Abstract
Rotaviruses infect mature, differentiated enterocytes of the small intestine and, by an unknown mechanism, escape the gastrointestinal tract and cause viremia. The neonatal rat model of rotavirus infection was used to determine the kinetics of viremia, spread, and pathology of rotavirus in extraintestinal organs. Five-day-old rat pups were inoculated intragastrically with an animal (RRV) or human (HAL1166) rotavirus or phosphate-buffered saline. Blood was collected from a subset of rat pups, and following perfusion to remove residual blood, organs were removed and homogenized to analyze rotavirus-specific antigen by enzyme-linked immunosorbent assay and infectious rotavirus by fluorescent focus assay or fixed in formalin for histology and immunohistochemistry. Viremia was detected following rotavirus infection with RRV and HAL1166. The RRV 50% antigenemia dose was 1.8 × 103 PFU, and the 50% diarrhea dose was 7.7 × 105 PFU, indicating that infection and viremia occurred in the absence of diarrhea and that detecting rotavirus antigen in the blood was a more sensitive measure of infection than diarrhea. Rotavirus antigens and infectious virus were detected in multiple organs (stomach, intestines, liver, lungs, spleen, kidneys, pancreas, thymus, and bladder). Histopathological changes due to rotavirus infection included acute inflammation of the portal tract and bile duct, microsteatosis, necrosis, and inflammatory cell infiltrates in the parenchymas of the liver and lungs. Colocalization of structural and nonstructural proteins with histopathology in the liver and lungs indicated that the histological changes observed were due to rotavirus infection and replication. Replicating rotavirus was also detected in macrophages in the lungs and blood vessels, indicating a possible mechanism of rotavirus dissemination. Extraintestinal infectious rotavirus, but not diarrhea, was observed in the presence of passively or actively acquired rotavirus-specific antibody. These findings alter the previously accepted concept of rotavirus pathogenesis to include not only gastroenteritis but also viremia, and they indicate that rotavirus could cause a broad array of systemic diseases in a number of different organs.
Rotaviruses, responsible for most cases of gastroenteritis in children under the age of five worldwide, have been thought to cause mucosal infections restricted to the mature, differentiated enterocytes of the small intestine. However, an increasing number of reports indicate that rotavirus escapes the gastrointestinal tract. Rotavirus antigen and RNA were detected in serum samples from approximately 65% of children with rotavirus diarrhea, indicating that antigenemia and possibly viremia occur during rotavirus infection (4, 7, 16). In other reports, rotavirus antigen and/or RNA was detected in the central nervous systems, spleens, hearts, kidneys, testes, and bladders of children who died during rotavirus infections (23, 29-32, 35, 42); in liver biopsies from infants with cholestatic disease (47); and in respiratory secretions, lung cells, or the microvasculature of hearts from children and adults with respiratory infections or cardiorespiratory failure (11, 41, 48, 56) and rotavirus gastroenteritis. These case reports support the concept that rotavirus can escape the intestine and possibly infect cells in a variety of organs, but the sites and prevalence of extraintestinal infection and whether rotavirus can be the etiologic agent of extraintestinal disease have not been established.
The detection of extraintestinal rotavirus has also been described for animals. Antigen or infectious rotavirus has been detected in the sera, nasal secretions, lungs, livers, kidneys, or brains of mice (26, 27, 50), rabbits, cows, rats (4), pigs (1), and monkeys (55). Since infectious rotavirus was detected in the blood or serum of many of these animals, detection of infectious virus in the various organs may have been due to contaminating blood in those organs. However, the development of a mouse model for rotavirus-induced biliary atresia (BA) and hepatitis suggests that rotavirus infection can cause clinical diseases other than gastroenteritis in animals (14, 43, 46, 50).
The detection of rotavirus viremia or antigenemia and rotavirus RNA or antigen in nonintestinal tissues in both children and animals and the ability of rotavirus to replicate in a variety of different cell types, including cells from the cervix, breasts, bone, lungs, prostate, and ovaries (9), led us to question if rotavirus infection and histopathology occur regularly in multiple tissues or organs in vivo outside the gastrointestinal tract. To address these questions, we used the neonatal rat model of rotavirus infection. The neonatal rat model is well suited for these studies because it resembles rotavirus-induced disease in children; rat pups can be infected with multiple human and animal rotavirus strains representing different serotypes and can be reinfected following a primary rotavirus inoculation (8; M. Ciarlet, S. E. Crawford, M. E. Conner, and M. K. Estes, submitted for publication). Following inoculation of neonatal rats with either a sialic acid-dependent simian (RRV) or a sialic acid-independent human (HAL1166) rotavirus, we examined the extents of antigenemia or viremia and the distributions and tissue and cell tropisms of rotavirus infections. We also examined if infectious virus could be detected extraintestinally following a second rotavirus infection in the presence of rotavirus-specific antibody.
MATERIALS AND METHODS
Viruses.
The simian rhesus rotavirus (RRV) (P5B[3], G3, isolated from the feces of a 3 1/2-month-old rhesus monkey with diarrhea) and human HAL1166 (P11[14], G8, isolated from an infant with diarrhea from Finland) strains were kindly supplied by H. B. Greenberg (Stanford University Medical School, Palo Alto, CA) and by Giuseppe Gerna (Istituto di Recovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy), respectively, and were cultivated in African green monkey kidney cells (MA104) and RAW 264.7 cells in the presence of trypsin as described previously (13).
Animals.
Two-month-old inbred Lewis (LEW/SsNHsd) or outbred Sprague-Dawley rat dams with 4-day-old litters were obtained from Harlan (Houston, TX). Rat pups from the different litters were randomly distributed among 40 dams, with seven or eight pups per dam, to reduce confounding effects of litter size and maternal care. Rat dams and control and inoculated pups were housed in microisolator cages in the same room under negative pressure in a biosafety level 2 containment facility at Baylor College of Medicine. Prior to inoculation, all rats were rotavirus antibody negative at a serum dilution of 1:50, as determined by enzyme-linked immunosorbent assay (ELISA) as previously described (12), but using rabbit anti-rat immunoglobulin-horseradish peroxidase (Sigma-Aldrich, St. Louis, Missouri) at a dilution of 1:3,000.
Animal inoculation.
Five-day-old pups were inoculated by oral gavage with 0.5 ml phosphate-buffered saline (PBS), 6.75 × 108 PFU of RRV, or 1.45 × 108 PFU of HAL1166 rotavirus. Control animals were always handled prior to virus-inoculated animals. To determine the serum 50% antigenemia dose (AD50) and the 50% diarrhea dose (DD50) of RRV, rat pups were inoculated with 0.5 ml of serial 10-fold dilutions of RRV. For a subset of experiments, the pups were examined daily for evidence of rotavirus-induced diarrhea by gentle abdominal palpation. Diarrhea was noted and scored from 0 to 4 based on stool color, amount, and consistency (2, 8). A score of 2 or greater was considered diarrhea.
To determine if extraintestinal infectious virus could be detected in the presence of rotavirus-specific maternal antibody, 5-day-old female pups were infected with RRV by oral gavage as described above. At approximately 60 days of age, the female rotavirus antibody-positive rats were bred, and their pups were inoculated by oral gavage with PBS or RRV as described above.
Sample collection and processing.
To determine the kinetics of antigenemia or viremia and tissue tropism, two RRV- or PBS-inoculated pups from each of 10 dams were decapitated at 48 and 96 h postinfection (hpi) and one RRV- or PBS-inoculated pup from each of 5 dams was decapitated at 24, 72, and 216 hpi, and blood was collected. Prior to dissection, the skin of each pup was washed with 70% ethanol to remove any contaminating fecal material or blood from the body of the pup. An initial incision was made to expose both the thoracic and abdominal cavities. Perfusion with PBS was performed to remove residual blood to the extent possible prior to the removal of organs by cross-clamping the neck of the pup to make an intact circulatory circuit, inserting a 23-gauge needle into a small incision in the left ventricle, and perfusing 20 ml of PBS through the circulatory system. Perfusion was deemed successful if the lungs and liver expanded and became pale. Following perfusion, the thymus, heart, and lungs were removed. Next, the abdominal organs were removed in the following order: pancreas, liver, spleen, and kidneys. In some experiments, urine was extracted from the bladder using a 23-gauge needle, and then the bladder was removed. The stomach and intestines were removed last. Samples were collected from PBS-inoculated rat pups prior to RRV-infected animals. New gloves were used to handle each PBS- or RRV-inoculated animal. The instruments were washed in ethanol and then in PBS after each organ was removed.
