multidrug susceptibility.
Mycobacteria.
Several efflux pumps of different classes have been described for Mycobacterium tuberculosis and/or M. smegmatis (e.g., see references 10, 36, 98, 154, 204, and 205). Several have also been shown to be involved in the transport of several different antibiotics, including fluoroquinolones, aminoglycosides, tetracycline, rifampin, and possibly isoniazid and ethambutol. However, it is unclear which of these are associated with antibiotic resistance in clinical isolates.
Evidence for Resistance in Clinical Isolates Mediated by Enhanced Efflux
It has been questioned whether the association between MDR and overexpression of an efflux pump (or disruption or deletion of an efflux pump gene giving consequent hyper-multidrug susceptibility and thus the assumption that overexpression would confer MDR) is significant in the antibiotic-resistant bacteria that are commonly isolated from humans and animals. Therefore, it is important to determine the prevalence of overexpression of efflux pump systems in clinical and veterinary isolates so that the clinical relevance of MDR conferred by specific efflux pumps can be established. Overexpression has usually been measured in two ways: (i) by measuring RNA expression or (ii) by performing Western blotting to measure protein expression. The latter method is more widely used, as it is easily available. However, the use of reverse transcriptase (RT) PCR has been questioned by some, with the suggestion that, as RT-PCR data does not determine protein expression, increased levels of RNA may not be reflected by increased protein expression. Few studies comparing the two data sets have been performed; however, recent proteomic data for S. enterica serovar Typhimurium efflux proteins (185) and OMPs (N. Coldham et al., unpublished data) confirmed that RT-PCR accurately predicted those proteins that were over- or underexpressed.
Ziha-Zarifi et al. (237) showed that 11 patients had MDR P. aeruginosa that overexpressed the MexAB-OprM pump. Oh et al. (146) showed that 17 of 20 fluoroquinolone-resistant clinical isolates of P. aeruginosa expressed high levels of MexB, MexD, MexF, or MexY. Hocquet et al. (62) also showed that 14 of 18 isolates overexpressed the MexAB-OprM pump. Wolter et al. (230) showed that six of seven gentamicin-resistant isolates overexpressed the MexXY system. These data indicate that when resistant clinical isolates are investigated, a considerable number overexpress one or more of the Mex pumps that have been associated with clinically relevant levels of MDR.
In 22 of 36 fluoroquinolone-resistant isolates of E. coli, enhanced efflux was suggested (44). Webber and Piddock (227) confirmed that 11 isolates overexpressed acrB. Mazzariol et al. (123) showed that 9 of 10 clinical isolates of E. coli overexpressed AcrA. Both cyclohexane tolerance and multiple antibiotic resistance have been attributed to overproduction of the AcrAB-TolC complex in E. coli, mediated by overexpression of a global regulator (marA or soxS) (6, 229). It has been observed that in E. coli there is a close correlation between organic solvent tolerance and low-level resistance to multiple antibiotics (9). Tolerance to this organic solvent has been used as a marker for multiple antibiotic resistance (MAR) and used to screen clinical isolates. Randall et al. (180) observed a similar association for different serovars of S. enterica. Kallman et al. (80) showed that cyclohexane-tolerant clinical isolates of E. coli were inhibited by significantly higher MICs of cefuroxime, suggesting that efflux contributed to resistance to this agent in E. coli. Schneiders et al. (201) showed that 5 of 10 organic solvent-tolerant MDR K. pneumoniae isolates overexpressed AcrA.
In 2000, overexpression of acrB was shown in three MDR human clinical isolates of S. enterica serovar Typhimurium (165). Phenotypic data supported the role of overexpression as being a primary mechanism of resistance. Efflux via AcrAB-TolC has also been shown to be important in the MDR of isolates of S. enterica serovar Typhimurium DT104 from cattle (14).
Pumbwe et al. (176) examined 32 isolates of C. jejuni from both humans and poultry that had an MDR phenotype. Nine isolates overexpressed cmeB, and three of these also overexpressed cmeF. All isolates that overexpressed cmeB accumulated low concentrations of ciprofloxacin, suggesting that the overexpression of cmeB was involved in the MDR.
Recently, Higgins et al. (60) investigated an outbreak of MDR A. baumannii in a German hospital and showed that a pretherapy isolate, U10247, was susceptible to netilmicin and meropenem but that a subsequent isolate, U11177, became resistant due to overexpression of AdeABC, such that the MICs were above the breakpoint concentrations for gentamicin and cefotaxime, both agents that are used clinically. Two of the outbreak strains were clones and expressed 20-fold-more adeB mRNA than a strain obtained earlier in the outbreak (60).
Kaczmarek et al. (79) showed that four high-level, ampicillin-resistant clinical isolates of H. influenzae contained frameshift insertions in acrR which were associated with MDR.
Few studies have examined many clinical isolates of S. aureus for the prevalence of overexpression of NorA; however, where investigated it has been shown that some norfloxacin-resistant clinical isolates overexpress norA (70, 77, 142). However, other studies have found little or no relationship between overexpression of norA and fluoroquinolone resistance (147, 200). This may be because NorA does not transport the agents investigated. Since 1990, fluoroquinolones have been increasingly used as a treatment for infections caused by MRSA. Many MRSA strains then evolved to become resistant to fluoroquinolones and became widely disseminated, such that for some countries the predominant clones of MRSA are often fluoroquinolone resistant (39). While most of this fluoroquinolone resistance has been deemed to be due to mutations in the genes encoding the target protein (either grlA or gyrA), overexpression of NorA may also play a role. To date there is still no clear evidence as to the cause of the rapid increase and clonal spread of MRSA in many hospitals in developed countries, despite the availability of the sequences of the genomes of seven different strains of S. aureus, including three MRSA strains; there are many hypotheses for the epidemic spread of MRSA and problems in eradication. It may be that overexpression of NorA, with its concomitant effect on biocide activity, plays a role.
Piddock et al. (163) also determined the prevalence of pmrA overexpression in clinical isolates of S. pneumoniae from several geographically distinct areas. The isolates were divided into four categories: (i) those isolates inhibited by ≥16 μg/ml norfloxacin and for which reserpine lowered the MIC of norfloxacin fourfold and where the MIC suggested that these isolates had a phenotype similar to that of strain R6N; (ii) isolates that were susceptible to norfloxacin but for which reserpine also lowered the MIC of norfloxacin; (iii) norfloxacin-resistant (MIC ≥ 16 μg/ml norfloxacin) isolates for which reserpine had no effect; and (iv) norfloxacin-susceptible isolates for which reserpine had no effect. Isolates from groups i and iii also contained mutations in topoisomerase genes (163). The level of expression of pmrA mRNA was measured by Northern blotting and quantitative competitive RT-PCR, and it was shown that there were isolates in all four groups that overexpressed pmrA. Three isolates that were phenotypically similar to R6N also had no detectable expression of pmrA. These data indicate that pmrA overexpression is not exclusively associated with MDR S. pneumoniae isolates, despite their MIC phenotype suggesting otherwise.
Taken together, all of these studies indicate that overexpression of an efflux pump is often found in antibiotic-resistant clinical isolates and therefore impacts the therapeutic options available.
REGULATION OF EFFLUX PUMPS IN CLINICAL ISOLATES
Although there have been many studies on the mechanisms of regulation of efflux pumps in laboratory-derived mutants, the mechanisms giving rise to increased efflux in clinical isolates have been shown to fall broadly into four groups: (i) mutations in the local repressor gene, (ii) mutations in a global regulatory gene, (iii) mutations in the promoter region of the transporter gene, and (iv) insertion elements upstream of the transporter gene.
Mutations in the Local Repressor Gene
Most RND MDR efflux pump genes are encoded by operons that are under the control of the local repressor gene, which is usually a TetR-type repressor; e.g., in E. coli, AcrA and AcrB are cotranscribed and under the control of acrR (148). The same is true for acr genes of other species. Mutations in acrR derepress expression of acrB, and acrS represses acrEF (96), giving rise to overexpression of the efflux pump. Such mutations have been found in acrR genes of clinical isolates of E. coli, S. enterica serovar Typhimurium, H. influenzae, and E. aerogenes (79, 149, 171, 227) (Table 9). Webber, Talukder, and Piddock (228) confirmed that a substitution of Cys for Arg45 in AcrR of E. coli gave rise to increased expression of acrB, concomitant MDR, and low accumulated concentrations of ciprofloxacin in six isolates of E. coli. Expression of acrB is also influenced by the quorum-sensing regulator SdiA (81, 179).
TABLE 9.
Substitutions in local repressor proteins
Mutations effecting expression of MexAB-OprM have been described at three loci: mexR (nalB), nalC (28, 105, 169), and nalD (206) (Table 9). Mutations identified in nfxB confer expression of mexCD-oprJ (203). Expression of mexEF-oprN is regulated by MexT (84). Mutations in mexS (PA2491) that give rise to overexpression of MexEF-OprN and MDR have also been identified (207). MexL is a transcriptional repressor of mexJK (33). Mutations in cmeR of C. jejuni (99, 176) and mtrR of N. gonorrhoeae (203) have been described. However, regulation of efflux in N. gonorrhoeae appears to be more complicated, and studies with laboratory-constructed mutants revealed that another gene, mtrF, is also required for high-level MDR (46).
Comparison of the mutations in the local repressor genes of various gram-negative species reveals that the majority of the substitutions in the proteins occur in the predicted helix-turn-helix motif involved in DNA binding to the target structural gene, i.e., the efflux pump gene, such as acrB (Table 9). Large deletions have also been observed and are predicted to render the repressor inactive.
The AdeABC efflux pump of A. baumannii is regulated by a two-component regulatory system encoded by AdeS and AdeR. Inactivation of AdeS gives rise to lower aminoglycoside MICs. Spontaneous gentamicin-resistant mutants contained substitutions in AdeS and in AdeR; these led to constitutive expression of the pump and MDR (115).
Expression of norA in S. aureus is via two systems, one a two-component regulatory system, ArlRS, the other MgrA (NorR) (219). Recently, MgrA has been shown also to regulate Tet38 and NorB (218). A MarR-type regulator, MepR, regulates expression of MepA (74). To date, no data for clinical isolates have been published, so it is not known what the contributions of these mechanisms are, if any, in conferring the overexpression of norA in clinical isolates.
Mutations in Global Regulator Genes
In E. coli, expression of acrAB is controlled by either acrR; the MarRAB operon, including MarA, a transcriptional activator; or the SoxRS operon (5, 50, 121, 128). In E. coli and other Enterobacteriaceae, there are porin proteins present in the outer membrane (139). Expression of porin proteins is also under the control of MarA and SoxS; when either of these transcriptional activators is overexpressed, an antisense RNA, micF (38), is produced, which in turn decreases expression of OmpF in the outer membrane, thereby reducing influx of some antimicrobial agents (148). These transcriptional activators also interact with acrAB, thereby increasing the amount of AcrAB produced and effectively enhancing efflux (148). There is significant homology at both the DNA and amino acid levels of the AcrAB-TolC pumps of S. enterica and E. coli, and so it is considered that much of the information obtained for E. coli AcrAB-TolC is directly relevant to S. enterica (Table 10). The marRAB and soxRS operons are also present on the genomes of S. enterica, and these too have significant homology with those of E. coli; thus, it is thought that regulation of efflux and influx occurs in salmonellae as in E. coli (214).
TABLE 10.
