Abstract
The intestinal tract is a complex ecosystem that combines resident microbiota and the cells of various phenotypes with complex metabolic activities that line the epithelial wall. The intestinal cells that make up the epithelium provide physical and chemical barriers that protect the host against the unwanted intrusion of microorganisms that hijack the cellular molecules and signaling pathways of the host and become pathogenic. Some of the organisms making up the intestinal microbiota also have microbicidal effects that contribute to the barrier against enteric pathogens. This review describes the two cell lineages present in the intestinal epithelium: the goblet cells and the Paneth cells, both of which play a pivotal role in the first line of enteric defense by producing mucus and antimicrobial peptides, respectively. We also analyze recent insights into the intestinal microbiota and the mechanisms by which some resident species act as a barrier to enteric pathogens. Moreover, this review examines whether the cells producing mucins or antimicrobial peptides and the resident microbiota act in partnership and whether they function individually and/or synergistically to provide the host with an effective front line of defense against harmful enteric pathogens.
INTRODUCTION
The mucosal surface of the intestinal tract is the largest body surface in contact with the external environment (200 to 300 m2). It is a complex ecosystem combining the gastrointestinal epithelium, immune cells and resident microbiota (249). The mucosa of the intestinal tract is exposed to various microbial pathogens. These potentially harmful enteric microorganisms can hijack the cellular molecules and signaling pathways of the host and become pathogenic. In the first step in the infectious process, some enteric bacterial pathogens adhere to the brush border of intestinal cells (46, 380), enabling them to exploit the underlying signaling pathways. Moreover, some enteric microbial pathogens have developed specialized systems that, after this essential step of adhesion, produce virulence factors. After normal host-cell processes have been subverted, these systems enable the pathogen to cross the epithelial barrier (74). The host cell cytoskeleton is commonly used and targeted by enteric microbial pathogens during the cell penetration step; it is exploited for purposes that include gaining entry into cells, moving within and between cells, and forming and remodeling vacuoles in order to create a specialized niche, which enhances the pathogen's chances of survival (131).
The host is protected from attack by potentially harmful enteric microorganisms by the physical and chemical barriers created by the intestinal epithelium (Fig. 1). The surface of the intestine is lined by a simple columnar epithelium that is folded to form a number of invaginations, or crypts, which are embedded in the connective tissue. The intestinal cells (192, 228, 271) that make up the epithelium provide a physical barrier that protects the host against the unwelcome intrusion of microorganisms (Fig. 1). The intestinal epithelium is a model of tissue renewal, since intestinal cells are constantly generated from a source of multipotent stem cells located in the crypts of Lieberkühn, and these provide new precursor cells permitting a high rate of cell turnover. In the intestinal villi, the polarized epithelial cells that form the epithelium separate two different compartments. This epithelial barrier is composed of four epithelial cell lineages, including the enterocytes, enteroendocrine, goblet, and Paneth cells present in the intestinal villi. In addition, M cells are present in the follicle-associated epithelia. The integrity of the layer of epithelial cells is maintained by intercellular junctional complexes composed of tight junctions (TJs), adherens junctions (AJs), and desmosomes, whereas gap junctions allow intercellular communication to occur. TJs, the most apical components of the junctional complex (9, 344), create a semipermeable diffusion barrier between individual cells, which can be regulated and serves as the permeability barrier. Forty different proteins have been shown to be located in TJs, including, for example, ZO-1, ZO-2, and ZO-3 proteins, members of the membrane-associated guanylate kinase protein family, occludin, claudins, cingulin, 7H6, and several unidentified phosphoproteins (59, 267). Interestingly, the biogenesis of the TJs appears to be regulated, in part, by classic signal transduction pathways, such as those involving heterotrimeric G proteins, Ca2+, and protein kinase C, and raft-like membrane microdomains that act as a platform for signaling molecules (81, 246, 286, 416). Downstream from the TJs are the AJs, composed of a cadherin-catenin complex and its associated proteins, and membrane and PDZ proteins (277, 377). In both TJs and AJs, interactions among their specific components seem to be dynamically regulated during the formation of the junctional complex in epithelial cells. Importantly, it has been noted that TJs and AJs play a pivotal role in maintaining cell polarization. Indeed, recent evidence suggests that cell polarization operates regardless of whether TJs are present, since they form an intramembrane barrier to diffusion that restricts mingling between the apical and basolateral membrane components (245, 382). It is of interest that many pathogenic enteric bacteria target and exploit the TJ domain to accomplish their pathogenic strategies by modulating intestinal permeability (105, 156). It has also been established that some enteric pathogens use the M cells overlying the organized mucosal-associated lymphoid system (204, 282) as a route of invasion and that after passing through these cells, the bacteria face phagocytic cells, particularly the macrophages that are present in the follicle dome (60, 186, 339).
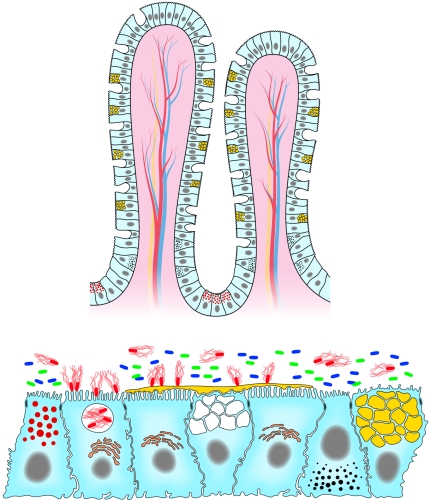
FIG. 1.
Architecture of the intestinal epithelium lining the intestinal tract. (A) Crypt-villus cell organization. The cell renewall is achieved from the pluripotent intestinal stems cells are up from the crypt base in the small intestinal and at the crypt base in the colon. Epithelial cells migrate up the crypt where they perform their differentiation, acquiring specific intestinal functions of absorption and secretion. Three cell types differentiate as they migrate: the predominant enterocytes, the mucus-secreting Goblet cells, and the peptide hormone-secreting enteroendocrine cells. Oppositely, the Paneth cells migrate down to the base of the crypt. (B) The assembly of the polarized epithelial-cell types results from an epithelium that provides a permeability barrier between the external and internal compartments. This barrier function is assumed by the junctional domain, including well-defined gap junctions, desmosomes, adherens junctions, and tight junctions. Four polarized epithelial cell lineages were present in the intestinal epithelium: the enterocytes expressing at the apical domain a dense, well-ordered brush border consisting of organized microvilli in the membrane of which oriented proteins support specific functions; the mucus-secreting goblet cells (cell with large yellow granules) producing membrane-bound mucins and containing mature storage granules in which secreted mucins are packaged; the enteroendocrine cells (cells with small, dark granules) containing small, oriented secretory granules in which different peptide hormones should be stored, although a same granule may store more than one peptide hormone; and the Paneth cells (cell with small, red granules) containing apically oriented granules in which AMPs and antimicrobial proteins were packaged as pro or mature forms. Enteric pathogens (red bacteria with flagella) interact with the intestinal epithelial cells, enter the cells, affect the cell architecture or organization, and disturb the cell functions. The commensal bacteria (blue and green bacteria) mainly reside in the lumen outside the mucus layer. Secreted mucins (in yellow, coating the epithelial surface) in association with the membrane-bound mucins act as a physicochemical barrier for the protection of the epithelial cell surface against undesirable harmful pathogens.
Host defense systems against unwelcome intrusion of pathogenic enteric microorganisms include adaptive and innate immunity. Adaptive immune responses are typically observed 4 to 7 days after infection, and this mechanism involves the generation of immunological memory and the expansion of receptors with relevant specificities. In contrast, the innate immune system is mobilized within the first few days in order to control infection (258). Unlike the adaptive immune system, which uses a clonal, random, and highly diverse repertoire, the innate immune system uses nonclonal sets of recognition molecules. The intestinal epithelium provides a surface where the host can sense the microbial environment in order to trigger a strong defense response, when this is required, by releasing signaling molecules such as cytokines and chemokines. These in turn trigger the recruitment of leukocytes and initiate the attraction of immune cells. However, the intestinal epithelium, unlike that of the lung, tolerates bacterial colonization by members of the resident microbiota. Indeed, although consistently exposed to commensal bacteria, the normal mucosa exhibits only a minimal inflammatory status in response to the abundant products of the normal flora triggered by resident gram-negative and gram-positive bacteria. These products include substances such as lipopolysaccharide (LPS) (for gram-negative bacteria) and lipoprotein and peptidoglycan (for gram-positive bacteria). Investigating how the host gut distinguishes between its commensal microbiota and unwelcome enterovirulent microorganisms has revealed that hosts possess highly sophisticated systems for detecting antigens (6, 14, 89). The endogenous bacterial species of the microbiota all share “self” signature molecules, known as microbe-associated molecular patterns. In contrast, following infection, the host innate mucosal immunity response is activated mainly as a result of the specific recognition by pattern recognition receptors of conserved “non-self” molecular structures found in large groups of pathogens, known as pathogen-associated molecular patterns (180). For example, epithelial cells sense the environment within the gut by means of their pattern recognition receptors, which include Toll-like receptors (TLRs) and the NOD (nucleotide-binding oligomerization domain) proteins (93, 181, 191, 281, 310). TLRs are evolutionary conserved proteins characterized by having an extracellular leucine-rich repeat domain involved in ligand recognition (1, 181, 257, 258) and an intracellular Toll/interleukin 1 (IL-1) receptor-like domain involved in signal transduction (4, 191). Moreover, two mammalian nucleotide-binding, leucine-rich, repeat proteins (NOD1 and NOD2) function as intracellular sensors of bacterial products in the induction of inflammatory responses (125, 155, 176). Biochemical studies have revealed that NOD2 is in fact a protein involved in the innate immune detection of bacterial products (201, 311). More specifically, NOD2 recognizes a fragment of peptidoglycan, known as muramyl dipeptide, which is found in the cell walls of both gram-negative and gram-positive bacteria.
The intestinal epithelium is not just a physical barrier that prevents unwanted bacteria from gaining access to essential organs; it also provides a surface covered by specialized cells producing mucus, antimicrobial peptides (AMPs), and antimicrobial molecules, such as lysozyme, which together with resident microbiota provide the front line of defense against pathogenic microorganisms (118). The aim of this review is to analyze what we know about this first line of defense. Our analysis focuses on the two cell lineages present in the intestinal epithelium: the goblet cells and the Paneth cells, both of which play a pivotal role in this front line of enteric defense (Fig. 1). We also discuss recent insights into the mechanisms by which the intestinal microbiota acts as a barrier to enteric pathogens.
MUCUS
The intestinal mucosa has a surface coating of mucus that is secreted by the specialized goblet cells, also known as mucin-secreting cells (72, 108, 207).
Mucin-Secreting Cells
Mucin-secreting cells have a polarized phenotype characterized by the fact that the apical and basolateral domains of the cell membrane are separated by TJs that are also involved in connections with adjacent cells (Fig. 1). In the apical domain, the mucin-secreting cells have a brush border, an ordered structure consisting of organized microvilli. As in the enterocytes, the microvillus of the brush border is organized by a cytoskeleton containing a bundle of actin filaments combined with various actin-bundling proteins, including villin and fimbrin. The cytoskeleton of the brush border plays a pivotal role in organizing and maintaining specialized intestinal functions in both enterocytes and mucin-secreting cells. The microtubule cytoskeleton localized within the cell is also specifically organized to facilitate vesicle trafficking between the Golgi network and the apical domain facing the luminal compartment (331), which is under the control of apical sorting signals. Mucin-secreting cells contain numerous high-electron-density, secretory granules containing packaged mucins located above the nucleus and below the brush border.
Mucin-secreting cells mature from the proliferative zone located at the base of the crypt, and they are all derived from stem cells located at the base of the crypt (18, 42, 241) (Fig. 1). Mucin-secreting cells mature as they migrate along the crypt-villus axis. The short-lived mucin-secreting cells ascend the villus, differentiate, and then exfoliate into the lumen within ∼5 to 7 days after they have been produced as a result of cell division, as do enterocytes and endocrine cells. Investigations of the regulation of stem cell proliferation and differentiation on the villus have revealed that they are controlled by several systems, including the Wnt and Hedgehog signaling pathways, the morphogenic proteins of bone, and intestinal transcription factors, CDX1, CDX2, and HNF1 (402, 403). For example, the canonical Wnt signaling cascade (312, 313) comprises 20 different secreted proteins, which interact with about 10 different Frizzled receptors. Wtn signaling is transduced via β-catenin/TCF4 (390) and is known to control multiple biological phenomena in vertebrates, including cell fate determination and maintaining stem/progenitor cells with predefined fates in specific compartments (241). Wnt signaling plays a key role in the intestinal epithelium (38, 202) in driving a stem cell/progenitor gene program that is crucial for maintaining undifferentiated progenitors near the bottom of the crypts of Lieberkühn. In addition, it has recently been reported that the Notch signaling (12) plays a critical role in intestinal development, since mucrosecreting goblet cells are severely depleted in the double transgenic Rosa-Notch/Cre+ mouse (110). Exfoliation of mature intestinal cells from the tip of the villi results from a particular cell death program, known as “anoikis,” that subject to both positive and negative control by focal adhesion kinase- or β1-integrin-related events, protein-kinase signaling pathways including phosphatidylinositol 3-kinase/Akt, mitogen-activated protein kinase, stress-activated protein kinase/Jun amino-terminal kinase, and certain Bcl-2 and Bcl-2-related proteins (113, 385, 423).
Changes in goblet cell function and in the chemical composition of the intestinal mucus have been detected in response to a broad range of luminal insults, including changes in the normal microbiota and the intrusion of harmful enteric pathogens, but the mechanisms involved are poorly understood (82). Studies have shown that germfree mice can exhibit changes in mucin gene expression, mucus composition, and mucus secretion in response to intestinal microbes or host-derived inflammatory mediators. For example, when germfree mice were conventionalized by the oral administration of microorganisms prepared from the feces of genetically identical mice, bacterial colonization led to a time-dependent change in the number of rectal goblet cells and mucin composition (115).
Mucins
Mucin-type molecules consist of a core protein moiety (apomucin) within which a number of carbohydrate chains are attached to serines, prolines, and threonines by glycoside bonds. O-linked and N-linked oligosaccharides form up to 80% of the molecule, and the lengths of the carbohydrate side chains range from 1 to more than 20 residues (348). Mucin-type oligosaccharides play a pivotal role in their hydroscopic properties, by binding various small molecules and proteins, and in specific ligand-receptor interactions. Mucins are synthesized as nascent peptides and form oligomers in the endoplasmic reticulum (108). Core- and end-glycosylations occur in the Golgi apparatus, and the mature mucins are then moved from the condensing granules to mature storage granules. Mucins can be divided into three distinct subfamilies on the basis of their structure: gel-forming, soluble, and membrane-bound mucins (Table 1). Moreover, the mucins secreted can be subdivided into two groups: gel-forming mucins and non-gel-forming mucins (51, 80, 121, 157). Eighteen genes encoding human mucin-type glycoproteins have so far been assigned to the MUC gene family, MUC1, MUC2, MUC3A, MUC3B, MUC4, MUC5B, MUC5AC, MUC6 through MUC13, and MUC15 through MUC17, with the approval of the Human Genome Organization Gene Nomenclature Committee (http://www.gene.ucl.ac.uk/nomenclature) (51, 79, 80, 269). A cluster of four mucin genes (MUC2, MUC5B, MUC5AC, and MUC6) located on chromosome 11p15.5 encodes secreted mucins. Nine genes, MUC1 (1q21), MUC3A (7q22), MUC3B (7q22), MUC4 (3q29), MUC11 (7q22), MUC12 (7q22), MUC13 (3q13), MUC16 (19p13.3), and MUC17 (7q22), encode membrane-associated mucins. There are also some products of MUC genes, including those of MUC7 (4q13 to 4q21), MUC8 (12q24), MUC9 (1p13), and MUC15 (11p14.3), that do not fit well into either class.
TABLE 1.
Membranous and secreted mucins
| Gene | Type | Expression | Reference(s)
|
|
|---|---|---|---|---|
| Mucins | Mucins/pathogens | |||
| MUC1 | Membranous | Gastrointestinal epithelium, genitous tract, ocular, respiratory tract | 51, 52, 182, 217, 222, 293, 356, 398, 405 | 52, 276, 396 |
| MUC3A | Membranous | Gastrointestinal epithelium, genitous tract, respiratory tract | 51, 75, 79, 205, 405, 411 | 210, 220, 231, 232 |
| MUC3B | Membranous | Gastrointestinal epithelium, genitous tract, respiratory tract | 51, 75, 79, 405, 411 | 210, 220, 231, 232 |
| MUC4 | Membranous | Gastrointestinal epithelium, genitous tract, ocular, respiratory tract | 50, 51, 54, 79, 187, 270, 285, 308, 316, 356, 358, 410, 424, 427 | 220 |
| MUC12 | Membranous | Gastrointestinal epithelium, genitous tract | 51, 220, 410 | 220 |
| MUC13 | Membranous | Gastrointestinal epithelium, genitous tract | 51, 73, 412 | |
| MUC17 | Membranous | Gastrointestinal epithelium | 51, 73, 134 | |
| MUC2 | Secreted | Gastrointestinal epithelium, genitous tract, respiratory tract | 51, 79, 133, 356, 378 | 216, 231, 232 |
| MUC5AC | Secreted | Gastrointestinal epithelium, genitous tract, respiratory tract | 50, 79, 220, 287, 316, 350, 359, 388, 390 | 52, 64, 67, 90, 220, 276, 321, 388, 390 |
| MUC5B | Secreted | Gastrointestinal epithelium, genitous tract, respiratory tract | 51, 79, 220, 316, 359 | |
| MUC6 | Secreted | Gastrointestinal epithelium, genitous tract, respiratory tract | 51, 79 | 52, 276 |
The secreted mucins MUC2, MUC5AC, MUC5B, and MUC6 assemble via interchain disulfide-forming, disulfide-linked oligomers/multimers with molecular weights in the millions (307). They express specific mucin domains (51, 157), including VNTE (variable number of tandem repeat) domains that are rich in serine, threonine, and proline residues; VWD sequences homologous to von Willebrand factor D domains (which are thought to be involved in the oligomerization of mucin to form gel); C-terminal CK (Cys-rich [cystin-rich]/CK [Cystin-Knot]) domains (which are thought to be involved in the initial dimerization of apomucin monomers); and VWC domains (homologous to von Willebrand factor C domains), which are thought to be involved in binding trefoil factors (315, 338).
The mechanism(s) by which the apical exocytosis of granule content occurs has not been fully elucidated. It has been proposed that mucus exocytosis may develop after the granule and plasma membrane fuse to form a fusion pore and that an expulsive force then extrudes the viscous mucins from the granules into the luminal space. It has also been suggested that electrolyte secretion may provide the osmotic driving forces (140, 261, 262). There are two possible secretory pathways for secreted mucins in intestinal mucin-secreting cells (108, 206, 207). The first of these is the regular vesicular constitutive pathway of mucin exocytosis, also known as baseline secretion, in which no storage occurs, since the small vesicles transporting the mucins through the constitutive pathway are guided directly to the cell surface via microtubules and undergo immediate exocytosis of their contents. The second pathway for mucin exocytosis involves the packaging and storage of mucins in large vesicles, from which mucin release is regulated by specific stimuli involving the activation of signaling pathways by a number of secretagogues, including neuroendocrine mediators (such as acetylcholine, vasoactive intestinal peptide, and neurotensin [15, 44, 208, 314]), and inflammatory/immune mediators (such as interleukin-1 [184] and nitrite oxide [48]). Purinergic stimulation by extracellular ATP leads to an increase in mucin secretion (33, 138, 260). Both Ca2+- and cAMP-mediated second messenger cascades acutely regulate mucin secretion from human colonic epithelial cells (44, 47, 183). Cholera toxin that binds with high affinity to apically localized receptors on mucin-secreting cells (215) is a strong activator of mucin exocytosis (96, 214, 272, 273, 284, 332). In contrast, Clostridium difficile toxin A is able directly to affect the intestinal epithelial barrier function and down-regulates stimulated mucin exocytosis (48).