Sera and brains were collected from only a subset of animals. Blood was obtained by heart puncture. Prior to removal of the brain, an incision was made to expose the carotid artery, the jugular vein was cut, and then 20 ml of PBS was perfused through the carotid artery and out the jugular vein.
In a subset of experiments, the excised organs were divided into two portions, one for homogenization and one for immunohistochemistry. The weight of the section destined for homogenization was determined prior to immediate freezing. Tissues were homogenized in PBS (1.6 ml/g for livers, 1 ml/g for lungs and brains, 1.25 ml/g for spleens, 2.5 ml/g for kidneys, thymuses, bladders, and pancreases, and 2 ml/g for hearts, stomachs, and intestines) containing protease inhibitors (1 μg/ml aprotinin, 1 mM benzamidine, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A) with a PCR tissue homogenizer (Cole-Parmer, Vernon Hills, IL). The organs from the PBS-inoculated pups were homogenized before the organs from the RRV-inoculated pups. A new homogenizer tip and vial were used for each organ.
Detection of rotavirus antigen by ELISA.
Samples were evaluated for the presence of rotavirus antigen by ELISA. ELISAs were performed in 96-well polyvinyl chloride microtiter plates (Dynatech, McLean, VA). The plates were coated with mouse ascites (diluted 1:20,000) containing anti-VP6 6E7 monoclonal antibody in carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at room temperature. The plates were blocked with 200 μl of 5% BLOTTO (5% [wt/vol] Carnation powdered milk in PBS) for 1 h at 37°C. Following removal of the BLOTTO, the plates were washed three times with 0.05% Tween 20 in PBS. The rat samples were treated with 50 mM EDTA at room temperature for 20 min prior to loading of 30 μl into each well. Hyperimmune guinea pig serum to ALA rotavirus diluted (1:2,000) in 0.5% BLOTTO was used as the detector antibody. Horseradish peroxidase-conjugated goat anti-guinea pig immunoglobulin G (Sigma) diluted (1:1,000) in 0.5% BLOTTO with 2.5% fetal calf serum was used as the conjugate. The substrate used in all assays was the TMB Microwell ELISA substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). The substrate was allowed to react for 10 min at room temperature, and the reaction was stopped by the addition of an equivalent volume of 1 M H3PO4. Optical densities (ODs) at 450 nm were determined with a Flow Titertech Multiscan Plus plate reader (Flow Laboratories, Inc., McLean, VA). An individual cutoff value was determined for each tissue as the mean of the ODs of the PBS control samples plus 3 standard deviations (see figures for values). A sample was considered positive if the OD of the well containing a sample of homogenized organ from an infected animal minus the OD of the well lacking antigen was greater than the cutoff value for that organ.
Detection of infectious virus by FFA.
Samples of serum or homogenized organs from PBS- or RRV-inoculated animals were treated with 10 μg/ml trypsin for 30 min at 37°C. Trypsin-activated samples were passaged once in MA104 cells or tested directly for infectious virus by a fluorescent focus assay (FFA) as previously described (10).
Evaluation of histology and immunocytochemistry.
To evaluate histopathology, each tissue was fixed in 10% formalin, transferred to 70% graded ethanol for dehydration, and subsequently embedded in paraffin. Sections of a pellet of RRV-infected MA104 cells embedded in paraffin were used as a positive control for all immunocytochemistry experiments (19). Conditions for immunocytochemistry were optimized for each tissue, using rabbit anti-NSP4 114-135 peptide or mouse anti-2/6-VLP serum. Macrophages were identified in sections, using mouse anti-rat CD68 (Serotec, Raleigh, NC) at a 1:1,000 dilution. Vectastain Elite ABC or alkaline phosphatase ABC kits (Vector Laboratories, Burlingame, CA) and liquid 3,3′-diaminobenzidine (BioGenex, San Ramon, CA) were used as recommended by the manufacturer.
Statistical analysis.
Statistical analyses were performed using SPSS, version 7.5, for Windows (SPSS, Inc., Chicago, IL). Correlation coefficients were calculated using Pearson's correlation coefficient.
RESULTS
Clinical disease in rat pups.
Rotavirus-induced diarrhea was assessed in rat pups by using a grading scale established previously for scoring stool consistency and color from 0 to 4, with a score of ≥2 indicating diarrhea (2, 8). Diarrhea was not observed in any of the PBS-inoculated rat pups. Following inoculation with 6.75 × 108 PFU of RRV, 89% of the RRV-inoculated rat pups had diarrhea between days 1 and 5, and 56% of those animals had diarrhea for more than 1 day. Fourteen percent of pups had a diarrhea score of 2 to 2.5, and 75% of pups had a diarrhea score of ≥3. None of the rat pups displayed any symptoms of encephalopathy, demonstrated convulsions, or showed any other discernible signs of systemic disease, although one of the RRV-inoculated pups appeared jaundiced (had yellow-tinted skin).
Detection of viral antigen and infectious virus in sera of RRV-inoculated rat pups.
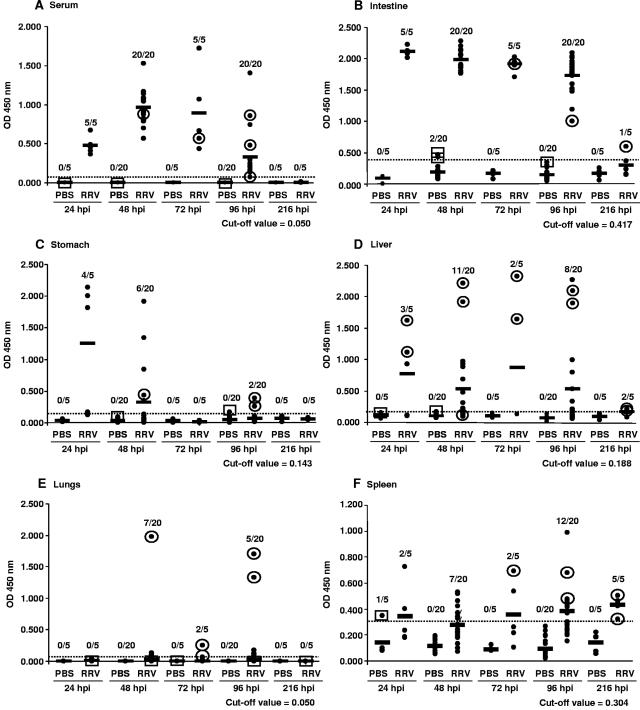
To determine if rotavirus was disseminated in the serum and organs, 5-day-old rat pups inoculated with 6.75 × 108 PFU of RRV or PBS were euthanized at 24, 48, 72, 96, or 216 hpi. Rotavirus antigen was detected by ELISA in the sera of the rotavirus-infected rat pups between 24 and 96 hpi (Fig. 1A). Rotavirus antigen was detected in the sera from all RRV-inoculated pups at 24 hpi. While the highest observed levels of antigen were detected at 48 to 72 hpi (P < 0.01, Mann-Whitney U test), antigen was still consistently detected at 96 hpi and was below the cutoff of detection by 216 hpi. Rotavirus antigen was not detected in sera from any of the PBS-inoculated pups. To determine if the detected antigen represented infectious virus, five serum samples from rotavirus-inoculated rat pups, including a sample from 96 hpi with a low but detectable level of rotavirus antigen, were assayed by FFA. Each of these samples contained infectious rotavirus, whereas infectious virus was not detected in any of three serum samples tested from PBS-inoculated rat pups, even after passage of the samples once in MA104 cells to allow amplification of possible small amounts of infectious virus (Fig. 1A). The titer of infectious virus, determined for two serum samples (OD, 0.56 at 72 hpi and 0.85 at 96 hpi) by FFA without passage of the virus through MA104 cells, was 103 FFU/ml. Rotavirus antigenemia was not rat strain specific, as outbred Sprague-Dawley rat pups also developed antigenemia between 24 and 72 hpi (data not shown).
FIG. 1.
Kinetics of rotavirus-specific antigen detection by ELISA and infectious virus detection by FFA in PBS- or RRV-inoculated rat pups. (A) Serum; (B) intestines; (C) stomach; (D) liver; (E) lungs; (F) spleen. Each dot represents an individual rat pup in each group, and each solid horizontal line represents the average OD value of each group at the indicated time point. The numbers above each column indicate the number of ELISA positive samples over the number of samples tested. The negative cutoff value for each organ, represented by the horizontal dotted line on each graph and the indicated numeric value, was determined by calculating 3 standard deviations above the mean of the OD values of the PBS control samples. The infectivities of samples were determined either by direct FFA or by passage of the sample on MA104 cells prior to FFA. Detection (circle around the dot) or no detection (box around the dot) of infectious virus is indicated for each sample tested.
DD50 versus AD50.