Comparison of identities and similarities of various efflux proteins made from pairwise alignments with Genedoc (Karl Nicholas)
| Proteins | % Identity | % Similarity | |
|---|---|---|---|
| MexAa | AcrAb | 56 | 71 |
| MexB | AcrBb | 69 | 83 |
| OprM | TolCb | 18 | 35 |
| MexC | MexA | 43 | 60 |
| MexC | MexE | 32 | 45 |
| MexD | MexB | 49 | 69 |
| MexD | MexF | 40 | 61 |
| OprN | OprM | 31 | 48 |
| AcrAb | AcrAc | 91 | 94 |
| AcrEb | AcrAb | 66 | 80 |
| AcrFb | AcrBb | 77 | 88 |
| AcrBb | AcrBc | 94 | 97 |
| AcrDb | AcrDc | 94 | 97 |
| CmeAd | MexA | 29 | 49 |
| CmeB | MexB | 41 | 62 |
| CmeC | OprM | 25 | 46 |
| CmeA | AcrAa | 31 | 51 |
| CmeB | AcrBa | 41 | 63 |
| CmeC | TolCa | 24 | 41 |
| Bmre | NorAf | 44 | 67 |
| Bmr | PmrAg | 33 | 42 |
| NorA | PmrA | 25 | 47 |
| AcrFb | AcrFc | 88 | 94 |
| TolCb | TolCc | 89 | 94 |
| AcrDc | AcrBc | 64 | 79 |
| AcrDc | AcrFc | 64 | 78 |
| AcrFc | AcrBb | 80 | 90 |
| AdeAh | MexA | 37 | 58 |
| AdeB | MexB | 47 | 67 |
| AdeC | OprM | 42 | 61 |
| AdeA | AcrAa | 38 | 55 |
| AdeB | AcrBa | 49 | 68 |
| AdeC | TolCa | 22 | 39 |
| MtrCi | MexA | 26 | 49 |
| MtrD | MexB | 49 | 68 |
| MtrE | OprM | 42 | 61 |
| MtrC | AcrAa | 29 | 47 |
| MtrD | AcrBa | 49 | 67 |
Mutations in clinical isolates of E. coli in those genes that are global regulators, including soxR and marR, have been described (67, 88, 145, 227). Constitutive overexpression of soxRS can also contribute to antibiotic resistance in clinically relevant S. enterica isolates (87).
Rob is also a member of the same AraC/XylS family of transcriptional regulators as MarA and SoxS (17). Overexpression of rob in E. coli produces both increased organic solvent tolerance and low-level resistance to multiple antimicrobial agents, due to increased expression of the AcrB-TolC system (229).
Other species of the genus or of the family Enterobacteriaceae possess homologues of the AcrAB-TolC MDR efflux pump, and so expression is also considered to be regulated as in E. coli. However, evidence is accumulating to suggest that the global regulator may not always be MarR or SoxR but RamA. Overexpression of this protein has been associated with MDR in S. enterica serovar Typhimurium, E. aerogenes, and K. pneumoniae (29, 49, 201, 222).
There are few global regulatory genes on the genome of P. aeruginosa, and regulation of the Mex pumps in P. aeruginosa is considered to be due to a mutation(s) in one of the local regulatory genes, such as mexR. Recently, however, SoxR, but not SoxS, in P. aeruginosa has been described (153). SoxR regulates a six-gene regulon including genes encoding putative efflux pumps and a pump involved in quorum-sensing signal homeostasis. Evidence that may support a global regulator involved in efflux-mediated antibiotic resistance in P. aeruginosa is provided by several studies with clinical isolates. First, an isolate for P. aeruginosa from a chronic obstructive airways disease patient overexpressed not only the MexAB-OprM efflux pump but also the MexEF-OprN pump and had decreased expression of a porin protein (OprF), plus a mutation in a topoisomerase gene (161, 173, 174). By complementing various genes, the role of each mechanism was determined. The net result was to yield a broadly resistant organism (Table 11). Le Thomas et al. (93) isolated a similar mutant from a surgical site. Llanes et al. (105) also showed that clinical isolates of P. aeruginosa can express two efflux pumps simultaneously and provided evidence for additional genes that regulate expression of mexAB-oprM and mexXY.
TABLE 11.
Susceptibility of P. aeruginosa overexpressing two Mex pumps and having other mechanisms that confer MDR
| P. aeruginosa characteristic or straina | MIC (μg/ml)b
|
||||||
|---|---|---|---|---|---|---|---|
| CIP | ENX | NAL | CHL | TET | CAR | CTX | |
| CLSI recommended breakpoint concn | 4 | 32 | 16 | 512 | 64 | ||
| BSAC recommended breakpoint concn | 8 | 16 | 8 | 2 | 256 | 16 | |
| NCTC 10662 (wild type) | 1 | 0.5 | 8 | 16 | 16 | 2 | 0.25 |
| G48 (d1) | 1 | 0.5 | 8 | 16 | 16 | 2 | 0.25 |
| G49 (d18) | 16 | 2 | 32 | 64 | 32 | 64 | 4 |
d1, day 1 of therapy; d18, day 18 of therapy. G49 overexpresses mexAB-oprM and mexEF-oprN, underexpresses OprF, and has a mutation in gyrA.
MtrA in N. gonorrhoeae is a transcriptional activator similar to other members of the AraC/XylS family (e.g., MarA) and is required for inducible resistance in this species (190).
Mutations in the Promoter Region of the Gene Encoding the Transporter
Mutations in the promoter region of norA of clinical isolates of S. aureus have been identified (73, 78, 141-143, 147). Recently, Kaatz et al. (78) showed that mutations in the +5 nucleotide of norA mRNA (flq mutations) of laboratory mutants gave rise to overexpression of norA; this could be reversed by overexpression of mgr. A study by Schmitz et al. (200) found that 36 of 42 norfloxacin-resistant isolates contained mutations in the promoter region of norA but that none were associated with resistance. However, it should be noted that various other mechanisms for the regulation of expression of norA in laboratory mutants of S. aureus, such as ArlRS and MgrA, have been described, and these too may play a role in clinical resistance (47, 48, 78, 218).
Insertion Sequences
The presence of insertion sequences (IS) upstream of the genes encoding a structural component of the efflux pump or inserted within the local repressor gene have been identified in some clinical isolates that overexpress MDR efflux pumps. Some IS elements have promoters or promoter sequences that can increase expression of a downstream efflux gene. First, an IS element was found inserted in acrR of a clinical isolate of E. coli (227). A spontaneous laboratory mutant of E. coli has also been selected with IS186 in acrR or IS2 upstream of acrEF (67). An IS element inserted in mexR has also been described for a clinical isolate of P. aeruginosa and for acrS (the putative repressor of acrEF) of S. enterica serovar Typhimurium DT204 (20, 150). In the latter case, the mutant was selected in vitro on high concentrations of fluoroquinolone from a veterinary isolate. The authors provided data to indicate that the overexpression of acrF was mediated solely by IS1 and IS10, and they hypothesized that the overexpression of acrEF may be additive to that conferred by overexpression of AcrAB. IS1 and IS10 are found on the chromosome of DT204 but not of the other epidemic S. enterica serovar Typhimurium strain, DT104, leading the authors to postulate that the presence of these IS elements may offer a selective advantage to DT204; they further emphasized the importance of IS transposable elements in the development of antimicrobial resistance.
Although N. meningitidis also possesses MtrCDE, expression is not regulated by MtrR or MtrA. Analysis of 12 isolates revealed the presence of an insertion sequence (otherwise known as a Correia element) in all isolates (191). One isolate also contained IS1301. The authors concluded that the Mtr efflux system of N. meningitidis was regulated by integration host factor and posttranscriptional regulation by cleavage in the inverted repeat of the Correia element.
MDR EFFLUX PUMPS AND DEVELOPMENT OF ANTIBIOTIC RESISTANCE
For some antibiotics in clinical use, the clinical relevance of overexpression of an efflux pump is that this confers MDR. However, there is also evidence to suggest that increased expression of efflux pumps may be the first step in which a bacterium becomes resistant to clinically relevant antimicrobials. It has been shown that reserpine (see “Inhibitors of MDR Efflux Pumps,” below) suppresses the in vitro emergence of norfloxacin-resistant S. aureus (117) and ciprofloxacin-resistant S. pneumoniae (116, 118). Lomovskaya et al. (106) also showed that the efflux pump inhibitor MC-207-110 suppressed the emergence of levofloxacin-resistant P. aeruginosa. This phenomenon has been confirmed and extended for S. pneumoniae and gemifloxacin and moxifloxacin (Garvey and Piddock, unpublished data). It was proposed that inhibition of efflux gave rise to high intracellular concentrations, such that for S. aureus the MICs afforded by mutations in the gene encoding the target topoisomerase are too low to allow survival (117).
It is also hypothesized that increased efflux of antimicrobials decreases the intracellular concentration, such that the bacterium can survive longer than may have been predicted from the MIC for that organism. During this time and within this population of bacteria, spontaneous mutants that contain mutations in genes encoding the target protein occur (e.g., gyrA of E. coli). Evidence in support of this hypothesis is that deletion of acrAB in E. coli with two mutations in gyrA resulted in the bacterium becoming hypersusceptible to fluoroquinolones, suggesting that a functional AcrAB MDR efflux pump is essential for resistance. Baucheron et al. (12) inactivated acrB in MDR S. enterica serovar Typhimurium DT204 (also containing a mutation[s] in topoisomerase genes), giving rise to low MICs, some of which were below the recommended breakpoint concentrations for these agents and this species. Luo et al. (108) obtained similar data when cmeB was deleted in C. jejuni containing a substitution in GyrA. Furthermore, S. enterica serovar Typhimurium strains that lack tolC do not give rise to ciprofloxacin-resistant mutants in vitro (185). S. enterica serovar Typhimurium lacking AcrB only gave rise to ciprofloxacin-resistant mutants when the concentration of bacteria was high (>1011), and even so the frequency of mutation to resistance was very low, at ∼1013 (Randall et al., unpublished data). Underlining the importance of the AcrAB-TolC pump in the development of ciprofloxacin resistance in S. enterica serovar Typhimurium, the strain lacking AcrB gave rise only to mutants containing a substitution in GyrA, whereas the parent strain containing AcrB gave rise to MDR mutants and those with a substitution in GyrA. These data indicate that the AcrAB system of E. coli and S. enterica serovar Typhimurium and the homologous system, CmeABC, in C. jejuni are important in the development of resistance to fluoroquinolones and some other agents. Use of fluoroquinolones in veterinary medicine has been a controversial issue for some years, as there are data to indicate that such use allows the selection of resistant bacteria that are transmitted to humans via the food chain. Use of efflux pump inhibitors concomitant with the use of antimicrobials may reduce the selection pressure, thereby allowing this valuable class of antimicrobials to continue to be used in animals.
