Abstract
Although the prognosis for patients with early-stage breast cancer has improved, the therapeutic options for patients with locally advanced and metastatic disease are limited. To improve the treatment of these patients, the molecular mechanisms underlying breast cancer invasion and metastasis must be understood. In this study, we report that signaling through the G12 family of heterotrimeric G proteins (Gα12 and Gα13) promotes breast cancer cell invasion. Moreover, we demonstrate that inhibition of G12 signaling reduces the metastatic dissemination of breast cancer cells in vivo. Finally, we demonstrate that the expression of Gα12 is significantly up-regulated in the earliest stages of breast cancer, implying that amplification of G12 signaling may be an early event in breast cancer progression. Taken together, these observations identify the G12 family proteins as important regulators of breast cancer invasion and suggest that these proteins may be targeted to limit invasion- and metastasis-induced patient morbidity and mortality.
Keywords: cadherin, Rho, G protein-coupled receptor
Heterotrimeric guanine nucleotide-binding proteins (G proteins) transmit extracellular signals from cell surface G protein-coupled receptors to intracellular effector molecules (1). Because of the array of extracellular signals that activate them and the large number of intracellular targets to which they signal, heterotrimeric G proteins have been implicated in many physiologic and pathophysiologic processes (2, 3). Heterotrimeric G proteins are classified into four subfamilies: Gs, Gi, Gq, and G12. The G12 subfamily has been of particular interest to cancer biologists since its discovery. Originally identified because of its sequence similarity to other G protein α-subunits (4) and as a sarcoma-associated oncogene (5), Gα12 and its sister protein Gα13 are the only heterotrimeric G proteins that are able to transform fibroblasts when overexpressed in their wild-type form (5, 6). As a result, it has been suggested that G12 signaling may promote tumorigenesis and tumor growth (7). However, to date, no role for G12 signaling in human cancers has been demonstrated.
We sought to explore the role of the G12 proteins in breast cancer, which, besides skin cancer, is the most common form of cancer in the world. Surprisingly, we found that activation of the G12 pathway did not promote growth in breast cancer cells. Instead, activation of G12 signaling induced a striking increase in breast cancer cell invasion in vitro and the inhibition of G12 signaling significantly reduced breast cancer metastasis in vivo. In addition, G12 expression was found to be markedly up-regulated in human breast cancer specimens, suggesting that G12 signaling is an important regulator of breast cancer invasion and metastasis.
Results
G12 Signaling Does Not Promote the Growth or Transformation of Breast Cancer Cells.
To assess the biological significance of G12 signaling in breast cancer, we first examined the effects of modulating the activity of G12 proteins on breast cancer cell proliferation. To stimulate G12 signaling in breast cancer cells, we expressed the constitutively active forms of Gα12 [Gα12(Q231L)] and Gα13 [Gα13(Q226L)] in several cell lines (Fig. 1A). Because most receptors that activate the G12 proteins also activate the Gq proteins (8), we also expressed the constitutively active form of Gαq [Gαq(Q209L)] in these lines as a control (Fig. 1A). To inhibit G12 signaling in breast cancer cells, we expressed the regulator of G protein signaling (RGS) domain of the p115 Rho-GEF (p115-RGS) (Fig. 1C). The expression of specific RGS domains is a well characterized method to selectively block signaling through different subfamilies of heterotrimeric G proteins (9–11), and the p115-RGS domain selectively binds and inactivates members of the G12 family (9, 10). In addition, to inhibit Gq signaling in breast cancer cells, we expressed RGS2, an RGS that selectively binds and inactivates members of the Gq family (11) (Fig. 1C).
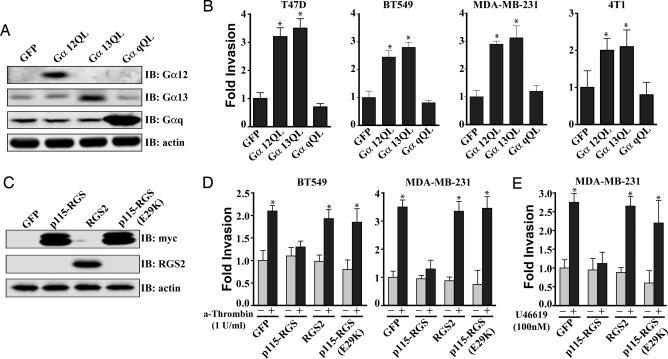
Fig. 1.
