Abstract
An oligonucleotide microarray detecting 189 Escherichia coli virulence genes or markers and 30 antimicrobial resistance genes was designed and validated using DNA from known reference strains. This microarray was confirmed to be a powerful diagnostic tool for monitoring emerging E. coli pathotypes and antimicrobial resistance, as well as for environmental, epidemiological, and phylogenetic studies including the evaluation of genome plasticity.
Escherichia coli, which is commonly found in the environment as well as in the intestinal tract of common animal species, including humans, is the causative agent of several diseases worldwide. Depending on their virulence properties and the types of clinical infection elicited, E. coli strains are classified into various pathotypes (17). Generally, strains belonging to the same pathotype possess the same virulence determinants, but some virulence factors can be associated with several pathotypes. Therefore, establishing the virulence gene content found within an E. coli isolate is critical in determining its pathogenic potential. As many virulence genes are located on mobile elements, such as plasmids, phages, or transposons (24), determining the virulence gene profile of a given E. coli isolate would help also in monitoring gene transfer between strains and would be consequently of great value in epidemiological and phylogenetic studies. Like virulence genes, many antimicrobial resistance genes can be acquired by horizontal transfer. Therefore, antimicrobial resistance profiling of a given E. coli isolate would be useful for antimicrobial resistance surveillance programs. It could also be of great diagnostic value and should be indispensable for designing effective antibiotic policies.
Although various molecular methods can be used to identify either virulence or antimicrobial resistance genes harbored by E. coli strains (11, 19, 25, 29, 31), there is still a lack of practical and cost-effective methods able to detect rapidly and simultaneously all these genes in a given isolate. Microarray technology offers a powerful alternative for determining simultaneously the presence of a wide diversity of genes within a given E. coli strain. DNA microarrays have been used successfully in various studies, involving taxonomy (9), genotyping of microbial strains (13), detection of environmentally important genes (28, 34), and, recently, detection of E. coli antimicrobial resistance genes (6, 16, 35) or virulence genes (1, 8, 18, 30). In this study, a DNA microarray was developed combining oligonucleotides designed to detect a complete set of virulence genes representative of all E. coli pathotypes and antimicrobial resistance genes representative of different antimicrobial families characteristically found in pathogenic E. coli strains (20, 22). The capacity of this microarray to detect all virulence and antimicrobial resistance genes in reference and clinical E. coli isolates was investigated, and the results were validated with other molecular techniques.
The microarray prototype was designed from a previous amplicon-based microarray developed in our laboratory (1). Oligonucleotides were preferred to longer double-stranded DNA amplicons due to the potential of the latter for cross-hybridization, while oligonucleotide probes, due to their short length, are generally considered to be more specific. Apart from the amplicon-to-oligonucleotide redesign, the prototype was also updated by adding oligonucleotides specific for recently characterized and new putative E. coli virulence genes and by coupling the determination of the virulence gene content with the detection of antimicrobial resistance genes. The newly designed microarray prototype was thus composed of 348 70-mer oligonucleotides that were designed either using the OligoPicker software program (32) or from published PCR primers (20) which were lengthened to 70 bases. Two hundred sixty-three of them correspond to 189 virulence genes or markers from all known E. coli pathotypes as well as, within some particular genes, to their genetic variants (see Table S1 in the supplemental material). Among these oligonucleotides, three were specific for the phylogenetic markers chuA, yjaA, and tspE4.C2 used in the PCR-based method described by Clermont et al. for the determination of the main E. coli phylogenetic groups (10). Thirty-three other oligonucleotides were designed to target 30 antimicrobial resistance genes conferring resistance to six well-known gram-negative antimicrobial families and the class 1 integron (see Table S2 in the supplemental material). The selectivity of each oligonucleotide sequence was individually verified through BLAST searches in GenBank and then simultaneously in the public BLAST server Goldorak (http://www.bioneq.qc.ca; BioneQ, Montréal, Québec) for a final global BLAST analysis. A complete array was composed of four subarrays, in which each oligonucleotide was printed in triplicate, as previously described (1), on Corning Ultra GAPS slides (Corning Canada, Whitby, Ontario). Positive and negative controls (see Table S1 in the supplemental material) as well as three printing buffer spots were added in each subarray (see Fig. S1 in the supplemental material). Three complete independent arrays were printed on the same slide, thus minimizing variations resulting from fluctuations in external parameters.