NATURAL ROLES OF MDR PUMPS
Bile Tolerance of Enteric Bacteria
It has long been considered that the natural physiological role for MDR efflux pumps in bacteria is in the export of noxious substances from the bacterial cell thereby allowing survival in a hostile environment. Efflux pumps predate the antibiotic era, so their natural role is unlikely to be related to antibiotic use; i.e., antibiotics such as fluoroquinolones are unlikely to have selected for pump evolution. The natural environment of enteric pathogens is rich in bile salts and fatty acids, suggesting that one of the many physiological functions of active efflux systems is both the secretion of intracellular metabolites and protection against a variety of substances in this environment. It has been shown for E. coli, S. enterica serovar Typhimurium, and C. jejuni that mutants lacking components of the AcrAB-TolC pump or the CmeABC pump are hypersusceptible to bile and bile salts (natural antimicrobial substances produced in the avian and mammalian gut as an antimicrobial defense to bacterial challenge) and that mutants that overexpress components of these pumps are resistant to high concentrations of bile and bile salts (16, 92, 100, 101, 111, 176, 217). Therefore, it has been suggested that the primary function of the E. coli AcrAB-TolC and C. jejuni CmeABC efflux pumps of these organisms is to allow enteric bacteria to survive in the presence of bile (101, 111). Induction of the E. coli AcrAB pump by bile salts is mediated by Rob (187), and exposure to bile salts gives rise to MDR. Thanassi et al. (217) showed that although the bile salts chenodeoxycholate and taurocholate are transported via the E. coli AcrAB and EmrAB pumps, their data indicated that another system also played a role in efflux of these agents. Microarray analysis of gene expression after exposure of S. enterica serovar Typhimurium to bile revealed that MarRAB is activated in a concentration-dependent manner and that AcrAB was also independently activated (172). For a recent review of the interaction of bacteria with bile, see Begley et al. (16).
Colonization, Invasion, and Survival in the Host
The physiological role of efflux pumps appears to be far more complex than merely that of an antibiotic export system, and data are emerging to suggest that RND systems in P. aeruginosa, N. gonorrhoeae, S. enterica, and C. jejuni are important in the pathogenicity of the organism and/or survival in their ecological niche.
Over the last 10 years there have been sporadic reports implicating components of efflux pumps in pathogenicity. Stone and Miller (213) showed that S. enterica serovar Enteritidis tolC Tn5 insertional mutants were avirulent for mice. Urban et al. (220) found that the phytopathogenic fungus Magnaporthe grisea requires the up-regulation of specific ABC transporters for pathogenesis. In 2002, Hirakata et al. (61) concluded that the P. aeruginosa MexAB-OprM efflux system exports virulence determinants that contribute to the virulence of this organism. Jerse et al. (68) reported that a functional MtrCDE efflux system in N. gonorrhoeae enhanced bacterial survival in a female mouse model of genital tract infection. These authors suggested that this was due to the bacteria being able to survive in the presence of hydrophobic mucosal substances. Lin et al. (101) demonstrated that the efflux pump CmeB confers resistance of C. jejuni to bile. They also suggested that resistance to bile improves C. jejuni survival in vivo in poultry and concluded that inhibition of CmeABC function not only may be involved in antibiotic resistance but also may prevent in vivo colonization by Campylobacter. Recently, Burse et al. (26) reported that the phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen Erwinia amylovora. Most recently, two groups have independently shown that the AcrAB-TolC system of S. enterica serovar Typhimurium is important in the colonization of chicks. Baucheron et al. (13) showed that mutant DT104 and DT204 that lacked TolC were unable to colonize the cecum, spleen, or liver. Buckley et al. (25) showed that S. enterica serovar Typhimurium lacking AcrB or TolC poorly colonized chickens and did not persist in the avian gut, whereas mutants with disrupted acrD or acrF colonized and persisted as well as the parent strain, SL1344. It may well be that the reduced colonization of chicks by S. enterica serovar Typhimurium in which marA has been inactivated (181) is due to decreased expression of acrB.
It has also been shown in tissue culture studies that components of RND efflux pumps are important in the invasion, adherence, and/or colonization of the host cell. Decreased cellular invasion was observed with mutant P. aeruginosa PAO1 with inactivated mexB (61). These authors also showed that a ΔmexAB-oprM double knockout of P. aeruginosa PAO1 had significantly reduced invasion in MDCK cells compared to the parent strain. Buckley et al. (25) have also shown that a S. enterica serovar Typhimurium mutant lacking tolC poorly adhered to both human embryonic intestine cells (INT-407) and mouse monocyte macrophages (RAW 264.7) and was unable to invade the macrophages. The acrB mutant adhered but did not invade macrophages. Taken together, these data suggest that efflux pump systems have a role in mediating adherence and uptake of bacteria into target host cells as well as in surviving noxious substances in the local environment.
Several extracellular virulence factors known to be regulated by quorum sensing appear to be substrates for the P. aeruginosa MexAB-OprM system (43, 166). It has been suggested that the reduced virulence of P. aeruginosa strains that overexpress efflux pumps may be due to enhanced efflux of quorum-sensing signals, thereby reducing the expression of those virulence determinants regulated by quorum-sensing molecules. Recently, overexpression of the MexCD-OprJ and MexEF-OprN systems (but not MexAB-OprM or MexXY) was associated with a reduction in the expression of genes encoding components of type III secretion by P. aeruginosa (102).
INHIBITORS OF MDR EFFLUX PUMPS
Most efflux pump systems, except for the ABC family, which utilizes ATP hydrolysis, utilize the PMF as an energy source to drive the export of substrates. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) and dinitrophenol (DNP) dissipate the PMF, thereby inhibiting efflux (35). However, these compounds are not inhibitors of efflux proteins.
Neyfakh et al. (135) showed that efflux-mediated MDR in B. subtilis had some similarities with that of PgP of mammalian cells, in that MDR was reversed in the presence of reserpine and verapamil, inhibitors of PgP. Reserpine, a plant alkaloid, interacts directly with the B. subtilis Bmr protein at amino acids phenylalanine 143, valine 286, and phenylalanine 306 (83). Although these amino acids are distant from each other, it is proposed that when the protein is folded into its tertiary structure, these amino acids form part of a reserpine binding pocket (83). It has been found that if valine 286 is replaced with another amino acid, the affinity of reserpine is increased or decreased. Replacement of valine 286 with the larger residue leucine results in a fourfold reduction in sensitivity of Bmr to reserpine. Substitution of amino acids at the other loci in Bmr also affect reserpine sensitivity and can also result in resistance to fluoroquinolones and other agents.
Neyfakh et al. (136) showed that reserpine inhibited NorA, and Kaatz and Seo (72) showed that reserpine potentiated the activity of norfloxacin for S. aureus. Alignment of PmrA with NorA indicates that there is overall homology between the amino acid sequences of the two proteins and that NorA contains two of the three amino acids important in binding reserpine in Bmr. Leucine 286 is found in NorA. Unfortunately, although reserpine has been used to treat hypertension, it cannot be used in combination with antibiotics for the treatment of staphylococcal infections, as the concentrations required to inhibit NorA are neurotoxic (117).
Based on the similarity between Bmr and the predicted amino acid sequence of PmrA (43% amino acid similarity with NorA and 42% similarity with Bmr), it has been assumed that reserpine inhibits Pmr in a manner similar to that of Bmr, and this gives rise to the synergistic effect observed in MIC studies between many fluoroquinolones and reserpine for S. pneumoniae. Alignment of PmrA with NorA and Bmr indicates that while there is overall homology between the amino acid sequences of the three proteins, there are differences in the putative reserpine binding site. PmrA does not contain the same residues as Bmr; instead, PmrA contains tyrosine 143 and glycine 306. These amino acids may not be employed in reserpine binding. PmrA also contains the larger residue tryptophan 286, which may significantly reduce the sensitivity of PmrA to reserpine. These data, coupled with the lack of effect of reserpine in the accumulation experiments with S. pneumoniae R6N (162), suggest that the synergistic effect seen in the MIC experiments with this strain is not due to interaction between a reserpine binding pocket in PmrA analogous to the pocket in Bmr (163). It would seem likely that reserpine interacts with another target in the bacterial cell, possibly another efflux pump protein, although the accumulation data do not support this hypothesis.
A search for compounds that interact with efflux pump proteins and can restore antimicrobial susceptibility has been ongoing for over a decade, initially focusing on S. aureus. Hsieh et al. (64) constructed a strain of S. aureus with norA disrupted and used this strain to screen natural compounds for enhanced activity compared with S. aureus with nondisrupted norA. Among the compounds identified were berberine and palmatine. Stermitz et al. (211) later showed that plant extracts contain an inhibitor of NorA (5′-MHC) that potentiates the activity of berberine. Synthetic derivatives of 5′-MHC were made, and structure-activity relationships were explored (57). It was suggested that plants could provide a rich source of MDR efflux pump inhibitors (EPIs) that could restore the activity of MDR efflux pump substrates even to synthetic agents such as fluoroquinolones (95). The search for natural agents that inhibit efflux by S. aureus has continued, and several compounds have been identified as potent inhibitors (131, 152, 209, 210, 212). Tegos and Lewis (216) determined the activity of several antimicrobials from plants, tetracycline and erythromycin, in the presence of two EPIs, MC207-110 (see below) and INF271 (118), for several species of bacteria, including P. aeruginosa, E. coli, S. enterica serovar Typhimurium, and S. aureus. Their data indicated that plants had evolved to produce active antimicrobials and EPIs and that these agents could form the basis for development of agents for use in human medicine. Markham et al. (118) screened 9,600 structurally diverse synthetic molecules for the ability to enhance (at lower concentrations than reserpine) the activity of ethidium bromide and ciprofloxacin against two strains of S. aureus, one of which overexpressed norA. Five putative inhibitors, including INF271, were identified. However, none of these compounds has so far entered full scale drug development; this is due to a variety of reasons, including stability, solubility issues, and potential toxicity. The search for compounds active against staphylococci using NorA-overexpressing strains has continued to focus on plant extracts and includes extracts from Hypericum (53), Lycopus europaeus (54), Rosmarinus officinalis (151), and Camilla sinensis (52).
Lomovskaya and colleagues screened a library of 200,000 synthetic compounds and natural product extracts for agents that could potentiate the activity of levofloxacin against P. aeruginosa (106, 183). From this screen, they identified MC-207,110 (Phe-Arg-β-naphthylamide). The mode of action of MC-207,110 as an inhibitor of the Mex pumps of P. aeruginosa was confirmed by microbiology and accumulation and efflux assays with a variety of different strains, including mutants with disrupted mex genes (107). However, despite the apparent good in vitro activity of this agent when combined with levofloxacin, this combination drug did not succeed to market due to phototoxicity issues. It has also been shown that the activity of the oxazolidonones for gram-negative bacteria can be improved by the addition of MC-207,110 or analogues (J. M. Buysse, personal communication). MC-207,110 and/or its analogues also enhance the activity of some antibiotics for other gram-negative bacteria, including the Enterobacteriaceae and H. influenzae (J. Blais, D. Cho, K. Tangen, C. Ford, A, Lee, O. Lomovskaya, and S. Chamberland, Prog. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1266, page 327, 1999), B. thetaiotaomicron (129), E. coli (123, 195), E. aerogenes (113), serovar Typhimurium (12, 41), Campylobacter spp. (114, 158), A. baumannii (A. E. Laudy, I. Wojtal, E. Witkowska, and B. J. Starosciak, Proc. 15th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P1213, 2005), and Stenotrophomonas maltophilia (184; Laudy et al., Proc. 15th Eur. Congr. Clin. Microbiol. Infect. Dis.) but not SmeDEF-overexpressing strains (198). Recently, a derivative of MC-207,110 has been used in a MIC screen to determine the prevalence of P. aeruginosa with a phenotype suggestive of overexpression of an efflux pump (89).
Several agents already used in human medicine, but for conditions other than treatment of infectious diseases, have also been shown to enhance the MICs of some antimicrobial agents against efflux pump-overexpressing strains. These agents and their targets include selective serotonin reuptake inhibitors and S. aureus (75), antipsychotic drugs (thioxanthenes) that block the postsynaptic receptors for dopamine and S. aureus (76, 90), and drugs (phenothiazenes) used to treat a variety of mental disorders and S. aureus (76) and M. tuberculosis (7). However, no biochemical data confirming a direct interaction with any efflux protein have yet been provided.