The 50% infectious dose of rotavirus for mouse and rat pups has historically been determined by the presence of diarrhea. However, the evaluation of diarrhea can be subjective. Following inoculation with 6.75 × 108 PFU of RRV, only 89% of the RRV-inoculated rat pups had diarrhea, whereas rotavirus antigen in blood was detected in 100% of these pups, indicating that all the pups were infected with rotavirus and that antigenemia might be a more sensitive measure of infection than the presence of diarrhea. We therefore determined the DD50 and the AD50 for RRV in rat pups.
To determine the DD50 and AD50, rat pups were inoculated with serial 10-fold dilutions of RRV. For the DD50, diarrhea was scored for the first 3 days postinoculation. For the AD50, serum was collected at 48 hpi and assayed for the presence of rotavirus antigen. Antigen and diarrhea were assessed only at these early time points to rule out possible secondary infections between littermates. Using the Reed-Muench calculation, the DD50 of the RRV preparation used in these experiments was 7.7 × 105 PFU, while the AD50 for the same virus preparation was over 2 logs lower, at 1.8 × 103 PFU.
Distribution of viral antigen in tissues of RRV-inoculated rat pups.
The detection of rotavirus antigen and infectious virus in the serum confirmed previous reports of antigenemia and possible viremia in mice (27), rats, rabbits, cows (4), pigs (1), and monkeys (55) and suggested that infectious virus could disseminate to and possibly infect a wide variety of organs. Therefore, the intestines, stomachs, livers, lungs, spleens, and kidneys from the rotavirus-infected and control animals previously tested for rotavirus antigenemia and viremia were homogenized and tested for rotavirus antigen by ELISA and infectious virus by FFA. Contaminating blood was removed from organs by perfusion prior to organ dissection from the animal. All nonintestinal organs were dissected and removed from the animals prior to removal of the stomach and intestines to reduce the possibility of viral contamination from the gastrointestinal tract. To allow comparison of the amounts of antigen present in each organ from the different animals, the amount of homogenization buffer added to each tissue was standardized according to the weight of the organ. Additionally, to determine if the antigen detected by ELISA represented infectious virus, the samples were passaged in MA104 cells and then assayed by FFA. In some cases, the samples were assayed directly, and a titer was obtained.
(i) Intestines.
High levels of rotavirus antigen were found in homogenates of combined small and large intestines in all the RRV-inoculated animals at 24 hpi, the levels remained high through 96 hpi (Fig. 1B), and by 216 hpi, antigen was only detected in 1/5 pups. Infectious virus was detected in the three intestinal samples tested from RRV-inoculated rat pups, including the one sample from 216 hpi that was positive for rotavirus antigen (Fig. 1B). Rotavirus antigen was not detected in any of the PBS-inoculated pups, with the exception of two animals whose samples at 48 hpi had OD values slightly above the established cutoff. Infectious virus was not detected in either of these samples or another sample tested from a control animal.
(ii) Stomach.
Viral antigen was detected in the stomachs of 4/5 of the RRV-inoculated animals at 24 hpi and was less consistently detected at 48 hpi (6/20 animals) (Fig. 1C). Detection of viral antigen at these early time points was most likely from the inoculum. No antigen was detected by 72 hpi; however, at 96 hpi, low levels of antigen were detected in the stomachs of 2/20 rat pups. Infectious rotavirus was detected in a sample from 48 hpi and in two samples from 96 hpi that were rotavirus antigen positive, but not in either of the two PBS-inoculated rat pups tested.
(iii) Liver.
Rotavirus antigen was detected in the liver at all time points (24 to 216 hpi), but only in approximately 50% of RRV-inoculated rat pups at each time point (Fig. 1D). The titers of infectious rotavirus in the liver ranged from 0.6 × 103 to 1.1 × 104 FFU/g for two samples from each of the 24-, 48-, 72-, and 96-hpi time points. Infectious rotavirus was also detected following passage in MA104 cells of samples collected from infected pups at 48 hpi and at 216 hpi that had ELISA OD values slightly below the established cutoff, but not in samples from PBS-inoculated pups.
(iv) Lungs.
Viral antigen was detected in the lungs from a subset of infected rat pups at 48, 72, and 96 hpi (Fig. 1E). It is unlikely that the high antigen levels detected at 96 hpi were due to blood contamination of the lungs because in addition to being perfused, these two pups had low serum antigen levels (ELISA ODs, 0.208 and 0.132). The three pups with high rotavirus antigen levels in the lungs at 48 and 96 hpi also had the highest antigen levels in the stomach at these time points (ELISA ODs, 1.910 and 1.975 for one pup at 48 hpi and 0.385 and 1.699 for one pup and 0.255 and 1.330 for one pup at 96 hpi [values for stomach and lungs, respectively]). However, at 24 hpi, when 3/5 pups had high levels of antigen in their stomachs, antigen was not detected in any of the lungs from these animals. Infectious virus was detected in the antigen-positive samples, and the titers of virus from the samples collected at 48 and 96 hpi with the highest levels of antigen were 4.4 × 103 and 0.8 ×103 FFU/g, respectively.
(v) Spleen.
Rotavirus antigen was detected in <50% of spleen samples at 24 to 72 hpi but in 60% (12/20) and 100% (5/5) of the samples at 96 and 216 hpi, respectively (Fig. 1F). Infectious virus was detected in spleen samples from the RRV- but not the PBS-inoculated pups.
(vi) Kidneys.
For the kidneys, viral antigen was detected in a subset of the pups at 24 to 96 hpi. By comparison to the cutoff value of 0.050, the numbers of positive samples/numbers of samples tested (OD ranges of positive samples and medians of positive OD values, respectively) were 1/5 (0.408) at 24 hpi, 13/20 (0.056 to 0.144, 0.098) at 48 hpi, 2/5 (0.088 and 0.238) at 72 hpi, and 3/20 (0.129 to 0.320, 0.195) at 96 hpi. Infectious virus was detected in the kidney samples with the highest OD values from RRV-inoculated pups at 48, 72, and 96 hpi (one, two, and one pup tested for each time point, respectively) following one blind passage in MA104 cells to amplify the virus in the samples (data not shown).
(vii) Brain.
The presence of rotavirus in the brains of infected rat pups was assessed at 48 and 96 hpi for five PBS- and RRV-inoculated pups. None of the brain samples from PBS- or RRV-inoculated pups had detectable viral antigen by ELISA or FFA after passage of the samples in MA104 cells to amplify possible infectious virus, although rotavirus antigen was detected in the serum of each of the same RRV-inoculated pups (data not shown).
(viii) Thymus, heart, pancreas, bladder, and urine.
Since antigen was detected in every organ tested, except the brain, in at least a subset of all the RRV-inoculated rat pups, an experiment was performed by inoculating 5-day-old rat pups with PBS or 6.75 × 108 PFU of RRV to test for antigen in other tissues and fluids (thymus, heart, pancreas, bladder, and urine) at 48 and 96 hpi. Rotavirus antigen detection was confirmed in the serum, liver, lungs, kidneys, and stomach for similar percentages of RRV-inoculated rat pups (Table 1) to those reported above. Additionally, rotavirus antigen was detected in the bladders, thymuses, hearts, and pancreases from RRV- but not PBS-inoculated rat pups. Urine could only be collected from six inoculated animals, and rotavirus antigen was detected in urine samples from 2/6 RRV-inoculated animals at 48 hpi. The urines from the two pups that tested positive for antigen had the highest antigen levels detected in the bladder at the same time point. The detection of rotavirus antigen in the urine suggested a route, other than fecal/oral, of rotavirus transmission. Samples from a subset of RRV- and PBS-inoculated pups were assayed for infectious virus after amplification once in MA104 cells. Infectious virus was detected in samples from the bladder, thymus, and pancreas (Table 1). No infectious virus was detected in the urine samples from the RRV-inoculated pups or in any of the samples from the PBS-inoculated pups.
TABLE 1.
Detection of rotavirus antigen or infectious virus in samples from rotavirus-infected rat pups
| Sample | No. of rotavirus-positive samples/no. of samples testeda
|
|||
|---|---|---|---|---|
| ELISA
|
FFA
|
|||
| 48 hpi | 96 hpi | 48 hpi | 96 hpi | |
| Serum | 10/10 | 10/10 | NT | NT |
| Liver | 5/10 | 3/10 | NT | NT |
| Lungs | 3/10 | 3/10 | NT | NT |
| Kidneys | 2/10 | 4/10 | NT | NT |
| Stomach | 3/10 | 0/10 | NT | NT |
| Bladder | 4/10 | 1/10 | 1/1 | 1/1 |
| Thymus | 2/10 | 0/10 | 1/1 | NT |
| Heart | 2/10 | 3/10 | NT | 0/1 |
| Pancreas | 2/10 | 3/10 | 1/1 | 0/1 |
| Urine | 2/6 | 0/2 | 0/2 | NT |
NT, not tested.