The three reference strains EDL933, CFT073, and MG1655, for which the genomes have been completely sequenced (4, 26, 33), and 18 well-characterized strains coming from a previous study performed in our laboratory (1) and representing most of the E. coli pathotypes were used to validate the specificity of the virulence oligonucleotides. A collection of 55 E. coli strains coming from studies performed by Maynard et al. (20, 22) was used for the validation of the antimicrobial resistance oligonucleotides. Citrobacter freundii strain ATCC 8090 was used as a negative control.
To validate the microarray prototype, three independent hybridizations were performed for all strains described above. A 2-μl sample of a lysate from an E. coli overnight culture in Luria-Bertani broth grown at 37°C under agitation was labeled with a simple random-priming protocol based on Invitrogen's Bioprime DNA labeling system (Invitrogen Life Technologies, Burlington, Ontario). In a total volume of 50 μl, 20 μl of a 2.5× random primer solution (from the kit) and 1 μl of high-concentration Klenow polymerase (40 U/μl) were added to 5 μl of a deoxynucleoside triphosphate mix (1.2 mM dATP, 1.2 mM dGTP, 1.2 mM dTTP, and 0.6 mM dCTP in 10 mM Tris [pH 8.0] and 1 mM EDTA). Two microliters of 1 mM Cy5-dCTP were added to fluorescently label the DNA. The reaction was incubated in the dark at 37°C for 2 h, and the labeled samples were then purified on QIAquick columns (QIAGEN Inc., Mississauga, Ontario) according to the manufacturer's protocol. Microarrays were hybridized overnight at 50°C in a slide hybridization chamber (Corning Canada) with 500 ng of labeled DNA previously resuspended in 6 μl of prewarmed (37°C) DIG Easy Hyb buffer (Roche Diagnostics, Laval, Quebec) and denatured by heating for 5 min at 95°C. Stringency washes, three in 0.1× SSC (15 mM NaCl and 1.5 mM trisodium citrate, pH 7.0) with 0.1% sodium dodecyl sulfate and one in 0.1× SSC, were then performed at 37°C for 5 min under agitation. Data acquisition and analysis were performed as previously described (21).
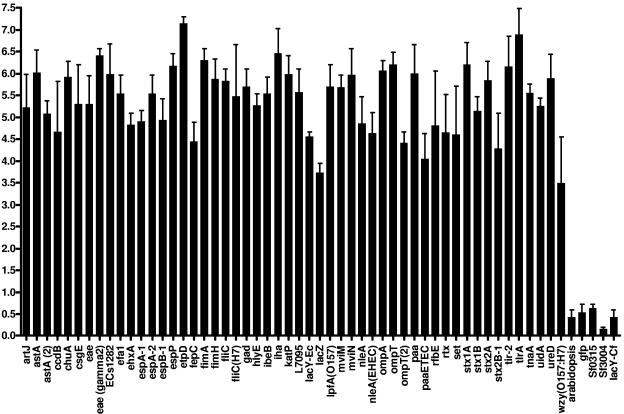
For all hybridizations performed with E. coli DNA, positive results (signal-to-noise fluorescence ratio of greater than 2.0 [21] [Fig. 1]) were obtained for all the oligonucleotides used as positive controls, and no fluorescence (signal-to-noise fluorescence ratio of less than 2.0 [21] [Fig. 1]) was observed for those used as negative controls. Expected results were also obtained for the C. freundii negative control strain. The microarray results were shown to be reproducible since, for each of the 76 E. coli strains tested, no missing spot was observed between the three independent replicate hybridizations. As shown in the example in Fig. 1, where the microarray results obtained for strain EDL933 are presented, oligonucleotides giving positive results always showed a signal-to-noise ratio clearly greater than 2.0 and, on the other hand, oligonucleotides giving negative results always