G12 signaling promotes breast cancer cell invasion. (A) Expression of the activated forms of Gα12 (Gα12QL) and Gα13 (Gα13QL) but not Gαq (GαqQL) induces breast cancer cell invasion in vitro. Cells were transduced with the indicated adenovirus, starved for 18 h, and then allowed to invade growth-factor reduced Matrigel (T47D, MDA-MB-231, and BT549) or collagen-coated (4T1) transwell filters for 30 h. (B) Immunoblot analysis showing expression levels of Gα12, Gα13, and Gαq in the T47D cell line after infection with the indicated adenovirus. Similar expression was observed in the other cells types. (C and D) Expression of p115-RGS blocks thrombin (C) and thromboxane A2-induced (U46619) (D) invasion of breast cancer cells, whereas expression of RGS2 or expression of an inactive form of the p115-RGS, p115-RGS(E29K), has no effect. Cells were transduced with the indicated adenovirus, starved for 18 h, then allowed to invade growth-factor-reduced Matrigel-coated transwell filters for 30 h in the presence of 1 unit/ml thrombin (A), 100 nM U46619 (B), or vehicle control. (E) Immunoblot analysis showing expression levels of p115-RGS, p115-RGS(E29K), and RGS2 in the MDA-MB-231 cell line after infection with the indicated adenovirus. Similar expression was observed in the BT549 cells. (A, C, and D) Experiments were performed in triplicate, and the results are presented as the fold increase over vehicle-treated GFP control. All results are presented as mean ± SE from a single experiment. All experiments were performed at least three times. ∗, P < 0.05 as determined by paired Student t test.
In contrast to previous studies using nontransformed cells (5, 6, 12, 13), expression of the activated forms of Gα12 and Gα13 did not promote, and in some cases even inhibited, in vitro proliferation of human breast cancer lines under either anchorage-dependent (Fig. 5A, which is published as supporting information on the PNAS web site) or -independent conditions (Fig. 5B). Furthermore, expression of p115-RGS and RGS2 had little to no effect on the cell proliferation (Fig. 5). Thus, although, in some cell types, Gα12 and Gα13 may be important promoters of cell growth, they do not appear to promote breast cancer cell growth.
G12 Signaling Promotes Breast Cancer Cell Invasion.
Notably, in addition to promoting cell growth, many of the signaling pathways downstream of the Gα12 and Gα13 have been implicated in cancer cell invasion and metastasis (7, 14). Therefore, we next sought to determine whether G12 proteins affect breast cancer invasion and metastasis. First, we examined the impact of G12 signaling on breast cancer cell invasion by using the transwell invasion assay (15). Expression of activated Gα12 or activated Gα13 in T47D, BT549, MDA-MB-231, and 4T1 (Fig. 1B) breast cancer cells significantly increased cellular invasion. In contrast, expression of activated Gαq had no effect (Fig. 1B). Thrombin stimulates cancer cell invasion and metastasis through protease-activated receptor-1 (PAR-1) (16, 17), and stimulation of PAR-1 activates both G12 and Gq proteins (8). Thrombin-induced invasion of the BT549 and MDA-MB-231 cell lines was blocked by inhibition of G12 signaling (by expression of p115-RGS), whereas the inhibition of Gq signaling (by expression of RGS2) had no effect (Fig. 1D). As an additional control, an adenovirus was engineered to express a disabled form of p115-RGS, p115-RGS(E29K). This point mutant of the p115-RGS neither binds to nor functions as a GTPase-activating protein for the G12 proteins and, thus, does not affect G12 signaling (18). Expression of this protein had no effect on thrombin-induced cell invasion (Fig. 1D), indicating that the inhibitory effects of p115-RGS are the result of its ability to bind and inactivate the G12 proteins. To determine whether these results were specific to PAR-1, we also assessed the impact of thromboxane A2 receptor stimulation on cancer cell invasion. Stimulation of this receptor, which also couples to both G12 and Gq proteins, markedly enhanced invasion of the MD-MBA-231 cells, and this effect was blocked by expression of p115-RGS but not RGS2 or the p115-RGS(E29K) (Fig. 1E). These data provide convincing evidence that stimulation of the G12 proteins is capable of promoting invasion of breast cancer cells.