Recently, another series of synthetic molecules, the arylpiperazines, have been shown to inhibit efflux in E. coli (18).
To date, no efflux pump inhibitor has been licensed for use in the treatment of bacterial infections in human or veterinary medicine, although the search continues. To be able to perform rational drug development and identify effective efflux pump inhibitors, an understanding of how a putative efflux pump inhibitor interacts with bacterial cells should be performed. With no clear indication as to the precise protein target(s) of efflux pump inhibitors or an understanding of the interaction with efflux pump proteins, it is difficult to see how rational drug development in this area can proceed. Advances in the structural biology of efflux pump proteins with and without substrates should provide this necessary information.
Numerous articles have been published for a variety of bacterial species showing MIC data of antibiotics with and without an EPI such as reserpine or MC-207,110; when such data reveal enhancement of antibiotic activity, this has been interpreted to indicate the presence of an efflux pump. For some species where there is clear biochemical and/or genetic data to confirm the MIC data, this interpretation is valid. However, there is also evidence that EPIs interact with more than one MDR efflux pump within the same bacterial species, e.g., MC-207,110 and P. aeruginosa. For those species where no direct interaction with the protein target(s) has been demonstrated, the use of EPIs in identifying isolates of bacteria that overexpress efflux pumps is questionable and may give rise to misleading data as to the prevalence of such strains in the clinical environment. In addition, some MIC data may have been misinterpreted, as Zloh et al. (238) have performed molecular modeling with inhibitors of MDR, suggesting that EPIs may have affinity for substrates of MDR transporters. This affinity may facilitate drug entry into cells, but a large inhibitor-drug complex may be a poor substrate for the MDR efflux pump. However, Yu et al. (233) recently determined the crystal structure of E. coli AcrB in the presence of several agents, including Phe-Arg-β-naphthylamide, and showed that it does indeed bind to this protein.
For articles focusing on EPIs, see the following reviews: Lomovskaya and Watkins (107), Kaatz (71), and Marquez (119).
BIOCIDES
There has been considerable controversy in the literature as to whether overexpression of MDR efflux pumps gives rise to biocide (disinfectant) resistance; this controversy has focused on triclosan. It is clear that when the MIC of a biocide (e.g., triclosan) is determined for those bacteria for which an efflux pump has been deleted, the organism has increased susceptibility to this biocide, and when the efflux pump is overexpressed, the MIC is increased (34, 94, 127). However, it has been argued that MICs bear little or no relevance to biocide use (192, 193). In addition, accurate MICs can be difficult to obtain with some biocides; e.g., triclosan precipitates at concentrations of >17 mg/liter. There is also no equivalent of a recommended breakpoint concentration for biocides.
The activity of disinfectants is determined in a variety of ways: first, activity is typically determined in the laboratory with bacteria growing in suspension; activity is then determined under conditions that represent practical use and simulated conditions of use, e.g., hand washing. To be able to put a value to biocide activity, two measures have been used, the D and E values. The D value is the time taken to reduce a population of indicator organisms either in suspension or attached to a surface (carrier test) by 90% (126). For instance, the D value of 0.025% sodium hypochlorite at pH 7 against E. coli is 6.1 min (126). Carrier tests are considered to be a more appropriate measure of biocide activity; these are tests in which the bacteria are attached to a surface, often after formation of a biofilm, and are considered to be less susceptible to antimicrobial compounds than bacteria grown in suspension (109). Another value of biocide activity is the E (European suspension test) value (157). This is the time taken for a biocide to demonstrate a ≥5-logarithmic reduction in numbers of bacteria grown in suspension when using the biocide under the conditions of use recommended by the manufacturer. Thus far, no D or E values under the concentration and conditions of use recommended by the manufacturer have been published for bacteria that overexpress efflux pumps. These values are especially pertinent for triclosan and E. coli, where there is good evidence to show that overexpression of AcrB, mediated by overexpression of MarA, typically confers a twofold decrease in susceptibility (127). Likewise, a MIC of 32 μg of triclosan/ml is required to inhibit S. enterica serovar Typhimurium strains that overexpress AcrB (25) and C. jejuni strains that overexpress CmeB (176). It has been shown that P. aeruginosa is intrinsically resistant to triclosan, due to the MexAB-OprM efflux pump, and requires >1,000 μg of triclosan/ml for inhibition; expression of MexCD-OprJ, MexEF-OprN, or MexJK-OprH can also confer triclosan resistance (31, 32, 34).
It is clear that only once D and E values (or similar data) are available will the controversy over the relevance of overexpression of MDR efflux pumps and biocide activity be resolved. However, there is the second issue that triclosan can select mutants that overexpress MDR efflux pumps that are resistant to multiple antibiotics. There is the worry that such bacteria could cause infections in humans. Again, there is controversy as to whether the threat is real or a laboratory phenomenon (193). Of particular concern are hospital-acquired pathogens and food-borne pathogens, where there is widespread use of biocides. Recently, MDR S. maltophilia was selected with triclosan, and 5 of 12 mutants were shown to overexpress SmeDEF (199).
CONCLUDING REMARKS
It is clear from the published literature that increased expression of efflux pumps increases the MICs of many antimicrobials, biocides (disinfectants), dyes, and detergents. However, these increased MICs are not always for clinically relevant antimicrobials or even above the recommended breakpoint concentrations. Genomics has revealed that most bacteria possess numerous efflux pumps, but data to date indicate that only a few per species confer resistance to agents used in clinical practice. Clearly, the role of efflux in microbial drug resistance is both drug and bacterial species dependent. However, a more fundamental role for bacterial efflux pumps may be their contribution to the survival of bacteria during exposure to antimicrobial agents that are substrates of MDR pumps, and these may contribute toward the survival of certain species within their ecological niche. While no efflux pump inhibitor has yet reached clinical practice, it is clear that this area of drug development offers considerable promise, as it will bring back into the antimicrobial armory several drugs that have previously been considered to be of great clinical value.
Acknowledgments
The writing of the manuscript was dogged with every conceivable delay and disaster and would not have been possible without the contributions of those listed below. I thank Jerry Buysse, Nick Coldham, Axel Cloekaert, Kim Lewis, Olga Lomovskaya, Hiroshi Nikaido, Marnie Petersen, Keith Poole, and William Shafer for providing helpful information. I also thank Mark Webber, from my research team, who helped to research material, provided data for Table 10, and proofread the manuscript. Thanks also to Andrew Bailey, Saba Ghori, and Vito Ricci. Finally, thanks to Melissa Brown for providing Fig. 1 and to Vassilis Koronakis for permitting reproduction of Fig. 3.
REFERENCES
- 1.Aires, J. R., T. Kohler, H. Nikaido, and P. Plesiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aires, J. R., and H. Nikaido. 2005. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. J. Bacteriol. 187:1923-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akama, H., M. Kanemaki, M. Yoshimura, T. Tsukihara, T. Kashiwagi, H. Yoneyama, S. Narita, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J. Biol. Chem. 279:52816-52819. [DOI] [PubMed] [Google Scholar]
- 4.Akama, H. T., T. Matsuura, S. Kashiwagi, H. Yoneyama, S. Narita, T. Tsukihara, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 279:25939-25942. [DOI] [PubMed] [Google Scholar]
- 5.Alekshun, M. A., and S. B. Levy. 2004. The Escherichia coli mar locus—antibiotic resistance and more. ASM News 70:451-456. [Google Scholar]
- 6.Alekshun, M. A., and S. B. Levy. 1997. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob. Agents Chemother. 41:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaral, L., M. Viveiros, and J. Molnar. 2004. Antimicrobial activity of phenothiazines. In Vivo 18:725-731. [PubMed] [Google Scholar]
- 8.Amyes, S. A. 2005. Treatment of staphylococcal infection. Br. Med. J. 330:976-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aono, R., M. Kobayashi, H. Nakajima, and H. Kobayashi. 1995. A close correlation between improvement of organic-solvent tolerance levels and alteration of resistance towards low-levels of multiple antibiotics in Escherichia coli. Biosci. Biotechnol. Biochem. 59:213-218. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee, S. K., K. Bhatt, P. Misra, and P. K. Chakraborti. 2000. Involvement of a natural transport system in the process of efflux-mediated drug resistance in Mycobacterium smegmatis. Mol. Gen. Genet. 262:949-956. [DOI] [PubMed] [Google Scholar]
- 11.Baranova, N. N., and A. A. Neyfakh. 1997. Apparent involvement of a multidrug transporter in the fluoroquinolone resistance of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:1396-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baucheron, S., H. Imberechts, E. Chaslus-Dancla, and A. Cloeckaert. 2002. The AcrB multidrug transporter plays a major role in high-level fluoroquinolone resistance in Salmonella enterica serovar Typhimurium phage type DT204. Microb. Drug Resist. 8:281-289. [DOI] [PubMed] [Google Scholar]
- 13.Baucheron, S., C. Mouline, K. Praud, E. Chaslus-Dancla, and A. Cloeckaert. 2005. TolC but not AcrB is essential for multidrug-resistant Salmonella enterica serotype Typhimurium colonization of chicks. J. Antimicrob. Chemother. 55:707-712. [DOI] [PubMed] [Google Scholar]
- 14.Baucheron, S., S. Tyler, D. Boyd, M. R. Mulvey, E. Chaslus-Dancla, and A. Cloeckaert. 2004. AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium DT104. Antimicrob. Agents Chemother. 48:3729-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baucheron, S. E., Chaslus-Dancla, and A. Cloeckaert. 2004. Role of TolC and parC mutation in high-level fluorquinolone resistance in Salmonella enterica serotype Typhimurium DT204. J. Antimicrob. Chemother. 53:657-659. [DOI] [PubMed] [Google Scholar]
- 16.Begley, M., C. G. M. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625-651. [DOI] [PubMed] [Google Scholar]
- 17.Bennik, M. H., P. J. Pomposiello, D. F. Thorne, and B. Demple. 2000. Defining a rob regulon in Escherichia coli by using transposon mutagenesis. J. Bacteriol. 182:3794-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohnert, J. A., and W. Kern. 2005. Selected arylpiperazines are capable of reversing multidrug resistance in Escherichia coli overexpressing RND efflux pumps. Antimicrob. Agents Chemother. 49:849-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borges-Walmsley, M. I., K. S. McKeegan, and A. R. Walmsley. 2003. Structure and function of efflux pumps that confer resistance to drugs. Biochem. J. 376:313-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutoille, D., S. Corvec, N. Caroff, C. Giraudeau, E. Espaze, J. Caillon, P. Plesiat, and A. Reynaud. 2004. Detection of an IS21 insertion sequence in the mexR gene of Pseudomonas aeruginosa increasing beta-lactam resistance. FEMS Microbiol. Lett. 230:143-146. [DOI] [PubMed] [Google Scholar]