showed a signal-to-noise ratio of less than 2.0. Expected results were obtained for the three reference strains MG1655, EDL933, and CFT073 and for the collection of 18 well-characterized E. coli isolates from the study of Bekal et al. (1). Among the 263 virulence-specific oligonucleotides, only the astA-specific probes gave unexpected positive results. BLAST searches in GenBank revealed the presence of a short truncation (24 nucleotides) in the astA sequences of various E. coli strains which cannot be differentiated from complete sequences with a 70-mer oligonucleotide probe. Consequently, positive results obtained with astA-specific oligonucleotides have to be confirmed by PCR with primers described previously (1). For all the other virulence-specific oligonucleotides, a perfect concordance was observed between microarray results and (i) BLAST searches against the sequenced genomes of the three reference strains and (ii) previous characterization of the 18 E. coli strains with an amplicon-based virulence microarray (1). The virulence gene content and the phylogenetic groups determined by our microarray of nine E. coli reference strains are presented in Table 1. For the 55 clinical E. coli isolates coming from the study of Maynard et al. (22), some discrepancies were observed comparing both microarray results and membrane hybridizations previously performed (22) (Table 2). Contrary to membrane hybridizations, where cross-hybridizations between tet(A) and tet(C) probes were observed (data not shown), the absence of such ambiguity with the microarray, as confirmed by PCR, underscores the high level of specificity. In the few other cases of discrepancies, PCR analysis and DNA sequencing performed as previously described (22) confirmed all the microarray results. Expected results were also obtained for the 29 positive control strains as well as the negative control reference strain MG1655, which lacks all the antimicrobial resistance genes targeted by the microarray.
FIG. 1.
Signal-to-noise fluorescence ratios obtained for the E. coli reference strain EDL933. The signal-to-noise ratios (log2) presented in this graph are the means of the ratios obtained by three independent replicate hybridizations performed with DNA from strain EDL933. All oligonucleotides which had a signal-to-noise fluorescence ratio of greater than 2.0 (log2 > 1) were considered positive. Oligonucleotides with a signal-to-noise ratio less than 2.0 (log2 < 1) were considered negative. For the negative results, only those for oligonucleotides specific to the green fluorescent protein (GFP) gene, Arabidopsis spp., Shigella flexneri, and C. freundii are shown.
TABLE 1.
Determination of the virulence gene content of E. coli reference strains and strains representing most E. coli pathotypes
| Strain identification | Pathotypea | Virulence genes for which a signal-to-noise fluorescence ratio of greater than 2.0 was obtainedb | Associated phylogenetic groupc | Reference |
|---|---|---|---|---|