Correlation of levels of antigen detected in different organs.
Detection of rotavirus antigen in sera from 100% of RRV-inoculated animals but in only a subset of extraintestinal organs from those same animals suggested a dissemination of rotavirus through the blood. Therefore, we determined whether the antigen levels at 48 or 96 hpi correlated between different samples (serum, intestine, liver, spleen, lung, kidney, and stomach samples from the two experiments; n = 30 per time point) to evaluate dissemination. This correlation was possible because all organs were homogenized with a standard concentration (vol/wt) of buffer. The level of rotavirus antigen detected in serum correlated weakly at 48 hpi but strongly at 96 hpi with the level of antigen detected in the liver and kidneys (correlation coefficient, 0.473 and 0.503, respectively, at 48 hpi and 0.906 and 0.937, respectively, at 96 hpi; P < 0.01). Correspondingly, antigen levels in the liver and kidneys also correlated with each other at 96 hpi (0.846; P < 0.01). These correlations suggest that virus may be disseminated through the blood to the liver and kidneys at each time point and, in addition, that the antigen detected at 96 hpi in the serum may be due to the release of virus replicating in the liver and kidneys into the circulatory system. The levels of rotavirus in the stomach correlated with the levels of antigen in the lungs at both 48 and 96 hpi (0.681 and 0.923, respectively; P < 0.01). This correlation suggests that rotavirus detected in the stomach was from the respiratory tract.
Histologic changes in organs from RRV-inoculated rat pups.
We next analyzed paraffin-embedded sections of organs from PBS- or RRV-inoculated pups containing the highest levels of rotavirus antigen detected by ELISA for the presence of histopathology by hematoxylin and eosin staining. Histological changes were observed in the liver and lungs (Fig. 2A to I) but not in other organs.
FIG. 2.
Histopathology and rotavirus antigen detection in the livers and lungs of PBS- and RRV-inoculated rat pups. Hematoxylin- and eosin-stained liver sections from RRV-inoculated rat pups at 48 hpi are shown in panels A to G. (A) Acute inflammation of the central vein (CV) and portal tract (portal vein [PV]); (B) increased magnification of panel A showing inflammation of the portal vein (PV), portal arteries (PA), and bile duct (BD); (C) microsteatosis in zone 3 (circled areas); (D) increased magnification of the boxed area of microsteatosis shown in panel C; (E) focal parenchymal necrosis; (F) serial section of the area of necrosis shown in panel E stained with anti-NSP4, with immunoperoxidase detection; (G) infiltrates of acute inflammatory cells in the necrotic parenchyma of the liver (arrows). Hematoxylin- and eosin-stained lung sections are shown in panels H and I. (H) Infiltrates of inflammatory cells in RRV-inoculated rat pups at 48 hpi; (I) normal lung morphology in PBS-inoculated rat pups at 48 hpi. Immunoperoxidase detection of rotavirus antigen is shown for serial sections of RRV-inoculated pup livers at 96 hpi stained with (J) anti-2/6-VLP and (K) anti-NSP4 antibody. The boxed area shows microsteatosis due to rotavirus infection, and the arrow indicates infiltrates of acute inflammatory cells. (L) Anti-NSP4-stained liver from PBS-inoculated pup at 48 hpi. Magnification: A, H, and I, ×50; B, ×400; C, ×100; D, ×1,000; E to G, ×250; J to L, ×10.
(i) Liver.
Histological changes were noted in all livers from eight (at 48 hpi [n = 6] and 96 hpi [n = 2]) RRV-inoculated rat pups (Fig. 2A to G) but in none of the livers from four (at 48 hpi [n = 2] and 96 hpi [n = 2]) PBS-inoculated rat pups (data not shown). Acute inflammation was observed in the portal tracts and bile ducts of RRV-inoculated rat pups (Fig. 2A and B). Microvesicular steatosis, necrosis, and an increase in inflammatory cells in the parenchyma of the liver were observed in RRV-infected rat pups at 48 and 96 hpi (Fig. 2C to G).
(ii) Lungs.
An increase in cellularity was observed in lung tissues from rotavirus-positive rat pups at 48 (n = 2) and 96 (n = 1) hpi compared to lung sections from two (48 hpi) PBS-inoculated pups (Fig. 2H and I, respectively [48 hpi]). The increased cellularity appeared to be due to infiltration of inflammatory cells, morphologically identified as lymphocytes and macrophages, into the alveolar interstitium.
Rotavirus replication in organs from RRV-inoculated rat pups.
The detection of rotavirus antigen or infectious virus in homogenized tissue suggested the presence of infectious rotavirus at extraintestinal sites. Therefore, we next examined the cellular localization of rotavirus and whether rotavirus was replicating in the heart, spleen, thymus, or stomach and was associated with the histopathology observed in the liver and lungs. To determine the distribution and localization of rotavirus antigen in ELISA-positive tissues, paraffin-embedded sections were stained with an anti-2/6-VLP serum to detect structural proteins or with an anti-NSP4 114-135 peptide serum to detect a nonstructural protein as an indicator of viral replication. Rotavirus antigen was detected in livers, lungs, hearts, spleens, and thymuses but not in stomachs or in any organs of PBS-inoculated controls (Fig. 2F and J to L and 3A to I and N).
FIG. 3.
Detection of rotavirus antigen in lungs, hearts, thymuses, spleens, macrophages in lungs, and livers and colocalization of rotavirus antigen and macrophages in PBS- or RRV-inoculated rat pups. Immunoperoxidase detection of rotavirus antigen by anti-NSP4 antibody was done for lung sections of (A) RRV- or (B) PBS-inoculated rat pups at 96 hpi (arrows indicate morphologically identified macrophages), heart sections from (C) RRV- (arrow indicates stained cells) or (D) PBS-inoculated rat pups at 96 hpi, thymus sections from (E) RRV- or (F) PBS-inoculated rat pups at 96 hpi, spleen sections from (G) RRV- or (H) PBS-inoculated rat pups at 96 hpi, and (I) a macrophage in a blood vessel in a section of thymus from an RRV-inoculated rat pup at 96 hpi (arrow). Immunoperoxidase detection of macrophages by anti-CD68 staining was done for lung sections from (J) RRV- or (K) PBS-inoculated rat pups at 96 hpi (arrows indicate macrophages) and liver sections from (L) RRV- or (M) PBS-inoculated rat pups at 96 hpi. (N) Immunofluorescence detection of NSP4 and the macrophage marker CD68 in a lung section from an RRV-inoculated rat pup at 96 hpi, pseudocolored for NSP4 in green, for macrophages in red, and for nuclei in blue. Colocalization of NSP4 and the macrophage marker is shown in yellow. Arrows show pneumocytes infected with RRV, as detected by NSP4 staining. Magnification: A and B, ×1,000; J and K, ×300; C, D, I, and N, ×500; E, F, G, and H, ×400; L and M, ×10.
(i) Liver and lungs.
Serial sections of liver from 5/5 RRV-inoculated rat pups showed similar patterns of staining with anti-2/6-VLP or anti-NSP4 serum (Fig. 2J and K, respectively), but no rotavirus antigen was detected in liver sections from PBS-inoculated pups (Fig. 2L). This demonstrated the specificity of staining and that rotavirus was replicating in the livers of RRV-inoculated rat pups. Hepatocytes surrounding the central vein and portal tracts were positive for rotavirus antigen (Fig. 2J and K). Rotavirus NSP4 antigen was detected in a serial section of liver with necrosis (Fig. 2F, which shows a serial section of Fig. 2E), microsteatosis, and increased inflammatory cells (Fig. 2K, boxed area and arrow, respectively), suggesting that these histological changes were the direct result of rotavirus replication in these cells. Structural and nonstructural proteins were also detected in lung sections from RRV-inoculated pups (Fig. 3A, only anti-NSP4 is shown) but not from PBS-inoculated rats (Fig. 3B), indicating the replication of rotavirus in lung cells and morphologically identified macrophages (Fig. 3A, arrows).
(ii) Heart.
NSP4 was detected in only a few cells in heart tissue from an RRV-inoculated (Fig. 3C) but not a PBS-inoculated (Fig. 3D) rat pup. The positive section was from the rat pup with the highest rotavirus antigen level determined by ELISA. No rotavirus antigen was detected in heart sections from a second RRV-inoculated rat pup with a high antigen level; the other rotavirus antigen-positive heart samples had ELISA readings just above the cutoff and were not tested.