- 21.Braibant, M. L., Guilloteau, and M. S. Zygmunt. 2002. Functional characterization of Brucella melitensis NorMI, an efflux pump belonging to the multidrug and toxic compound extrusion family. Antimicrob. Agents Chemother. 46:3050-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenwald, N. P., M. J. Gill, and R. Wise. 1997. The effect of reserpine, an inhibitor of multi-drug efflux pumps, on the in vitro susceptibilities of fluoroquinolone-resistant strains of Streptococcus pneumoniae to norfloxacin. J. Antimicrob. Chemother. 40:458-460. [DOI] [PubMed] [Google Scholar]
- 23.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brosky, J., K. Coleman, M. N. Gwynn, L. McClosky, C. Traini, L. Voelker, and R. Warren. 2000. Efflux and target mutations as quinolone resistance mechanisms in clinical isolates of Streptococcus pneumoniae. J. Antimicrob. Chemother. 45(Suppl. 1):95-99. [DOI] [PubMed] [Google Scholar]
- 25.Buckley, A., S. Cooles, L. Randall, R. M., La Ragione, M. Woodward, and L. J. V. Piddock. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell. Microbiol., in press. [DOI] [PubMed]
- 26.Burse, E., H. Weingart, and M. S. Ullrich. 2004. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen, Erwinia amylovora. Mol. Plant-Microbe Interact. 17:43-54. [DOI] [PubMed] [Google Scholar]
- 27.Cagliero, C., L. Cloix, S. Payot, and A. Cloeckaert. 2004. High genetic variation of the multidrug transporter gene cmeB in Campylobacter jejuni and Campylobacter coli. Int. J. Antimicrob. Agents 24:S107. [DOI] [PubMed] [Google Scholar]
- 28.Cao, L., R. Srikumar, and K. Poole. 2004. MexAB-OprM hyperexpression in NalC-type nultidrug resistant Pseudomonal aeruginosa: identification and chracterization of the nalC gene encoding a repressor of PA3720-PA3719. Mol. Microbiol. 53:1423-1436. [DOI] [PubMed] [Google Scholar]
- 29.Chollet, R., J. Chevalier, C. Bollet, J. M. Pages, and A. Davin-Regli. 2004. RamA is an alternate activator of the multidrug resistance cascade in Enterobacter aerogenes. Antimicrob. Agents Chemother. 48:2518-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chollet, R., J. Chevalier, A. Bryskier, and J. M. Pages. 2004. The AcrAB-TolC pump is involved in macrolide resistance but not in telithromycin efflux in Enterobacter aerogenes and Escherichia coli. Antimicrob. Agents Chemother. 48:3621-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuanchuen, R., T. Murata, N. Gotoh, and H. P. Schweizer. 2005. Substrate-dependent utilization of OprM or OpmH by the Pseudomonas aeruginosa MexJK efflux pump. Antimicrob. Agents Chemother. 49:2133-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuanchuen, R., C. T. Narasaki, and H. P. Schweizer. 2002. The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J. Bacteriol. 184:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuanchuen, R., J. B. Gaynor, R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. Molecular characterization of MexL, the transcriptional repressor of the mexJK multidrug efflux operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:1844-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuanchuen, R., R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2003. High-level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. Am. J. Infect. Control 31:124-127. [DOI] [PubMed] [Google Scholar]
- 35.Cohen, S. P., L. M. McMurry, D. C. Hooper, J. S. Wolfson, and S. B. Levy. 1989. Cross-resistance to fluoroquinolones in multiple antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob. Agents Chemother. 33:1318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colangeli, R., D. Helb, S. Sridharan, J. Sun, M. Varma-Basil, M. H. Hazbon, R. Harbacheuski, N. J. Megjugorac, W. R. Jacobs, A. Holzenburg, J. C. Sacchettimi, and D. Alland. 2005. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol. Microbiol. 55:1829-1840. [DOI] [PubMed] [Google Scholar]
- 37.Dean, C. R., M. A. Visalli, S. J. Projan, P. E. Sum, and P. A. Bradford. 2003. Efflux-mediated resistance to tigecylcine (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 47:972-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delihas, N., and S. Forst. 2001. MicF: an antisense RNA gene involved in response of Escherichia coli to global stress factors. J. Mol. Biol. 313:1-12. [DOI] [PubMed] [Google Scholar]
- 39.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and the SENTRY Participants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY antimicrobial surveillance program, 1997-1999. Clin. Infect. Dis. 32:S114-S132. [DOI] [PubMed] [Google Scholar]
- 39a.Dupont, P., D. Hocquet, K. Jeannot, P. Chavanet, and P. Plesiat. 2005. Bacteriostatic and bactericidal activities of eight fluoroquinolones against MexAB-OprM-overproducing strains of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 55:518-522. [DOI] [PubMed] [Google Scholar]
- 40.Eaves, D. J., V. Ricci, and L. J. Piddock. 2004. Expression of acrB, acrF, acrD, marA, and soxS in Salmonella enterica serovar Typhimurium: role in multiple antibiotic resistance. Antimicrob. Agents Chemother. 48:1145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escribano, I., J. C. Rodriguez, L. Cebrian, and G. Royo. 2004. The importance of active efflux systems in the quinolone resistance of clinical isolates of Salmonella spp. Int. J. Antimicrob. Agents 24:428-432. [DOI] [PubMed] [Google Scholar]
- 42.Eswaran, J., E. Koronakis, M. K. Higgins, C. Hughes, and V. Koronakis. 2004. Three's company: component structures bring a closer view of tripartite drug efflux pumps. Curr. Opin. Struct. Biol. 14:741-747. [DOI] [PubMed] [Google Scholar]
- 43.Evans, K., L. Passador, R. Srikumar, E. Tsang, J. Nezezon, and K. Poole. 1998. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 180:5443-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everett, M. J., Y.-F. Jin, V. Ricci, and L. J. V. Piddock. 1996. Contribution of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli isolates from humans and animals. Antimicrob. Agents Chemother. 40:2380-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandes, P., B. S. Ferreira, and J. M. Cabral. 2003. Solvent tolerance in bacteria: role of efflux pumps and cross-resistance with antibiotics. Int. J. Antimicrob. Agents 22:211-216. [DOI] [PubMed] [Google Scholar]
- 46.Folster, J. P., and W. M. Shafer. 2005. Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance. J. Bacteriol. 187:3713-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fournier, B., R. Aras, and D. C. Hooper. 2000. Expression of the multidrug resistance transporter NorA from Staphylococcus aureus is modified by a two-component regulatory system. J. Bacteriol. 182:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fournier, B., Q. C. Truong-Bolduc, X. Zhang, and D. C. Hooper. 2001. A mutation in the 5′ untranslated region increases stability of norA mRNA, encoding a multidrug resistance transporter of Staphylococcus aureus. J. Bacteriol. 183:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George, A. M., R. M. Hall, and H. W. Stokes. 1995. Multidrug resistance in Klebsiella pneumoniae: a novel gene, ramA, confers a multidrug resistance phenotype in Escherichia coli. Microbiology 141:1909-1920. [DOI] [PubMed] [Google Scholar]
- 50.George, A. M., and S. B. Levy. 1983. Gene in the major co-transduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J. Bacteriol. 155:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerken, H., and R. Misra. 2004. Genetic evidence for functional interactions between TolC and AcrA proteins of a major antibiotic efflux pump of Escherichia coli. Mol. Microbiol. 54:620-631. [DOI] [PubMed] [Google Scholar]
- 52.Gibbons, S., E. Moser, and G. W. Kaatz. 2004. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta Med. 70:1240-1242. [DOI] [PubMed] [Google Scholar]
- 53.Gibbons, S., B. Ohlendorf, and I. Johnsen. 2002. The genus Hypericum—a valuable resource of anti-Staphylococcal leads. Fitoterapia 73:300-304. [DOI] [PubMed] [Google Scholar]
- 54.Gibbons, S., M. Oluwatuyi, N. C. Veitch, and A. I. Gray. 2003. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 62:83-87. [DOI] [PubMed] [Google Scholar]
- 55.Gill, M. J., N. P. Brenwald, and R. Wise. 1999. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giraud, E., A. Cloeckaert, D. Kerboeuf, and E. Chaslus-Dancla. 2000. Evidence for active efflux as the primary mechanism of resistance to ciprofloxacin in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 44:1223-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guz, N. R., F. R. Stermitz, J. B. Johnson, T. D. Beeson, S. Willen, J.-F. Hsiang, and K. Lewis. 2001. Flavonolignan and flavone inhibitors of a Staphylococcus aureus multidrug resistance pump: structure-activity relationships. J. Med. Chem. 44:261-268. [DOI] [PubMed] [Google Scholar]
- 58.He, G. X., T. Kuroda, T. Mima, Y. Morita, T. Mizushima, and T. Tsuchiya. 2004. An H+-coupled multidrug efflux pump, PmpM, a member of the MATE family of transporters, from Pseudomonas aeruginosa. J. Bacteriol. 186:262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higgins, M. K., E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis. 2004. Structure of the periplasmic component of a bacterial efflux pump. Proc. Natl. Acad. Sci. USA 101:9994-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higgins, P. G., H. Wisplingnoff, D. Stefanik, and H. Seifert. 2004. Selection of topoisomerase mutations and overexpresssion of adeB mRNA transcripts during an outbreak of Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:821-823. [DOI] [PubMed] [Google Scholar]
- 61.Hirakata, Y., R. Srikumar, K. Poole, N. Gotoh, T. Suematsu, S. Kohno, S. Kamihira, R. E. Hancock, and D. P. Speert. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 196:109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hocquet, D., X. Bertand, T. Kohler, D. Talon, and P. Plesiat. 2003. Genetic and phenotypic variations of a resistant Pseudomonas aeruginosa epidemic clone. Antimicrob. Agents Chemother. 47:1887-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hooper, D. C. 2005. Efflux pumps and nosocomial antibiotic resistance: a primer for hospital epidemiologists. Clin. Infect. Dis. 40:1811-1817. [DOI] [PubMed] [Google Scholar]
- 64.Hsieh, P.-C., S. A. Siegel, B. Rogers, D. Davis, and K. Lewis. 1998. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc. Natl. Acad. Sci. USA 95:6602-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huda, M. N., J. Chen, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Gene cloning and characterization of VcrM, Na+-coupled multidrug efflux pump, from Vibrio cholerae non-O1. Microbiol. Immunol. 47:419-427. [DOI] [PubMed] [Google Scholar]
- 66.Huda, M. N., Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2001. Na+-driven multidrug efflux pump VcmA from Vibrio cholerae non-O1, a non-halophilic bacterium. FEMS Microbiol. Lett. 203:235-239. [DOI] [PubMed] [Google Scholar]
- 67.Jellen-Ritter, A. S., and W. V. Kern. 2001. Enhanced expression of the multidrug efflux pumps AcrAB and AcrEF associated with insertion element transposition in Escherichia coli mutants selected with a fluoroquinolone. Antimicrob. Agents Chemother. 45:1467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jerse, A. E., N. D. Sharma, A. N. Simms, E. T. Crow, L. A. Snyder, and W. M. Shafer. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71:5576-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson, K. W., D. Lofland, and H. E. Moser. 2005. PDF inhibitors: an emerging class of antibacterial drugs. Curr. Drug Targets Infect. Disord. 5:39-52. [DOI] [PubMed] [Google Scholar]
- 70.Jones, M. E., N. M. Boenink, J. Verhoef, K. Kohrer, and F.-J. Schmitz. 2000. Multiple mutations conferring ciprofloxacin resistance in Staphylococcus aureus demonstrate the long term stability in an antibiotic free environment. J. Antimicrob. Chemother. 45:353-356. [DOI] [PubMed] [Google Scholar]