| EDL933d | EHEC (O157H7) | stx1A, stx1B, stx2A, stx2B-1, eae, eae(gamma2), espA1, espA2, espB1, tir2, ehxA, nleA, paa, wzy(O157H7), artJ, astA, ccdB, chuA, csgE, Ecs1282, efa1, espP, etpD, fepC, fimA, fimH, fliC, fliC(H7), gad, hlyE, ibeB, iha, katP, l7095, lacY-Ec, lacZ, lpfA(O157), mviM, mviN, ompA, ompT, rfbE, rtx, set, tlrA, tnaA, uidA, ureD | D | 26 |
| CFT073d | UPEC | papA(7-1), papA(7-2), papC, papGII, chuA, fepC, irp1, irp2, fyuA, iroN, usp, agn43, artJ, astA, b1121, b1432, ce1a, csgE, fimA, fimH, fliC, focA, focG, gad, hlyA, hlyE, ibeB, iha, iss, iucD, kpsM-II, lacY-Ec, lacZ, malX, mchB, mviM, mviN, ompA, ompT, pic, sat, sfaD, tnaA, tspE4.C2, uidA, wzx(O6), yjaA | B2 | 33 |
| MG1655d | K12 | agn43, artJ, b1121, csgE, fimA, fimH, fliC, gad, hlyE, ibeB, lacY-Ec, lacZ, mviM, mviN, ompA, ompT, tnaA, uidA, yjaA | A | 4 |
| E2348/69 | EPEC | eae, eae(alpha), espA1, espB2, tir3, bfpA, bfpA(alpha), artJ, astA, b1121, ccdB, chuA, csgE, eaf, efa1, espC, fepC, fimA, fimH, fliC, gad, hlyE, ibeB, lacY-Ec, lacZ, malX, mviM, mviN, ompA, ompT, set, tnaA, traT, uidA, yjaA | B2 | 14 |
| H-10407 | ETEC | esta1, st, toxA, toxB, tia, tibA, leoA, agn43, artJ, astA, b1121, cfaB, csgE, fimA, fimH, flmA54, fyuA, gad, hlyE, ibeB, irp1, irp2, lacY-Ec, lacZ, mviM, mviN, ompA, tnaA, uidA, yjaA | A | 23 |
| 17.2 | EAEC | capU, shf, virK, aap, aggA, agn43, artJ, astA, b1121, ccdB, csgE, fimA, fimH, fyuA, gad, hlyA, hlyE, ibeB, iha, irp1, irp2, iss, iucD, kpsMII, lacY-Ec, lacZ, mviM, mviN, ompA, papA(16), papC, papGII, sat, tnaA, uidA, yjaA | A | 27 |
| J96 | UPEC | papA(13), papC, papGI, papGIII, chuA, fepC, irp1, irp2, fyuA, iroN, usp, agn43, artJ, b1121, b1432, ce1a, cnf1, csgE, fimA, fimH, fliC, focA, focG, gad, hlyA, hlyE, hra1, ibeB, iss, kpsMIII, lacY-Ec, lacZ, malX, mchB, mviM, mviN, ompA, ompT, rfc, sfaD, tnaA, uidA, yjaA | B2 | 5 |
| 2787 | DAEC | aidaI, agn43, artJ, astA, b1121, ccdB, csgE, fimA, fimH, flmA54, fyuA, gad, hlyE, ibeB, irp1, irp2, lacY-Ec, lacZ, mviM, mviN, ompA, tnaA, traT, uidA, yjaA | A | 2 |
| 31A | ExPEC (septicemia) | papA(11), papC, irp1, irp2, fyuA, iucD, iutA, agn43, artJ, b1121, clpG, csgE, espP, f165(1)A, F17cA, fimA, fimH, fliC, gad, gafD, hlyE, hra1, ibeB, iss, lacY-Ec, lacZ, lpfA(O113), lpfA, mviM, mviN, ompA, ompT, tnaA, traT, uidA, yjaA | A | 3 |
A pathotype is attributed to clinical strains according to their set of virulence genes or markers: EHEC (Shiga-like toxin-encoding genes, genes from the LEE, ehxA), EPEC (genes from the LEE, bfpA), ETEC (heat-stable and heat-labile toxin-encoding genes, F4 and F18 fimbria-encoding genes), EAEC (capU, shf, virK, aggregative adherence fimbria-encoding genes), DAEC (aidaI), UPEC (P pili-encoding genes, chuA, fepC, irp1, irp2, fyuA, iroN, usp).
All virulence genes targeted in this study are described in an exhaustive list in Table S1 in the supplemental material.
As previously described, according to the presence or absence of the three phylogenetic markers chuA, yjaA, and tspE4.C2 (10).
TABLE 2.