(iii) Spleen and thymus.
Rotavirus antigen was also detected in the lymphoid organs, i.e., spleens and thymuses, of RRV-inoculated pups (Fig. 3E and G, respectively) but not in organs from PBS-inoculated rats (Fig. 3F and H, respectively), indicating that rotavirus can also replicate in these tissues.
(iv) Stomach, pancreas, and bladder.
No NSP4 was detected in stomach tissues from the rotavirus ELISA-positive samples tested (data not shown). Rotavirus antigen was detected in the pancreas and bladder by ELISA, but due to their small size, these organs were not processed for immunohistochemical evaluation.
Immunohistochemical detection of macrophages.
Rotavirus antigen was observed by immunohistochemistry in morphologically identified macrophages in a blood vessel in a section of thymus and in the lungs (Fig. 3I and A, respectively, indicated by arrows) of RRV-inoculated rat pups. To confirm the infiltration of macrophages into the lungs of RRV-inoculated rat pups, lung sections from three PBS- and three RRV-inoculated rat pups were stained with antibody against a marker for macrophages (CD68), and the alveolar and interstitial macrophages from six different areas of the stained sections were quantified. Compared to PBS-inoculated rat lungs (Fig. 3K) (number of macrophages in alveolar spaces, 15.8 ± 4.7; number of macrophages in interstitium, 1.4 ± 1.5), a decrease in the number of macrophages in the alveolar spaces and an increase in the number of macrophages in the alveolar interstitium were observed in lung sections from RRV-inoculated rats (Fig. 3J) (number of macrophages in alveolar spaces, 3.2 ± 4.8; number of macrophages in interstitium, 31.7 ± 10.9; P > 0.001, t test). A twofold increase in the total number of macrophages was observed for the RRV-inoculated rat pup lungs compared to the PBS-inoculated rat lungs (P > 0.001 by a t test). Since an increase in inflammatory cells was seen in liver sections from RRV-inoculated rat pups, liver sections were evaluated for macrophages. Infiltrates of macrophages were seen in sections from 1/3 livers from RRV-inoculated rats (Fig. 3L), whereas normal numbers of Kupffer cells were seen in liver sections from PBS-inoculated rats (Fig. 3M).
To determine if rotavirus antigen was present in the lung macrophages of RRV-inoculated pups, immunohistochemistry was performed on lung sections with antibodies against NSP4 and macrophages. NSP4 colocalized with the macrophage cell marker CD68 in the majority of macrophages in the alveolar interstitia of lung sections (Fig. 3N). Many pneumocytes adjacent to these macrophages were stained only with antibody against NSP4, indicating that rotavirus was replicating in the pneumocytes as well as in macrophages (Fig. 3N, arrows indicate infected pneumocytes).
Rotavirus infects cultured mouse macrophages.
To determine if cultured macrophages could be infected with rotavirus, confluent monolayers of mouse RAW 264.7 macrophages were infected with the sialic acid-dependent simian RRV (P5B[3], G3), bovine NCDV (P6[1], G6), or porcine OSU (P9[7], G5) strain or the sialic acid-independent human DS-1 (P1B[4], G2), P (P1A[8], G3), 69M (P4[10], G8), VA70 (P1A[8], G4), YO (P1A[8], G3), or K8 (P3A[9], G1) rotavirus strain (10) or were mock infected. Replicating virus was detected using the NSP4 antibody in cells infected with each of the virus strains (Fig. 4; data not shown for strains RRV, NCDV, OSU, P, 69M, and VA70).
FIG. 4.
Detection of rotavirus NSP4 antigen in mouse macrophages. Confluent monolayers of mouse RAW 264.7 macrophages were infected with human rotavirus strain K8 (P3A[9], G1), DS-1 (P1B[4], G2), or YO (P1A[8], G3) or were mock infected. Replicating virus was detected using NSP4 antibody and Alexa 488 anti-rabbit IgG.
Human rotavirus infection also induces viremia in rat pups.
In the preceding experiments, rat pups were inoculated with RRV, an animal-specific, sialic acid-dependent rotavirus. However, most rotaviruses, including human rotaviruses, are sialic acid independent (10). To determine whether viremia was limited to sialic acid-dependent strains of rotavirus, rat pups were inoculated intragastrically with HAL1166, a human sialic acid-independent rotavirus strain, and the kinetics of rotavirus antigen and infectious virus accumulation in the serum and liver were evaluated. Rotavirus antigen and infectious virus were detected in the sera of all rat pups inoculated with HAL1166 (Fig. 5A), indicating that rotavirus viremia is not restricted by sialic acid dependence. In contrast to that for RRV-inoculated rat pups, the duration of antigenemia in the HAL1166-inoculated rat pups was short. Virus was detected in all rat pups at 24 and 48 hpi, but only low levels of antigen were detected in a subset of pups at 96 and 216 hpi (Fig. 5A).
FIG. 5.
Kinetics of rotavirus-specific antigen detection by ELISA and infectious virus detection by FFA in PBS- or HAL1166-inoculated rat pups. (A) Serum; (B) liver. Each diamond represents an individual rat pup in each group, and each solid horizontal line represents the average OD value of each group at the indicated time point. The numbers above each column indicate the number of ELISA positive samples over the number of samples tested. The negative cutoff value for each organ, represented by the horizontal dotted line on each graph and the indicated numeric value, was determined by calculating 3 standard deviations above the mean of the OD values of the PBS control samples. The infectivities of samples were determined either by direct FFA or by passage of the sample on MA104 cells prior to FFA. Detection (circle around the diamond) or no detection (box around the diamond) of infectious virus is indicated for each sample tested.
We next determined whether HAL1166 spread into extraintestinal tissues by examining the liver at 48, 72, and 216 hpi; no other time points were examined. Rotavirus antigen was present in the livers of two of five pups at 72 hpi and four of five pups at 216 hpi (Fig. 5B). Infectious virus was detected in the livers of the subset of pups tested. These results indicate that a human sialic acid-independent rotavirus can also cause viremia and replicate in an extraintestinal site during rotavirus infection.
Detection of extraintestinal rotavirus in pups born to rotavirus-seropositive dams.
The previous studies showed that viremia and extraintestinal viral infection occur in immunocompetent, rotavirus-naïve rats following rotavirus infection. However, rotavirus infections of children often occur in the presence of maternal antibody, and this antibody might prevent viremia or extraintestinal spread of rotavirus. Therefore, we next evaluated if infectious virus could be detected in an extraintestinal organ in animals infected in the presence of maternally derived rotavirus antibody. Seven female rats that had previously been inoculated with RRV as 5-day-old rat pups were bred at approximately 60 days of age. The rotavirus-specific antibody titer was determined for each of the rat dams prior to or at the time of giving birth (Table 2); six of the seven dams had detectable rotavirus antibody. All pups produced by these dams were inoculated with RRV or PBS at 5 days of age, and at 3 dpi, a subset of pups from each litter were bled, euthanized, and perfused, and the livers were collected, homogenized, and tested for infectious virus. Serum from each pup was tested by ELISA to confirm the rotavirus antibody status. On the day of euthanasia, the rat pups had rotavirus-specific antibody titers similar to those of their respective dams (data not shown). Infectious virus was detected in the livers of 4/5 RRV-inoculated pups but not in any of four PBS-inoculated rat pups. These results indicate that low levels of maternally acquired rotavirus-specific antibody did not prevent the extraintestinal dissemination of rotavirus in rotavirus-infected pups.
TABLE 2.
Detection of infectious virus in livers of rat pups following primary and secondary rotavirus infections of pups born to rotavirus-seropositive dams
| Dam | Dam rotavirus antibody titer
|
Pup | Inoculum | Infectious virus in pup liver following rotavirus or PBS inoculation (72 hpi)
|
||
|---|---|---|---|---|---|---|
| Prea | Postb | Primaryc | Secondaryd | |||
| 1 | 0 | 3,200 | A | RRV | + | |
| B | RRV | + | ||||
| C | RRV | NT | + | |||
| D | RRV | NT | + | |||
| 2 | 200 | 3,200 | A | RRV | + | |
| B | RRV | NT | − | |||
| C | RRV | NT | − | |||
| 3 | 100 | 6,400 | A | RRV | NT | + |
| B | RRV | NT | + | |||
| 4 | 800 | ND | A | RRV | + | |
| B | RRV | − | ||||
| 5 | 1,600 | 6,400 | A | RRV | NT | + |
| B | RRV | NT | + | |||
| 6 | 400 | 400 | A | PBS | − | |
| B | PBS | − | ||||
| C | PBS | NT | − | |||
| D | PBS | NT | − | |||
| 7 | 800 | 800 | A | PBS | − | |
| B | PBS | − | ||||
| C | PBS | NT | − | |||
| D | PBS | NT | − | |||
Approximately 60-day-old female rats that had been inoculated with RRV at 5 days of age were bred. Prior to or following pup delivery, sera from the dams were tested for rotavirus-specific antibody responses by ELISA.