Membrane-bound mucins MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC16, and MUC17 are associated with the cell membrane by an integral transmembrane domain and are characterized by having relatively short cytoplasmic tails that associate with cell cytoskeletal proteins. Membrane-bound mucins express specific mucins domains, including EGF (Epidermal Growth Factor)-like domains, the SEA (Sea urchin sperm protein, Enterokinase, and Agrin) domain, and the tandem repeat domain rich in serine, threonine and praline residues. Matsuo et al. (244) reported two distinct mucus layers. An elegant model of the functional organization of the mucus layer associating secreted mucins and membrane-bound mucins, has recently been proposed by Hollingsworth and Swanson (157). Membrane-bound mucins associate with the secreted mucins by both covalent and noncovalent bonds in order to create a high local concentration of specific molecular structures and to develop functions including binding sites for lectins, selectin, and adhesion molecules, stoichiometric power that enables them to exclude larger molecules and microorganisms, hygroscopic effects that influence the degree of hydration at the cell surface, ion exchange effects, and an area in which growth factors, cytokines, and chemokines are sequestered. Recent studies have also implicated membrane-bound mucins in cellular signaling, suggesting that they may have an important function as sensor mechanisms in response to invasion or damage of the epithelia (55). In this function, the cytoplasmic tails of membrane-bound mucins associate with adaptator proteins in the cytosol. For example, MUC4 acts as a receptor ligand and MUC1 as a docking protein for signaling molecules. MUC1 has been found to be associated with lipid rafts that function as a platform for signaling molecules. It expresses a highly conserved cytoplasmic tail, which binds beta-catenin, a key component of adherens junctions and a regulator of transcription, in a process that is tightly regulated by MUC1 phosphorylation. MUC4 is a novel intramembrane ligand for the receptor tyrosine kinase ErbB2/HER2/Neu, triggering specific phosphorylation of the ErbB2 in the absence of other ErbB ligands, and potentiating phosphorylation and signaling through the ErbB2/ErbB3 heterodimeric receptor complex that is formed in the presence of neuregulin.
Some of the MUC7, MUC8, MUC9, and MUC15 mucins do not fit easily into either the secreted or membrane-bound class but do share some characteristics of these classes. For example, MUC15 has a transmembrane domain and a cytoplasmic tail.
Barrier Effect against Pathogens
For a long time, it was thought that the sole function of mucins was to protect and lubricate the epithelial surfaces (72); however, it has recently been established that they are also involved in other important functions, such as growth, and are directly implicated in fetal development, epithelial renewal, differentiation and integrity, carcinogenesis, and metastasis (71, 269). The mucus gel could be useful to enteric bacteria in at least two ways. First, the intestinal mucus offers numerous ecological advantages for both resident microbiotic bacteria and some pathogenic bacteria present within the lumen and in the intestinal epithelium, since it can provide nutrients for bacterial growth, thus promoting intestinal colonization by the adhering bacteria, which have the ability to survive and multiply in the outer regions of the mucus layer (11). Mucins do indeed provide a source of energy by producing the saccharides used for the sustained growth of both the indigenous enteric microbiota (27, 230) and the pathogens that adhere to the mucus (151, 197, 222, 323, 395). The second role played by the mucus layer is linked to its generally accepted role in cytoprotection (392). A discontinuous, thinner layer of mucus gel covers the epithelial cells that line the epithelium of the small intestine. Mucus thicknesses differ in the large intestine, gradually increasing from the colon to the rectum, and Peyer's patches apparently have no mucus covering (244). The mucus layer creates a physical barrier that acts as a dynamic defense barrier against enteric microbial pathogens (Fig. 1) (268). Consistent with this, bacteria associated with the outer layer of mucus have been observed. Several gastrointestinal pathogens have developed specific pathogenic factors and/or ways of interfering with mucin production in order to enable them to cross the mucus barrier. The prototype of such pathogens is Helicobacter pylori, which colonizes the gastric mucous gel layer by means of a very close association with MUC5AC mucin (388, 389) and probably also with the membrane-bound mucin MUC1 (396). H. pylori uses its flagella for motility within the mucus layer in the acid-secreting stomach (296). In addition, H. pylori reduces mucin exocytosis (264), decreases gastric mucin synthesis by inhibiting UDP-galactosyltransferase (374), and causes an aberrant expression of the gastric mucins MUC1, MUC5AC, and MUC6 (52, 276). It is interesting that mucins play also a role in Pseudomonas aeruginosa pathogenesis since an upregulated transcription of the MUC2 (216) and MUC5AC (90) mucin genes follows infection. The fact that upregulation of the MUC5AC gene can be mimicked by LPS indicates that there must be a general mechanism by which epithelial cells respond to the presence of bacteria by increasing mucin synthesis.
Secreted mucus has already been reported to act as a barrier to enteroinvasive Yersinia enterocolitica (239), rhesus rotavirus (58), and Shigella flexneri (287). It has also been reported that the bovine, mammary-associated, serum amyloid A3 increases the membrane-bound mucin MUC3, which in turn inhibits the adherence of enteropathogenic Escherichia coli (EPEC) (210). Resident intestinal bacteria are able to inhibit the adherence of pathogenic bacteria to intestinal epithelial cells as a result of their ability to increase the production of intestinal mucins. For example, Lactobacillus plantarum strain 299v increases the levels of expression of the mRNA of mucins MUC2 and MUC3, thus in turn inhibiting the cell attachment of EPEC, an effect that can be mimicked by adding purified exogenous MUC2 and MUC3 mucins (231, 232). Moreover, it has been observed that LPS of gram-negative bacteria increases the expression of the mRNA of MUC5AC and MUC5B and stimulates the secretion of MUC5AC and MUC5B mucins (359). It has been recently demonstrated that the secreted mucins including MUC5AC together with membrane-bound mucins, contributes to host defense by preventing bacterial invasion of the intestinal cells. Indeed, both in vivo (321) and in vitro (64) infections by the gram-positive, facultative intracellular human pathogen Listeria monocytogenes are associated with the massive release of mucus by goblet cells. This increase in mucin secretion develops through a listeriolysin-dependent mechanism that appears to be related to the binding of the toxin to multiple membrane-associated lipid receptors, which allows the toxin monomers to oligomerize and requires the toxin to be internalized through the caveolae (67). Listeriolysin also increases the transcription of the MUC3, MUC4, and MUC12 genes that encode membrane-bound mucins (220). In contrast, the MUC5AC gene encoding a secreted mucin is not upregulated. Whereas secreted mucins or membrane-bound mucins alone were unable to prevent the cell entry of L. monocytogenes, both secreted and membrane-bound mucins have been shown to be necessary to inhibit cell entry (221). This is consistent with the fact that membrane-bound mucins, including MUC3, MUC4, and MUC12, are associated with secreted mucins, in particular, with the gel-forming mucin MUC5AC, by both covalent and noncovalent bonds (157). The fact that the MUC5AC gene can be upregulated by LPS (90) but not by L. monocytogenes (220) suggests that for this MUC gene, epithelial cells respond to the presence of gram-negative bacteria by a general mechanism.
ANTIMICROBIAL PEPTIDES
It is known that both nonvertebrates, such as insects and plants, and vertebrates, ranging from fish and frogs to humans, produce AMPs and that these peptides are the effectors of the innate immune response. The AMPs present in the gastrointestinal tract of the host constitute one of the partners involved in the front line of chemical defense against harmful microorganisms (Fig. 2) (35, 93, 117, 154, 172, 212, 235, 345, 379). This chemical antimicrobial defense system functions in the airways, gingival epithelium, cornea, reproductive tract, and urinary and gastrointestinal tracts. AMPs play a major role in the innate immune system, enabling it to respond in a matter of hours, well before the adaptive immune system can be sufficiently mobilized. The main advantage of the innate immune system is that it permits the host to curb, delay, or avoid the growth of undesirable intruding bacteria shortly after an infection, in a way that is not highly specific and does not involve memory. AMPs were first identified in polymorphonuclear neutrophils and macrophages. AMPs are gene-encoded peptides that have a broad spectrum of antibiotic activity.
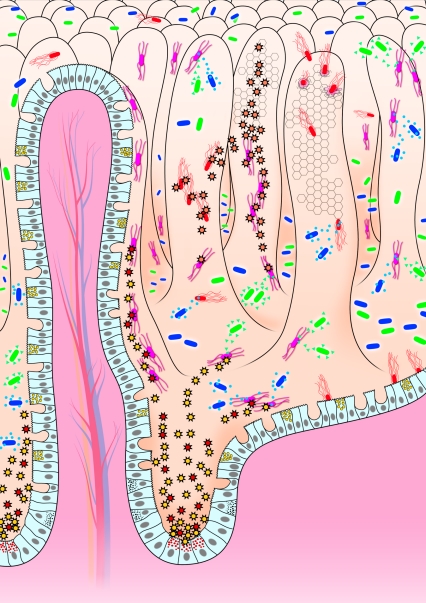
FIG. 2.
The chemical front line of enteric host defense against unwelcome intrusion of harmful bacterial pathogens. Enteric invasive and noninvasive bacterial pathogens (red bacteria) expressing pathogenic factors (adhesive factors, invasines, and toxins, etc.) interact with the host epithelial cells lining the villi. At the base of the crypt, the Paneth cells containing antimicrobial-rich granules, released AMPs (red and yellow spike rings) upon exposure of intestinal epithelium to undesirable harmful pathogens and/or their bacterial products (LPS and toxins, etc.). Moreover, other intestinal cells lining the villi also secreted antimicrobial proteins (orange spike rings). In parallel, the commensal gram-negative (green bacteria) and gram-positive (blue bacteria) intestinal bacteria that reside in the lumen produced antibacterial molecules (green triangles and blue circles).
Intestinal Cells That Produce Antimicrobial Peptides
AMPs are produced by specialized cells known as Paneth cells (Fig. 1 and 2) (303, 347). These cells, one of the four major epithelial cell lineages present in the intestine, are present at the base of the crypts of Lieberkühn in mammals and play a pivotal role in the enteric defense against pathogenic harmful bacterial intruders. Paneth cells, like the enterocytes, goblet cells, and enteroendocrine cells, originate from intestinal epithelial stem cells (18, 42, 241). The maturation of Paneth cells has been investigated in mice. Recently, it has been demonstrated that the canonical Wnt signaling cascade (312, 313) plays a pivotal role in the maturation of Paneth cells (391). In addition, it has been reported recently that, consistent with the fact that Notch signaling (12) plays a critical role in intestinal development, the double transgenic Rosa-Notch/Cre+ mouse exhibits compromised differentiation of the Paneth cells (110). Paneth cells are pyramid-shaped, columnar, exocrine cells, and they have been identified within a few days after birth in mice and as early as 24 weeks of gestation in humans. The ultrastructure of Paneth cells (317, 340) shows that they have a basally located nucleus with a nucleolus, a perinuclear region containing the rough endoplasmic reticulum and Golgi apparatus, and a supranuclear region containing numerous high-electron dense, apically located, eosinophilic secretory granules containing AMPs and other antimicrobial molecules, including lysozyme, phospholipase A2, α1-antitrypsin, and AMPs (91, 116, 212, 300) (Fig. 1). One of the functions attributed to Paneth cells is the control of the bacterial milieu in the intestine (16). It is possible that AMPs may influence the composition of the enteric microbial flora under physiological conditions, but this remains to be demonstrated (297). Moreover, because certain AMPs stimulate cultured epithelial cells to secrete the chloride ion, these peptides appear to be capable of interacting directly with the apical membranes of neighboring cells and, perhaps, of influencing crypt physiology (298). Under physiological conditions, the continual release of preformed AMPs allows the chemical defense system to contribute directly to the innate immunity of the crypt microenvironment, and it probably also does this by diffusing the peptides secreted into the lumen (Fig. 2). Interestingly, it has been reported that AMP activity can be compromised by inadequate dissolution of Paneth cell granules within the crypt lumina (61). Moreover, the maintenance of the release of granule constituents into the lumen of the crypt is important, since it has been recently demonstrated that compromised Paneth cell function is detrimental to host defense against E. coli infection in the neonatal small intestine (351).
Other observations have suggested that AMPs could be produced by intestinal cells other than Paneth cells lining the epithelium (Fig. 2). Cunliffe et al. (77) have identified dispersed epithelial cells expressing AMPs that resemble goblet cells. Little is known about the relationship between the expression of AMPs and the differentiation of polarized intestinal epithelial cells. Alteration in enterovirulent, diffusely adhering E. coli C1845 has been observed following the infection of human enterocyte-like Caco-2 and HT-29 Glc−/+ cells (obtained by culturing the parental HT-29 cell line in culture medium deprived of glucose and then being adapted for growth in the presence of glucose), whereas this phenomenon is not observed in infected human, embryonic undifferentiated INT407 cells (31). Hase et al. (148) reported that hLL-37 mRNA and protein expression paralleled the spontaneous differentiation of Caco-2 human colon epithelial cells. Moreover, in HCA-7 human colon epithelial cells treated with the cell differentiation-inducing agent sodium butyrate, there is an increase in the expression of hLL-37 mRNA and protein (148). Similarly, sodium butyrate increased the level of hLL-37 transcripts in both colon and epithelial SW620 and SW480 cells, that do not express hLL-37, and in colon carcinoma Gek-12 and HT-29 cells, which do exhibit a basal level of hLL-37 expression (342).
Antimicrobial Peptides
AMPs are small peptides, 20 to 40 amino acids in length (Table 2). Two major families of AMPs have been identified: the defensins (119, 213) and the cathelicidins (419). Defensins were first identified by Ouellete (298) in mouse small intestinal cells. The mouse cryptin gene family encodes at least 19 different cryptdin proteins. The first murine Paneth cell defensin, known as cryptdin-1, which displays anti-Salmonella activity, has been identified by Ouellette (301). The mouse cryptdins (cryptdin-1 to cryptdin-5 and cryptdin-16) have been particularly investigated (167, 297-299, 302). In mammals, defensins are found in the phagocytic leukocytes and in various epithelial cells, including Paneth cells (35, 37, 41, 116).
TABLE 2.
Human intestinal AMPs
| AMP | Family | Expression | Storage/processing | References |
|---|---|---|---|---|
| HD-5 | α-Defensin | Paneth cells | Propeptide; during or after release | 76, 77, 223, 318, 319 |
| HD-6 | α-Defensin | Paneth cells | 36, 100, 223, 237, 409 | |
| hBD-1 | β-Defensin | Intestinal epithelial cells | 26, 93, 188, 223, 294, 406, 425, 428 | |
| hBD-2 | β-Defensin | Intestinal epithelial cells | Mature peptide, endoplasmic reticulum? | 21, 93, 100, 143, 223, 291, 294, 346, 372, 383, 400, 401, 404, 406-408 |
| hBD-3 | β-Defensin | Gastrointestinal cells | 120, 145 | |
| hLL37 | Cathelicidin | Intestinal epithelial cells | Propeptide; during secretion process | 22, 23, 148, 149, 152, 153, 295, 342, 417, 418 |
AMPs have been classified on the basis of their secondary structure. Magainins and numerous cathelicidins (419) contain an α-helical structure (α-defensins), other AMPs have a β-sheet that contains three disulfide bonds (β-defensins), and the first circular AMP has recently been identified (θ-defensins) (Table 2). Cathelicidins comprise mammalian proteins that are expressed by mammalian leukocytes (23, 203, 212, 324, 355, 418). The cathelicidin-derived AMPs are generally characterized by conserved propeptide sequences, include α-helicoidal, proline-rich, disulfide bonds, and/or a β-sheet, and tryptophan-rich peptides, but cathelicidins themselves have a linear, non-α-helical structure (Table 2) (212, 418).
To date, α-defensins (HD) and β-defensins (hBD) (35, 76, 117), as well as cathelicidins (23, 212, 418-420), in humans have been identified (Table 2). In contrast, θ-defensins are not expressed, although humans express mRNA encoding θ-defensin orthologs and mutations that introduce stop codons abolish peptide production. Certain defensin genes are expressed in phagocytic cells of hematopoietic origin, whereas others are expressed in Paneth cells, and in the epithelial cells of the small intestine. The genes encoding the α- and β-defensins are located in a cluster at chromosome 8p23 (223). For example, the gene encoding hBD-1 has been mapped to chromosomal region 8p23.1-8p23.2, which is in close proximity (within 100 to 150 kb) to the gene for the human neutrophil α-defensin HNP-1 (224). α-Defensins are small polypeptides with 29 to 35 residues and a six-cysteine motif that forms three intramolecular disulfide bounds (Cys1-Cys6, Cys2-Cys4, and Cys3-Cys5). Among the six α-defensins identified, four designated as human neutrophil peptides (HNPs) 1, 2, 3, and 4 form part of the armory of the neutrophils, where they are involved in systemic innate immunity. The remaining HDs (HD-5 and HD-6) are expressed in intestinal cells and contribute to the innate defense of the intestinal mucosal surface. The levels of HD-5 and HD-6 transcripts are not high in the duodenum and increase distally (36). HD-5 is expressed in Paneth cells and also in some villous epithelial cells in healthy duodenum, jejunum, and ileum, but in contrast, it is not expressed in the healthy stomach or colon (318). β-Defensins differ from α-defensins in size (38 to 42 amino acid residues) and cysteine motifs (Cys1-Cys5, Cys2-Cys4, and Cys3-Cys6). Six β-defensins (hBD-1 to hBD-6) have been identified in humans. Human β-defensin-1 (hBD-1), consisting of a short basic peptide of 36 amino acid residues containing six cysteines forming three intramolecular disulfide bonds, has been found in epithelial cells of the small and large intestine (425). β-Defensin-2 (hBD-2) has been isolated from the skin and is expressed mainly in the respiratory tract (146) but also in the epithelial cells of both the small and large intestine (21). β-Defensin-3 (hBD-3), which exhibits microbicidal activity against E. coli, has been detected in the epithelia of the gastrointestinal tract (120, 145). Consistent with the fact that the α- and β-defensins are located in a cluster at chromosome 8p23 (223), β-defensin-4 (hBD-4) has been recently identified by screening genomic sequences and found to be highly expressed in the testis and gastric antrum (120). In addition, it has recently been reported that polypeptides have been isolated from the human colon: three antimicrobials had previously been identified as ribosomal polypeptides (L30 and ubiquicidin), and two were members of the histone family (H1.5 and H2B) that exhibited bactericidal activity against E. coli (168). The levels of HD-5 and HD-6 transcripts are not high in the duodenum and increase distally. Both hBD-1 and hBD-2 mRNAs have been detected in some, but not all, biopsy specimens from healthy small intestines (86). HD-5 is expressed in Paneth cells and also in some villous epithelial cells in healthy duodenum, jejunum, and ileum, but in contrast, it is not expressed in the healthy stomach or colon (318). The cathelicidin hLL-37 has been shown to be expressed within epithelial cells located at the surface and upper crypts of healthy human colon (148) and gastric cells (149).
All AMPs are generated as prepropeptides, and all need to be processed to be activated. However, some are processed intracellularly and packaged in their processed forms, while others are processed after being secreted. It has been reported that some AMPs need to be processed to be activated. For example, HD-5 is present in Paneth cells only in the form of a precursor that does not have any antimicrobial activity against a defensin-sensitive Salmonella sp. and is processed to reach its mature form by a trypsin-dependent mechanism during and/or after being secreted inside Paneth cells (77, 124). Like defensins, some cathelicidins are fully processed before storage, whereas others are stored as precursors that still require further processing (212). Indeed, some cathelicidins are produced as inactive precursors containing a C-terminal cationic antimicrobial domain that becomes active after being freed from the N-terminal cathelin portion of the holoprotein. Signal peptidase removes the N-terminal signal sequence, whereas peptidylglycine α-amidating monooxygenase often amidates and cleaves the C-terminal region. Removal of the cathelin domain liberates the active antimicrobial peptide. The hLL-37/hCAP-18 propeptide is present in the secondary granules, specific to neutrophils, and its C-terminal antimicrobial peptide, hLL-37, is liberated by proteinase 3 during degranulation and secretion. The bactericidal activity of cryptdins requires proteolytic activation of precursors by matrix metalloproteinase-7 (matrilysin), which is present in Paneth cells and known to be involved in innate host defense, since matrilysin-null mice have an impaired ability to activate prodefensins and to kill exogenous bacteria in their small intestines (413).
Antimicrobial Activities
AMPs have a wide spectrum of microbicidal activities against a wide variety of gram-negative and gram-positive bacteria, fungi, protozoa, and even enveloped viruses. AMPs either induce membrane damage that is a lethal event for target bacteria or bind to several targets in the cytoplasmic region of the bacteria. All the evidence indicates that the action of the AMPs does not involve stereospecific protein-receptor recognition, since the interactions of AMPs with their targets are generally considered to be nonspecific. To a large extent, biophysical studies have been performed using membrane model systems demonstrating that AMPs use several distinctive different mechanisms to kill bacteria (185, 226, 305). The amino acid composition, amphipathicity, cationic charge, and size of AMPs allow them to attach to and insert into membrane bilayers to form pores by “barrel-stave,” “carpet-like,” or “toroidal-pore” mechanisms. It has been demonstrated that the tridisulfide structure of mature α- and β-defensins was essential for the microbicidal activity of the folded molecules. These defensins are microbicidal at concentrations in a range of 0.5 to 5 μM. Various isoforms of hBD-1 showing bactericidal or basteriostatic activities exist. Studies of the microbicidal effect of α-defensins HNP1 to HNP3 have provided evidence that bacterial inner and outer membranes are permeabilized as the consequence of voltage-dependent channels created by the AMP. LL-37, a cationic, amphipathic α-helical AMP, targets the bacterial membrane, destroys the chemical gradients over the membrane by forming stable or transient pores (152, 153) and produces a detergent-like effect via a “carpet-like” mechanism (295). However, it has recently been speculated that transmembrane pore formation may not be the only mechanism by which AMPs kill microbes. In fact, several observations suggest that translocated AMPs can alter cytoplasmic membrane septum formation, reduce the synthesis of the cell wall, nucleic acid, and protein, and inhibit enzymatic activity. It should be noted that some AMPs also display lytic activity against various eukaryotic cells, but these AMPs have two distinct physical states of binding to lipid bilayers (169).