Determination of the antimicrobial resistance gene content of clinical E. coli extraintestinal isolates by microarray and comparison with membrane hybridizations
| Strain identification | Origin | Microarray result(s)a,b | Membrane hybridizationsa,b |
|---|---|---|---|
| 01-8344-0611 | Caribou | blaTEM, aadA1d, tet(A), tet(B), dhfrI, sulI, sulII, class 1 integron | blaTEM, tet(A), tet(B), tet(C), dhfrI, dhfrXV, sulI, sulII, class 1 integron |
| EcL1329 (01-D913)c | Pig | blaTEM, aadA1d, aphA1, tet(A), dhfrV, sulII | blaTEM, aphA1, tet(A), tet(C), dhfrV, sulII |
| EcL10038 (02-779-175)c | Pig | blaTEM, aadA1d, tet(A), sulI, class 1 integron | blaTEM, tet(A), tet(C), sulI, class 1 integron |
| EcL10040 (02-839)c | Pig | aadA1d, tet(A), sulI, class 1 integron | tet(A), tet(C), sulI, class 1 integron |
| EcL10044 (02-1273-175)c | Cattle | blaTEM, tet(A) | blaTEM, tet(A), tet(C) |
| EcL1335 (02-1902-2)c | Pig | blaTEM, aadA1d, aphA1, tet(A), dhfrV, sulI, sulII, class 1 integron | blaTEM, aphA1, tet(A), tet(C), dhfrV, sulI, sulII, class 1 integron |
| 02-1926 | Avian | blaTEM, aadA1d, tet(A), sulI, sulII, class 1 integron | blaTEM, tet(A), tet(C), sulI, sulII, class 1 integron |
| EcL10106 (02-1940-1)c | Pig | blaTEM, aadA1d, aphA1, tet(A), dhfrV, sulI, sulII, class 1 integron | blaTEM, aphA1, tet(A), tet(C), dhfrV, sulI, sulII, class 1 integron |
| 02-6386 | Cattle | blaTEM, tet(A), sulII | blaTEM, tet(A), tet(C), sulII |
| 1182768 | Human | blaTEM, aadA1d, tet(A), sulI, class 1 integron | blaTEM, tet(A), tet(C), sulI, class 1 integron |
| 67101013-1 | Human | blaTEM, aadA1d, tet(A), catI, sulI, class 1 integron | blaTEM, tet(A), tet(C), catI, sulI, class 1 integron |
| 67170713-3 | Human | aadA1d, tet(A), sulI, class 1 integron | tet(A), tet(C), sulI, class 1 integron |
| 67170751-1 | Human | blaTEM, aadA1d, aadB1d, aphA1, tet(A), sulI, sulII, class 1 integron | blaTEM, aphA1, tet(A), tet(C), sulI, sulII, class 1 integron |
| 67300471-1 | Human | blaTEM, aadA1d, tet(A), dhfrI, sulI, sulII, class 1 integron | blaTEM, tet(A), tet(B), tet(C), dhfrI, dhfrXV, sulI, sulII, class 1 integron |
| 67310973-1 | Human | aadA1d, tet(A), sulI, class 1 integron | tet(A), tet(C), sulI, class 1 integron |
| 68030679-1 | Human | blaTEM, tet(A), sulI | blaTEM, tet(A), tet(C), sulI |
| 68070861-1 | Human | blaTEM, tet(A), tet(B), catI, sulII | blaTEM, blaSHV, tet(A), tet(B), tet(C), catI, sulII |
| EcL8134 (98-2453)c | Dog | tet(A) | tet(A), tet(B), tet(C) |
| EcL1333 (P02-014-1)c | Pig | blaTEM, aadA1d, tet(A), sulI, class 1 integron | blaTEM, tet(A), tet(B), tet(C), sulI, class 1 integron |
All antimicrobial resistance genes targeted in this study are described in an exhaustive list in Table S2 in the supplemental material.
Discrepancies are indicated in boldface. For these genes, PCR and DNA sequencing performed with primers previously described (22) validated the microarray results.
Previous nomenclature.
Gene not tested by membrane hybridization.
In comparison with previous microarray-based studies (6-8, 16, 18, 30), we have developed a powerful molecular tool by coupling the detection of an exhaustive set of virulence genes and the detection of numerous antimicrobial resistance genes. This oligonucleotide microarray is a valuable tool not only for the assessment of the pathotype and the determination of the pathogenic potential of E. coli strains, but also for monitoring the transfer of virulence genes between strains (15). It should thus facilitate the identification of emerging pathotypes as well as the evaluation of genome plasticity by investigating the capacity of a strain to acquire virulence genes from other pathotypes, as shown in previous studies (1, 12, 18). This microarray also represents a valuable tool for diagnostic-based studies, surveillance programs of antimicrobial resistance, and monitoring of resistance gene dissemination between E. coli isolates. The presence in the microarray of oligonucleotides specific for the three phylogenetic markers used by Clermont et al. (10) for the determination of the main E. coli phylogenetic groups is of great help in epidemiological and phylogenetic studies (K. Hamelin et al., unpublished data). Finally, this microarray should not only be applicable in veterinary and medical diagnosis but should also find widespread use in microbial quality control of food and water and in environmental studies (K. Hamelin et al., unpublished data).