Rotavirus-specific antibody responses determined by ELISA 21 days following primary rotavirus or PBS inoculation of pups from each dam. ND, not done.
All rat pups were inoculated with RRV or PBS at 5 days of age. A subset of pups were sacrificed at 72 hpi. NT, not tested.
Rat pups inoculated with RRV or PBS at 5 days of age were inoculated again with RRV or PBS at 26 days of age.
We next evaluated whether an active immune response generated by a primary infection would protect rats from rotavirus viremia and extraintestinal spread. Primary rotavirus infection in neonatal rats, as in children, does not confer sterilizing immunity, despite the development of active immune responses, and rats shed rotavirus following a second rotavirus infection (8; Ciarlet et al., submitted for publication). To determine if infectious rotavirus could be detected in an extraintestinal organ following a second rotavirus infection, the remaining rat pups which were given a primary RRV infection in the previous experiment were reinoculated 21 days after primary infection with RRV or PBS, and 3 days after the secondary infection, two grown pups from each dam were bled, euthanized, and perfused, and their livers were collected, homogenized, and tested for infectious virus by FFA following amplification in MA104 cells. The induction of rotavirus-specific active immunity in the 26-day-old grown pups was confirmed by the increase in rotavirus-specific antibody titers in the dams (Table 2) and grown pups (data not shown) among the RRV- but not PBS-inoculated animals compared to the titers before the primary challenge. Infectious rotavirus was detected in the livers of the majority of the grown rat pups (6/8) undergoing secondary rotavirus infection at 26 days of age but not in four PBS-inoculated grown rat pups of the same age. Thus, extraintestinal spread of rotavirus occurred in rats during a secondary rotavirus infection and in the presence of circulating passively and actively acquired rotavirus-specific antibodies.
DISCUSSION
The detection of rotavirus antigenemia in children and of viremia in animal models and in tissues of infected children and adults led us to examine the kinetics of viremia and extraintestinal spread, cell and tissue tropism, and whether rotavirus infection of extraintestinal organs leads to histopathological changes. We confirmed previous reports of rotavirus antigenemia in animals, detecting antigenemia in both inbred and outbred rat strains. Antigenemia was detected in blood from 100% of the pups inoculated intragastrically with either a sialic acid-dependent (RRV) or -independent human (HAL1166) rotavirus strain, indicating that the escape of rotavirus from the gastrointestinal tract is efficient, occurs soon after infection, and is independent of sialic acid receptor usage. In fact, the detection of RRV antigen in the blood of infected rats was a more sensitive readout for infection than the commonly used measurement of diarrhea, since the AD50 was >2 log lower than the DD50. We also found that viremia and dissemination to an extraintestinal organ occurred in the presence of maternal antibody or active immunity.
Since evidence is increasing that rotavirus escapes the gastrointestinal tract and that infectious virus is present in the blood (1, 4, 7, 16, 27, 55), perfusion was performed in an attempt to remove, to the extent possible, potentially rotavirus-contaminated blood from the circulatory systems and organs of infected rat pups prior to examining tissues for virus. We found that rotavirus was able to disseminate to and replicate in a variety of organs, including the liver, lungs, spleen, kidneys, thymus, heart, pancreas, and bladder. The detection of rotavirus antigen, but not infectious virus, in urine indicated that even though rotavirus antigen can be excreted in the urine, this may not be a likely mode of virus transmission. Further studies are required to determine if the pH or other components of urine inactivate virus infectivity or if excreted virus might retain infectivity under some disease conditions. We did not detect rotavirus in the brains of infected pups, as previously reported for mice (14, 27). However, Kraft suggested that dissemination of rotavirus to the brain in mice is age restricted, and our lack of detection of rotavirus in the brain may indicate that infection of 5-day-old rat pups is past the restricted age (27). Why the dissemination of rotavirus to the brain is age restricted remains unknown.
The detection of rotavirus in the liver confirms earlier studies with mice and children (19, 26, 50). Replicating rotavirus was detected in areas of the liver exhibiting microsteatosis and necrosis and in regions containing infiltrates of inflammatory cells, indicating that this histopathology occurred as a result of rotavirus infection. Inflammatory cells were also detected in the bile duct and portal triad, which has been observed in 1- to 3-day-old mice infected with both human and animal rotavirus strains that eventually develop biliary atresia (BA) (46, 50). We did not observe BA in the RRV-inoculated rat pups, but rotavirus-induced BA in mice only occurs at a high frequency in pups inoculated 1 to 3 days after birth, and this apparent age-restricted induction of biliary atresia (BA) suggests that other host responses or factors may dictate the pathogenesis of infection of the biliary tree.
Viral factors appear to dictate rotavirus extraintestinal dissemination, based on differences in the kinetics of antigenemia and virus detection in different organs between RRV and HAL1166. RRV antigenemia was consistently present in all pups between 24 and 96 hpi, the levels of RRV antigen in the serum and liver were correlated, and RRV was detected in the livers of ∼50% of pups at all time points. In contrast, infectious HAL1166 was detected early in the blood but was below the cutoff of detection at 72 hpi, at which time antigen was first detected in the liver. Low levels of antigen were subsequently detected in the blood at 96 hpi. Future studies with larger groups of animals are needed to determine if the rotavirus antigen being detected in the blood at later time points was (i) the result of reinfection of enterocytes and release into the blood, (ii) from virus replication and release from extraintestinal sites, or (iii) simply the result of a lack of detecting antigen in the serum and liver at these time points due to the small numbers of animals used in this study. It will be of interest to determine whether the biphasic rotavirus shedding that has been observed in mouse pups infected with some strains of rotavirus (5, 26) corresponds with a biphasic pattern of antigenemia that may denote a cyclic pattern of infection-reinfection. Such an occurrence may be responsible for the prolonged virus shedding observed in some children (45).
Both RRV and HAL1166 replicated in the liver, but the kinetics of virus dissemination to the liver differed between these two strains, suggesting that different rotavirus strains have different phenotypes with regard to virus dissemination to the liver. RRV and HAL1166 differ in sialic acid receptor usage and dependency: RRV is sialic acid dependent, and HAL1166 is sialic acid independent. This difference in receptor usage may be responsible for the observed differences in kinetics of virus dissemination. Differences in sialic acid binding of congenic strains of reovirus result in different kinetics of reovirus dissemination to extraintestinal organs (3). The sialic acid-binding reovirus strain spreads more rapidly from the intestine to extraintestinal sites and replicates to higher titers, although both virus strains result in equivalent mortality rates. Alternatively, other viral genes or differences in infectious doses may play a role, alone or together, in dissemination. A study of mice with SA11/RRV rotavirus reassortants has identified NSP3 and VP6 as viral determinants for rotavirus dissemination to the liver (36, 37).
Rotavirus was found to disseminate to and infect and replicate in the lungs in a limited number of rat pups. The presence of replicating rotavirus in the lungs suggests several possible consequences that may help to explain clinical data for children and is consistent with experimental data in animal models. One consequence is the possibility that rotavirus can be released into respiratory secretions. Rotavirus has been detected in respiratory secretions from 30 to 60% of children with rotavirus-positive stool or diarrhea (18, 20, 28, 56) and in 100% of nasal swabs from rotavirus-infected pigs (1), suggesting that the rotavirus detected in respiratory secretions could be due to rotavirus infection of epithelial cells in the upper and/or lower respiratory tract. Our data support rotavirus replication in the lower respiratory tract and, coupled with the strong correlation of antigen levels in the lungs and stomach, suggest that rotavirus is released into respiratory secretions and swallowed, thus providing the potential for intestinal reinfection and prolonging the rotavirus infection. Another consequence of rotavirus replication and release in the respiratory tract is the possibility of transmission. Transmission of rotavirus by the respiratory route has been suspected based on epidemiologic analyses of rapid rotavirus spread similar to that of respiratory viruses (17), rotavirus RNA detection in air samples from a hospital ward with rotavirus-infected children (15), survival studies using aerosolized bovine and murine rotaviruses (24, 25), and the abilities to prevent rotavirus spread in mice by the use of microfiltration cages (27) and to infect mice with rotavirus by small-particle aerosolization (44). These potential consequences of rotavirus replication in the respiratory tract indicate a diagnostic gap in rotavirus disease identification and management, and further studies are required to determine if rotavirus causes clinically significant respiratory disease in children.