Recent observations indicate that in response to attack by pathogenic bacteria, the host engages its front line of chemical defense by increasing the production of AMPs, such as the α- and β-defensins (16, 17). Ayabe et al. (17) report that LPS, LTA, lipid A and muramyl dipeptide were all able to elicit cryptdin secretion. In HD-5 transgenic mice, in which endogenous enteric defensin gene expression has been found in Paneth cells, there is a marked resistance to an oral challenge with virulent S. enterica serovar Typhimurium (337). It has been recently reported that expression of LL-37/hCAP-18, a human cathelicidin antimicrobial peptide, by gene transfer into C57BL/6 mice results in an increase in the innate immune response, providing support for the hypothesis that vertebrate antimicrobial peptides provide protection against microorganisms in vivo (22). The cathelicidin-related antimicrobial peptide, the only murine cathelicidin to be expressed in the intestinal tract, displays antimicrobial activity against the murine enteric pathogen Citrobacter rodentium, which produces lesions in the intestinal cells similar to those produced by EPEC and enterohemorrhagic E. coli (EHEC) (173). Indeed, greater penetration of C. rodentium into the colonic mucosa occurs in cathelicidin-knockout mice. Moreover, infection of HCA-7 cells with S. enterica serovar Dublin or enteroinvasive E. coli modestly upregulated hLL-37 mRNA expression (148). The expression, regulation, and production of AMPs in human intestinal epithelial cells are modulated in response to LPS and enteric pathogens. Although TLR-mediated β-defensin expression has been best investigated in lung tissues (114), LPS- and peptidoglycan-stimulated hBD-2 production by activation of TLR4 and TLR2 in cell lines that constitutively or transgenically express TLRs has been reported (399). Moreover, S. enterica serovar Enteritidis flagellin using TLR5 and gangliosodes as coreceptors increases hBD-2 expression in Caco-2 cells (291, 292, 372). A mutation in the NF-κB or AP-1 site within the hBD-2 promoter eliminated this response. In addition, inhibition of Jun kinase prevents the up-regulation of hBD-2 protein expression in response to LPS. It has been found that human colon epithelial cell lines constitutively express hBD-1 mRNA and protein but not hBD-2 (294). In contrast, the expression of cathelicidin hLL-37 mRNA is not upregulated in response to tumor necrosis factor alpha (TNF-α), IL-1α, gamma interferon, LPS, or IL-6 (148). Caco-2 cells produce two hBD-1 isoforms and an hBD-2 peptide that is bigger than previously reported hBD-2 isoforms. Interestingly, hBD-2 expression is rapidly induced by infecting human colon epithelial Caco-2 cells with S. enterica serovar Enteritidis, S. enterica serovar Typhimurium, and S. enterica serovar Typhi. S. enterica serovar Dublin induced hBD-2 mRNA expression in human carcinoma cells, and hBD-2 expression, but not hBD-1, is up-regulated in xenografts infected intraluminally with Salmonella (291, 294). The flagellar filament structural protein FliC of S. enteritidis has been identified as inducing hBD-2 expression in Caco-2 cells via NF-κB activation (291, 292, 372). The myeloid ELF-1-like factor (MEF) is involved in innate immunity responses, such as the activation of perforin and lysozyme transcription (368, 370), and also increased the level of endogenous hBD-2 transcription (229, 369). In addition, it is interesting that elevated levels of hBD-2 and hBD-3 transcripts have been found in Helicobacter pylori-infected gastric cells (20, 122, 143, 400, 401, 408).
Enteric pathogens have developed sophisticated strategies to survive in the gastrointestinal tract by evading the host defenses. It is significant that some of the major enteric pathogens have developed resistance to AMPs as a way of evading innate mucosal defenses. Bacterial pathogens have evolved counter-measures to limit the effectiveness of AMPs, including the repulsion of AMPs by reducing the net negative charge of the bacterial cell envelope through covalent modification of anionic molecules; expelling AMPs by means of energy-dependent pumps; altering membrane fluidity; and cleaving AMPs with proteases (309). Oral inoculation of mice with wild-type S. enterica serovar Typhimurium results in a decrease in the expression of α-defensins and lysozyme (336). Moreover, the expression of antibacterial peptides LL-37 and hBD-1 has been found to be reduced in biopsy specimens from patients with bacillary dysenteries and in Shigella-infected cultures of epithelial cells (177). Moreover, the intracellular survival of Salmonella depends on the bacterium's ability to resist the activity of cationic AMPs within the phagolysosome (128, 137, 266). Indeed, S. enterica serovar Typhimurium can sense sublethal concentrations of AMPs and induces various mechanisms of AMP resistance. The Salmonella PhoP/PhoQ regulators sense host environments to promote remodeling of the bacterial envelope that results in the modification in LPS-promoting bacterial survival by increasing resistance to AMPs, and by altered host recognition of LPS (97, 135, 136, 138, 139, 279, 353, 354). In particular, it has been observed that sublethal concentrations of AMPs activate the PhoP/PhoQ and RpoS virulence regulons, while repressing the transcription of genes required for flagellum synthesis, for the invasion-associated, type III secretion system, and for inducing RpoS-dependent protection against hydrogen peroxide (19). It should be noted that the intestinal production of the antimicrobial agent nitric oxide (104) generated by the inducible nitric oxide synthase that mediated the conversion of l-arginine to l-citrulline (95) is stimulated following infection by certain enteric pathogen including invasive E. coli and S. enterica serovar Dublin (414). Interestingly, it has been recently demonstrated that EPEC infection in Caco-2 cells can inhibit the inducible nitric oxide synthase expression at transcriptional and posttranscriptional levels by direct and indirect type III secretion system-dependent mechanisms (240).
RESIDENT MICROBIOTA
The gastrointestinal tract is a complex ecosystem that associates a resident microbiota (27, 230, 422) and cells of various phenotypes lining the epithelial wall (Fig. 1). The term “microbiota” was defined by Savage (341) to describe the collective societies of bacteria assembled on the mucosal surfaces of an individual. Mammals are born without these microorganisms (233).
Species Composition
The resident microbiota in the digestive tract constitutes a heterogeneous microbial ecosystem containing up to 1 × 1014 CFU of bacteria (27, 144, 230, 274, 376, 394). Resident bacteria localize “off-shore” from the epithelial cells within the mucus and seem to be content to catabolize mucin components (Fig. 1). Aerobic, facultative, and anaerobic bacteria all form part of the gastrointestinal microbiota. The microbial profile of the digestive tract is typified by the absence of anaerobic microorganisms in the stomach and, conversely, their overwhelming predominance in the distal colon. The proportion of anaerobic bacteria gradually increases from the proximal to distal regions, and 99% of the inhabitants located in the large intestine are anaerobes. Moreover, facultative anaerobes tend to associate along the epithelial layer, where oxygen diffusing from the tissues can be efficiently utilized. This is crucial for E. coli and probably also for other organisms. Different microbial communities may be located in the intestinal lumen, in the mucus covering the epithelium, in the crypt spaces and in the various cells lining the epithelium, and in addition, some species adhere, whereas others do not. It has been estimated that there are about more than 400 bacterial species in the intestinal microbiota. Currently, only 20 to 40% of the bacterial species present in the gastrointestinal tract have been cultured or characterized due in particular to the precise oxygen requirements of some species, the largely unknown nutrient requirements for growth and the fact that some species develop for growth a high level of mutualism, since in the microbiota, they live in close proximity and benefit from one another. Molecular biological methods help in analyzing the structural and functional complexity of the microflora and in identifying its components. Identification of the species present in the gastrointestinal microbiota is in progress, as a result of the introduction of higher resolution molecular techniques based on 16S rRNA or rRNA genes, and of technological innovations, such as the selective media that now make it possible to grow bacteria that could not previously be cultured (243, 376).
The colonization of gastrointestinal tract starts immediately at birth. In adults, the intestinal microbiota consists of an enormous biomass of >100,000 billion bacteria. The compositions of the bifidobacterial microbiotas differ in infants and adults and indeed during other stages in the host's life (166). For example, the fecal microbiota of children has been found to be bacteriologically less complex, whereas advancing age is associated with a decrease in bifidobacteria and increasing species diversity of the Bacteroides genus. It has been postulated that changes in the microbial composition of the gut with age may alter the metabolic capacity of the gut microbiota and that this has important implications for the occurrence of disease. The intestinal microbiota, which can be considered to be a postnatally acquired organ, is composed of a wide diversity of bacteria that perform important functions for the host and can be modulated by environmental factors, such as nutrition (40, 87, 94, 103). The first bacteria to colonize the gut originate in the birth canal, and include both aerobic and anaerobic bacteria, such as E. coli, Clostridium spp., Streptococcus spp., Lactobacillus spp., Bacteroides spp., and Bifidobacterium spp. The upper part of the small intestine has relatively low bacterial densities and the distal portion of the small intestine, the ileum, shows higher bacterial densities. The lower intestine is colonized predominantly by anaerobes, particularly the Bacteroides spp., bifidobacteria, fusobacteria, and peptostreptococci, and aerobes and facultative aerobes such as Enterobacteriaceae and lactobacilli are present at moderate densities. Analyzing the E. coli commensal microbiota, Escobar-Paramo et al. (98) have observed that the E. coli isolates of intercontinental populations distribute into the four phylogenetic groups A, B1, D, and B2 with major differences between the geographical populations. Lactobacillus and Bifidobacterium spp., all of which are autochthonous species in the intestinal microbiota, have attracted interest (248, 274, 375). Reuter (326) has recently gained new insights into the species of these microorganisms that are present within the human intestinal microbiota. In humans, the autochthonous Lactobacillus and Bifidobacterium remain stable throughout life. Lactobacillus gasseri and L. reuteri are predominant autochthonous Lactobacillus species in both infants and adults. Marked interindividual variations have been found in microbial composition at the genus and species levels (166). The compositions of the bifidobacterial microbiota differ in infants and adults and during different stages of the host's life (326). Species typically found in infants are Bifidobacterium bifidum, B. infantis, B. breve, and B. parvulorum. According to Matsuki et al. (243), the Bifidobacterium catenulatum group is the most commonly found taxon, followed by B. longum and B. adolescentis, in the adult intestinal bifidobacterial flora, and B. breve, B. infantis, and B. longum are frequently found in the intestinal tracts of infants.
Intestinal Functions
The intestinal microbiota plays an important role in normal gut function and in maintaining host health. All the components of the gastrointestinal ecosystem seem to be necessary for the gut to develop its specific intestinal functions (249, 422). Little is known about how members of the indigenous microbiota interact with their mammalian hosts to establish mutually beneficial relationships. Midtvedt et al. and Gordon et al. (49, 102, 158-165, 227, 365, 415) have recently gained important new insights into the mechanism by which members of the intestinal microbiota influence intestinal functions by means of cross talk with epithelial cells. For example, some observations lend support to the hypothesis that the capacity for synthesizing diverse carbohydrate structures may have arisen in part from our need both to evade pathogenic relationships and to coevolve in symbiotic relationships with our nonpathogenic resident microbes (161). The intraluminal microbiota influences the release of biologically active gastrointestinal peptides, and contributes to regulating gastrointestinal endocrine cells and the epithelial structure (384). Bacteroides thetaiotaomicron is one such bacterial symbiont that is a dominant member of the intestinal microbiota of mammals, including human beings (70, 159). Colonization of germfree mice by B. thetaiotaomicron VPI-5482, a component of the intestinal flora, has revealed that this commensal bacterium modulates the expression of genes involved in several important intestinal functions, including nutrient absorption, mucosal barrier fortification, xenobiotic metabolism, angiogenesis, and postnatal intestinal maturation (160, 164). The colonization of germfree mice with the VPI-5482 strain of B. thetaiotaomicron restored the fucosylation program, whereas an isogenic strain carrying a transposon insertion that disrupts its ability to use l-fucose as a carbon source did not (49, 165). Colonization of germfree mice with B. thetaiotaomicron has shown how this anaerobe modifies many aspects of intestinal cellular differentiation/gene expression to the benefit of both the host and the microbe (162). In line with this observation, comparison of gut glycosylation patterns in germfree and conventional mice have revealed both quantitative and qualitative differences in the cellular and subcellular distribution of glycans (111). It has been observed that this strain also has the capacity for changing the galactosylation process in cultured human mucin-secreting HT29-MTX cells as a result of posttranslational regulation, via a mechanism that involves a soluble, heat-labile, low-molecular-weight factor (112). Interestingly, in colonized germfree mice, a strain of B. thetaiotaomicron increased the production of matrilysin (227), a matrix metalloprotease expressed in Paneth cells and shown to be involved in innate host defense, as matrilysin-null mice have an impaired ability to activate prodefensins and to kill exogenous bacteria in their small intestines (413). It has also been reported that the normal colonization of the mammalian intestine with commensal microbes influences the development of the humoral and cellular mucosal immune systems during neonatal life and maintains the physiologically normal steady state of inflammation in the gut throughout life (56, 373). In connection with microbiota, it has been observed that the introduction of germfree mice into a conventional environment results in the enhanced expression and secretion of the goblet cell-specific protein RELMβ, providing evidence that colon-specific gene expression can be regulated by colonization with normal enteric bacteria (150).
The host is highly adapted to the presence of commensal intestinal bacteria by a phenomenon termed “mucosal immune adaptation.” In addition, a second adaptive phenomenon termed “systemic immune ignorance” has been investigated (196, 251, 253, 254). McPherson and Uhr (256) have showed that commensal bacteria are rapidly killed by macrophages and intestinal dendritic cells (DCs) can retain small numbers of live commensals for several days. This allows DCs to selectively induce immunoglobulin A through a pathway that was independent of T-cell help and of follicular lymphoid tissue organization, which helps protect against mucosal penetration by commensals and the specific anticommensal immunoglobulin A induction (251, 256). Because DCs loaded with commensal bacteria do not penetrate further than the mesenteric lymph nodes, immune induction to commensals is confined to the mucosa, which ensures that immune responses to commensal bacteria are induced locally, without potentially damaging systemic immune responses (252, 255). However, the resident microflora contains a number of components able to activate innate and adaptive immunity (288). In consequence, immune responses to mucosal microbiota require a precise regulatory control and unlimited immune activation in response to signals from commensal bacteria could pose the risk of inflammation (193, 194, 196, 250). Importantly, resident microbiota bacteria are recognized to suppress unnecessary inflammatory response, thereby helping to maintain immune homeostasis (194). An improved understanding of commensal bacteria-host interactions has been obtained employing germfree animal models with selective colonization strategies combined with modern molecular techniques. For example, the potential role of the intestinal microbiota in facilitating the development of tissue injury and systemic inflammation has been examined by Souza et al. (360) showing that there was marked edema formation, hemorrhage, and production of tumor necrosis factor alpha (TNF-α) and monocyte chemoattractant protein 1 in intestine of conventional mice compared with germfree mice. Moreover, pathogenic E. coli organisms, including EPEC (426), enteroaggregative E. coli (147, 198), and EHEC (28, 198), and nonpathogenic organisms, including diffusely adhering E. coli (34), commensal E. coli strain MG1655 (24), and B. vulgatus (142), have been observed to be able to promote activation of NF-κB nuclear translocation and, thereafter, proinflammatory gene expression in intestinal cells. Generally, only Lactobacillus spp. were not able to promote proinflammatory response; however, in the presence of underlying leukocytes, challenge of Caco-2 cells with L. sakei induces expression of IL-8, monocyte chemoattractant protein 1, IL-1β, and TNF-α mRNA (141). Interestingly, it has been recently demonstrated that commensal bacteria could inhibit the proinflammatory responses (40, 343). For example, Kelly et al. (193, 195) have shown that B. thetaiotaomicron inhibits proinflammatory cytokine IL-8 expression (195) and attenuates the flagellated pathogen-induced proinflammatory cytokine expression by promoting nuclear export of NF-κB subunit RelA, through a peroxisome proliferator-activated receptor-γ-dependent pathway (193). Similar inhibition has been observed with the Lactobacillus acidophilus strain LB of intestinal microbiota origin against the Salmonella-induced IL-8 expression (66). Moreover, the B. breve strain BbC50 isolated from the fecal flora of a healthy breast fed infant has been found able to display a TNF-α inhibitory capacity (259). The host appears also adapted to the deleterious effects promoted by commensal intestinal bacteria. Indeed, alterations in the intestinal barrier that resemble those promoted by enteric pathogens have been observed induced by species of the intestinal microbiota. For example, the E. coli strain EM0, a human fecal strain expressing hemolysin and cytotoxic necrotising factor, induced a lytic effect against cultured human intestinal cells (170). The prototype translocating E. coli strain C25 isolated from human feces, induces a loss of transepithelial electrical resistance, changes in distribution of TJ-associated proteins ZO-1 and claudin-4, and vacuolation of mitochondria (421). Observation that these deleterious effects were not promoted by the commensal E. coli strain F18 (421) is indicative that only certain strains of the intestinal microbiota have the capacity for developing pathogen-like effects. It is possible that species of the intestinal microbiota, including Lactobacillus, function as regulators against the pathogen-like commensal strains since, as described below, they have the capacity for blocking the pathogen-induced deleterious effects in host cells.
Barrier Effect against Pathogens
One of the basic physiological functions of the resident microbiota is that of providing a microbial barrier against microbial pathogens (Table 3). For exemple, Nicaise et al. (283) have recently documented the mechanism(s) of the immune response of the intestinal microbiota by examining the regulation of interleukin-1 (IL-1), IL-6, TNF-α, and IL-12 production in macrophages from germfree and from flora-associated mice, conventional, conventionalized and E. coli-monoassociated mice. The findings show that the intestinal flora can modulate bone marrow and spleen macrophage cytokine production in a differential manner. Enhanced IL-12 production in the spleen by the intestinal flora is also potentially important, since this cytokine is implicated in determining the relative levels of Th1 and Th2 responses, and plays a pivotal role in defending the host against intracellular microorganisms. Recent reports have provided new insights into how members of the intestinal microbiota develop a barrier effect and produce antimicrobial activity against enteropathogens.
TABLE 3.
Bacterial strains of microbiota origin exerting antibacterial effects against intestinal pathogens
| Strain(s) | Reference(s) |
|---|---|
| E. coli Nissle 1917 producing microcins | 7, 43 |
| E. coli strains producing microcins | 2, 25, 39, 84, 320, 328, 333, 335, 397 |
| E. coli strains not producing microcins | 170 |
| Peptostreptococcus strain | 325 |
| Ruminococcus gnavus E1 | 78, 126 |
| Clostridium cocleatum | 45 |
| L. acidophilus LB | 57, 62, 63, 65, 66, 68, 219 |
| L. johnsonii La1 | 30, 32, 106 |
| L. casei DN-114001 | 106, 132, 175, 304 |
| L. casei Shirota YT9029 | 13, 85, 106, 289, 290 |
| L. rhamnosus GG | 106, 171, 211, 232, 357 |
| L. plantarum 299v | 232, 238 |
| L. acidophilus HN017 | 127 |
| L. rhamnosus DR20 | 127 |
| Bifidobacterium strains | 29, 69, 218 |
Cecal microflora of hamster is able to develop an anti-C. difficile barrier effect (367). Interestingly, a C. cocleatum strain has been found involved in this anti-C. difficile barrier effect (45). Ramare et al. (325) have observed that when a human intestinal strain of Peptostreptococcus colonized the gut of gnotobiotic rats, it produced an antibacterial substance that was active against several gram-positive bacteria, including potentially pathogenic Clostridium spp. such as C. perfringens, C. difficile, C. butyricum, C. septicum, and C. sordellii. Similarly, the E1 strain of Ruminococcus gnavus, a gram-positive strictly anaerobic strain isolated from a human fecal sample, was able to produce an antibacterial substance, known as ruminococcin A, that is also active against various pathogenic clostridia (78, 126). As previously reported for some AMPs, including HD-5 (77, 124) and some cathelicidins (212), it is interesting that two antibacterial substances produced by bacterial species in the intestinal microbiota, Peptostreptococcus sp. (325) and the R. gnavus E1 strain (78, 126), require processing to be activated, after the proforms have been cleaved by trypsin.