Work is under way to further improve the performance of this microarray (i) by coupling microarray technology with bioinformatics software to automate pathotype determination from an isolate's virulence gene content directly from the hybridization image and (ii) through array updating by adding oligonucleotides specific for newly recognized virulence genes.
Supplementary Material
Acknowledgments
We thank Miria Elias, Tracey Rigby, Mélanie Arbour, and Julie Champagne for their excellent technical support and Caroline Sotomey for her work on this project. We are also very grateful to Clarisse Désautels and John Fairbrother from the E. coli laboratory of the Faculté de Médecine Vétérinaire of the Université de Montréal for all the information on E. coli strains.
The financial support of the Natural Sciences and Engineering Research Council of Canada (NSERC) under the research work unit of the Canadian Research Network on Bacterial Pathogens of Swine (grant 225155) is also gratefully acknowledged.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bekal, S., R. Brousseau, L. Masson, G. Prefontaine, J. Fairbrother, and J. Harel. 2003. Rapid identification of Escherichia coli pathotypes by virulence gene detection with DNA microarrays. J. Clin. Microbiol. 41:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. [DOI] [PubMed] [Google Scholar]
- 3.Bertin, Y., C. Martin, J. P. Girardeau, P. Pohl, and M. Contrepois. 1998. Association of genes encoding P fimbriae, CS31A antigen and EAST 1 toxin among CNF1-producing Escherichia coli strains from cattle with septicemia and diarrhea. FEMS Microbiol. Lett. 162:235-239. [DOI] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Blum, G., V. Falbo, A. Caprioli, and J. Hacker. 1995. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and alpha-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol. Lett. 126:189-195. [DOI] [PubMed] [Google Scholar]
- 6.Call, D. R., M. K. Bakko, M. J. Krug, and M. C. Roberts. 2003. Identifying antimicrobial resistance genes with DNA microarrays. Antimicrob. Agents Chemother. 47:3290-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S., S. Zhao, P. F. McDermott, C. M. Schroeder, D. G. White, and J. Meng. 2005. A DNA microarray for identification of virulence and antimicrobial resistance genes in Salmonella serovars and Escherichia coli. Mol. Cell. Probes 19:195-201. [DOI] [PubMed] [Google Scholar]
- 8.Chizhikov, V., A. Rasooly, K. Chumakov, and D. D. Levy. 2001. Microarray analysis of microbial virulence factors. Appl. Environ. Microbiol. 67:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho, J. C., and J. M. Tiedje. 2001. Bacterial species determination from DNA-DNA hybridization by using genome fragments and DNA microarrays. Appl. Environ. Microbiol. 67:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colom, K., J. Perez, R. Alonso, A. Fernandez-Aranguiz, E. Larino, and R. Cisterna. 2003. Simple and reliable multiplex PCR assay for detection of blaTEM, blaSHV and blaOXA-1 genes in Enterobacteriaceae. FEMS Microbiol. Lett. 223:147-151. [DOI] [PubMed] [Google Scholar]
- 12.Dobrindt, U., F. Agerer, K. Michaelis, A. Janka, C. Buchrieser, M. Samuelson, C. Svanborg, G. Gottschalk, H. Karch, and J. Hacker. 2003. Analysis of genome plasticity in pathogenic and commensal Escherichia coli isolates by use of DNA arrays. J. Bacteriol. 185:1831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougherty, J. D., and D. H. Geschwind. 2002. Subtraction-coupled custom microarray analysis for gene discovery and gene expression studies in the CNS. Chem. Senses 27:293-298. [DOI] [PubMed] [Google Scholar]
- 14.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Paramo, P., O. Clermont, A. B. Blanc-Potard, H. Bui, C. Le Bouguenec, and E. Denamur. 2004. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 21:1085-1094. [DOI] [PubMed] [Google Scholar]