- 71.Kaatz, G. W. 2005. Bacterial efflux pump inhibition. Curr. Opin. Investig. Drugs 6:191-198. [PubMed] [Google Scholar]
- 72.Kaatz, G. W., and S. M. Seo. 1995. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 39:260-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaatz, G. W., and S. M. Seo. 1997. Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaatz, G. W., F. McAleese, and S. M. Seo. 2005. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 49:1857-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaatz, G. W., V. V. Moudgal, S. M. Seo, J. B. Hansen, and J. E. Kristiansen. 2003. Phenothylpiperadine selective serotonin reuptake inhibitors interfere with multidrug efflux pump activity in Staphylococcus aureus. Int. J. Antimicrob. Agents 22:254-261. [DOI] [PubMed] [Google Scholar]
- 76.Kaatz, G. W., V. V. Moudgal, S. M. Seo, and J. E. Kristiansen. 2003. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:719-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaatz, G. W., S. M. Seo, and C. A. Ruble. 1993. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 37:1086-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaatz, G. W., R. V. Thyagarajan, and S. M. Seo. 2005. Effect of promoter region mutations and mgrA overexpression on transcription of norA, which encodes a Staphylococcus aureus multidrug efflux transporter. Antimicrob. Agents Chemother. 49:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaczmarek, F. S., T. D. Gootz, F. Dib-Hajj, W. Shang, S. Hallowell, and M. Cronan. 2004. Genetic and molecular characterization of β-lactamase-negative ampicillin-resistant Haemophilus influenzae with unusually high resistance to ampicillin. Antimicrob. Agents Chemother. 48:1630-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kallman, O., F. Fendukly, I. Karlsson, and G. Kronvall. 2003. Contribution of efflux to cefuroxime resistance in clinical isolates of Escherichia coli. Scand. J. Infect. Dis. 35:464-470. [DOI] [PubMed] [Google Scholar]
- 81.Kanamaru, K., I. Tatsuno, T. Tobe, and C. Sasakawa. 2000. SdiA, an Escherichia coli homologue of quorum-sensing regulators, controls the expression of virulence factors in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 38:805-816. [DOI] [PubMed] [Google Scholar]
- 82.Kim, S. H., A. B. Chang, and M. H. Saier. 2004. Sequence similarity between multidrug resistance efflux pumps of the ABC and RND superfamilies. Microbiology 150:2493-2495. [DOI] [PubMed] [Google Scholar]
- 83.Klyachko, K. A., S. Schuldiner, and A. A. Neyfakh. 1997. Mutations affecting substrate specificity of the Bacillus subtilis multidrug transporter Bmr. J. Bacteriol. 179:2189-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kohler, T., S. F. Epp, L. K. Curty, and J. C. Pechere. 1999. Chracterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 181:6300-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koronakis, V., J. Eswaran, and C. Hughes. 2004. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu. Rev. Biochem. 73:467-489. [DOI] [PubMed] [Google Scholar]
- 86.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multi-drug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 87.Koutsolioutsou, A. E., A. Martins, D. G. White, S. B. Levy, and B. Demple. 2001. A soxRS-constitutive mutation contributing to antibiotic resistance in a clinical isolate or Salmonella enterica (serovar Typhimurium). Antimicrob. Agents Chemother. 45:38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koutsolioutsou, A. E., S. Peña-Llopis, and B. Demple. 2005. Constitutive soxR mutations contribute to multiple-antibiotic resistance in clinical Escherichia coli isolates. Antimicrob. Agents Chemother. 49:2746-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kriengkauykiat, J., E. Porter, O. Lomovskaya, and A. Wong-Beringer. 2005. Use of an efflux pump inhibitor to determine the prevalence of efflux pump-mediated fluoroquinolone resistance and multidrug resistance in Pseudomonas aeruginosa. Antimicrob. Agents. Chemother. 49:565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kristiansen, M. M., C. Leandro, D. Ordway, M. Martins, M. Viveiros, T. Pacheco, J. E. Kristiansen, and L. Amaral. 2003. Phenothiazines alter resistance of methicillin-resistant strains of Staphylococcus aureus (MRSA) to oxacillin in vitro. Int. J. Antimicrob. Agents 22:250-253. [DOI] [PubMed] [Google Scholar]
- 91.Kumar, A., and E. A. Worobec. 2005. Cloning, sequencing and characterization of the SdeAB multidrug efflux pump of Serratia marcescens. Antimicrob. Agents Chemother. 49:1495-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lacroix, F. J.-C., A. Cloeckaert, O. Grepinet, C. Pinault, M. Y. Popoff, H. Waxin, and P. Pardon. 1996. Salmonella typhimurium acrB-like gene: identification and role in resistance to biliary salts and detergents and in murine infection. FEMS Microbiol. Lett. 135:161-167. [DOI] [PubMed] [Google Scholar]
- 93.Le Thomas, I., G. Couetdic, O. Clermont, N. Brahimi, P. Plesiat, and E. Bingen. 2001. In vivo selection of a target/efflux double mutant of Pseudomonas aeruginosa by ciprofloxacin therapy. J. Antimicrob. Chemother. 48:553-555. [DOI] [PubMed] [Google Scholar]
- 94.Levy, S. B. 2002. Active efflux, a common mechanism for biocide and antibiotic resistance. J. Appl. Microbiol. 92:65S-71S. [PubMed] [Google Scholar]
- 95.Lewis, K. 2001. In search of natural substrates and inhibitors of MDR pumps. J. Mol. Microbiol. Biotechnol. 3:247-254. [PubMed] [Google Scholar]
- 96.Li, X., and H. Nikaido. 2004. Efflux mediated drug resistance in bacteria. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 97.Li, X. Z., D. M. Livermore, and H. Nikaido. 1994. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob. Agents Chemother. 38:1732-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li, X. Z., L. Zhang, and H. Nikaido. 2004. Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 48:2415-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin, J., M. Akiba, O. Sahin, and Q. Zhang. 2005. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob. Agents Chemother. 49:1067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin, J., L. M. Overbye, and Q. Zhang. 2002. CmeABC functions as a multidrug efflux system in Campylobater jejuni. Antimicrob. Agents Chemother. 46:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin, J., O. Sahin, L. O. Michel, and Q. Zhang,. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 71:4250-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Linares, J. F., J. A. Lopez, E. Camafeita, J. P. Albar, F. Rojo, and J. L. Martinez. 2005. Overexpression of the multidrug efflux pumps MexCD-OprJ and MexEF-OprN is associated with a reduction of type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 187:1384-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Livermore, D. L. 2003. Linezolid in vitro: mechanism and antibacterial spectrum. J. Antimicrob. Chemother. 51(Suppl. 2):9-16. [DOI] [PubMed] [Google Scholar]
- 104.Livermore, D. M., and K. W. M. Davy. 1991. Invalidity for Pseudomonas aeruginosa of an accepted model of bacterial permeability to β-lactam antibiotics. Antimicrob. Agents Chemother. 35:916-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Llanes, C., D. Hocquet, C. Vogne, D. Benali-Baitich, C. Neuwirth, and P. Plesiat. 2004. Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously. Antimicrob. Agents Chemother. 48:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lomovskaya, O., M. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lomovskaya, O., and W. Watkins. 2001. Inhibition of efflux pumps as a novel approach to combat drug resistance in bacteria. J. Mol. Microbiol. Biotechnol. 3:225-236. [PubMed] [Google Scholar]
- 108.Luo, N., O. Sahin, J. Lin, L. O. Michel, and Q. Zhang. 2003. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob. Agents Chemother. 47:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luppens, S. B. I., M. W. Reij, R. W. L. van der Heijden, F. M. Rombouts, and T. Abee. 2002. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 68:4194-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. M. Nikaido, and E. Hearst. 1993. Molecular cloning of acrA and acrE genes of Escherichia coli. J. Bacteriol. 175:6299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 112.Magnet, S., P. Courvalin, and T. Lambert. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Malléa, M., J. Chevalier, A. Eyraud, and J.-M. Pagès. 2002. Inhibitors of antibiotic efflux pump in resistant Enterobacter aerogenes strains. Biochem. Biophys. Res. Commun. 293:1370-1373. [DOI] [PubMed] [Google Scholar]
- 114.Mamelli, L., J.-P. Amoros, J.-M. Pagès, and J.-M. Bolla. 2003. A phenylalanine arginine beta-naphthylamide sensitive multidrug efflux pump involved in intrinsic and acquired resistance of Campylobacter to macrolides. Int. J. Antimicrob. Agents 22:237-241. [DOI] [PubMed] [Google Scholar]
- 115.Marchand, I., L. Damier-Piolle, P. Courvalin, and T. Lambert. 2004. Expression of the RND-type efflux pump AdeACB in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 48:3298-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Markham, P. N. 1999. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob. Agents Chemother. 43:988-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Markham, P. N., and A. Neyfakh. 1996. Inhibition of the multi-drug transporter norA prevents emergence of norfloxacin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 40:2673-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Markham, P. N., E. Westhaus, K. Klyachko, M. E. Johnson, and A. A. Neyfakh. 1999. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marquez, B. Bacterial efflux systems and efflux pump inhibitors. Biochimie, in press. [DOI] [PubMed]
- 120.Marrer, E., T. Satoh, M. M. Johnson, L. J. V. Piddock, and M. G. P. Page. 2006. Global transcriptome analysis of the responses of a fluoroquinolone-resistant Streptococcus pneumoniae mutant and its parent to ciprofloxacin. Antimicrob. Agents Chemother. 50:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin, R. G., W. K. Gillette, and J. L. Rosner. 2000. Promoter discrimination by the related transcriptional activators MarA and SoxS: differential regulation by differential binding. Mol. Microbiol. 35:623-634. [DOI] [PubMed] [Google Scholar]
- 122.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mazzariol, A., Y. Tokue, T. M. Kanegawa, G. Cornaglia, and H. Nikaido. 2000. High-level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein AcrA. Antimicrob. Agents Chemother. 44:3441-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mazzariol, A., Y. Tokue, T. M. Kanegawa, G. Cornaglia, and H. Nikaido. 2001. High-level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein AcrA. Antimicrob. Agents Chemother. 45:647. (Erratum.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mazzariol, A., J. Zuliani, G. Cornaglia, G. M. Rossolini, and R. Fontana. 2002. AcrAB efflux system: expression and contribution to fluoroquinolone resistance in Klebsiella spp. Antimicrob. Agents Chemother. 46:3984-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mazzola, P. G., T. C. V. Penna, and A. M. Martins. 2003. Determination of decimal reduction time (D value) of chemical agents in hospitals for disinfectant purposes. BMC Infect. Dis. 3:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McMurray, L. M., M. Oethinger, and S. B. Levy. 1998. Over-expression of marA, soxS, or acrB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS Microbiol. Lett. 166:305-309. [DOI] [PubMed] [Google Scholar]