It has been demonstrated that strains of E. coli of intestinal microbiota origin have the capacity for protecting mice against bacterial infection (Table 3). E. coli contributes to the antibacterial defense by producing antibacterial proteins, known as colicins and microcins (83, 327). Microcins are a miscellaneous group of low-molecular-mass antibiotics (molecular mass less than 10 kDa), whereas colicins are much bigger, from 25 to 80 kDa. All colicins and some microcins are encoded by gene clusters organized in operons, whereas other microcins are encoded on the chromosome of produced bacteria (275). All the bacteria encoding microcins or colicins have immunity towards the antibiotics that they produce. Colicin immunity is specific, but in some cases, other mechanisms are also involved, such as pumping microcin out of the cells. The bactericidal spectrum of activity was found to be restricted to Enterobacteriaceae and specifically directed against Escherichia (333) and Salmonella (320, 397) species. The microcin inserts into the inner membrane, whereupon the potential becomes destabilized due to pore formation that leads to depolarization and permeabilization of the E. coli cytoplasmic membrane (25, 84, 328). Another mechanism of antibacterial activity has been reported for E. coli strain Nissle 1917 (129) that produces microcins (7). This E. coli strain induces the expression of hBD-2 in Caco-2 intestinal epithelial cells in a time- and dose-dependent manner (407). This induction results of the activation of the hBD-2 promoter involving functional binding sites for NF-κB and AP-1 via a signaling pathway involving c-Jun N-terminal kinase, p38 mitogen-activated protein kinase, and signal-regulated kinase 1/2. It is interesting that, as reported above for AMPs, microcins have generated mechanisms of resistance in Salmonella (53, 109). It should be noted that Hudault et al. (170) have shown that resident E. coli that did not produce microcin had also a barrier effect when colonizing the gut of gnotobiotic C3H/He/Oujco mice orally infected by a lethal strain of S. enterica serovar Typhimurium.
Lactobacillus and Bifidobacterium spp. of human intestinal microbiotic origin produce antimicrobial substances that are active in vitro and in vivo against enterovirulent microorganisms involved in diarrhea disorders (Table 3) (349). For example, Lactobacillus acidophilus LB, L. johnsonii La1, L. rhamnosus GG, L. casei Shirota YT9029, L. casei DN-114 001, L. acidophilus HN017, and L. rhamnosus DR20 strains produced antibacterial components that are active against a wide range of gram-negative and gram-positive pathogens, such as EPEC, EHEC, L. monocytogenes, S. enterica serovar Typhimurium, and S. flexneri (32, 63, 65, 106, 127, 171). Moreover, antibacterial components produced by L. acidophilus strain LB were able to inhibit the growth of S. enterica serovar Typhimurium residing intracellularly in a vacuole in infected intestinal Caco-2 cells (66). These components, although not characterized at the molecular level, do not share the characteristics of bacteriocins and are different from lactic acid (106). L. rhamnosus GG secretes a low-molecular-mass, heat-stable, inhibitory substance which is distinct from lactic and acetic acids (357). The molecules that support the antibacterial activity of L. acidophilus LB and L. johnsonii La1 have a low molecular mass and are heat stable and insensitive to proteases (32, 65). An antibacterial component produced by human Bifidobacterium sp. CA1 and F9 strains has been found to consist of one or more lipophilic molecule(s) with a molecular mass of less than 3,500 Da (218). A mechanism by which non-lactic acid molecules secreted by Lactobacillus may kill gram-negative pathogens has recently been identified (68). Evidence showing that the bacterial membrane damage induced by the nonbacteriocin, non-lactic acid molecule(s) produced by the L. acidophilus LB of human intestinal microbiotal origin are lethal for S. enterica serovar Typhimurium has been provided. The mechanism of action includes (i) the depletion of intracellular ATP, (ii) an increase in membrane permeabilization, (iii) the release of LPS from the bacterial membrane, and (iv) the sensitization of the bacterial membrane towards the lytic action of detergent. The mechanism by which L. acidophilus LB kills S. enterica serovar Typhimurium resembles the mechanism by which AMPs and several classes of antibiotics kill bacteria. Indeed, intracellular K+ and ATP depletion have also been observed in EHEC strain O157:H7 subjected to AMPs (10). Moreover, it has been reported that a release of LPS from the membrane of gram-negative pathogens is triggered by several antibiotics (99, 179, 278, 381, 393). Since AMPs are discharged from Paneth cells at effective microbiocidal concentrations into the small intestinal crypts (116-118), it is tempting to suggest that some commensal intestinal bacteria, including E. coli and Lactobacillus, may discharge antimicrobial substance(s) into ecological niches within the intestine and thus also contribute to the front line of the chemical defense against enteric pathogens. In addition, metabolic end products of resident microbiotic bacteria could have an antimicrobial effect and so may potentiate the effects of other enteric antimicrobial substances, such as those produced by members of the microbiota and/or AMPs.
Importantly, it has been demonstrated that Lactobacillus and Bifidobacterium strains of intestinal microbiota origin that exert in vitro antimicrobicidal activities have the capacity for combatting infection in rodent models infected with human enterovirulent bacteria. The first model used is that of gnotobiotic mice, in which the microbiota is missing and the epithelium is not fully differentiated. The L. johnsonii La1 (32) and GG (171) strains, which colonize the gut of gnotobiotic C3H/He/Oujco mice, develop antibacterial activity when the mice are orally infected by S. enterica serovar Typhimurium C5, and this increases the survival of the mice. The human Bifidobacterium sp. CA1 and F9 bacteria that colonize the intestinal tract of axenic C3/He/Oujco mouse protect the mice against a lethal infection of S. enterica serovar Typhimurium C5 (218). The second model used is that of conventional mice, which have both a microbiota and a fully differentiated epithelium. In this mouse model, the spent culture supernatant of the human L. acidophilus strain LB, which contains an antibacterial molecule(s), given daily following infection is active against S. enterica serovar Typhimurium C5 infection in conventional C3H/He/Oujco mice, reducing the levels of viable Salmonella in the feces (65; D. Fayol-Messaoudi, M.-H. Coconnier-Polter, V. Lievin-Le Moal, C. N. Berger, and A. L. Servin, unpublished data).
Inhibition of Pathogen-Host Cell Interactions and Pathogen-Induced Cell Injuries
Bacteria that originated from the intestinal microbiota have the capacity for interfering with or block the process of pathogenicity of enteric bacterial pathogens. E. coli strain Nissle 1917 that produces microcin, was able to inhibit invasion of epithelial intestinal INT407 cells by Salmonella spp., S. flexneri, and L. monocytogenes without affecting the viability of the invasive bacteria (7). This E. coli strain, independently of the microcin production, is able to block the invasion process of the Crohn's disease-associated adherent-invasive E. coli LF82 (43). Similarly, the DN-114 001 strain of L. casei, independently of this bactericidal effect, strongly inhibits interaction of adherent-invasive E. coli LF82 with intestinal epithelial cells (175). It has been reported that Lactobacillus inhibited the internalization of S. enterica serovar Typhimurium within human intestinal cells, and this effect had been attributed to a secreted molecule(s) that could affect the virulence factors involved in cell entry and/or block the host cell signal transduction necessary for bacterial internalization (32, 63, 65, 66). An identical effect has been reported for Bifidobacterium strains isolated from stools of infants (29, 218). The mechanism by which some of the molecules produced by Lactobacillus strains impair the internalization process has been recently identified (Fayol-Messaoudi et al., unpublished). Indeed, compound(s) secreted by L. johnsonii La1 and L. casei Shirota YT9029 strains impair flagellum motility function in S. enterica serovar Typhimurium, and this in turn reduces the capacity of the pathogen to penetrate into human intestinal cells. This finding is consistent with the fact that the flagella that provide the motility of Salmonella (5, 234) have been found to be involved in bacterial internalization within eukaryotic cells (88, 189, 209, 225, 306, 329, 330, 386, 387). It has been observed that the expression of the FliC protein composing the full-length filament of the flagellum (234) is not modified, suggesting that the molecule(s) produced by Lactobacillus affect(s) the functionality of S. enterica serovar Typhimurium flagella. Interestingly, the Salmonella flagellum-dependent production of the proinflammatory cytokine IL-8 (88, 92, 123) is blocked by L. acidophilus LB.
Lactobacillus strains of intestinal microbiota origin have the capacity for inhibiting the cellular lesions induced by enteric pathogens within the intestinal epithelial barrier. For example, L. acidophilus LB (219) and L. helveticus R0052 (352) antagonized the cytoskeleton rearrangements produced by enterovirulent E. coli in T84 and Caco-2 cells. The decrease in brush border expression of sucrase-isomaltase, dipeptidylpeptidase IV, alkaline phosphatase, and fructose transporter induced by the diffusely adhering Afa/Dr E. coli C1845 in Caco-2 cells was inhibited by the L. acidophilus strain LB (219). L. helveticus strain R0052 (352), L. plantarum 299v (238, 263), and L. casei DN-114 001 (304) all reduce the pathogen-induced drop in transepithelial electrical resistance in cultured colonic T84 cells forming monolayer infected by EHEC and EPEC.
CONCLUDING REMARKS
Mucins, AMPs, and members of the intestinal microbiota all separately provide an effective front line of intestinal defense against unwelcome harmful microorganisms (Fig. 1 and 2). Mucins create a dynamic physical barrier, while Paneth cells and intestinal microbiota produce AMPs and antimicrobial molecules, respectively, which have the effect of killing enteric pathogens and inhibiting pathogen-host cell interaction. Whether these systems of defense act in partnership, and whether they function synergistically to provide the host with an efficient front line of defense against harmful, enteric pathogens, remains currently poorly documented.
It is conceivable that the resident intestinal bacteria may affect goblet cell dynamics and the mucus layer both directly, via the local release of bioactive factors, and indirectly, by activating host cells. This hypothesis has been investigated in a few studies. For example, it has been observed that the LPS of gram-native bacteria increases the expression of the mRNA of MUC5AC and MUC5B and stimulates the secretion of MUC5AC and MUC5B mucins (359). The quorum-sensing signal molecule [N-(3-oxododecanoyl) homoserine lactone (3O-C12-HSL)] of gram-negative bacteria could stimulate the production of a major mucin core protein, MUC5AC (174). Moreover, Lactobacillus organisms, subdominant species of the microbiota, increase the levels of expression of the mRNA of mucins MUC2 and MUC3 (231, 232). It seems likely that the AMPs may influence mucin secretion, since AMPs stimulate the secretion of chloride ions (298), and both chloride secretion and mucin exocytosis have been observed to be stimulated in mucin-secreting cells (140, 260, 262).
How the microbiota can influence AMP production remains controversial. Some reports suggest that in fact the microbiota has no influence. Indeed, in the intestine of germfree mice, the same set of mature enteric defensins (defensins 1, 2, 3, 4, and 6) has been found as in mice colonized by a normal microbiota (322). Moreover, the expression of cathelicidin hLL-37 by the colonic epithelium does not require the presence of commensal bacteria, since the peptide is produced with a similar pattern of expression by epithelial cells in human colon xenografts that have no luminal microbiota (148). In contrast, it has been reported that the intestinal commensal bacteria can influence gut microbial ecology and shape innate immunity. Indeed, angiogenin-4, a molecule with bactericidal activity produced by mouse Paneth cells, is induced by B. thetaiotaomicron, a dominant member of the gut microbiota (163). The B. thetaiotaomicron strain that colonizes the intestine of germfree mice increased the production of matrilysin (227), a matrix metalloprotease expressed in Paneth cells, and has been shown to be involved in innate host defense (413). It has recently been observed that Bifidobacterium or its cell wall proteins can induce AMP hBD-2 gene expression in cultured human intestinal epithelial cells (404). In response to components of gram-negative bacteria, such as LPS and peptidoglycan, hBD-2 expression is increased (399). Moreover, the flagellum filament structural protein FliC of gram-negative bacteria has been found to induce hBD-2 expression via an NF-κB-dependent mechanism (291, 292, 372). It remains to be determined whether activity by LPS or flagella of the resident intestinal E. coli could contribute to activating the production of AMPs, in turn regulating the intestinal microbiota.
It has recently been established that gram-negative pathogenic bacteria are able to sense both the cell density and the metabolic potential of their environment by a quorum-sensing system, which is a cell density-dependent signaling system used by bacteria to coordinate gene expression within a population (3, 101, 265, 371). In particular, in pathogenic E. coli, quorum-sensing involves a transcription regulator (LuxR homologue) and an autoinducer, either AI-2 or AI-3, depending on the function encoded by the luxS gene (8, 130, 190, 361-364). A quorum-sensing system in gram-positive bacteria has been identified (366). Autoinducing peptides are involved in intercellular communication in gram-positive bacteria, and many of these peptides are exported by dedicated systems and finally sensed by other cells via membrane-located receptors. The production-biosynthesis, maturation, and secretion of colicins or microcins (83, 327) into the medium by E. coli are encoded by gene clusters organized in operons that are silent/repressed during exponential growth and are induced/derepressed when cells sense nutrient starvation and stop their exponential growth (275). It could be of interest in the future to find out whether the E. coli strains that are resident in the intestinal microbiota possess a quorum-sensing system that senses the presence of these pathogens and in turn controls the production and secretion of antimicrobial molecules. For gram-positive bacterial members of the intestinal microbiota an identical investigation is of interest considering that the production of bacteriocins, a type of bactericidal proteinaceous molecules produced by Lactobacillus (178, 199, 247, 280, 334), has been found to be regulated at the transcriptional level in a manner dependent on cell-density (200) and appears to be controlled by a peptide-based, quorum-sensing system that drives strong, regulated promoters (236, 242). In addition, it has been reported recently that the production of nonbacteriocin, non-lactic acid antibacterial molecules that are active against gram-negative enteric pathogens is temperature controlled (106).
Further progress is required to enable us to understand how the three components of the front line of defense against enteric pathogens are coordinated and whether the various components modulate the functions of the others and/or act synergically.
Acknowledgments
We express our sincere thanks especially to Marie-Hélène Coconnier-Polter and all the members, past and present, of INSERM Unité 510, “Pathogènes et Fonctions des Cellules Epithéliales Polarisées,” for their contribution to elucidating the mechanisms of action of two of the three components of the front line of intestinal defense—mucins and microbiota—against enteric pathogens.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar, A., J. C. Perez-Diaz, F. Baquero, and C. Asensio. 1982. Microcin 15m from Escherichia coli: mechanism of antibiotic action. Antimicrob. Agents Chemother. 21:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmer, B. M. 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol. Microbiol. 52:933-945. [DOI] [PubMed] [Google Scholar]
- 4.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 5.Aldridge, P., and K. T. Hughes. 2002. Regulation of flagellar assembly. Curr. Opin. Microbiol. 5:160-165. [DOI] [PubMed] [Google Scholar]
- 6.Aldridge, P. D., M. A. Gray, B. H. Hirst, and C. M. Anjam Khan. 2005. Who's talking to whom? Epithelial-bacterial pathogen interactions. Mol. Microbiol. 55:655-663. [DOI] [PubMed] [Google Scholar]
- 7.Altenhoefer, A., S. Oswald, U. Sonnenborn, C. Enders, J. Schulze, J. Hacker, and T. A. Oelschlaeger. 2004. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 40:223-229. [DOI] [PubMed] [Google Scholar]
- 8.Anand, S. K., and M. W. Griffiths. 2003. Quorum sensing and expression of virulence in Escherichia coli O157:H7. Int. J. Food Microbiol. 85:1-9. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, J. M., M. S. Balda, and A. S. Fanning. 1993. The structure and regulation of tight junctions. Curr. Opin. Cell Biol. 5:772-778. [DOI] [PubMed] [Google Scholar]
- 10.Appendini, P., and J. H. Hotchkiss. 1999. Antimicrobial activity of a 14-residue peptide against Escherichia coli O157:H7. J. Appl. Microbiol. 87:750-756. [DOI] [PubMed] [Google Scholar]
- 11.Aristoteli, L. P., and M. D. Willcox. 2003. Mucin degradation mechanisms by distinct Pseudomonas aeruginosa isolates in vitro. Infect. Immun. 71:5565-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artavanis-Tsakonas, S., M. D. Rand, and R. J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science 284:770-776. [DOI] [PubMed] [Google Scholar]
- 13.Asahara, T., K. Nomoto, M. Watanuki, and T. Yokokura. 2001. Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob. Agents Chemother. 45:1751-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Athman, R., and D. Philpott. 2004. Innate immunity via Toll-like receptors and Nod proteins. Curr. Opin. Microbiol. 7:25-32. [DOI] [PubMed] [Google Scholar]