The presence of replicating rotavirus in the lungs was associated with an increase in cellularity that appeared to be at least partially due to an infiltration of macrophages into the alveolar and interalveolar septae. Replicating rotavirus was detected in these macrophages and adjacent pneumocytes (Fig. 3N), suggesting that the antigen detected in the macrophages might be due to (i) engulfment of rotavirus-containing cells adjacent to the macrophages, (ii) infection of macrophages by virus released by the pneumocytes, or (iii) infection of circulating monocytes/macrophages, as seen in Fig. 3I, and the subsequent infection of pneumocytes by virus released from the infected monocytes/macrophages. The presence of replicating rotavirus in lung macrophages and the correlation of detecting rotavirus in both the lungs and hearts of RRV-inoculated rat pups (P < 0.001; r = 0.801 [Pearson's correlation coefficient]) are of interest. In cases of fatal pneumonitis, myocarditis, or sudden, unexpected death in adults and children, rotavirus antigen or RNA was detected in infiltrating macrophages, endothelial cells, and pneumocytes (11, 35, 41). Altogether, our data indicate that detection of rotavirus in the heart and lungs appears to be a rare event, but further studies are warranted to assess the involvement of rotavirus with pulmonary complications and myocarditis.
Macrophages were first suggested by Brown and Offit (6) as a vehicle for viral dissemination and infection of other organs due to the detection of rotavirus-specific proteins in murine macrophages in intestinal and extraintestinal lymphoid tissues. Our detection of NSP4 antigen in macrophages in several tissues and a blood vessel, together with our observation that many sialic acid-dependent and -independent strains of rotavirus can replicate in cultured mouse macrophages (Fig. 4), points to macrophages as a possible vehicle for viral dissemination. However, extraintestinal spread may occur through multiple routes, including the lymphatics or entry and transport through M cells in Peyer's patches (36). Further studies are needed to evaluate these possible mechanisms.
Our understanding of rotavirus involvement in nongastrointestinal diseases is limited. However, a wide spectrum of clinical manifestations have been associated with rotavirus infection in children, including neurological complications, such as benign to severe convulsions, encephalitis, meningitis, and cerebellitis (23, 31, 40, 42, 49, 51-53); hemophagocytic lymphohistiocytosis (49); and various upper and lower respiratory tract infections, including otitis media, laryngitis, pharyngitis, and pneumonia (28, 34, 39, 41, 48). Other reports have cited Kawasaki syndrome (33), sudden infant death syndrome (54), a hepatic abscess (21), pancreatitis (38), and diabetes (22) in association with rotavirus infection. In most of these reports, rotavirus was only one of many organisms tested to seek the etiologic pathogen responsible for the disease. It is not surprising that rotavirus has not been identified more often as an agent associated with nongastrointestinal diseases since in the absence of diarrhea and the possible presence of rotavirus-specific antibody, rotavirus might not be suspected and therefore not sought. Our results clearly show that (i) rotavirus has a viremic phase that can occur and be detected in the presence of maternal or acquired antibody, (ii) viremia can occur in the absence of diarrheal disease, (iii) rotavirus can replicate in the parenchymas of multiple organs and in macrophages, and (iv) replication in the liver and lungs can be associated with histological lesions. Our findings provide support for the plausibility of rotavirus as an etiologic cause for the clinical manifestations reported to be associated with rotavirus infection and highlight the need to pursue studies to determine the involvement of rotavirus as a cause of nongastrointestinal diseases.
Acknowledgments
We are grateful to Alese McKinstry and Fred Basile for expert tissue culture technical assistance and animal care.
This work was supported by Public Health Service grants AI 24998 and DK30144, by Texas Gulf Coast Digestive Diseases Center grant DK56338, by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and by training grant DK07664 (D.G.P.).
REFERENCES
- 1.Azevedo, A. S., L. Yuan, K. I. Jeong, A. González, T. V. Nguyen, S. Pouly, M. Gochnauer, W. Zhang, A. Azevedo, and L. J. Saif. 2005. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J. Virol. 79:5428-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball, J. M., P. Tian, C. Q. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. [DOI] [PubMed] [Google Scholar]
- 3.Barton, E. S., B. E. Youree, D. H. Ebert, J. C. Forrest, J. L. Connolly, T. Valyi-Nagy, K. Washington, J. D. Wetzel, and T. S. Dermody. 2003. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Investig. 111:1823-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blutt, S. E., C. D. Kirkwood, V. Parreño, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 5.Boshuizen, J. A., J. H. J. Reimerink, A. M. Korteland-van Male, V. J. J. van Ham, M. P. G. Koopmans, H. A. Büller, J. Dekker, and A. W. C. Einerhand. 2003. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J. Virol. 77:13005-13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, K. A., and P. A. Offit. 1998. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb. Pathog. 24:327-331. [DOI] [PubMed] [Google Scholar]
- 7.Chiappini, E., C. Azzari, M. Moriondo, L. Galli, and M. de Martino. 2005. Viraemia is a common finding in immunocompetent children with rotavirus infection. J. Med. Virol. 76:265-267. [DOI] [PubMed] [Google Scholar]
- 8.Ciarlet, M., M. E. Conner, M. J. Finegold, and M. K. Estes. 2002. Group A rotavirus infection and age-dependent diarrheal disease in rats: a new animal model to study the pathophysiology of rotavirus infection. J. Virol. 76:41-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciarlet, M., S. E. Crawford, E. Cheng, S. E. Blutt, D. A. Rice, J. M. Bergelson, and M. K. Estes. 2002. VLA-2 (alpha2beta1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J. Virol. 76:1109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciarlet, M., and M. K. Estes. 1999. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity. J. Gen. Virol. 80:943-948. [DOI] [PubMed] [Google Scholar]
- 11.Cioc, A. M., and G. J. Nuovo. 2002. Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod. Pathol. 15:914-922. [DOI] [PubMed] [Google Scholar]
- 12.Conner, M. E., M. A. Gilger, M. K. Estes, and D. Y. Graham. 1991. Serologic and mucosal immune response to rotavirus infection in the rabbit model. J. Virol. 65:2562-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford, S. E., A. K. Mukherjee, M. K. Estes, J. A. Lawton, A. L. Shaw, R. F. Ramig, and B. V. Prasad. 2001. Trypsin cleavage stabilizes the rotavirus VP4 spike. J. Virol. 75:6052-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czech-Schmidt, G., W. Verhagen, P. Szavay, J. Leonhardt, and C. Petersen. 2001. Immunological gap in the infectious animal model for biliary atresia. J. Surg. Res. 101:62-67. [DOI] [PubMed] [Google Scholar]
- 15.Dennehy, P. H. 2000. Transmission of rotavirus and other enteric pathogens in the home. Pediatr. Infect. Dis. 19:S103-S105. [DOI] [PubMed] [Google Scholar]
- 16.Fischer, T. K., D. Ashely, K. Kerin, E. Reynolds-Hedmann, J. Gentsch, M.-A. Widdowson, L. Westerman, N. Ruhr, R. M. Turcios, and R. I. Glass. 2005. Rotavirus antigenemia in patients with acute gastroenteritis. J. Infect. Dis. 192:913-919. [DOI] [PubMed] [Google Scholar]
- 17.Foster, S. O., E. L. Palmer, G. W. Gary, Jr., M. L. Marin, K. L. Herrnamm, P. Beasley, and J. Sampson. 1980. Gastroenteritis due to rotavirus in an isolated Pacific island group; an epidemic of 3,439 cases. J. Infect. Dis. 141:32-39. [DOI] [PubMed] [Google Scholar]
- 18.Fragoso, M., A. Kumar, and D. L. Murray. 1986. Rotavirus in nasopharyngeal secretions of children with upper respiratory tract infections. Diagn. Microbiol. Infect. Dis. 4:87-88. [DOI] [PubMed] [Google Scholar]