- 128.Miller, P. F., L. F. Gambino, M. C. Sulavik, and S. J. Gracheck. 1994. Genetic relationship between soxRS and mar loci in promoting multiple antibiotic resistance in Escherichia coli. Antimicrob. Agents Chemother. 38:1773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miyamae, A., O. Ueda, F. Yoshimura, J. Hwang, Y. Tanaka, and H. Nikaido. 2001. A MATE family multidrug efflux transporter pumps out fluoroquinolones in Bacteroides thetaiotaomicron. Antimicrob. Agents Chemother. 45:3341-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Molbak, K., D. L. Baggesen, F. M. Aarestrup, J. M. Ebbesen, J. Engberg, K. Frydendahl, P. Gernersmidt, A. M. Peterson, and H. C. Wegener. 1999. An outbreak of multidrug-resistant, quinolone-resistant, Salmonella enterica serotype typhimurium DT104. N. Engl. J. Med. 341:1420-1425. [DOI] [PubMed] [Google Scholar]
- 131.Morel, C., F. R. Stermitz, G. Tegos, and K. Lewis. 2003. Isoflavones as potentiators of antibacterial activity. J. Agric. Food Chem. 51:5677-5679. [DOI] [PubMed] [Google Scholar]
- 132.Morita, Y., K. Kodama, S. Shiota, T. Mine, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. NorM, a putative multidrug efflux protein of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 42:1778-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murakami, S., R. Nakashima, E. Yamashita, and A. Yamaguchi. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587-593. [DOI] [PubMed] [Google Scholar]
- 134.Neyfakh, A. A. 1992. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob. Agents Chemother. 36:484-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neyfakh, A. A., V. E. Bidnenko, and L. Bo Chen. 1991. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA 88:4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neyfakh, A. A., C. M. Borsch, and G. W. Kaatz. 1993. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 37:128-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ng, E. Y., M. Truckis, and D. C. Hooper. 1994. Quinolone resistance mediated by NorA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob. Agents Chemother. 38:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nikaido, H. 1998. Multiple antibiotic resistance and efflux. Curr. Opin. Microbiol. 1:515-523. [DOI] [PubMed] [Google Scholar]
- 139.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nishino, K., and A. Yamaguchi. 2001. Analysis of the complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Noguchi, N., M. Hase, M. Kitta, M. Sasatsu, K. Deguchi, and M. Kono. 1999. Antiseptic susceptibility and distribution of antiseptic-resistance genes in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 172:247-253. [DOI] [PubMed] [Google Scholar]
- 142.Noguchi, N., H. Okada, K. Narui, and M. Sasatsu. 2004. Comparison of the nucleotide sequence and expression of norA genes and microbial susceptibility in 21 strains of Staphylococcus aureus. Microb. Drug Resist. 10:197-203. [DOI] [PubMed] [Google Scholar]
- 143.Noguchi, N., T. Okihara, Y. Namiki, Y Kumaki, Y. Yamanaka, M. Koyama, K. Wakasugi, and M. Sasatsu. 2005. Susceptibility and resistance genes to fluoroquinolones in methicillin-resistant Staphylococcus aureus isolated in 2002. Int. J. Antimicrob. Agents 25:374-379. [DOI] [PubMed] [Google Scholar]
- 144.Oethinger, M., W. V. Kern, A. S. Jellen-Ritter, L. M. McMurry, and S. B. Levy. 2000. Ineffectiveness of topoisomerase mutations in mediating clinically significant fluoroquinolone resistance in Escherichia coli in the absence of the AcrAB efflux pump. Antimicrob. Agents Chemother. 44:10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oethinger, M., I. Podglajen, W. V. Kern, and S. B. Levy. 1998. Overexpression of the marA or soxS regulatory gene in clinical topoisomerase mutants of Escherichia coli. Antimicrob. Agents Chemother. 42:2089-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oh, H., J. Stenhoff, S. Jalal, and B. Wretlind. 2003. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb. Drug Resist. 9:323-328. [DOI] [PubMed] [Google Scholar]
- 147.Oizumi, N., S. Kawabata, M. Hirao, K. Watanabe, S. Okuno, T. Fujiwara, and M. Kikuchi. 2001. Relationship between mutations in the DNA gyrase and topoisomerase IV genes and nadifloxacin resistance in clinically isolated quinolone-resistant Staphylococcus aureus. J. Infect. Chemother. 7:191-194. [DOI] [PubMed] [Google Scholar]
- 148.Okusu, H., D. Ma, and D. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Olliver, A., M. Valle, E. Chaslus-Dancla, and A. Cloeckaert. 2004. Role of an acrR mutation in multidrug resistance of in vitro selected fluoroquinolone-resistant mutants of Salmonella enterica serovar Typhimurium. FEMS Microbiol. Lett. 238:267-272. [DOI] [PubMed] [Google Scholar]
- 150.Olliver, A., M. Valle, E. Chaslus-Dancla, and A. Cloeckaert. 2005. Overexpression of the multidrug efflux operon acrEF by insertional activation with IS1 or IS10 elements in Salmonella enterica serovar Typhimurium DT204 acrB mutants selected with fluoroquinolones. Antimicrob. Agents Chemother. 49:289-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oluwatuyi, M., G. W. Kaatz, and S. Gibbons. 2004. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 65:3249-3254. [DOI] [PubMed] [Google Scholar]
- 152.Ordway, D., J. Hohmann, M. Viveiros, A. Viveiros, J. Molnar, C. Leandro, M. J. Arroz, M. A. Gracio, and L. Amaral. 2003. Carpobrotus edulis methanol extract inhibits the MDR efflux pumps, enhances killing of phagocytosed S. aureus and promotes immune modulation. Phytother. Res. 17:512-519. [DOI] [PubMed] [Google Scholar]
- 153.Palma, M., J. Zurita, J. A. Ferreras, S. Worgall, D. H. Larone, L. Shi, F. Campagne, and L. E. Quadri. 2005. Pseudomonas aeruginosa SoxR does not conform to the archetypal paradigm for SoxR-dependent regulation of the bacterial oxidative stress adaptive response. Infect. Immun. 73:2958-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pasca, M. R., P. Guglierame, F. Arcesi, M. Bellinzoni, E. De Rossi, and G. Riccardi. 2004. Rv2686c-Rv2687c-Rv2688c, an ABC fluoroquinolone efflux pump in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48:3175-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Paulsen, I. T. 2003. Multidrug efflux pumps and resistance: regulation and evolution. Curr. Opin. Microbiol. 6:446-451. [DOI] [PubMed] [Google Scholar]
- 156.Paulsen, I. T., J. Chen, K. E. Nelson, and M. H. Saier. 2001. Comparative genomics of microbial drug efflux systems. J. Mol. Microbiol. Biotechnol. 3:145-150. [PubMed] [Google Scholar]
- 157.Payne, D. N., J. R. Babb, and C. R. Bradley. 1999. An evaluation of the suitability of the European suspension test to reflect in vitro activity of antiseptics against clinically significant organisms. Lett. Appl. Microbiol. 28:7-12. [DOI] [PubMed] [Google Scholar]
- 158.Payot, S., L. Avrain, C. Magras, K. Praud, A. Cloeckaert, and E. Chaslus-Dancla. 2004. Relative contribution of target gene mutation and efflux to fluoroquinolone and erythromycin resistance in French poultry and pig isolates of Campylobacter coli. Int. J. Antimicrob. Agents 23:468-472. [DOI] [PubMed] [Google Scholar]
- 159.Peric, M., B. Bozdogan, M. R. Jacobs, and P. C. Appelbaum. 2003. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob. Agents Chemother. 47:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Piddock, L. J. V., D. J. Griggs, M. C. Hall, and Y. F. Jin. 1993. Ciprofloxacin resistance in clinical isolates of Salmonella typhimurium obtained from two patients. Antimicrob. Agents Chemother. 37:662-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Piddock, L. J. V., M. C. Hall, F. Bellido, M. Bains, and R. E. Hancock. 1992. A pleiotropic, posttherapy, enoxacin-resistant mutant of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Piddock, L. J. V., and M. M. Johnson. 2002. Accumulation of ten fluoroquinolones by wild-type and efflux mutant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Piddock, L. J. V., M. M. Johnson, S. Simjee, and L. Pumbwe. 2002. Expression of efflux pump gene pmrA in fluoroquinolone-resistant and -susceptible clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Piddock, L. J. V., M. Johnson, V. Ricci, and S. L. Hill. 1998. Activities of new fluoroquinolones against fluoroquinolone-resistant pathogens of the lower respiratory tract. Antimicrob. Agents Chemother. 42:2956-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Piddock, L. J. V., D. G. White, K. Gensberg, L. Pumbwe, and D. J. Griggs. 2000. Evidence for an efflux pump mediating multiple antibiotic resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 44:3118-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Poole, K. 2005. Efflux mediated antimicrobial resistance. J. Antimicrob. Chemother. 56:20-51. [DOI] [PubMed] [Google Scholar]
- 167.Poole, K. 2004. Efflux mediated multi-resistance in Gram-negative bacteria. Clin. Microbiol. Infect. 10:12-26. [DOI] [PubMed] [Google Scholar]
- 168.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Poole, K., and R. Srikumar. 2001. Multidrug efflux in Pseudomonas aeruginosa: components, mechanisms and clinical significance. Curr. Top. Med. Chem. 1:59-71. [DOI] [PubMed] [Google Scholar]
- 170.Pos, K. M., A. Schiefner, M. A. Seeger, and K. Diedrichs. 2004. Crystallographic analysis of AcrB. FEBS Lett. 564:333-339. [DOI] [PubMed] [Google Scholar]
- 171.Pradel, E., and J. M. Pagès. 2002. The AcrAB-TolC efflux pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob. Agents Chemother. 46:2640-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Prouty, A. M., I. E. Brodsky, S. Falkow, and J. S. Gunn. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 150:775-783. [DOI] [PubMed] [Google Scholar]
- 173.Pumbwe, L., M. J. Everett, R. E. W. Hancock, and L. J. V. Piddock. 1996. Role of gyrA mutation and loss of OprF in the multiple antibiotic resistance (MAR) phenotype of Pseudomonas aeruginosa G49. FEMS Microbiol. Lett. 143:25-28. [DOI] [PubMed] [Google Scholar]
- 174.Pumbwe, L., and L. J. V. Piddock. 2000. Two efflux systems expressed simultaneously in multidrug-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:2861-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pumbwe, L., and L. J. V. Piddock. 2002. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol. Lett. 206:185-189. [DOI] [PubMed] [Google Scholar]
- 176.Pumbwe, L., L. P. Randall, M. J. Woodward, and L. J. V. Piddock. 2004. Expression of the efflux pump genes cmeB, cmeF and the porin gene porA in multiply antibiotic-resistant Campylobacter spp. J. Antimicrob. Chemother. 54:341-347. [DOI] [PubMed] [Google Scholar]
- 177.Pumbwe, L., L. P. Randall, M. J. Woodward, and L. J. V. Piddock. 2005. Evidence for multiple antibiotic resistance in Campylobacter jejuni not mediated by CmeB or CmeF. Antimicrob. Agents Chemother. 49:1289-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Putman, M., H. W. Van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rahmati, S., S. Yang, A. L. Davidson, and E. L. Zechiedrich. 2002. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol. Microbiol. 43:677-685. [DOI] [PubMed] [Google Scholar]
- 180.Randall, L. P., S. W. Cooles, A. Sayers, and M. Woodward. 2001. Association between cyclohexane resistance in Salmonella of different serovars and increased resistance to multiple antibiotics, disinfectants and dyes. J. Med. Microbiol. 50:919-924. [DOI] [PubMed] [Google Scholar]
- 181.Randall, L. P., and M. J. Woodward. 2001. Multiple antibiotic resistance (mar) locus in Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 67:1190-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ren, Q., K. H. Kang, and I. T. Paulsen. 2004. TransportDB: a relational database of cellular membrane transport systems. Nucleic Acids Res. 32:D284-D288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Renau, T. E., R. Leger, E. M. Flamme, J. Sangalang, M. W. She, R. Yen, C. L. Gannon, D. Griffith, S. Chamberland, O. Lomovskaya, S. J. Hecker, V. J. Lee, T. Ohta, and K. Nakayama. 1999. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42:4928-4931. [DOI] [PubMed] [Google Scholar]
- 184.Ribera, A., J. Ruiz, M. T. Jiminez de Anta, and J. Vila. 2002. Effect of an efflux pump inhibitor on the MIC of nalidixic acid for Acinetobacter baumannii and Stenotrophomonas maltophilia clinical isolates. J. Antimicrob. Chemother. 49:697-698. [DOI] [PubMed] [Google Scholar]