- 15.Augeron, C., T. Voisin, J. J. Maoret, B. Berthon, M. Laburthe, and C. L. Laboisse. 1992. Neurotensin and neuromedin N stimulate mucin output from human goblet cells (Cl. 16E) via neurotensin receptors. Am. J. Physiol. 262:G470-G476. [DOI] [PubMed] [Google Scholar]
- 16.Ayabe, T., T. Ashida, Y. Kohgo, and T. Kono. 2004. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol. 12:394-398. [DOI] [PubMed] [Google Scholar]
- 17.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 18.Bach, S. P., A. G. Renehan, and C. S. Potten. 2000. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis 21:469-476. [DOI] [PubMed] [Google Scholar]
- 19.Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50:219-230. [DOI] [PubMed] [Google Scholar]
- 20.Bajaj-Elliott, M., P. Fedeli, G. V. Smith, P. Domizio, L. Maher, R. S. Ali, A. G. Quinn, and M. J. Farthing. 2002. Modulation of host antimicrobial peptide (beta-defensins 1 and 2) expression during gastritis. Gut 51:356-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bals, R., D. J. Weiner, A. D. Moscioni, R. L. Meegalla, and J. M. Wilson. 1999. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 67:6084-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bals, R., and J. M. Wilson. 2003. Cathelicidins-a family of multifunctional antimicrobial peptides. Cell. Mol. Life Sci. 60:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bambou, J. C., A. Giraud, S. Menard, B. Begue, S. Rakotobe, M. Heyman, F. Taddei, N. Cerf-Bensussan, and V. Gaboriau-Routhiau. 2004. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J. Biol. Chem. 279:42984-42992. [DOI] [PubMed] [Google Scholar]
- 25.Bellomio, A., R. G. Oliveira, B. Maggio, and R. D. Morero. 2005. Penetration and interactions of the antimicrobial peptide, microcin J25, into uncharged phospholipid monolayers. J. Colloid Interface Sci. 285:118-124. [DOI] [PubMed] [Google Scholar]
- 26.Bensch, K. W., M. Raida, H. J. Magert, P. Schulz-Knappe, and W. G. Forssmann. 1995. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 368:331-335. [DOI] [PubMed] [Google Scholar]
- 27.Berg, R. D. 1996. The indigenous gastrointestinal microflora. Trends Microbiol. 4:430-435. [DOI] [PubMed] [Google Scholar]
- 28.Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-kappaB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 4:635-648. [DOI] [PubMed] [Google Scholar]
- 29.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1993. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl. Environ. Microbiol. 59:4121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1994. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Differentiation-associated antimicrobial functions in human colon adenocarcinoma cell lines. Exp. Cell Res. 226:80-89. [DOI] [PubMed] [Google Scholar]
- 32.Bernet-Camard, M. F., V. Lievin, D. Brassart, J. R. Neeser, A. L. Servin, and S. Hudault. 1997. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 63:2747-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertrand, C. A., C. L. Laboisse, and U. Hopfer. 1999. Purinergic and cholinergic agonists induce exocytosis from the same granule pool in HT29-Cl. 16E monolayers. Am. J. Physiol. 276:C907-C914. [DOI] [PubMed] [Google Scholar]
- 34.Betis, F., P. Brest, V. Hofman, J. Guignot, M. F. Bernet-Camard, B. Rossi, A. Servin, and P. Hofman. 2003. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect. Immun. 71:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bevins, C. L. 2004. The Paneth cell and the innate immune response. Curr. Opin. Gastroenterol. 20:572-580. [DOI] [PubMed] [Google Scholar]
- 36.Bevins, C. L., D. E. Jones, A. Dutra, J. Schaffzin, and M. Muenke. 1996. Human enteric defensin genes: chromosomal map position and a model for possible evolutionary relationships. Genomics 31:95-106. [DOI] [PubMed] [Google Scholar]
- 37.Bevins, C. L., E. Martin-Porter, and T. Ganz. 1999. Defensins and innate host defence of the gastrointestinal tract. Gut 45:911-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 39.Blond, A., M. Cheminant, D. Destoumieux-Garzon, I. Segalas-Milazzo, J. Peduzzi, C. Goulard, and S. Rebuffat. 2002. Thermolysin-linearized microcin J25 retains the structured core of the native macrocyclic peptide and displays antimicrobial activity. Eur. J. Biochem. 269:6212-6222. [DOI] [PubMed] [Google Scholar]
- 40.Blum, S., and E. J. Schiffrin. 2003. Intestinal microflora and homeostasis of the mucosal immune response: implications for probiotic bacteria? Curr. Issues Intest. Microbiol. 4:53-60. [PubMed] [Google Scholar]
- 41.Boman, H. G. 2000. Innate immunity and the normal microflora. Immunol. Rev. 173:5-16. [DOI] [PubMed] [Google Scholar]
- 42.Booth, C., and C. S. Potten. 2000. Gut instincts: thoughts on intestinal epithelial stem cells. J. Clin. Investig. 105:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudeau, J., A. L. Glasser, S. Julien, J. F. Colombel, and A. Darfeuille-Michaud. 2003. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment. Pharmacol. Ther. 18:45-56. [DOI] [PubMed] [Google Scholar]
- 44.Bou-Hanna, C., B. Berthon, L. Combettes, M. Claret, and C. L. Laboisse. 1994. Role of calcium in carbachol- and neurotensin-induced mucin exocytosis in a human colonic goblet cell line and cross-talk with the cyclic AMP pathway. Biochem. J. 299:579-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boureau, H., D. Decre, J. P. Carlier, C. Guichet, and P. Bourlioux. 1993. Identification of a Clostridium cocleatum strain involved in an anti-Clostridium difficile barrier effect and determination of its mucin-degrading enzymes. Res. Microbiol. 144:405-410. [DOI] [PubMed] [Google Scholar]
- 46.Boyle, E. C., and B. B. Finlay. 2003. Bacterial pathogenesis: exploiting cellular adherence. Curr. Opin. Cell Biol. 15:633-639. [DOI] [PubMed] [Google Scholar]
- 47.Bradbury, N. A. 2000. Protein kinase-A-mediated secretion of mucin from human colonic epithelial cells. J. Cell. Physiol. 185:408-415. [DOI] [PubMed] [Google Scholar]
- 48.Branka, J. E., G. Vallette, A. Jarry, C. Bou-Hanna, P. Lemarre, P. N. Van, and C. L. Laboisse. 1997. Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology 112:1887-1894. [DOI] [PubMed] [Google Scholar]
- 49.Bry, L., P. G. Falk, T. Midtvedt, and J. I. Gordon. 1996. A model of host-microbial interactions in an open mammalian ecosystem. Science 273:1380-1383. [DOI] [PubMed] [Google Scholar]
- 50.Buisine, M. P., P. Desreumaux, E. Leteurtre, M. C. Copin, J. F. Colombel, N. Porchet, and J. P. Aubert. 2001. Mucin gene expression in intestinal epithelial cells in Crohn's disease. Gut 49:544-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrd, J. C., and R. S. Bresalier. 2004. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 23:77-99. [DOI] [PubMed] [Google Scholar]
- 52.Byrd, J. C., C. K. Yunker, Q. S. Xu, L. R. Sternberg, and R. S. Bresalier. 2000. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology 118:1072-1079. [DOI] [PubMed] [Google Scholar]
- 53.Carlson, S. A., T. S. Frana, and R. W. Griffith. 2001. Antibiotic resistance in Salmonella enterica serovar Typhimurium exposed to microcin-producing Escherichia coli. Appl. Environ. Microbiol. 67:3763-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carraway, K. L., A. Perez, N. Idris, S. Jepson, M. Arango, M. Komatsu, B. Haq, S. A. Price-Schiavi, J. Zhang, and C. A. Carraway. 2002. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog. Nucleic Acid Res. Mol. Biol. 71:149-185. [DOI] [PubMed] [Google Scholar]
- 55.Carraway, K. L., V. P. Ramsauer, B. Haq, and C. A. Carothers Carraway. 2003. Cell signaling through membrane mucins. Bioessays 25:66-71. [DOI] [PubMed] [Google Scholar]
- 56.Cebra, J. J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S-1051S. [DOI] [PubMed] [Google Scholar]
- 57.Chauviere, G., M. H. Coconnier, S. Kerneis, A. Darfeuille-Michaud, B. Joly, and A. L. Servin. 1992. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from human enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol. Lett. 70:213-217. [DOI] [PubMed] [Google Scholar]
- 58.Chen, C. C., M. Baylor, and D. M. Bass. 1993. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology 105:84-92. [DOI] [PubMed] [Google Scholar]
- 59.Citi, S., and M. Cordenonsi. 1998. Tight junction proteins. Biochim. Biophys. Acta 1448:1-11. [DOI] [PubMed] [Google Scholar]
- 60.Clark, M. A., and M. A. Jepson. 2003. Intestinal M cells and their role in bacterial infection. Int. J. Med. Microbiol. 293:17-39. [DOI] [PubMed] [Google Scholar]
- 61.Clarke, L. L., L. R. Gawenis, E. M. Bradford, L. M. Judd, K. T. Boyle, J. E. Simpson, G. E. Shull, H. Tanabe, A. J. Ouellette, C. L. Franklin, and N. M. Walker. 2004. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am. J. Physiol. Gastr. Liver Physiol. 286:G1050-G1058. [DOI] [PubMed] [Google Scholar]
- 62.Coconnier, M. H., M. F. Bernet, G. Chauviere, and A. L. Servin. 1993. Adhering heat-killed human Lactobacillus acidophilus, strain LB, inhibits the process of pathogenicity of diarrhoeagenic bacteria in cultured human intestinal cells. J. Diarrhoeal Dis. Res. 11:235-242. [PubMed] [Google Scholar]
- 63.Coconnier, M. H., M. F. Bernet, S. Kerneis, G. Chauviere, J. Fourniat, and A. L. Servin. 1993. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 110:299-305. [DOI] [PubMed] [Google Scholar]
- 64.Coconnier, M. H., E. Dlissi, M. Robard, C. L. Laboisse, J. L. Gaillard, and A. L. Servin. 1998. Listeria monocytogenes stimulates mucus exocytosis in cultured human polarized mucosecreting intestinal cells through action of listeriolysin O. Infect. Immun. 66:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coconnier, M. H., V. Lievin, M. F. Bernet-Camard, S. Hudault, and A. L. Servin. 1997. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob. Agents Chemother. 41:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coconnier, M. H., V. Lievin, M. Lorrot, and A. L. Servin. 2000. Antagonistic activity of Lactobacillus acidophilus LB against intracellular Salmonella enterica serovar Typhimurium infecting human enterocyte-like Caco-2/TC-7 cells. Appl. Environ. Microbiol. 66:1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coconnier, M. H., M. Lorrot, A. Barbat, C. Laboisse, and A. L. Servin. 2000. Listeriolysin O-induced stimulation of mucin exocytosis in polarized intestinal mucin-secreting cells: evidence for toxin recognition of membrane-associated lipids and subsequent toxin internalization through caveolae. Cell. Microbiol. 2:487-504. [DOI] [PubMed] [Google Scholar]
- 68.Coconnier-Polter, M. H., V. Lievin-Le Moal, and A. L. Servin. 2005. A Lactobacillus acidophilus strain of human gastrointestinal microbiota origin elicits killing of the enterovirulent Salmonella enterica serovar Typhimurium by triggering lethal bacterial membrane damage. Appl. Environ. Microbiol. 71:6115-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collado, M. C., A. Gonzalez, R. Gonzalez, M. Hernandez, M. A. Ferrus, and Y. Sanz. 2005. Antimicrobial peptides are among the antagonistic metabolites produced by Bifidobacterium against Helicobacter pylori. Int. J. Antimicrob. Agents 25:385-391. [DOI] [PubMed] [Google Scholar]
- 70.Comstock, L. E., and M. J. Coyne. 2003. Bacteroides thetaiotaomicron: a dynamic, niche-adapted human symbiont. Bioessays 25:926-929. [DOI] [PubMed] [Google Scholar]
- 71.Corfield, A. P., D. Carroll, N. Myerscough, and C. S. Probert. 2001. Mucins in the gastrointestinal tract in health and disease. Front. Biosci. 6:D1321-D1357. [DOI] [PubMed] [Google Scholar]
- 72.Corfield, A. P., N. Myerscough, R. Longman, P. Sylvester, S. Arul, and M. Pignatelli. 2000. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut 47:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corrales, R. M., D. J. Galarreta, J. M. Herreras, M. Calonge, and F. J. Chaves. 2003. Normal human conjunctival epithelium expresses MUC13, MUC15, MUC16 and MUC17 mucin genes. Arch. Soc. Esp. Oftalmol. 78:375-381. [PubMed] [Google Scholar]
- 74.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. [DOI] [PubMed] [Google Scholar]
- 75.Crawley, S. C., J. R. Gum, Jr., J. W. Hicks, W. S. Pratt, J. P. Aubert, D. M. Swallow, and Y. S. Kim. 1999. Genomic organization and structure of the 3′ region of human MUC3: alternative splicing predicts membrane-bound and soluble forms of the mucin. Biochem. Biophys. Res. Commun. 263:728-736. [DOI] [PubMed] [Google Scholar]
- 76.Cunliffe, R. N. 2003. Alpha-defensins in the gastrointestinal tract. Mol. Immunol. 40:463-467. [DOI] [PubMed] [Google Scholar]
- 77.Cunliffe, R. N., F. R. Rose, J. Keyte, L. Abberley, W. C. Chan, and Y. R. Mahida. 2001. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 48:176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dabard, J., C. Bridonneau, C. Phillipe, P. Anglade, D. Molle, M. Nardi, M. Ladire, H. Girardin, F. Marcille, A. Gomez, and M. Fons. 2001. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl. Environ. Microbiol. 67:4111-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Debailleul, V., A. Laine, G. Huet, P. Mathon, M. C. d'Hooghe, J. P. Aubert, and N. Porchet. 1998. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. An improved method to analyze large mRNAs. J. Biol. Chem. 273:881-890. [DOI] [PubMed] [Google Scholar]
- 80.Dekker, J., J. W. Rossen, H. A. Buller, and A. W. Einerhand. 2002. The MUC family: an obituary. Trends Biochem. Sci. 27:126-131. [DOI] [PubMed] [Google Scholar]
- 81.Denker, B. M., and S. K. Nigam. 1998. Molecular structure and assembly of the tight junction. Am. J. Physiol. 274:F1-F9. [DOI] [PubMed] [Google Scholar]
- 82.Deplancke, B., and H. R. Gaskins. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73:1131S-1141S. [DOI] [PubMed] [Google Scholar]
- 83.Destoumieux-Garzon, D., J. Peduzzi, and S. Rebuffat. 2002. Focus on modified microcins: structural features and mechanisms of action. Biochimie 84:511-519. [DOI] [PubMed] [Google Scholar]
- 84.Destoumieux-Garzon, D., X. Thomas, M. Santamaria, C. Goulard, M. Barthelemy, B. Boscher, Y. Bessin, G. Molle, A. M. Pons, L. Letellier, J. Peduzzi, and S. Rebuffat. 2003. Microcin E492 antibacterial activity: evidence for a TonB-dependent inner membrane permeabilization on Escherichia coli. Mol. Microbiol. 49:1031-1041. [DOI] [PubMed] [Google Scholar]
- 85.de Waard, R., J. Garssen, G. C. Bokken, and J. G. Vos. 2002. Antagonistic activity of Lactobacillus casei strain shirota against gastrointestinal Listeria monocytogenes infection in rats. Int. J. Food Microbiol. 73:93-100. [DOI] [PubMed] [Google Scholar]
- 86.Dhaliwal, W., M. Bajaj-Elliott, and P. Kelly. 2003. Intestinal defensin gene expression in human populations. Mol. Immunol. 40:469-475. [DOI] [PubMed] [Google Scholar]
- 87.Diaz, R. L., L. Hoang, J. Wang, J. L. Vela, S. Jenkins, R. Aranda, and M. G. Martin. 2004. Maternal adaptive immunity influences the intestinal microflora of suckling mice. J. Nutr. 134:2359-2364. [DOI] [PubMed] [Google Scholar]
- 88.Dibb-Fuller, M. P., E. Allen-Vercoe, C. J. Thorns, and M. J. Woodward. 1999. Fimbriae- and flagella-mediated association with and invasion of cultured epithelial cells by Salmonella enteritidis. Microbiology 145:1023-1031. [DOI] [PubMed] [Google Scholar]
- 89.Didierlaurent, A., J. C. Sirard, J. P. Kraehenbuhl, and M. R. Neutra. 2002. How the gut senses its content. Cell. Microbiol. 4:61-72. [DOI] [PubMed] [Google Scholar]
- 90.Dohrman, A., S. Miyata, M. Gallup, J. D. Li, C. Chapelin, A. Coste, E. Escudier, J. Nadel, and C. Basbaum. 1998. Mucin gene (MUC 2 and MUC 5AC) upregulation by gram-positive and gram- negative bacteria. Biochim. Biophys. Acta 1406:251-259. [DOI] [PubMed] [Google Scholar]
- 91.Dommett, R., M. Zilbauer, J. T. George, and M. Bajaj-Elliott. 2005. Innate immune defence in the human gastrointestinal tract. Mol. Immunol. 42:903-912. [DOI] [PubMed] [Google Scholar]
- 92.Eaves-Pyles, T., K. Murthy, L. Liaudet, L. Virag, G. Ross, F. G. Soriano, C. Szabo, and A. L. Salzman. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248-1260. [DOI] [PubMed] [Google Scholar]
- 93.Eckmann, L. 2005. Defence molecules in intestinal innate immunity against bacterial infections. Curr. Opin. Gastroenterol. 21:147-151. [DOI] [PubMed] [Google Scholar]
- 94.Edwards, C. A., and A. M. Parrett. 2002. Intestinal flora during the first months of life: new perspectives. Br. J. Nutr. 88(Suppl. 1):S11-S18. [DOI] [PubMed] [Google Scholar]
- 95.Elliott, S. N., and J. L. Wallace. 1998. Nitric oxide: a regulator of mucosal defense and injury. J. Gastroenterol. 33:792-803. [DOI] [PubMed] [Google Scholar]
- 96.Epple, H. J., K. M. Kreusel, C. Hanski, J. D. Schulzke, E. O. Riecken, and M. Fromm. 1997. Differential stimulation of intestinal mucin secretion by cholera toxin and carbachol. Pflugers Arch. 433:638-647. [DOI] [PubMed] [Google Scholar]
- 97.Ernst, R. K., T. Guina, and S. I. Miller. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 3:1327-1334. [DOI] [PubMed] [Google Scholar]
- 98.Escobar-Paramo, P., K. Grenet, A. Le Menac'h, L. Rode, E. Salgado, C. Amorin, S. Gouriou, B. Picard, M. C. Rahimy, A. Andremont, E. Denamur, and R. Ruimy. 2004. Large-scale population structure of human commensal Escherichia coli isolates. Appl. Environ. Microbiol. 70:5698-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans, M. E., and M. Pollack. 1993. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J. Infect. Dis. 167:1336-1343. [DOI] [PubMed] [Google Scholar]
- 100.Fahlgren, A., S. Hammarstrom, A. Danielsson, and M. L. Hammarstrom. 2003. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin. Exp. Immunol. 131:90-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Falcao, J. P., F. Sharp, and V. Sperandio. 2004. Cell-to-cell signaling in intestinal pathogens. Curr. Issues Intest. Microbiol. 5:9-17. [PubMed] [Google Scholar]
- 102.Falk, P. G., L. V. Hooper, T. Midtvedt, and J. I. Gordon. 1998. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 62:1157-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fanaro, S., R. Chierici, P. Guerrini, and V. Vigi. 2003. Intestinal microflora in early infancy: composition and development. Acta Paediatr. Suppl. 91:48-55. [DOI] [PubMed] [Google Scholar]
- 104.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fasano, A., and J. P. Nataro. 2004. Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins. Adv. Drug Deliv. Rev. 56:795-807. [DOI] [PubMed] [Google Scholar]
- 106.Fayol-Messaoudi, D., C. N. Berger, M.-H. Coconnier-Polter, V. Lievin-Le Moal, and A. L. Servin. 2005. pH-, lactic acid- and non-lactic acid-dependent activities against Salmonella enterica serovar Typhimurium by probiotic strains of Lactobacillus. Appl. Environ. Microbiol. 71:6008-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reference deleted.