The Role of Rho G Proteins in G12-Promoted Breast Cancer Cell Invasion.
In most cell types, stimulation of the G12 proteins results in the activation of the RhoA/B/C family of monomeric G proteins (7). Many studies have demonstrated that the Rho family of proteins plays a significant role in breast cancer invasion (19). Thus, we examined the role of Rho signaling in G12-induced breast cancer invasion. First, we confirmed that the G12 proteins are able to activate Rho in breast cancer cell lines. Expression of the activated forms of Gα12 and Gα13 in the MDA-MB-231 (Fig. 2A), T47D (Fig. 6A, which is published as supporting information on the PNAS web site), and 4T1 (Fig. 6B) cell lines induced a significant increase in the levels of GTP-bound Rho. Interestingly and consistent with recent reports (20), the activated form of Gαq also induced a small increase in the level of GTP-bound Rho (Fig. 2A). In addition, stimulation of the MDA-MB-231 cell line with thrombin resulted in ≈5-fold increase in the levels of GTP-bound Rho (Fig. 2B). This stimulation was inhibited by expression of the p115-RGS but not by expression of the p115-RGS(E29K) or RGS2 (Fig. 2B). Taken together these data confirm that G12 signaling is able to activate the Rho proteins in breast cancer cells.
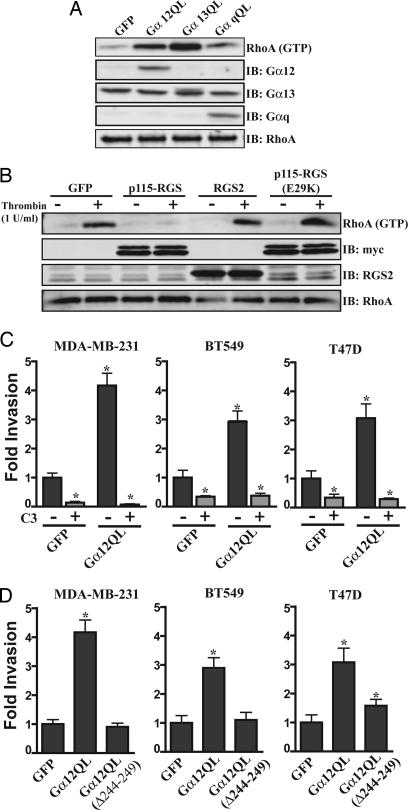
Fig. 2.
G12 signaling is able to promote breast cancer cell invasion through the activation of the Rho family of monomeric G proteins. (A) Expression of Gα12QL and Gα13QL induces RhoA activation in the MDA-MB-231 cell line. Cells were transduced with the indicated adenovirus and then starved for 18 h. Cells were lysed, and the lysates were subjected to pull-down assays using a GST fusion of the activated RhoA-binding domain of rhotekin. Levels of precipitated RhoA were determined by immunoblot analysis using anti-RhoA antibody. Levels of total RhoA, Gα12, Gα13, and Gαq also were determined. All lanes are representative of two or more separate experiments. (B) Thrombin-stimulated RhoA activation in MDA-MB-231 cells is inhibited by expression of p115-RGS but not by expression of RGS2 or p115-RGS(E29K). Cells were transduced with the indicated adenovirus, starved for 18 h, and then stimulated with thrombin (1 unit/ml) or a vehicle control for 5 min. Levels of activated Rho were determined as in A. Levels of total RhoA, myc-p115-RGS, and RGS2 also were determined. All lanes are representative of two or more separate experiments. (C) Gα12-mediated breast cancer cell invasion requires the activity of the Rho family of G proteins. Cells were transduced with the indicated adenovirus, starved for 18 h in the presence and absence of C3 toxin as indicated, and then allowed to invade growth-factor-reduced Matrigel-coated transwell filters for 30 h. Experiments were performed in duplicate, and the results are presented as the fold increase over that observed with GFP control. All experiments were performed at least three times. All results are presented as mean ± SE. ∗, P < 0.05 as determined by paired Student t test. (D) The effect of the expression of Gα12QL (Δ244–249), a mutant of Gα12QL that is functionally uncoupled from the Rho axis but is still able to interact with other putative G12 effectors, on breast cancer cell invasion. Experiments were performed as in C. Experiments were preformed in duplicate, and the results are presented as the fold increase over that observed with GFP control. All experiments were performed at least three times. All results are presented as mean ± SE. ∗, P < 0.05 as determined by paired Student t test.