- 19.Gilger, M. A., D. O. Matson, M. E. Conner, H. M. Rosenblatt, M. J. Finegold, and M. K. Estes. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120:912-917. [DOI] [PubMed] [Google Scholar]
- 20.Goldwater, P. N., I. L. Chrystie, and J. E. Banatvala. 1979. Rotaviruses and the respiratory tract. Br. Med. J. 2:1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunow, J. E., S. F. Dunton, and J. L. Waner. 1985. Human rotavirus-like particles in a hepatic abscess. J. Pediatr. 106:73-76. [DOI] [PubMed] [Google Scholar]
- 22.Honeyman, M. C., B. S. Coulson, N. L. Stone, S. A. Gellert, P. N. Goldwater, C. E. Steele, J. J. Couper, B. D. Tait, P. G. Colman, and L. C. Harrison. 2000. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49:1319-1324. [DOI] [PubMed] [Google Scholar]
- 23.Hongou, K., T. Konishi, S. Yagi, K. Araki, and T. Miyawaki. 1998. Rotavirus encephalitis mimicking afebrile benign convulsions in infants. Pediatr. Neurol. 18:354-357. [DOI] [PubMed] [Google Scholar]
- 24.Ijaz, M. K., Y. G. Karim, S. A. Sattar, and C. M. Johnson-Lussenburg. 1987. Development of methods to study the survival of airborne viruses. J. Virol. Methods 18:87-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ijaz, M. K., S. A. Sattar, T. Alkarmi, F. K. Dar, A. R. Bhatti, and K. M. Elhag. 1994. Studies on the survival of aerosolized bovine rotavirus (Uk) and a murine rotavirus. Comp. Immunol. Microbiol. Infect. Dis. 17:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaimes, M. C., N. Feng, and H. B. Greenberg. 2005. Characterization of homologous and heterologous rotavirus-specific T-cell responses in infant and adult mice. J. Virol. 79:4568-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraft, L. M. 1958. Observations on the control and natural history of epidemic diarrhea of infant mice (EDIM). Yale J. Biol. Med. 31:122-136. [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis, H. M., J. V. Parry, H. A. Davies, R. P. Parry, A. Mott, R. R. Dourmashkin, P. J. Sanderson, D. A. Tyrrell, and H. B. Valman. 1979. A year's experience of the rotavirus syndrome and its association with respiratory illness. Arch. Dis. Child. 54:339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, N., Y. Yao, and Q. Ou. 2001. Preliminary investigation of the relationship between liver lesion and relevant factors in young children with rotavirus diarrhea. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1:51-54. [PubMed] [Google Scholar]
- 30.Li, N., and Z. Y. Wang. 2003. Viremia and extraintestinal infections in infants with rotavirus diarrhea. Di Yi Jun Yi Da Xue Xue Bao 7:643-648. [PubMed] [Google Scholar]
- 31.Lynch, M., B. Lee, P. Azimi, J. Gentsch, C. Glaser, S. Gilliam, H. G. Chang, R. Ward, and R. I. Glass. 2001. Rotavirus and central nervous system symptoms: cause or contaminant? Case reports and review. Clin. Infect. Dis. 33:932-938. [DOI] [PubMed] [Google Scholar]
- 32.Lynch, M., W. J. Shieh, K. Tatti, J. R. Gentsch, T. F. Harris, B. M. Jiang, J. Guarner, J. S. Bresee, M. Greenwald, S. Cullen, H. D. Davies, C. Trevenen, S. R. Zaki, and R. I. Glass. 2003. The pathology of rotavirus-associated deaths, using new molecular diagnostics. Clin. Infect. Dis. 37:1327-1333. [DOI] [PubMed] [Google Scholar]
- 33.Matsuno, S., E. Utagawa, and A. Sugiura. 1983. Association of rotavirus infection with Kawasaki syndrome. J. Infect. Dis. 148:177. [DOI] [PubMed] [Google Scholar]
- 34.McCormack, J. G. 1982. Clinical features of rotavirus gastroenteritis. J. Infect. 4:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, C., T. Gilson, and G. J. Nuovo. 2001. Histologic distribution of fatal rotaviral infection: an immunohistochemical and reverse transcriptase in situ polymerase chain reaction analysis. Hum. Pathol. 32:216-221. [DOI] [PubMed] [Google Scholar]
- 36.Mossel, E. C., and R. F. Ramig. 2003. A lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouse. J. Virol. 77:12352-12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nigro, G. 1991. Pancreatitis with hypoglycemia-associated convulsions following rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 12:280-282. [DOI] [PubMed] [Google Scholar]
- 39.Nigro, G., and M. Midulla. 1983. Acute laryngitis associated with rotavirus gastroenteritis. J. Infect. 7:81-82. (Letter.) [DOI] [PubMed] [Google Scholar]
- 40.Nigrovic, L. E., C. Lumeng, C. Landrigan, and V. W. Chiang. 2002. Rotavirus cerebellitis? Clin. Infect. Dis. 34:130. [DOI] [PubMed] [Google Scholar]
- 41.Nuovo, G. J., G. Owor, T. Andrew, and C. Magro. 2002. Histologic distribution of fatal rotaviral pneumonitis: an immunohistochemical and RT in situ PCR analysis. Diagn. Mol. Pathol. 11:140-145. [DOI] [PubMed] [Google Scholar]
- 42.Pager, C., D. Steele, P. Gwamanda, and M. Driessen. 2000. A neonatal death associated with rotavirus infection—detection of rotavirus dsRNA in the cerebrospinal fluid. S. Afr. Med. J. 90:364-365. [PubMed] [Google Scholar]
- 43.Petersen, C., M. Kuske, E. Bruns, D. Biermanns, P. von Wussow, and H. Mildenberger. 1998. Progress in developing animal models for biliary atresia. Eur. J. Pediatr. Surg. 8:137-141. [DOI] [PubMed] [Google Scholar]
- 44.Prince, D. S., C. Astry, S. Vonderfecht, G. Jakab, F. M. Shen, and R. H. Yolken. 1986. Aerosol transmission of experimental rotavirus infection. Pediatr. Infect. Dis. 5:218-222. [DOI] [PubMed] [Google Scholar]
- 45.Richardson, S., K. Grimwood, R. Gorrell, E. Palombo, G. Barnes, and R. Bishop. 1998. Extended excretion of rotavirus after severe diarrhoea in young children. Lancet 351:1844-1848. [DOI] [PubMed] [Google Scholar]
- 46.Riepenhoff-Talty, M., K. Schaekel, H. F. Clark, W. Mueller, I. Uhnoo, T. Rossi, J. Fisher, and P. L. Ogra. 1993. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr. Res. 33:394-399. [DOI] [PubMed] [Google Scholar]
- 47.Riepenhoff-Talty, M., V. Gouvea, M. J. Evans, L. Svensson, E. Hoffenberg, R. J. Sokol, I. Uhnoo, S. J. Greenberg, K. Schakel, G. Zhaori, J. Fitzgerald, S. Chong, M. el-Yousef, A. Nemeth, M. Brown, D. Piccoli, J. Hyams, D. Ruffin, and T. Rossi. 1996. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J. Infect. Dis. 174:8-15. [DOI] [PubMed] [Google Scholar]
- 48.Santosham, M., R. H. Yolken, E. Quiroz, L. Dillman, G. Oro, W. C. Reeves, and R. B. Sack. 1983. Detection of rotavirus in respiratory secretions of children with pneumonia. J. Pediatr. 103:583-585. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi, S., J. Oki, A. Miyamoto, S. Koyano, K. Ito, H. Azuma, and A. Okuno. 1999. Encephalopathy associated with haemophagocytic lymphohistiocytosis following rotavirus infection. Eur. J. Pediatr. 158:133-137. [DOI] [PubMed] [Google Scholar]
- 50.Uhnoo, I., M. Riepenhoff-Talty, T. Dharakul, P. Chegas, J. E. Fisher, H. B. Greenberg, and P. L. Ogra. 1990. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J. Virol. 64:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong, C. J., Z. Price, and D. A. Bruckner. 1984. Aseptic meningitis in an infant with rotavirus gastroenteritis. Pediatr. Infect. Dis. 3:244-246. [DOI] [PubMed] [Google Scholar]
- 52.Wong, V. 2001. Acute gastroenteritis-related encephalopathy. J. Child Neurol. 16:906-910. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto, H., T. Yamano, S. Niijima, J. Kohyama, and H. Yamanouchi. 2004. Spontaneous improvement of intractable epileptic seizures following acute viral infections. Brain Dev. 26:377-379. [DOI] [PubMed] [Google Scholar]
- 54.Yolken, R., and M. Murphy. 1982. Sudden infant death syndrome associated with rotavirus infection. J. Med. Virol. 10:291-296. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, W., M. J. Xia, T. Bridges-Malveo, M. Cantu, M. M. McNeal, A. H. Choi, R. L. Ward, and K. Sestak. 2005. Evaluation of rotavirus dsRNA load in specimens and body fluids from experimentally infected juvenile macaques by real-time PCR. Virology 341:248-256. [DOI] [PubMed] [Google Scholar]
- 56.Zheng, B. J., R. X. Chang, G. Z. Ma, J. M. Xie, Q. Liu, X. R. Liang, and M. H. Ng. 1991. Rotavirus infection of the oropharynx and respiratory tract in young children. J. Med. Virol. 34:29-37. [DOI] [PubMed] [Google Scholar]