- 108.Forstner, G. 1995. Signal transduction, packaging and secretion of mucins. Annu. Rev. Physiol. 57:585-605. [DOI] [PubMed] [Google Scholar]
- 109.Frana, T. S., S. A. Carlson, D. C. Rauser, B. D. Jones, B. J. Fergen, and R. W. Griffith. 2004. Effects of microcin 24-producing Escherichia coli on shedding and multiple-antimicrobial resistance of Salmonella enterica serotype typhimurium in pigs. Am. J. Vet. Res. 65:1616-1620. [DOI] [PubMed] [Google Scholar]
- 110.Fre, S., M. Huyghe, P. Mourikis, S. Robine, D. Louvard, and S. Artavanis-Tsakonas. 2005. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435:964-968. [DOI] [PubMed] [Google Scholar]
- 111.Freitas, M., L. G. Axelsson, C. Cayuela, T. Midtvedt, and G. Trugnan. 2002. Microbial-host interactions specifically control the glycosylation pattern in intestinal mouse mucosa. Histochem. Cell Biol. 118:149-161. [DOI] [PubMed] [Google Scholar]
- 112.Freitas, M., C. Cayuela, J. M. Antoine, F. Piller, C. Sapin, and G. Trugnan. 2001. A heat labile soluble factor from Bacteroides thetaiotaomicron VPI-5482 specifically increases the galactosylation pattern of HT29-MTX cells. Cell. Microbiol. 3:289-300. [DOI] [PubMed] [Google Scholar]
- 113.Frisch, S. M., and R. A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13:555-562. [DOI] [PubMed] [Google Scholar]
- 114.Froy, O. 2005. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell. Microbiol. 7:1387-1397. [DOI] [PubMed] [Google Scholar]
- 115.Fukushima, K., I. Sasaki, H. Ogawa, H. Naito, Y. Funayama, and S. Matsuno. 1999. Colonization of microflora in mice: mucosal defense against luminal bacteria. J. Gastroenterol. 34:54-60. [DOI] [PubMed] [Google Scholar]
- 116.Ganz, T. 2005. Defensins and other antimicrobial peptides: a historical perspective and an update. Comb. Chem. High Throughput Screen. 8:209-217. [DOI] [PubMed] [Google Scholar]
- 117.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 118.Ganz, T. 2002. Epithelia: not just physical barriers. Proc. Natl. Acad. Sci. USA 99:3357-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ganz, T., M. E. Selsted, D. Szklarek, S. S. Harwig, K. Daher, D. F. Bainton, and R. I. Lehrer. 1985. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 76:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia, J. R., F. Jaumann, S. Schulz, A. Krause, J. Rodriguez-Jimenez, U. Forssmann, K. Adermann, E. Kluver, C. Vogelmeier, D. Becker, R. Hedrich, W. G. Forssmann, and R. Bals. 2001. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell. Tissue Res. 306:257-264. [DOI] [PubMed] [Google Scholar]
- 121.Gendler, S. J., and A. P. Spicer. 1995. Epithelial mucin genes. Annu. Rev. Physiol. 57:607-634. [DOI] [PubMed] [Google Scholar]
- 122.George, J. T., P. K. Boughan, H. Karageorgiou, and M. Bajaj-Elliott. 2003. Host anti-microbial response to Helicobacter pylori infection. Mol. Immunol. 40:451-456. [DOI] [PubMed] [Google Scholar]
- 123.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 124.Ghosh, D., E. Porter, B. Shen, S. K. Lee, D. Wilk, J. Drazba, S. P. Yadav, J. W. Crabb, T. Ganz, and C. L. Bevins. 2002. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat. Immunol. 3:583-590. [DOI] [PubMed] [Google Scholar]
- 125.Girardin, S. E., P. J. Sansonetti, and D. J. Philpott. 2002. Intracellular vs. extracellular recognition of pathogens-common concepts in mammals and flies. Trends Microbiol. 10:193-199. [DOI] [PubMed] [Google Scholar]
- 126.Gomez, A., M. Ladire, F. Marcille, and M. Fons. 2002. Trypsin mediates growth phase-dependent transcriptional regulation of genes involved in biosynthesis of ruminococcin A, a lantibiotic produced by a Ruminococcus gnavus strain from a human intestinal microbiota. J. Bacteriol. 184:18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gopal, P. K., J. Prasad, J. Smart, and H. S. Gill. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 67:207-216. [DOI] [PubMed] [Google Scholar]
- 128.Groisman, E. A., C. Parra-Lopez, M. Salcedo, C. J. Lipps, and F. Heffron. 1992. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11939-11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grozdanov, L., C. Raasch, J. Schulze, U. Sonnenborn, G. Gottschalk, J. Hacker, and U. Dobrindt. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 186:5432-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gruenheid, S., and B. B. Finlay. 2000. Crowd control: quorum sensing in pathogenic E. coli. Trends Microbiol. 8:442-443. [DOI] [PubMed] [Google Scholar]
- 131.Gruenheid, S., and B. B. Finlay. 2003. Microbial pathogenesis and cytoskeletal function. Nature 422:775-781. [DOI] [PubMed] [Google Scholar]
- 132.Guerin-Danan, C., J. C. Meslin, A. Chambard, A. Charpilienne, P. Relano, C. Bouley, J. Cohen, and C. Andrieux. 2001. Food supplementation with milk fermented by Lactobacillus casei DN-114 001 protects suckling rats from rotavirus-associated diarrhea. J. Nutr. 131:111-117. [DOI] [PubMed] [Google Scholar]
- 133.Gum, J. R., Jr. 1995. Human mucin glycoproteins: varied structures predict diverse properties and specific functions. Biochem. Soc. Trans. 23:795-799. [DOI] [PubMed] [Google Scholar]
- 134.Gum, J. R., Jr., S. C. Crawley, J. W. Hicks, D. E. Szymkowski, and Y. S. Kim. 2002. MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 291:466-475. [DOI] [PubMed] [Google Scholar]
- 135.Gunn, J. S. 2001. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 7:57-62. [PubMed] [Google Scholar]
- 136.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 137.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 139.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 140.Guo, X. W., D. Merlin, C. Laboisse, and U. Hopfer. 1997. Purinergic agonists, but not cAMP, stimulate coupled granule fusion and Cl- conductance in HT29-Cl. 16E. Am. J. Physiol. 273:C804-C809. [DOI] [PubMed] [Google Scholar]
- 141.Haller, D., C. Bode, W. P. Hammes, A. M. Pfeifer, E. J. Schiffrin, and S. Blum. 2000. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 47:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haller, D., and C. Jobin. 2004. Interaction between resident luminal bacteria and the host: can a healthy relationship turn sour? J. Pediatr. Gastroenterol. Nutr. 38:123-136. [DOI] [PubMed] [Google Scholar]
- 143.Hamanaka, Y., M. Nakashima, A. Wada, M. Ito, H. Kurazono, H. Hojo, Y. Nakahara, S. Kohno, T. Hirayama, and I. Sekine. 2001. Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut 49:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hao, W. L., and Y. K. Lee. 2004. Microflora of the gastrointestinal tract: a review. Methods Mol. Biol. 268:491-502. [DOI] [PubMed] [Google Scholar]
- 145.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 146.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 147.Harrington, S. M., M. C. Strauman, C. M. Abe, and J. P. Nataro. 2005. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell. Microbiol. 7:1565-1578. [DOI] [PubMed] [Google Scholar]
- 148.Hase, K., L. Eckmann, J. D. Leopard, N. Varki, and M. F. Kagnoff. 2002. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect. Immun. 70:953-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hase, K., M. Murakami, M. Iimura, S. P. Cole, Y. Horibe, T. Ohtake, M. Obonyo, R. L. Gallo, L. Eckmann, and M. F. Kagnoff. 2003. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 125:1613-1625. [DOI] [PubMed] [Google Scholar]
- 150.He, W., M. L. Wang, H. Q. Jiang, C. M. Steppan, M. E. Shin, M. C. Thurnheer, J. J. Cebra, M. A. Lazar, and G. D. Wu. 2003. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology 125:1388-1397. [DOI] [PubMed] [Google Scholar]
- 151.Helander, A., G. C. Hansson, and A. M. Svennerholm. 1997. Binding of enterotoxigenic Escherichia coli to isolated enterocytes and intestinal mucus. Microb. Pathog. 23:335-346. [DOI] [PubMed] [Google Scholar]
- 152.Henzler Wildman, K. A., D. K. Lee, and A. Ramamoorthy. 2003. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 42:6545-6558. [DOI] [PubMed] [Google Scholar]
- 153.Henzler-Wildman, K. A., G. V. Martinez, M. F. Brown, and A. Ramamoorthy. 2004. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry 43:8459-8469. [DOI] [PubMed] [Google Scholar]
- 154.Hiemstra, P. S. 2001. Epithelial antimicrobial peptides and proteins: their role in host defence and inflammation. Paediatr. Respir. Rev. 2:306-310. [DOI] [PubMed] [Google Scholar]
- 155.Hisamatsu, T., M. Suzuki, H. C. Reinecker, W. J. Nadeau, B. A. McCormick, and D. K. Podolsky. 2003. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124:993-1000. [DOI] [PubMed] [Google Scholar]
- 156.Hofman, P. 2003. Pathological interactions of bacteria and toxins with the gastrointestinal epithelial tight junctions and/or the zonula adherens: an update. Cell Mol. Biol. (Noisy-le-grand) 49:65-75. [PubMed] [Google Scholar]
- 157.Hollingsworth, M. A., and B. J. Swanson. 2004. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 4:45-60. [DOI] [PubMed] [Google Scholar]
- 158.Hooper, L. V., L. Bry, P. G. Falk, and J. I. Gordon. 1998. Host-microbial symbiosis in the mammalian intestine: exploring an internal ecosystem. Bioessays 20:336-343. [DOI] [PubMed] [Google Scholar]
- 159.Hooper, L. V., P. G. Falk, and J. I. Gordon. 2000. Analyzing the molecular foundations of commensalism in the mouse intestine. Curr. Opin. Microbiol. 3:79-85. [DOI] [PubMed] [Google Scholar]
- 160.Hooper, L. V., and J. I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science 292:1115-1118. [DOI] [PubMed] [Google Scholar]
- 161.Hooper, L. V., and J. I. Gordon. 2001. Glycans as legislators of host-microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology 11:1R-10R. [DOI] [PubMed] [Google Scholar]
- 162.Hooper, L. V., T. Midtvedt, and J. I. Gordon. 2002. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 22:283-307. [DOI] [PubMed] [Google Scholar]
- 163.Hooper, L. V., T. S. Stappenbeck, C. V. Hong, and J. I. Gordon. 2003. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 4:269-273. [DOI] [PubMed] [Google Scholar]
- 164.Hooper, L. V., M. H. Wong, A. Thelin, L. Hansson, P. G. Falk, and J. I. Gordon. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881-884. [DOI] [PubMed] [Google Scholar]
- 165.Hooper, L. V., J. Xu, P. G. Falk, T. Midtvedt, and J. I. Gordon. 1999. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc. Natl. Acad. Sci. USA 96:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hopkins, M. J., R. Sharp, and G. T. Macfarlane. 2002. Variation in human intestinal microbiota with age. Dig. Liver Dis. 34(Suppl. 2):S12-S18. [DOI] [PubMed] [Google Scholar]
- 167.Hornef, M. W., K. Putsep, J. Karlsson, E. Refai, and M. Andersson. 2004. Increased diversity of intestinal antimicrobial peptides by covalent dimer formation. Nat. Immunol. 5:836-843. [DOI] [PubMed] [Google Scholar]
- 168.Howell, S. J., D. Wilk, S. P. Yadav, and C. L. Bevins. 2003. Antimicrobial polypeptides of the human colonic epithelium. Peptides 24:1763-1770. [DOI] [PubMed] [Google Scholar]
- 169.Huang, H. W. 2000. Action of antimicrobial peptides: two-state model. Biochemistry 39:8347-8352. [DOI] [PubMed] [Google Scholar]
- 170.Hudault, S., J. Guignot, and A. L. Servin. 2001. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut 49:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hudault, S., V. Lievin, M. F. Bernet-Camard, and A. L. Servin. 1997. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol. 63:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 173.Iimura, M., R. L. Gallo, K. Hase, Y. Miyamoto, L. Eckmann, and M. F. Kagnoff. 2005. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 174:4901-4907. [DOI] [PubMed] [Google Scholar]
- 174.Imamura, Y., K. Yanagihara, Y. Mizuta, M. Seki, H. Ohno, Y. Higashiyama, Y. Miyazaki, K. Tsukamoto, Y. Hirakata, K. Tomono, J. Kadota, and S. Kohno. 2004. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrob. Agents Chemother. 48:3457-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ingrassia, I., A. Leplingard, and A. Darfeuille-Michaud. 2005. Lactobacillus casei DN-114 001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn's disease patients to adhere to and to invade intestinal epithelial cells. Appl. Environ. Microbiol. 71:2880-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 177.Islam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7:180-185. [DOI] [PubMed] [Google Scholar]
- 178.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jackson, J. J., and H. Kropp. 1992. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J. Infect. Dis. 165:1033-1041. [DOI] [PubMed] [Google Scholar]
- 180.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 181.Janssens, S., and R. Beyaert. 2003. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jarrard, J. A., R. I. Linnoila, H. Lee, S. M. Steinberg, H. Witschi, and E. Szabo. 1998. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Res. 58:5582-5589. [PubMed] [Google Scholar]
- 183.Jarry, A., D. Merlin, U. Hopfer, and C. L. Laboisse. 1994. Cyclic AMP-induced mucin exocytosis is independent of Cl− movements in human colonic epithelial cells (HT29-Cl. 16E). Biochem. J. 304:675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jarry, A., G. Vallette, J. E. Branka, and C. Laboisse. 1996. Direct secretory effect of interleukin-1 via type I receptors in human colonic mucous epithelial cells (HT29-C1.16E). Gut 38:240-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jelinek, R., and S. Kolusheva. 2005. Membrane interactions of host-defense peptides studied in model systems. Curr. Protein Pept. Sci. 6:103-114. [DOI] [PubMed] [Google Scholar]
- 186.Jepson, M. A., and M. A. Clark. 1998. Studying M cells and their role in infection. Trends Microbiol. 6:359-365. [DOI] [PubMed] [Google Scholar]
- 187.Jepson, S., M. Komatsu, B. Haq, M. E. Arango, D. Huang, C. A. Carraway, and K. L. Carraway. 2002. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene 21:7524-7532. [DOI] [PubMed] [Google Scholar]
- 188.Jia, H. P., T. Starner, M. Ackermann, P. Kirby, B. F. Tack, and P. B. McCray, Jr. 2001. Abundant human beta-defensin-1 expression in milk and mammary gland epithelium. J. Pediatr. 138:109-112. [DOI] [PubMed] [Google Scholar]
- 189.Jones, B. D., C. A. Lee, and S. Falkow. 1992. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect. Immun. 60:2475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kanamaru, K., I. Tatsuno, T. Tobe, and C. Sasakawa. 2000. SdiA, an Escherichia coli homologue of quorum-sensing regulators, controls the expression of virulence factors in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 38:805-816. [DOI] [PubMed] [Google Scholar]
- 191.Kawai, T., and S. Akira. 2005. Toll-like receptor downstream signaling. Arthritis Res. Ther. 7:12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kedinger, M., I. Duluc, C. Fritsch, O. Lorentz, M. Plateroti, and J. N. Freund. 1998. Intestinal epithelial-mesenchymal cell interactions. Ann. N. Y. Acad. Sci. 859:1-17. [DOI] [PubMed] [Google Scholar]
- 193.Kelly, D., J. I. Campbell, T. P. King, G. Grant, E. A. Jansson, A. G. Coutts, S. Pettersson, and S. Conway. 2004. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat. Immunol. 5:104-112. [DOI] [PubMed] [Google Scholar]
- 194.Kelly, D., and S. Conway. 2005. Bacterial modulation of mucosal innate immunity. Mol. Immunol. 42:895-901. [DOI] [PubMed] [Google Scholar]
- 195.Kelly, D., and S. Conway. 2001. Genomics at work: the global gene response to enteric bacteria. Gut 49:612-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kelly, D., S. Conway, and R. Aminov. 2005. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 26:326-333. [DOI] [PubMed] [Google Scholar]
- 197.Kerneis, S., M. F. Bernet, M. H. Coconnier, and A. L. Servin. 1994. Adhesion of human enterotoxigenic Escherichia coli to human mucus secreting HT-29 cell subpopulations in culture. Gut 35:1449-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Khan, M. A., J. Kang, and T. S. Steiner. 2004. Enteroaggregative Escherichia coli flagellin-induced interleukin-8 secretion requires Toll-like receptor 5-dependent p38 MAP kinase activation. Immunology 112:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 200.Kleerebezem, M. 2004. Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. Peptides 25:1405-1414. [DOI] [PubMed] [Google Scholar]
- 201.Kobayashi, K. S., M. Chamaillard, Y. Ogura, O. Henegariu, N. Inohara, G. Nunez, and R. A. Flavell. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731-734. [DOI] [PubMed] [Google Scholar]
- 202.Korinek, V., N. Barker, P. Moerer, E. van Donselaar, G. Huls, P. J. Peters, and H. Clevers. 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19:379-383. [DOI] [PubMed] [Google Scholar]
- 203.Kougias, P., H. Chai, P. H. Lin, Q. Yao, A. B. Lumsden, and C. Chen. 2005. Defensins and cathelicidins: Neutrophil peptides with roles in inflammation, hyperlipidemia and atherosclerosis. J. Cell. Mol. Med. 9:3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kraehenbuhl, J. P., and M. R. Neutra. 2000. Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol. 16:301-332. [DOI] [PubMed] [Google Scholar]
- 205.Kyo, K., T. Muto, H. Nagawa, G. M. Lathrop, and Y. Nakamura. 2001. Associations of distinct variants of the intestinal mucin gene MUC3A with ulcerative colitis and Crohn's disease. J. Hum. Genet. 46:5-20. [DOI] [PubMed] [Google Scholar]
- 206.Laboisse, C., A. Jarry, J. E. Branka, D. Merlin, C. Bou-Hanna, and G. Vallette. 1996. Recent aspects of the regulation of intestinal mucus secretion. Proc. Nutr. Soc. 55:259-264. [DOI] [PubMed] [Google Scholar]
- 207.Laboisse, C., A. Jarry, J. E. Branka, D. Merlin, C. Bou-Hanna, and G. Vallette. 1995. Regulation of mucin exocytosis from intestinal goblet cells. Biochem. Soc. Trans. 23:810-813. [DOI] [PubMed] [Google Scholar]
- 208.Laburthe, M., C. Augeron, C. Rouyer-Fessard, I. Roumagnac, J. J. Maoret, E. Grasset, and C. Laboisse. 1989. Functional VIP receptors in the human mucus-secreting colonic epithelial cell line CL. 16E. Am. J. Physiol. 256:G443-G450. [DOI] [PubMed] [Google Scholar]
- 209.La Ragione, R. M., W. A. Cooley, P. Velge, M. A. Jepson, and M. J. Woodward. 2003. Membrane ruffling and invasion of human and avian cell lines is reduced for aflagellate mutants of Salmonella enterica serotype Enteritidis. Int. J. Med. Microbiol. 293:261-272. [DOI] [PubMed] [Google Scholar]
- 210.Larson, M. A., S. H. Wei, A. Weber, D. R. Mack, and T. L. McDonald. 2003. Human serum amyloid A3 peptide enhances intestinal MUC3 expression and inhibits EPEC adherence. Biochem. Biophys. Res. Commun. 300:531-540. [DOI] [PubMed] [Google Scholar]
- 211.Lee, Y. K., K. Y. Puong, A. C. Ouwehand, and S. Salminen. 2003. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 52:925-930. [DOI] [PubMed] [Google Scholar]
- 212.Lehrer, R. I., and T. Ganz. 2002. Cathelicidins: a family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 9:18-22. [DOI] [PubMed] [Google Scholar]
- 213.Lehrer, R. I., and T. Ganz. 1992. Defensins: endogenous antibiotic peptides from human leukocytes. Ciba Found. Symp. 171:276-293. [DOI] [PubMed] [Google Scholar]
- 214.Leitch, G. J. 1988. Cholera enterotoxin-induced mucus secretion and increase in the mucus blanket of the rabbit ileum in vivo. Infect. Immun. 56:2871-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lencer, W. I., F. D. Reinhart, and M. R. Neutra. 1990. Interaction of cholera toxin with cloned human goblet cells in monolayer culture. Am. J. Physiol. 258:G96-G102. [DOI] [PubMed] [Google Scholar]
- 216.Li, J. D., A. F. Dohrman, M. Gallup, S. Miyata, J. R. Gum, Y. S. Kim, J. A. Nadel, A. Prince, and C. B. Basbaum. 1997. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc. Natl. Acad. Sci. USA 94:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li, X., L. Wang, D. P. Nunes, R. F. Troxler, and G. D. Offner. 2003. Pro-inflammatory cytokines up-regulate MUC1 gene expression in oral epithelial cells. J. Dent. Res. 82:883-887. [DOI] [PubMed] [Google Scholar]
- 218.Lievin, V., I. Peiffer, S. Hudault, F. Rochat, D. Brassart, J. R. Neeser, and A. L. Servin. 2000. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 47:646-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lievin-Le Moal, V., R. Amsellem, A. L. Servin, and M. H. Coconnier. 2002. Lactobacillus acidophilus (strain LB) from the resident adult human gastrointestinal microflora exerts activity against brush border damage promoted by a diarrhoeagenic Escherichia coli in human enterocyte-like cells. Gut 50:803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lievin-Le Moal, V., G. Huet, J. P. Aubert, J. Bara, M. E. Forgue-Lafitte, A. L. Servin, and M. H. Coconnier. 2002. Activation of mucin exocytosis and upregulation of MUC genes in polarized human intestinal mucin-secreting cells by the thiol-activated exotoxin listeriolysin O. Cell. Microbiol. 4:515-529. [DOI] [PubMed] [Google Scholar]