Next we sought to determine the contribution of signaling through the Rho proteins in G12-induced breast cancer cell invasion. Treatment of the MDA-MB-231, BT549, T47D (Fig. 2C), and 4T1 (Fig. 6C) cell lines with C3 toxin, a specific and irreversible inhibitor of RhoA/B/C, blocked Gα12(QL)-induced invasion. These data demonstrate that Rho signaling is required for G12-stimulated invasion. However, given that C3 toxin treatment of these breast cancer cells also reduced basal invasion, it was not clear from these data whether G12-stimulated invasion simply requires Rho signaling or whether G12 must activate Rho to induce invasion. To address these possibilities, we used a mutant form of Gα12 [Gα12 (Δ244–249)] that is unable to activate Rho but is still able to interact with other putative G12 effectors (21). Expression of the constitutively active form of this protein [Gα12QL (Δ244–249)] in the MDA-MB-231, BT549 (Fig. 2D), or 4T1 (Fig. 6D) cell lines did not promote invasion. These results suggest that that G12 must activate the Rho pathway to induce invasion in these cell types. Interestingly, however, when Gα12QL (Δ244–249) was expressed in T47D cell line, although it did not activate Rho (Fig. 6A), it did induce a small but significant increase in invasion (Fig. 2D). This finding suggests that, although maximal G12-stimulated breast cancer invasion requires Rho activation, in some cell types G12 may be able to promote invasion in a Rho-independent pathways (7, 14, 22).
G12 Signaling Promotes Cancer Metastasis.
Although in vitro assays can provide mechanistic insight into cancer progression and spread, the complexity of the metastatic process demands in vivo experimentation for accurate modeling (23). For this reason, we decided to employ the 4T1 cell line (24). When implanted in the mammary fat pad of recipient mice, 4T1 cells grow and metastasize in a manner similar to that of human breast cancer (24–26). We engineered 4T1 cells to stably express p115-RGS, p115-RGS (E29K), or RGS2 and implanted these cells in the mammary fat pad of 6-week-old BALB/c mice. Consistent with our in vitro results, the expression of p115-RGS, p115-RGS(E29K), or RGS2 had no measurable effect on the rate of tumor growth compared with control tumors (Fig. 7, which is published as supporting information on the PNAS web site). Moreover, the inhibition of G12 or Gq signaling led to no detectable differences among the tumors upon histopathologic examination in proliferative index, as determined by Ki-67 staining; rate of apoptosis, as determined by TUNEL staining; nor vascular density, as determined by CD31 staining (data not shown). Thus, inhibition of G12 signaling had no measurable effect on the growth of the 4T1 primary tumors.
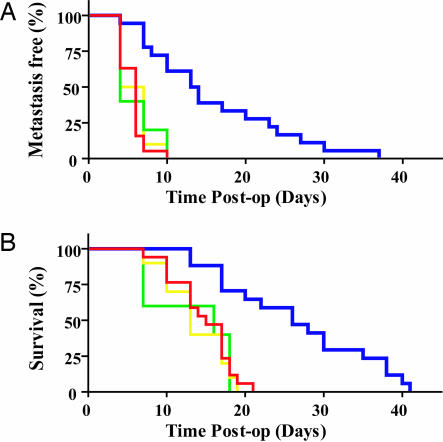
To track the metastatic spread of the 4T1 cells in live animals, the 4T1 cells were also engineered to stably express firefly luciferase (26, 27). After 3 weeks of growth, the primary 4T1 tumors were removed and metastasis was visualized with the IVIS imaging system (26). We defined a metastatic event as any detectable luciferase signal above background and away from the primary tumor site to prevent confusion resulting from primary tumor recurrence. Strikingly, inhibition of G12 signaling with p115-RGS (n = 16) resulted in a significant increase in metastasis-free survival compared with the p115-RGS(E29K) (n = 5; P < 0.0013), RGS2 (n = 11; P < 0.001), or vector control (n = 17; P < 0.0001) groups (Fig. 3A). Moreover, inhibition of G12 signaling with p115-RGS (n = 16) resulted in a significant increase in overall survival compared with the p115-RGS(E29K) (n = 5; P < 0.002), RGS2 (n = 11; P < 0.001), and vector control (n = 17; P < 0.0001) groups (Fig. 3B). These data indicate that G12 signaling is required for efficient metastatic spread of 4T1 cancer cells in vivo.