- 185.Ricci, V., P. Tzakas, A. Buckley, N. Coldham, and L. J. V. Piddock. 2006. Ciprofloxacin-resistant Salmonella enterica serovar Typhimurium strains are difficult to select in the absence of AcrB and TolC. Antimicrob. Agents. Chemother. 50:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Robertson, G. T., T. B. Doyle, and A. S. Lynch. 2005. Use of an efflux-deficient Streptococcus pneumoniae strain panel to identify ABC-class multidrug transporters involved in intrinsic resistance to antimicrobial agents. Antimicrob. Agents Chemother. 49:4781-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rosenberg, E. Y., D. Bertenthal, M. L. Nilles, K. P. Bertrand, and H. Nikaido. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 48:1609-1619. [DOI] [PubMed] [Google Scholar]
- 188.Rosenberg, E. Y., D. Ma, and H. Nikaido. 2000. AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 182:1754-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rouquette-Loughlin, C., S. A. Dunham, M. Kuhn, J. T. Balthazar, and W. M. Shafer. 2003. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J. Bacteriol. 185:1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rouquette-Loughlin C., J. B. Harmon, and W. M. Shafer. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol. Microbiol. 33:651-658. [DOI] [PubMed] [Google Scholar]
- 191.Rouquette-Loughlin, C. E., J. T. Balthazar, S. A. Hill, and W. M. Shafer. 2004. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol. Microbiol. 54:731-741. [DOI] [PubMed] [Google Scholar]
- 192.Russell, A. D. 2003. Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 3:794-803. [DOI] [PubMed] [Google Scholar]
- 193.Russell, A. D. 2004. Whither triclosan? J. Antimicrob. Chemother. 53:693-695. [DOI] [PubMed] [Google Scholar]
- 194.Ruzin, A., D. Keeney, and P. A. Bradford. 2005. AcrAB efflux pump plays a role in decreased susceptibility to tigecycline in Morganella morganii. Antimicrob. Agents Chemother. 49:791-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saenz, Y., J. Ruiz, M. Zarazaga, M. Teixido, C. Torres, and J. Vila. 2004. Effect of the efflux pump inhibitor Phe-Arg-beta-naphthylamide on the MIC values of the quinolones tetracycline and chloramphenicol in Escherichia coli isolates of different origin. J. Antimicrob. Chemother. 53:544-545. [DOI] [PubMed] [Google Scholar]
- 196.Saier, M. H., Jr., and I. T. Paulsen. 2001. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 12:205-213. [DOI] [PubMed] [Google Scholar]
- 197.Sanchez, L., W. Pan, M. Vinas, and H. Nikaido. 1997. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J. Bacteriol. 179:6855-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sanchez, P., U. Le, and J. L. Martinez. 2003. The efflux pump inhibitor Phe-Arg-beta-naphthylamide does not abolish the activity of the Stenotrophomonas maltophilia SmeDEF multidrug efflux pump. J. Antimicrob. Chemother. 51:1042-1045. [DOI] [PubMed] [Google Scholar]
- 199.Sanchez, P., E. Moreno, and J. L. Martinez. 2005. The biocide triclosan selects Stenotrophomonas maltophilia mutants that overproduce the SmeDE multidrug efflux pump. Antimicrob. Agents Chemother. 49:781-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schmitz, F.-J., B. Hofmann, S. Scheuring, J. Verhoef, A. C. Fluit, H.-P. Heinz, K. Kohrer, and M. E. Jones. 1998b. Relationship between mutations in the coding and promoter regions of the norA genes in 42 unrelated clinical isolates of Staphylococcus aureus and the MICs of norfloxacin for these strains. J. Antimicrob. Chemother. 42:561-563. [DOI] [PubMed] [Google Scholar]
- 201.Schneiders, T., S. G. Amyes, and S. B. Levy. 2003. Role of AcrR and RamA in fluoroquinolone resistance in clinical Klebsiella pneumoniae isolates from Singapore. Antimicrob. Agents Chemother. 47:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shafer, W. M., J. T. Balthazar, K. E. Hagman, and S. A. Morse. 1995. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 141:907-911. [DOI] [PubMed] [Google Scholar]
- 203.Shiba, T., K. Ishiguro, N., Takemoto, H. Koibuchi, and K. Sugimoto. 1995. Purification and characterization of the Pseudomonas aeruginosa NfxB protein, the negative regulator of the nfxB gene. J. Bacteriol. 177:5872-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Siddiqi, N., R. Das, N. Pathak, S. Banerjee, N. Ahmed, V. M. Katoch, and S. E. Hasnain. 2004. Mycobacterium tuberculosis isolate with a distinct genomic identity overexpresses a tap-like efflux pump. Infection 32:109-111. [DOI] [PubMed] [Google Scholar]
- 205.Silva, P. E., F. Bigi, M. de la Paz Santangelo, M. I. Romano, C. Martin, A. Cataldi, and J. A. Ainsa. 2001. Characterization of P55, a multidrug efflux pump in Mycobacterium bovis and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 45:800-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sobel, M. L., D. Hocquet, L. Cao, P. Plesiat, and K. Poole. 2005. Mutations in PA3574 (nalD) lead to increased MexAB-OprM expression and multidrug resistance in laboratory and clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:1782-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sobel, M. L., S. Neshat, and K. Poole. 2005. Mutations in PA2491 (mexS) promote MexT-dependent mexEF-oprN expression and multidrug resistance in a clinical strain of Pseudomonas aeruginosa. J. Bacteriol. 187:1246-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Srikumar, R., T, Kon, N. Gotoh, and K. Poole. 1998. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob. Agents Chemother. 42:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stermitz, F. R., T. D. Beeson, P. J. Mueller, J. Hsiang, and K. Lewis. 2001. Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensu strictu) species. Biochem. Syst. Ecol. 29:793-798. [DOI] [PubMed] [Google Scholar]
- 210.Stermitz, F. R., K. K. Cashman, K. M. Halligan, C. Morel, G. P. Tegos, and K. Lewis. 2003. Polyacylated neohesperidosides from Geranium caespitosum: bacterial multidrug resistance pump inhibitors. Bioorg. Med. Chem. Lett. 13:1915-1918. [DOI] [PubMed] [Google Scholar]
- 211.Stermitz, F. R., P. Lorenz, J. N. Tawara, L. A. Zenewicz, and K. Lewis. 2000. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 97:1433-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Stermitz, F. R., L. N. Scriven, G. Tegos, and K. Lewis. 2002. Two flavonols from Artemisa annua which potentiate the activity of berberine and against a norfloxacin resistant strain of Staphylococcus aureus. Planta Med. 68:1140-1141. [DOI] [PubMed] [Google Scholar]
- 213.Stone, B. J., and V. L. Miller. 1995. Salmonella enteritidis has a homologue of tolC that is required for virulence in BALB/c mice. Mol. Microbiol. 17:701-712. [DOI] [PubMed] [Google Scholar]
- 214.Sulavik, M. C., M. Dazer, and P. F. Miller. 1997. The Salmonella typhimurium mar locus: molecular and genetic analyses and assessment of its role in virulence. J. Bacteriol. 179:1857-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and S. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tegos, G., F. R. Stermitz, O. Lomovskaya, and K. Lewis. 2002. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 46:3133-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Truong-Bolduc, Q. C., P. M. Dunman, J. Strahilevitz, S. J. Projan, and D. C. Hooper. 2005. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 187:2395-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Truong-Bolduc, Q. C., X. Zhang, and D. C. Hooper. 2003. Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus. J. Bacteriol. 185:3127-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Urban, M., T. Bhargava, and J. E. Hamer. 1999. An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J. 18:512-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van Amsterdam, K., A. Bart, and A. van der Ende. 2005. A Helicobacter pylori TolC efflux pump confers resistance to metronidazole. Antimicrob. Agents Chemother. 49:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van der Straaten, T., R. Janssen, D. J. Mevius, and J. T. van Dissel. 2004. Salmonella gene rma (ramA) and multiple-drug-resistant Salmonella enterica Serovar Typhimurium. Antimicrob. Agents Chemother. 48:2292-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.van Veen, H. W., K. Venema, H. Bolhuis, I. Oussenko, J. Kok, B. Poolman, A. J. Driessen, and W. N. Konings. 1996. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc. Natl. Acad. Sci. USA 93:10668-10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Veal, W. L., R. A. Nicholas, and W. M. Shafer. 2002. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J. Bacteriol. 184:5619-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Visalli, M. A., E. Murphy, S. J. Projan, and P. A. Bradford. 2003. AcrAB multidrug efflux pump is associated with reduced levels of susceptibility to tigecycline (GAR-936) in Proteus mirabilis. Antimicrob. Agents Chemother. 47:665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wain, J., N. T. Hoa, N. T. Chinh, H. Vinh, M. J. Everett, T. S. Diep, N. P. Day, T. Solomon, N. J. White, L. J. V. Piddock, and C. M. Parry. 1997. Quinolone-resistant Salmonella typhimurium in Viet Nam: basis of resistance and clinical response to treatment. Clin. Infect. Dis. 25:1404-1410. [DOI] [PubMed] [Google Scholar]
- 227.Webber, M. A., and L. J. V. Piddock. 2001. Absence of mutations in marAB or soxRS in acrB-overexpressing fluoroquinolone-resistant clinical and veterinary isolates of Escherichia coli. Antimicrob. Agents Chemother. 45:1550-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Webber, M. A., A. Talukder, and L. J. V. Piddock. 2005. Contribution of mutation at amino acid 45 of AcrR to acrB expression and ciprofloxacin resistance in clinical and veterinary Escherichia coli isolates. Antimicrob. Agents Chemother. 49:4390-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.White, D. G., J. D. Goldman, B. Demple, and S. B. Levy. 1997. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J. Bacteriol. 179:6122-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wolter, D. J., E. Smith-Moland, R. V. Goering, N. D. Hanson, and P. D. Lister. 2004. Multidrug resistance associated with mexXY expression in clinical isolates of Pseudomonas aeruginosa from a Texas hospital. Diagn. Microbiol. Infect. Dis. 50:43-50. [DOI] [PubMed] [Google Scholar]
- 231.Xu, X. J., X. Z. Su, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Molecular cloning of the HmrM multidrug efflux pump from Haemophilus influenzae Rd. Microbiol. Immunol. 47:937-943. [DOI] [PubMed] [Google Scholar]
- 232.Yoshida, H., M. Bogaki, S. Nakamura, K. Ubukata, and M. Konno. 1990. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 172:6942-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yu, E. W., J. R. Aires, G. McDermott, and H. Nikaido. 2005. A periplasmic drug-binding site of the AcrB multidrug efflux pump: a crystallographic and site-directed mutagenesis study. J. Bacteriol. 187:6804-6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yu, E. W., J. R. Aires, and H. Nikaido. 2003. AcrB multidrug efflux pump of Escherichia coli: composite substrate-binding cavity of exceptional flexibility generates its extremely wide substrate specificity. J. Bacteriol. 185:5657-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yu, E. W., G. McDermott, H. I. Zgurskaya, H. Nikaido, and D. E. Koshland, Jr. 2003. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300:976-980. [DOI] [PubMed] [Google Scholar]
- 236.Zgurskaya, H. I., and H. Nikaido. 2000. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37:219-225. [DOI] [PubMed] [Google Scholar]
- 237.Ziha-Zarifi, I., C. Llanes, T. Köhler, J.-C. Pechère, and P. Plésiat. 1999. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob. Agents Chemother. 43:287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zloh, M., G. W. Kaatz, and S. Gibbons. 2004. Inhibitors of multidrug resistance (MDR) have affinity for MDR substrates. Bioorg. Med. Chem. Lett. 14:881-885. [DOI] [PubMed] [Google Scholar]