- 221.Lievin-Le Moal, V., A. L. Servin, and M.-H. Coconnier-Polter. 2005. The increase in mucin exocytosis and the up-regulation of MUC genes encoding for membrane-bound mucins induced by the thiol-activated exotoxin listeriolysin O is a host cell defence response that inhibits the cell-entry of Listeria monocytogenes. Cell. Microbiol. 7:1035-1048. [DOI] [PubMed] [Google Scholar]
- 222.Lillehoj, E. P., S. W. Hyun, B. T. Kim, X. G. Zhang, D. I. Lee, S. Rowland, and K. C. Kim. 2001. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 280:L181-L187. [DOI] [PubMed] [Google Scholar]
- 223.Linzmeier, R., C. H. Ho, B. V. Hoang, and T. Ganz. 1999. A 450-kb contig of defensin genes on human chromosome 8p23. Gene 233:205-211. [DOI] [PubMed] [Google Scholar]
- 224.Liu, L., C. Zhao, H. H. Heng, and T. Ganz. 1997. The human beta-defensin-1 and alpha-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics 43:316-320. [DOI] [PubMed] [Google Scholar]
- 225.Lockman, H. A., and R. Curtiss III. 1990. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect. Immun. 58:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lohner, K., and S. E. Blondelle. 2005. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 8:241-256. [DOI] [PubMed] [Google Scholar]
- 227.Lopez-Boado, Y. S., C. L. Wilson, L. V. Hooper, J. I. Gordon, S. J. Hultgren, and W. C. Parks. 2000. Bacterial exposure induces and activates matrilysin in mucosal epithelial cells. J. Cell Biol. 148:1305-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Louvard, D., M. Kedinger, and H. P. Hauri. 1992. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu. Rev. Cell Biol. 8:157-195. [DOI] [PubMed] [Google Scholar]
- 229.Lu, Z., K. A. Kim, M. A. Suico, T. Shuto, J. D. Li, and H. Kai. 2004. MEF up-regulates human beta-defensin 2 expression in epithelial cells. FEBS Lett. 561:117-121. [DOI] [PubMed] [Google Scholar]
- 230.Lupp, C., and B. B. Finlay. 2005. Intestinal microbiota. Curr. Biol. 15:R235-R236. [DOI] [PubMed] [Google Scholar]
- 231.Mack, D. R., S. Ahrne, L. Hyde, S. Wei, and M. A. Hollingsworth. 2003. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52:827-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mack, D. R., S. Michail, S. Wei, L. McDougall, and M. A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941-G950. [DOI] [PubMed] [Google Scholar]
- 233.Mackie, R. I., A. Sghir, and H. R. Gaskins. 1999. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69:1035S-1045S. [DOI] [PubMed] [Google Scholar]
- 234.Macnab, R. M. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77-100. [DOI] [PubMed] [Google Scholar]
- 235.Mahida, Y. R., and R. N. Cunliffe. 2004. Defensins and mucosal protection. Novartis Found. Symp. 263:71-77. [DOI] [PubMed] [Google Scholar]
- 236.Maldonado, A., R. Jimenez-Diaz, and J. L. Ruiz-Barba. 2004. Induction of plantaricin production in Lactobacillus plantarum NC8 after coculture with specific gram-positive bacteria is mediated by an autoinduction mechanism. J. Bacteriol. 186:1556-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mallow, E. B., A. Harris, N. Salzman, J. P. Russell, R. J. DeBerardinis, E. Ruchelli, and C. L. Bevins. 1996. Human enteric defensins. Gene structure and developmental expression. J. Biol. Chem. 271:4038-4045. [DOI] [PubMed] [Google Scholar]
- 238.Mangell, P., P. Nejdfors, M. Wang, S. Ahrne, B. Westrom, H. Thorlacius, and B. Jeppsson. 2002. Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig. Dis. Sci. 47:511-516. [DOI] [PubMed] [Google Scholar]
- 239.Mantle, M., L. Basaraba, S. C. Peacock, and D. G. Gall. 1989. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect. Immun. 57:3292-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Maresca, M., D. Miller, S. Qittard, P. Dean, and B. Kenny. 2005. Enteropathogenic Escherichia coli (EPEC) effector-mediated suppression of antimicrobial nitric oxide production in a small intestinal epithelial model system. Cell. Microbiol. 7:1749-1762. [DOI] [PubMed] [Google Scholar]
- 241.Marshman, E., C. Booth, and C. S. Potten. 2002. The intestinal epithelial stem cell. Bioessays 24:91-98. [DOI] [PubMed] [Google Scholar]
- 242.Mathiesen, G., H. M. Namlos, P. A. Risoen, L. Axelsson, and V. G. Eijsink. 2004. Use of bacteriocin promoters for gene expression in Lactobacillus plantarum C11. J. Appl. Microbiol. 96:819-827. [DOI] [PubMed] [Google Scholar]
- 243.Matsuki, T., K. Watanabe, R. Tanaka, M. Fukuda, and H. Oyaizu. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matsuo, K., H. Ota, T. Akamatsu, A. Sugiyama, and T. Katsuyama. 1997. Histochemistry of the surface mucous gel layer of the human colon. Gut 40:782-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Matter, K., and M. S. Balda. 2003. Functional analysis of tight junctions. Methods 30:228-234. [DOI] [PubMed] [Google Scholar]
- 246.Matter, K., and M. S. Balda. 2003. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 4:225-236. [DOI] [PubMed] [Google Scholar]
- 247.McAuliffe, O., R. P. Ross, and C. Hill. 2001. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 25:285-308. [DOI] [PubMed] [Google Scholar]
- 248.McCartney, A. L., W. Wenzhi, and G. W. Tannock. 1996. Molecular analysis of the composition of the bifidobacterial and lactobacillus microflora of humans. Appl. Environ. Microbiol. 62:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.McCracken, V. J., and R. G. Lorenz. 2001. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 3:1-11. [DOI] [PubMed] [Google Scholar]
- 250.McDonald, T. T., and S. Pettersson. 2000. Bacterial regulation of intestinal immune responses. Inflamm. Bowel Dis. 6:116-122. [DOI] [PubMed] [Google Scholar]
- 251.Mcpherson, A. J., D. Gatto, E. Sainsbury, G. R. Harriman, H. Hengartner, and R. M. Zinkernagel. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288:2222-2226. [DOI] [PubMed] [Google Scholar]
- 252.Mcpherson, A. J., M. B. Geuking, and K. D. McCoy. 2005. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology 115:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mcpherson, A. J., and N. L. Harris. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4:478-485. [DOI] [PubMed] [Google Scholar]
- 254.Mcpherson, A. J., M. M. Martinic, and N. Harris. 2002. The functions of mucosal T cells in containing the indigenous commensal flora of the intestine. Cell. Mol. Life Sci. 59:2088-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mcpherson, A. J., and T. Uhr. 2004. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann. N. Y. Acad. Sci. 1029:36-43. [DOI] [PubMed] [Google Scholar]
- 256.Mcpherson, A. J., and T. Uhr. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662-1665. [DOI] [PubMed] [Google Scholar]
- 257.Means, T. K., D. T. Golenbock, and M. J. Fenton. 2000. Structure and function of Toll-like receptor proteins. Life Sci. 68:241-258. [DOI] [PubMed] [Google Scholar]
- 258.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 259.Menard, S., C. Candalh, J. C. Bambou, K. Terpend, N. Cerf-Bensussan, and M. Heyman. 2004. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53:821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Merlin, D., C. Augeron, X. Y. Tien, X. Guo, C. L. Laboisse, and U. Hopfer. 1994. ATP-stimulated electrolyte and mucin secretion in the human intestinal goblet cell line HT29-Cl. 16E. J. Membr. Biol. 137:137-149. [DOI] [PubMed] [Google Scholar]
- 261.Merlin, D., X. Guo, C. L. Laboisse, and U. Hopfer. 1995. Ca2+ and cAMP activate different K+ conductances in the human intestinal goblet cell line HT29-Cl. 16E. Am. J. Physiol. 268:C1503-C1511. [DOI] [PubMed] [Google Scholar]
- 262.Merlin, D., X. Guo, K. Martin, C. Laboisse, D. Landis, G. Dubyak, and U. Hopfer. 1996. Recruitment of purinergically stimulated Cl- channels from granule membrane to plasma membrane. Am. J. Physiol. 271:C612-C619. [DOI] [PubMed] [Google Scholar]
- 263.Michail, S., and F. Abernathy. 2002. Lactobacillus plantarum reduces the in vitro secretory response of intestinal epithelial cells to enteropathogenic Escherichia coli infection. J. Pediatr. Gastroenterol. Nutr. 35:350-355. [DOI] [PubMed] [Google Scholar]
- 264.Micots, I., C. Augeron, C. L. Laboisse, F. Muzeau, and F. Megraud. 1993. Mucin exocytosis: a major target for Helicobacter pylori. J. Clin. Pathol. 46:241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 266.Miller, S. I., W. S. Pulkkinen, M. E. Selsted, and J. J. Mekalanos. 1990. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect. Immun. 58:3706-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mitic, L. L., and J. M. Anderson. 1998. Molecular architecture of tight junctions. Annu. Rev. Physiol. 60:121-142. [DOI] [PubMed] [Google Scholar]
- 268.Moncada, D. M., S. J. Kammanadiminti, and K. Chadee. 2003. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 19:305-311. [DOI] [PubMed] [Google Scholar]
- 269.Moniaux, N., F. Escande, N. Porchet, J. P. Aubert, and S. K. Batra. 2001. Structural organization and classification of the human mucin genes. Front. Biosci. 6:D1192-D1206. [DOI] [PubMed] [Google Scholar]
- 270.Moniaux, N., S. Nollet, N. Porchet, P. Degand, A. Laine, and J. P. Aubert. 1999. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem. J. 338:325-333. [PMC free article] [PubMed] [Google Scholar]
- 271.Montgomery, R. K., A. E. Mulberg, and R. J. Grand. 1999. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 116:702-731. [DOI] [PubMed] [Google Scholar]
- 272.Moore, B. A., K. A. Sharkey, and M. Mantle. 1993. Neural mediation of cholera toxin-induced mucin secretion in the rat small intestine. Am. J. Physiol. 265:G1050-G1056. [DOI] [PubMed] [Google Scholar]
- 273.Moore, B. A., K. A. Sharkey, and M. Mantle. 1996. Role of 5-HT in cholera toxin-induced mucin secretion in the rat small intestine. Am. J. Physiol. 270:G1001-G1009. [DOI] [PubMed] [Google Scholar]
- 274.Morelli, L., C. Cesena, C. de Haen, and L. Gozzini. 1998. Taxonomic Lactobacillus composition of feces from human newborns during the first few days. Microb. Ecol. 35:205-212. [DOI] [PubMed] [Google Scholar]
- 275.Moreno, F., J. E. Gonzalez-Pastor, M. R. Baquero, and D. Bravo. 2002. The regulation of microcin B, C and J operons. Biochimie 84:521-529. [DOI] [PubMed] [Google Scholar]
- 276.Morgenstern, S., R. Koren, S. F. Moss, G. Fraser, E. Okon, and Y. Niv. 2001. Does Helicobacter pylori affect gastric mucin expression? Relationship between gastric antral mucin expression and H. pylori colonization. Eur. J. Gastroenterol. Hepatol. 13:19-23. [DOI] [PubMed] [Google Scholar]
- 277.Nagafuchi, A. 2001. Molecular architecture of adherens junctions. Curr. Opin. Cell Biol. 13:600-603. [DOI] [PubMed] [Google Scholar]
- 278.Nasu, T., K. Okamoto, T. Nakanishi, and T. Nishino. 1999. In vitro antibacterial activity of faropenem, a novel oral penem antibiotic, against enterohemorrhagic Escherichia coli O157 strains. Jpn. J. Antibiot. 52:541-553. [PubMed] [Google Scholar]
- 279.Navarre, W. W., T. A. Halsey, D. Walthers, J. Frye, M. McClelland, J. L. Potter, L. J. Kenney, J. S. Gunn, F. C. Fang, and S. J. Libby. 2005. Co-regulation of Salmonella enterica genes required for virulence and resistance to antimicrobial peptides by SlyA and PhoP/PhoQ. Mol. Microbiol. 56:492-508. [DOI] [PubMed] [Google Scholar]
- 280.Nes, I. F., and H. Holo. 2000. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 55:50-61. [DOI] [PubMed] [Google Scholar]
- 281.Netea, M. G., C. van der Graaf, J. W. Van der Meer, and B. J. Kullberg. 2004. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J. Leukoc. Biol. 75:749-755. [DOI] [PubMed] [Google Scholar]
- 282.Neutra, M. R., N. J. Mantis, and J. P. Kraehenbuhl. 2001. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2:1004-1009. [DOI] [PubMed] [Google Scholar]
- 283.Nicaise, P., A. Gleizes, C. Sandre, R. Kergot, H. Lebrec, F. Forestier, and C. Labarre. 1999. The intestinal microflora regulates cytokine production positively in spleen-derived macrophages but negatively in bone marrow-derived macrophages. Eur. Cytokine Netw. 10:365-372. [PubMed] [Google Scholar]
- 284.Njoku, O. O., and G. J. Leitch. 1983. Separation of cholera enterotoxin-induced mucus secretion from electrolyte secretion in rabbit ileum by acetazolamide, colchicine, cycloheximide, cytochalasin B and indomethacin. Digestion 27:174-184. [DOI] [PubMed] [Google Scholar]
- 285.Nollet, S., N. Moniaux, J. Maury, D. Petitprez, P. Degand, A. Laine, N. Porchet, and J. P. Aubert. 1998. Human mucin gene MUC4: organization of its 5′-region and polymorphism of its central tandem repeat array. Biochem. J. 332:739-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nusrat, A., C. A. Parkos, P. Verkade, C. S. Foley, T. W. Liang, W. Innis-Whitehouse, K. K. Eastburn, and J. L. Madara. 2000. Tight junctions are membrane microdomains. J. Cell Sci. 113:1771-1781. [DOI] [PubMed] [Google Scholar]
- 287.Nutten, S., P. Sansonetti, G. Huet, C. Bourdon-Bisiaux, B. Meresse, J. F. Colombel, and P. Desreumaux. 2002. Epithelial inflammation response induced by Shigella flexneri depends on mucin gene expression. Microbes Infect. 4:1121-1124. [DOI] [PubMed] [Google Scholar]
- 288.Ogawa, H., K. Fukushima, I. Sasaki, and S. Matsuno. 2000. Identification of genes involved in mucosal defense and inflammation associated with normal enteric bacteria. Am. J. Physiol. Gastr. Liver Physiol. 279:G492-G499. [DOI] [PubMed] [Google Scholar]
- 289.Ogawa, M., K. Shimizu, K. Nomoto, M. Takahashi, M. Watanuki, R. Tanaka, T. Tanaka, T. Hamabata, S. Yamasaki, and Y. Takeda. 2001. Protective effect of Lactobacillus casei strain Shirota on Shiga toxin-producing Escherichia coli O157:H7 infection in infant rabbits. Infect. Immun. 69:1101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ogawa, M., K. Shimizu, K. Nomoto, R. Tanaka, T. Hamabata, S. Yamasaki, T. Takeda, and Y. Takeda. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68:135-140. [DOI] [PubMed] [Google Scholar]
- 291.Ogushi, K., A. Wada, T. Niidome, N. Mori, K. Oishi, T. Nagatake, A. Takahashi, H. Asakura, S. Makino, H. Hojo, Y. Nakahara, M. Ohsaki, T. Hatakeyama, H. Aoyagi, H. Kurazono, J. Moss, and T. Hirayama. 2001. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J. Biol. Chem. 276:30521-30526. [DOI] [PubMed] [Google Scholar]
- 292.Ogushi, K., A. Wada, T. Niidome, T. Okuda, R. Llanes, M. Nakayama, Y. Nishi, H. Kurazono, K. D. Smith, A. Aderem, J. Moss, and T. Hirayama. 2004. Gangliosides act as co-receptors for Salmonella enteritidis FliC and promote FliC induction of human beta-defensin-2 expression in Caco-2 cells. J. Biol. Chem. 279:12213-12219. [DOI] [PubMed] [Google Scholar]
- 293.Ohgami, A., T. Tsuda, T. Osaki, T. Mitsudomi, Y. Morimoto, T. Higashi, and K. Yasumoto. 1999. MUC1 mucin mRNA expression in stage I lung adenocarcinoma and its association with early recurrence. Ann. Thorac. Surg. 67:810-814. [DOI] [PubMed] [Google Scholar]
- 294.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163:6718-6724. [PubMed] [Google Scholar]
- 295.Oren, Z., J. C. Lerman, G. H. Gudmundsson, B. Agerberth, and Y. Shai. 1999. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 341:501-513. [PMC free article] [PubMed] [Google Scholar]
- 296.Ottemann, K. M., and A. C. Lowenthal. 2002. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 70:1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ouellette, A. J. 2004. Defensin-mediated innate immunity in the small intestine. Best Pract. Res. Clin. Gastroenterol. 18:405-419. [DOI] [PubMed] [Google Scholar]
- 298.Ouellette, A. J. 1999. IV. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. Am. J. Physiol. 277:G257-G261. [DOI] [PubMed] [Google Scholar]
- 299.Ouellette, A. J. 1997. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology 113:1779-1784. [DOI] [PubMed] [Google Scholar]
- 300.Ouellette, A. J., and C. L. Bevins. 2001. Paneth cell defensins and innate immunity of the small bowel. Inflamm. Bowel Dis. 7:43-50. [DOI] [PubMed] [Google Scholar]
- 301.Ouellette, A. J., S. I. Miller, A. H. Henschen, and M. E. Selsted. 1992. Purification and primary structure of murine cryptdin-1, a Paneth cell defensin. FEBS Lett. 304:146-148. [DOI] [PubMed] [Google Scholar]
- 302.Ouellette, A. J., and M. E. Selsted. 1996. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 10:1280-1289. [DOI] [PubMed] [Google Scholar]
- 303.Paneth, J. 1988. Ueber die secernierenden Zellen des Duenndarmepithels. Arch. Mikriskop. Anat. 31:113-191. [Google Scholar]
- 304.Parassol, N., M. Freitas, K. Thoreux, G. Dalmasso, R. Bourdet-Sicard, and P. Rampal. 2005. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res. Microbiol. 156:256-262. [DOI] [PubMed] [Google Scholar]
- 305.Park, Y., and K. S. Hahm. 2005. Antimicrobial peptides (AMPs): peptide structure and mode of action. J. Biochem. Mol. Biol. 38:507-516. [DOI] [PubMed] [Google Scholar]
- 306.Parker, C. T., and J. Guard-Petter. 2001. Contribution of flagella and invasion proteins to pathogenesis of Salmonella enterica serovar Enteritidis in chicks. FEMS Microbiol. Lett. 204:287-291. [DOI] [PubMed] [Google Scholar]
- 307.Perez-Vilar, J., and R. L. Hill. 1999. The structure and assembly of secreted mucins. J. Biol. Chem. 274:31751-31754. [DOI] [PubMed] [Google Scholar]
- 308.Perrais, M., P. Pigny, M. P. Ducourouble, D. Petitprez, N. Porchet, J. P. Aubert, and I. Van Seuningen. 2001. Characterization of human mucin gene MUC4 promoter. Importance of growth factors and pro-inflammatory cytokines for its regulation in pancreatic cancer cells. J. Biol. Chem. 19:19. [DOI] [PubMed] [Google Scholar]
- 309.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 310.Philpott, D. J., and S. E. Girardin. 2004. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol. Immunol. 41:1099-1108. [DOI] [PubMed] [Google Scholar]
- 311.Philpott, D. J., and J. Viala. 2004. Towards an understanding of the role of NOD2/CARD15 in the pathogenesis of Crohn's disease. Best Pract. Res. Clin. Gastroenterol. 18:555-568. [DOI] [PubMed] [Google Scholar]
- 312.Pinto, D., and H. Clevers. 2005. Wnt, stem cells and cancer in the intestine. Biol. Cell 97:185-196. [DOI] [PubMed] [Google Scholar]
- 313.Pinto, D., A. Gregorieff, H. Begthel, and H. Clevers. 2003. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17:1709-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Plaisancie, P., A. Bosshard, J. C. Meslin, and J. C. Cuber. 1997. Colonic mucin discharge by a cholinergic agonist, prostaglandins, and peptide YY in the isolated vascularly perfused rat colon. Digestion 58:168-175. [DOI] [PubMed] [Google Scholar]
- 315.Podolsky, D. K., K. Lynch-Devaney, J. L. Stow, P. Oates, B. Murgue, M. DeBeaumont, B. E. Sands, and Y. R. Mahida. 1993. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J. Biol. Chem. 268:6694-6702. [PubMed] [Google Scholar]
- 316.Porchet, N., P. Pigny, M. P. Buisine, V. Debailleul, P. Degand, A. Laine, and J. P. Aubert. 1995. Human mucin genes: genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem. Soc. Trans. 23:800-805. [DOI] [PubMed] [Google Scholar]
- 317.Porter, E. M., C. L. Bevins, D. Ghosh, and T. Ganz. 2002. The multifaceted Paneth cell. Cell Mol. Life Sci. 59:156-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Porter, E. M., L. Liu, A. Oren, P. A. Anton, and T. Ganz. 1997. Localization of human intestinal defensin 5 in Paneth cell granules. Infect. Immun. 65:2389-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Porter, E. M., E. van Dam, E. V. Valore, and T. Ganz. 1997. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect. Immun. 65:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Portrait, V., S. Gendron-Gaillard, G. Cottenceau, and A. M. Pons. 1999. Inhibition of pathogenic Salmonella enteritidis growth mediated by Escherichia coli microcin J25 producing strains. Can. J. Microbiol. 45:988-994. [DOI] [PubMed] [Google Scholar]
- 321.Pron, B., C. Boumaila, F. Jaubert, S. Sarnacki, J. P. Monnet, P. Berche, and J. L. Gaillard. 1998. Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system. Infect. Immun. 66:747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Putsep, K., L. G. Axelsson, A. Boman, T. Midtvedt, S. Normark, H. G. Boman, and M. Andersson. 2000. Germ-free and colonized mice generate the same products from enteric prodefensins. J. Biol. Chem. 275:40478-40482. [DOI] [PubMed] [Google Scholar]
- 323.Rajkumar, R., H. Devaraj, and S. Niranjali. 1998. Binding of Shigella to rat and human intestinal mucin. Mol. Cell. Biochem. 178:261-268. [DOI] [PubMed] [Google Scholar]
- 324.Ramanathan, B., E. G. Davis, C. R. Ross, and F. Blecha. 2002. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 4:361-372. [DOI] [PubMed] [Google Scholar]
- 325.Ramare, F., J. Nicoli, J. Dabard, T. Corring, M. Ladire, A. M. Gueugneau, and P. Raibaud. 1993. Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl. Environ. Microbiol. 59:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Reuter, G. 2001. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr. Issues Intest. Microbiol. 2:43-53. [PubMed] [Google Scholar]