Fig. 3.
Inhibition of G12 signaling reduces metastatic dissemination of the 4T1 cell line orthotopically implanted in the mouse mammary fat pad. The mammary fat pads of BALB/c mice were injected with 5 × 105 4T1-Luc cells (red; n = 17), 4T1-Luc cells expressing p115-RGS (blue; n = 16), 4T1-Luc cells expressing RGS2 (yellow; n = 11), or 4T1-Luc cells expressing p115-RGS(E29K) (green; n = 5). Tumors were grown for 21 days and then excised as described in Methods and Materials. (A) Metastasis-free survival after primary tumor resection. A metastatic event was defined as any detectable bioluminescent signal above background away from the primary site. Mice that received p115-RGS-expressing 4T1 cells had significantly longer metastasis-free survival times than did mice that received control 4T1 cells (P < 0.0001), RGS2-expressing cells (P < 0.001), or p115-RGS(E29K)-expressing cells (P < 0.0013). Statistical analyses were performed by using the log-rank test. (B) Overall mouse survival. Mice that received p115-RGS-expressing 4T1 cells survived significantly longer than did mice that received control 4T1 cells (P < 0.0001), RGS2-expressing cells (P < 0.001), or p115-RGS(E29K)-expressing cells (P < 0.002). Statistical analyses were performed by using the log-rank test.
To further dissect the role of G12 signaling in metastasis, we injected 4T1 cells expressing the p115-RGS (n = 5) or vector control (n = 6) into the tail vein of BALB/c mice (23, 26). When seeded directly into the blood stream in this manner, no difference was seen in the metastatic dissemination of the cancer cells (Fig. 8A, which is published as supporting information on the PNAS web site) or the overall survival of the mice (Fig. 8B). These results imply that G12 function is not required in the later steps of the metastatic cascade. Taken together, these in vivo results correlate well with our in vitro findings and suggest that G12 signaling promotes metastasis by stimulating invasion by the primary tumor.
The Gα12 Protein Is Up-Regulated in Carcinoma of the Breast.
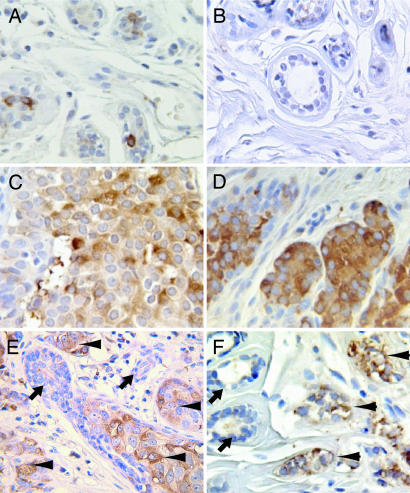
Because G12 signaling promoted breast cancer cell invasion both in vitro and in vivo, we sought to elucidate the physiological relevance of the G12 proteins in tumor invasion and metastasis by assessing its expression in breast biopsies obtained from patients with breast cancer. Immunohistochemical detection of Gα12 in sections of both ductal carcinoma of the breast and lobular carcinoma of the breast revealed that breast cancer cells consistently expressed higher levels of Gα12 compared with benign breast epithelial cells within the same tissue section (Fig. 4). Gα12 staining could be completely blocked by preincubation of the antibody with its blocking peptide, demonstrating antibody specificity (Fig. 9, which is published as supporting information on the PNAS web site). Interestingly, no increase in Gαq expression was seen in these specimens (Fig. 10, which is published as supporting information on the PNAS web site), suggesting that this elevated expression is specific to Gα12.
Fig. 4.
Gα12 protein levels are up-regulated in carcinoma in situ and in invasive adenocarcinoma of the breast. Sections of formalin-fixed paraffin-embedded breast tissue were stained for Gα12 with anti-G12 anti-sera (Santa Cruz Biotechnology) as described in Methods and Materials. Original images were taken with a ×40 objective. (A and B) Benign breast tissue. (C) Ductal carcinoma in situ of the breast. (D) Lobular carcinoma in situ of the breast. (E) Section containing benign breast epithelium, carcinoma in situ, and invasive ductal carcinoma of the breast. (F) Section containing both benign breast epithelium and invasive lobular carcinoma of the breast. (E and F) Arrowheads indicate cancer; arrows indicate benign epithelium.