- 16.Grimm, V., S. Ezaki, M. Susa, C. Knabbe, R. D. Schmid, and T. T. Bachmann. 2004. Use of DNA microarrays for rapid genotyping of TEM beta-lactamases that confer resistance. J. Clin. Microbiol. 42:3766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 18.Korczak, B., J. Frey, J. Schrenzel, G. Pluschke, R. Pfister, R. Ehricht, and P. Kuhnert. 2005. Use of diagnostic microarrays for determination of virulence gene patterns of Escherichia coli K1, a major cause of neonatal meningitis. J. Clin. Microbiol. 43:1024-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mabilat, C., and S. Goussard. 1993. PCR detection and identification of genes for extended-spectrum beta-lactamases, p. 553-559. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 20.Maynard, C., S. Bekal, F. Sanschagrin, R. C. Levesque, R. Brousseau, L. Masson, S. Larivière, and J. Harel. 2004. Heterogeneity among virulence and antimicrobial resistance gene profiles of extraintestinal Escherichia coli isolates of animal and human origin. J. Clin. Microbiol. 42:5444-5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maynard, C., F. Berthiaume, K. Lemarchand, J. Harel, P. Payment, P. Bayardelle, L. Masson, and R. Brousseau. 2005. Waterborne pathogen detection by use of oligonucleotide-based microarrays. Appl. Environ. Microbiol. 71:8548-8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maynard, C., J. M. Fairbrother, S. Bekal, F. Sanschagrin, R. C. Levesque, R. Brousseau, L. Masson, S. Larivière, and J. Harel. 2003. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrob. Agents Chemother. 47:3214-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moseley, S. L., M. Samadpour-Motalebi, and S. Falkow. 1983. Plasmid association and nucleotide sequence relationships of two genes encoding heat-stable enterotoxin production in Escherichia coli H-10407. J. Bacteriol. 156:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhldorfer, I., and J. Hacker. 1994. Genetic aspects of Escherichia coli virulence. Microb. Pathog. 16:171-181. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Pérez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 27.Rich, C., S. Favre-Bonte, F. Sapena, B. Joly, and C. Forestier. 1999. Characterization of enteroaggregative Escherichia coli isolates. FEMS Microbiol. Lett. 173:55-61. [DOI] [PubMed] [Google Scholar]
- 28.Small, J., D. R. Call, F. J. Brockman, T. M. Straub, and D. P. Chandler. 2001. Direct detection of 16S rRNA in soil extracts by using oligonucleotide microarrays. Appl. Environ. Microbiol. 67:4708-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toma, C., Y. Lu, N. Higa, N. Nakasone, I. Chinen, A. Baschkier, M. Rivas, and M. Iwanaga. 2003. Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J. Clin. Microbiol. 41:2669-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Ijperen, C., P. Kuhnert, J. Frey, and J. P. Clewley. 2002. Virulence typing of Escherichia coli using microarrays. Mol. Cell. Probes 16:371-378. [DOI] [PubMed] [Google Scholar]
- 31.Vidal, R., M. Vidal, R. Lagos, M. Levine, and V. Prado. 2004. Multiplex PCR for diagnosis of enteric infections associated with diarrheagenic Escherichia coli. J. Clin. Microbiol. 42:1787-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, X., and B. Seed. 2003. Selection of oligonucleotide probes for protein coding sequences. Bioinformatics 19:796-802. [DOI] [PubMed] [Google Scholar]
- 33.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, L., D. K. Thompson, G. Li, R. A. Hurt, J. M. Tiedje, and J. Zhou. 2001. Development and evaluation of functional gene arrays for detection of selected genes in the environment. Appl. Environ. Microbiol. 67:5780-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu, X., M. Susa, C. Knabbe, R. D. Schmid, and T. T. Bachmann. 2004. Development and validation of a diagnostic DNA microarray to detect quinolone-resistant Escherichia coli among clinical isolates. J. Clin. Microbiol. 42:4083-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.