- 327.Riley, M. A., and J. E. Wertz. 2002. Bacteriocin diversity: ecological and evolutionary perspectives. Biochimie 84:357-364. [DOI] [PubMed] [Google Scholar]
- 328.Rintoul, M. R., B. F. de Arcuri, R. A. Salomon, R. N. Farias, and R. D. Morero. 2001. The antibacterial action of microcin J25: evidence for disruption of cytoplasmic membrane energization in Salmonella newport. FEMS Microbiol. Lett. 204:265-270. [DOI] [PubMed] [Google Scholar]
- 329.Robertson, J. M., G. Grant, E. Allen-Vercoe, M. J. Woodward, A. Pusztai, and H. J. Flint. 2000. Adhesion of Salmonella enterica var. Enteritidis strains lacking fimbriae and flagella to rat ileal explants cultured at the air interface or submerged in tissue culture medium. J. Med. Microbiol. 49:691-696. [DOI] [PubMed] [Google Scholar]
- 330.Robertson, J. M., N. H. McKenzie, M. Duncan, E. Allen-Vercoe, M. J. Woodward, H. J. Flint, and G. Grant. 2003. Lack of flagella disadvantages Salmonella enterica serovar Enteritidis during the early stages of infection in the rat. J. Med. Microbiol. 52:91-99. [DOI] [PubMed] [Google Scholar]
- 331.Rodriguez-Boulan, E., G. Kreitze, and A. Musch. 2005. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6:233-247. [DOI] [PubMed] [Google Scholar]
- 332.Roomi, N., M. Laburthe, N. Fleming, R. Crowther, and J. Forstner. 1984. Cholera-induced mucin secretion from rat intestine: lack of effect of cAMP, cycloheximide, VIP, and colchicine. Am. J. Physiol. 247:G140-G148. [DOI] [PubMed] [Google Scholar]
- 333.Sable, S., A. M. Pons, S. Gendron-Gaillard, and G. Cottenceau. 2000. Antibacterial activity evaluation of microcin J25 against diarrheagenic Escherichia coli. Appl. Environ. Microbiol. 66:4595-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sablon, E., B. Contreras, and E. Vandamme. 2000. Antimicrobial peptides of lactic acid bacteria: mode of action, genetics and biosynthesis. Adv. Biochem. Eng. Biotechnol. 68:21-60. [DOI] [PubMed] [Google Scholar]
- 335.Salomon, R. A., and R. N. Farias. 1992. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J. Bacteriol. 174:7428-7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Salzman, N. H., M. M. Chou, H. de Jong, L. Liu, E. M. Porter, and Y. Paterson. 2003. Enteric Salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect. Immun. 71:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522-526. [DOI] [PubMed] [Google Scholar]
- 338.Sands, B. E., and D. K. Podolsky. 1996. The trefoil peptide family. Annu. Rev. Physiol. 58:253-273. [DOI] [PubMed] [Google Scholar]
- 339.Sansonetti, P. J., and A. Phalipon. 1999. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin. Immunol. 11:193-203. [DOI] [PubMed] [Google Scholar]
- 340.Satoh, Y., M. Yamano, M. Matsuda, and K. Ono. 1990. Ultrastructure of Paneth cells in the intestine of various mammals. J. Electron Microsc. Tech. 16:69-80. [DOI] [PubMed] [Google Scholar]
- 341.Savage, D. C. 1977. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31:107-133. [DOI] [PubMed] [Google Scholar]
- 342.Schauber, J., C. Svanholm, S. Termen, K. Iffland, T. Menzel, W. Scheppach, R. Melcher, B. Agerberth, H. Luhrs, and G. H. Gudmundsson. 2003. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut 52:735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Schiffrin, E. J., and S. Blum. 2002. Interactions between the microbiota and the intestinal mucosa. Eur. J. Clin. Nutr. 56(Suppl. 3):S60-S64. [DOI] [PubMed] [Google Scholar]
- 344.Schneeberger, E. E., and R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell. Physiol. 286:C1213-C1228. [DOI] [PubMed] [Google Scholar]
- 345.Schneider, J. J., A. Unholzer, M. Schaller, M. Schafer-Korting, and H. C. Korting. 2005. Human defensins. J. Mol. Med. 83:587-595. [DOI] [PubMed] [Google Scholar]
- 346.Schroder, J. M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 347.Schwalbe, G. 1872. Beitraege zur Kenntris der Druesen in den Darmwandungen, in'sBesondere der Brunner'schen Druesen. Arch. Mikroskop. Anat. 8:92-140. [Google Scholar]
- 348.Seregni, E., C. Botti, S. Massaron, C. Lombardo, A. Capobianco, A. Bogni, and E. Bombardieri. 1997. Structure, function and gene expression of epithelial mucins. Tumori 83:625-632. [DOI] [PubMed] [Google Scholar]
- 349.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 350.Sheehan, J. K., C. Brazeau, S. Kutay, H. Pigeon, S. Kirkham, M. Howard, and D. J. Thornton. 2000. Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem. J. 347:37-44. [PMC free article] [PubMed] [Google Scholar]
- 351.Sherman, M. P., S. H. Bennett, F. F. Hwang, J. Sherman, and C. L. Bevins. 2005. Paneth cells and antibacterial host defense in neonatal small intestine. Infect. Immun. 73:6143-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sherman, P. M., K. C. Johnson-Henry, H. P. Yeung, P. S. Ngo, J. Goulet, and T. A. Tompkins. 2005. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect. Immun. 73:5183-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Shi, Y., M. J. Cromie, F. F. Hsu, J. Turk, and E. A. Groisman. 2004. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53:229-241. [DOI] [PubMed] [Google Scholar]
- 354.Shi, Y., T. Latifi, M. J. Cromie, and E. A. Groisman. 2004. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J. Biol. Chem. 279:38618-38625. [DOI] [PubMed] [Google Scholar]
- 355.Shinnar, A. E., K. L. Butler, and H. J. Park. 2003. Cathelicidin family of antimicrobial peptides: proteolytic processing and protease resistance. Bioorg. Chem. 31:425-436. [DOI] [PubMed] [Google Scholar]
- 356.Shirazi, T., R. J. Longman, A. P. Corfield, and C. S. Probert. 2000. Mucins and inflammatory bowel disease. Postgrad. Med. J. 76:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Silva, M., N. Jacobus, C. Deneke, and S. L. Gorbach. 1987. Antimicrobial substance from a human Lactobacillus strain. Antimicrob. Agents Chemother. 31:1231-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Singh, A. P., N. Moniaux, S. C. Chauhan, J. L. Meza, and S. K. Batra. 2004. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 64:622-630. [DOI] [PubMed] [Google Scholar]
- 359.Smirnova, M. G., L. Guo, J. P. Birchall, and J. P. Pearson. 2003. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 221:42-49. [DOI] [PubMed] [Google Scholar]
- 360.Souza, D. G., A. T. Vieira, A. C. Soares, V. Pinho, J. R. Nicoli, L. Q. Vieira, and M. M. Teixeira. 2004. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J. Immunol. 173:4137-4146. [DOI] [PubMed] [Google Scholar]
- 361.Sperandio, V., C. C. Li, and J. B. Kaper. 2002. Quorum-sensing Escherichia coli regulator A: a regulator of the LysR family involved in the regulation of the locus of enterocyte effacement pathogenicity island in enterohemorrhagic E. coli. Infect. Immun. 70:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 183:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 365.Stappenbeck, T. S., L. V. Hooper, and J. I. Gordon. 2002. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl. Acad. Sci. USA 99:15451-15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sturme, M. H., M. Kleerebezem, J. Nakayama, A. D. Akkermans, E. E. Vaugha, and W. M. de Vos. 2002. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Leeuwenhoek 81:233-243. [DOI] [PubMed] [Google Scholar]
- 367.Su, W. J., P. Bourlioux, M. Bournaud, M. O. Besnier, and J. Fourniat. 1986. Transfer of the cecal flora of the hamster to the germfree C3H mouse: use of this model to study the flora of the anti-Clostridium difficile barrier. Can. J. Microbiol. 32:132-136. [PubMed] [Google Scholar]
- 368.Suico, M. A., T. Koga, T. Shuto, A. Hisatsune, Z. Lu, C. Basbaum, T. Okiyoneda, and H. Kai. 2004. Sp1 is involved in the transcriptional activation of lysozyme in epithelial cells. Biochem. Biophys. Res. Commun. 324:1302-1308. [DOI] [PubMed] [Google Scholar]
- 369.Suico, M. A., Z. Lu, T. Shuto, T. Koga, T. Uchikawa, H. Yoshida, K. Matsuzaki, M. Nakao, J. D. Li, and H. Kai. 2004. The regulation of human beta-defensin 2 by the ETS transcription factor MEF (myeloid Elf-1-like factor) is enhanced by promyelocytic leukemia protein. J. Pharmacol. Sci. 95:466-470. [DOI] [PubMed] [Google Scholar]
- 370.Suico, M. A., H. Yoshida, Y. Seki, T. Uchikawa, Z. Lu, T. Shuto, K. Matsuzaki, M. Nakao, J. D. Li, and H. Kai. 2004. Myeloid Elf-1-like factor, an ETS transcription factor, up-regulates lysozyme transcription in epithelial cells through interaction with promyelocytic leukemia protein. J. Biol. Chem. 279:19091-19098. [DOI] [PubMed] [Google Scholar]
- 371.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Takahashi, A., A. Wada, K. Ogushi, K. Maeda, T. Kawahara, K. Mawatari, H. Kurazono, J. Moss, T. Hirayama, and Y. Nakaya. 2001. Production of beta-defensin-2 by human colonic epithelial cells induced by Salmonella enteritidis flagella filament structural protein. FEBS Lett. 508:484-488. [DOI] [PubMed] [Google Scholar]
- 373.Talham, G. L., H. Q. Jiang, N. A. Bos, and J. J. Cebra. 1999. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 67:1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Tanaka, S., M. Mizuno, T. Maga, F. Yoshinaga, J. Tomoda, J. Nasu, H. Okada, K. Yokota, K. Oguma, Y. Shiratori, and T. Tsuji. 2003. H. pylori decreases gastric mucin synthesis via inhibition of galactosyltransferase. Hepatogastroenterology 50:1739-1742. [PubMed] [Google Scholar]
- 375.Tannock, G. W. 2002. The bifidobacterial and Lactobacillus microflora of humans. Clin. Rev. Allergy Immunol. 22:231-253. [DOI] [PubMed] [Google Scholar]
- 376.Tannock, G. W. 2001. Molecular assessment of intestinal microflora. Am. J. Clin. Nutr. 73:410S-414S. [DOI] [PubMed] [Google Scholar]
- 377.Tepass, U. 2002. Adherens junctions: new insight into assembly, modulation and function. Bioessays 24:690-695. [DOI] [PubMed] [Google Scholar]
- 378.Thornton, D. J., I. Carlstedt, M. Howard, P. L. Devine, M. R. Price, and J. K. Sheehan. 1996. Respiratory mucins: identification of core proteins and glycoforms. Biochem. J. 316:967-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Tollin, M., P. Bergman, T. Svenberg, H. Jornvall, G. H. Gudmundsson, and B. Agerberth. 2003. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 24:523-530. [DOI] [PubMed] [Google Scholar]
- 380.Torres, A. G., X. Zhou, and J. B. Kaper. 2005. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 73:18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Trautmann, M., R. Zick, T. Rukavina, A. S. Cross, and R. Marre. 1998. Antibiotic-induced release of endotoxin: in-vitro comparison of meropenem and other antibiotics. J. Antimicrob. Chemother. 41:163-169. [DOI] [PubMed] [Google Scholar]
- 382.Tsukita, S., M. Furuse, and M. Itoh. 2001. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2:285-293. [DOI] [PubMed] [Google Scholar]
- 383.Uehara, N., A. Yagihashi, K. Kondoh, N. Tsuji, T. Fujita, H. Hamada, and N. Watanabe. 2003. Human beta-defensin-2 induction in Helicobacter pylori-infected gastric mucosal tissues: antimicrobial effect of overexpression. J. Med. Microbiol. 52:41-45. [DOI] [PubMed] [Google Scholar]
- 384.Uribe, A., M. Alam, O. Johansson, T. Midtvedt, and E. Theodorsson. 1994. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 107:1259-1269. [DOI] [PubMed] [Google Scholar]
- 385.Valentijn, A. J., N. Zouq, and A. P. Gilmore. 2004. Anoikis. Biochem. Soc. Trans. 32:421-425. [DOI] [PubMed] [Google Scholar]
- 386.Van Asten, F. J., H. G. Hendriks, J. F. Koninkx, B. A. Van der Zeijst, and W. Gaastra. 2000. Inactivation of the flagellin gene of Salmonella enterica serotype Enteritidis strongly reduces invasion into differentiated Caco-2 cells. FEMS Microbiol. Lett. 185:175-179. [DOI] [PubMed] [Google Scholar]
- 387.van Asten, F. J., H. G. Hendriks, J. F. Koninkx, and J. E. van Dijk. 2004. Flagella-mediated bacterial motility accelerates but is not required for Salmonella serotype Enteritidis invasion of differentiated Caco-2 cells. Int. J. Med. Microbiol. 294:395-399. [DOI] [PubMed] [Google Scholar]
- 388.Van de Bovenkamp, J. H., J. Mahdavi, A. M. Korteland-Van Male, H. A. Buller, A. W. Einerhand, T. Boren, and J. Dekker. 2003. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter 8:521-532. [DOI] [PubMed] [Google Scholar]
- 389.Van den Brink, G. R., K. M. Tytgat, R. W. Van der Hulst, C. M. Van der Loos, A. W. Einerhand, H. A. Buller, and J. Dekker. 2000. H. pylori colocalises with MUC5AC in the human stomach. Gut 46:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A. P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241-250. [DOI] [PubMed] [Google Scholar]
- 391.van Es, J. H., P. Jay, A. Gregorieff, M. E. van Gijn, S. Jonkheer, P. Hatzis, A. Thiele, M. van den Born, H. Begthel, T. Brabletz, M. M. Taketo, and H. Clevers. 2005. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 7:381-386. [DOI] [PubMed] [Google Scholar]
- 392.Van Klinken, B. J., J. Dekker, H. A. Buller, and A. W. Einerhand. 1995. Mucin gene structure and expression: protection vs. adhesion. Am. J. Physiol. 269:G613-G627. [DOI] [PubMed] [Google Scholar]
- 393.van Langevelde, P., K. M. Kwappenberg, P. H. Groeneveld, H. Mattie, and J. T. van Dissel. 1998. Antibiotic-induced lipopolysaccharide (LPS) release from Salmonella typhi: delay between killing by ceftazidime and imipenem and release of LPS. Antimicrob. Agents Chemother. 42:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Vaughan, E. E., F. Schut, H. G. Heilig, E. G. Zoetendal, W. M. de Vos, and A. D. Akkermans. 2000. A molecular view of the intestinal ecosystem. Curr. Issues Intest. Microbiol. 1:1-12. [PubMed] [Google Scholar]
- 395.Vimal, D. B., M. Khullar, S. Gupta, and N. K. Ganguly. 2000. Intestinal mucins: the binding sites for Salmonella typhimurium. Mol. Cell. Biochem. 204:107-117. [DOI] [PubMed] [Google Scholar]
- 396.Vinall, L. E., M. King, M. Novelli, C. A. Green, G. Daniels, J. Hilkens, M. Sarner, and D. M. Swallow. 2002. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology 123:41-49. [DOI] [PubMed] [Google Scholar]
- 397.Vincent, P. A., M. A. Delgado, R. N. Farias, and R. A. Salomon. 2004. Inhibition of Salmonella enterica serovars by microcin J25. FEMS Microbiol. Lett. 236:103-107. [DOI] [PubMed] [Google Scholar]
- 398.von Mensdorff-Pouilly, S., F. G. Snijdewint, A. A. Verstraeten, R. H. Verheijen, and P. Kenemans. 2000. Human MUC1 mucin: a multifaceted glycoprotein. Int. J. Biol. Markers 15:343-356. [DOI] [PubMed] [Google Scholar]
- 399.Vora, P., A. Youdim, L. S. Thomas, M. Fukata, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, A. Wada, T. Hirayama, M. Arditi, and M. T. Abreu. 2004. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J. Immunol. 173:5398-5405. [DOI] [PubMed] [Google Scholar]
- 400.Wada, A., N. Mori, K. Oishi, H. Hojo, Y. Nakahara, Y. Hamanaka, M. Nagashima, I. Sekine, K. Ogushi, T. Niidome, T. Nagatake, J. Moss, and T. Hirayama. 1999. Induction of human beta-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem. Biophys. Res. Commun. 263:770-774. [DOI] [PubMed] [Google Scholar]
- 401.Wada, A., K. Ogushi, T. Kimura, H. Hojo, N. Mori, S. Suzuki, A. Kumatori, M. Se, Y. Nakahara, M. Nakamura, J. Moss, and T. Hirayama. 2001. Helicobacter pylori-mediated transcriptional regulation of the human beta-defensin 2 gene requires NF-kappaB. Cell. Microbiol. 3:115-123. [DOI] [PubMed] [Google Scholar]
- 402.Walters, J. R. 2004. Cell and molecular biology of the small intestine: new insights into differentiation, growth and repair. Curr. Opin. Gastroenterol. 20:70-76. [DOI] [PubMed] [Google Scholar]
- 403.Walters, J. R. 2005. Recent findings in the cell and molecular biology of the small intestine. Curr. Opin. Gastroenterol. 21:135-140. [DOI] [PubMed] [Google Scholar]
- 404.Wang, G., Y. Feng, Y. Wang, N. Huang, Q. Wu, and B. Wang. 2003. Bifidobacterium cell wall proteins induced beta-defensin 2 mRNA expression in human intestinal epithelial cells. Sichuan Da Xue Xue Bao Yi Xue Ban 34:622-624. [PubMed] [Google Scholar]
- 405.Wang, R. Q., and D. C. Fang. 2003. Alterations of MUC1 and MUC3 expression in gastric carcinoma: relevance to patient clinicopathological features. J. Clin. Pathol. 56:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wehkamp, J., K. Fellermann, K. R. Herrlinger, S. Baxmann, K. Schmidt, B. Schwind, M. Duchrow, C. Wohlschlager, A. C. Feller, and E. F. Stange. 2002. Human beta-defensin 2 but not beta-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 14:745-752. [DOI] [PubMed] [Google Scholar]
- 407.Wehkamp, J., J. Harder, K. Wehkamp, B. Wehkamp-von Meissner, M. Schlee, C. Enders, U. Sonnenborn, S. Nuding, S. Bengmark, K. Fellermann, J. M. Schroder, and E. F. Stange. 2004. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect. Immun. 72:5750-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Wehkamp, J., K. Schmidt, K. R. Herrlinger, S. Baxmann, S. Behling, C. Wohlschlager, A. C. Feller, E. F. Stange, and K. Fellermann. 2003. Defensin pattern in chronic gastritis: HBD-2 is differentially expressed with respect to Helicobacter pylori status. J. Clin. Pathol. 56:352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Wehkamp, J., B. Schwind, K. R. Herrlinger, S. Baxmann, K. Schmidt, M. Duchrow, C. Wohlschlager, A. C. Feller, E. F. Stange, and K. Fellermann. 2002. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig. Dis. Sci. 47:1349-1355. [DOI] [PubMed] [Google Scholar]
- 410.Williams, S. J., M. A. McGuckin, D. C. Gotley, H. J. Eyre, G. R. Sutherland, and T. M. Antalis. 1999. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 59:4083-4089. [PubMed] [Google Scholar]
- 411.Williams, S. J., D. J. Munster, R. J. Quin, D. C. Gotley, and M. A. McGuckin. 1999. The MUC3 gene encodes a transmembrane mucin and is alternatively spliced. Biochem. Biophys. Res. Commun. 261:83-89. [DOI] [PubMed] [Google Scholar]
- 412.Williams, S. J., D. H. Wreschner, M. Tran, H. J. Eyre, G. R. Sutherland, and M. A. McGuckin. 2001. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 276:18327-18336. [DOI] [PubMed] [Google Scholar]
- 413.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 414.Witthoft, T., L. Eckmann, J. M. Kim, and M. F. Kagnoff. 1998. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am. J. Physiol. 275:G564-G571. [DOI] [PubMed] [Google Scholar]
- 415.Xu, J., M. K. Bjursell, J. Himrod, S. Deng, L. K. Carmichael, H. C. Chiang, L. V. Hooper, and J. I. Gordon. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299:2074-2076. [DOI] [PubMed] [Google Scholar]
- 416.Zahraoui, A., D. Louvard, and T. Galli. 2000. Tight junction, a platform for trafficking and signaling protein complexes. J. Cell Biol. 151:F31-F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Zaiou, M., V. Nizet, and R. L. Gallo. 2003. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Investig. Dermatol. 120:810-816. [DOI] [PubMed] [Google Scholar]
- 418.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 419.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]
- 420.Zanetti, M., R. Gennaro, M. Scocchi, and B. Skerlavaj. 2000. Structure and biology of cathelicidins. Adv. Exp. Med. Biol. 479:203-218. [DOI] [PubMed] [Google Scholar]
- 421.Zareie, M., J. Riff, K. Donato, D. M. McKay, M. Perdue, J. D. Soderlholm, M. Karmali, M. B. Cohen, J. Hawkins, and P. M. Sherman. 2005. Novel effects of the prototype translocating Escherichia coli, strain C25 on intestinal epithelial structure and barrier function. Cell. Microbiol. 7:1782-1797. [DOI] [PubMed] [Google Scholar]
- 422.Zboril, V. 2002. Physiology of microflora in the digestive tract. Vnitr. Lek. 48:17-21. [PubMed] [Google Scholar]
- 423.Zhan, M., H. Zhao, and Z. C. Han. 2004. Signalling mechanisms of anoikis. Histol. Histopathol. 19:973-983. [DOI] [PubMed] [Google Scholar]
- 424.Zhang, J., and K. L. Carraway. 2002. A new monoclonal antibody demonstrates that MUC4, the intramembrane ligand for ErbB2/HER2/Neu, is overexpressed in multiple carcinomas. Sci. World J. 2(Suppl. 2):140-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Zhao, C., I. Wang, and R. I. Lehrer. 1996. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 396:319-322. [DOI] [PubMed] [Google Scholar]
- 426.Zhou, X., J. A. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Zhu, X., S. A. Price-Schiavi, and K. L. Carraway. 2000. Extracellular regulated kinase (ERK)-dependent regulation of sialomucin complex/rat Muc4 in mammary epithelial cells. Oncogene 19:4354-4361. [DOI] [PubMed] [Google Scholar]
- 428.Zucht, H. D., J. Grabowsky, M. Schrader, C. Liepke, M. Jurgens, P. Schulz-Knappe, and W. G. Forssmann. 1998. Human beta-defensin-1: A urinary peptide present in variant molecular forms and its putative functional implication. Eur. J. Med. Res. 3:315-323. [PubMed] [Google Scholar]