To broaden the analysis of Gα12 expression in primary human cancers, a tissue microarray of 80 breast biopsy samples containing ductal carcinoma in situ and invasive carcinoma with matched benign breast tissue was stained for Gα12 expression and graded 0–4+ based on signal intensity. This analysis demonstrated that Gα12 expression is significantly increased in both in situ and invasive ductal carcinomas of the breast (staining intensity: normal breast epithelium, 1.2 ± 0.1; ductal carcinoma in situ, 2.3 ± 0.4; and invasive ductal carcinoma, 2.1 ± 0.1) (Table 1). These data provide evidence that Gα12 expression is increased early in breast cancer progression, before the tumors become invasive. Thus, these findings are consistent with our results showing G12 signaling as an important regulator of breast cancer invasion and suggest that Gα12 up-regulation may serve as an early marker for the development of a more aggressive, invasive phenotype.
Table 1.
Distribution of breat speciman subtypes by Gα12 staining
| Specimen | N | Staining intensity | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Avg. ± SEM | ||
| Benign breast epithelium | 39 | 5 | 19 | 9 | 2 | 1.2 ± 0.1 |
| Ductal carcinoma in situ | 6 | 0 | 2 | 0 | 4 | 2.3 ± 0.4* |
| Invasive lobular carcinoma | 40 | 1 | 10 | 15 | 15 | 2.1 ± 0.1** |
*, P < 0.05 for ductal carcinoma in situ vs. benign breast epithelium.
**, P < 0.0001 for invasive lobular carcinoma vs. benign breast epithelium. Avg., average.
Discussion
In this report, we demonstrate that G12 is markedly up-regulated in adenocarcinoma of the breast and identify G12 signaling as an important regulator of breast cancer invasion and metastasis. Previous studies have shown that G12 signaling is able to promote cell growth in some cell lines (5, 6, 12, 13). Thus, our results that G12 signaling did not promote tumor cell growth were surprising. However, it is interesting to note that much of the previous work on the mitogenic effects of G12 signaling was done in nontransformed, fibroblast-derived cell lines (5, 6, 12, 13). Because our studies used transformed cells of epithelial origin, it is possible that the discrepancy results from fundamental differences between normal and transformed cells and/or between cells of mesenchymal and epithelial origin. Thus, although it is clear that in some cell types Gα12 and Gα13 may be important promoters of cell growth, they do not appear to promote breast cancer cell growth.
In contrast to the data on proliferation, this study provides compelling evidence for a role for G12 signaling in breast cancer cell invasion. Activation of the pathway by expression of the activated forms of the G12 proteins or by receptor stimulation of the endogenous G12 proteins resulted in a significant increase in the in vitro invasiveness of several breast cancer cell lines. Although this finding is novel in the field of cancer biology, studies in other systems have implicated G12 signaling in the related biologies of cell migration and extracellular matrix adhesion. In particular, studies in neutrophils have demonstrated that the G12 proteins are critically important in establishing cell polarity (28). Developmental studies in Drosophila (29) and in zebrafish (30) have demonstrated that G12 signaling is critical for cell migration during gastrulation. Furthermore, genetic ablation of G13 in mice impairs the organization of the vascular system (31). Embryonic fibroblasts cultured from these mice display a reduced chemokinetic response to several G protein-coupled receptor ligands, and Offermanns et al. speculated that this defect in cell migration may underlie the failed angiogenesis (31).
Although the results from the in vitro assays used in this study strongly suggest a role for G12 in tumor invasion, the most compelling data supporting this hypothesis comes from the in vivo studies with the 4T1 mammary carcinoma line. In these experiments, inhibition of G12 signaling resulted in a significant decrease in metastasis from the mammary fat pad but did not affect either primary tumor growth or seeding of the lungs when cancer cells were introduced via the tail vein. Recent evidence suggests that 4T1 cells metastasize to the lung and, possibly, other organs by embolizing to the capillary bed and then growing in the intravascular space (32). This phenomenon of metastasis without extravasation does not appear to be limited to the 4T1 cells (32). However, exiting the vascular bed does appear to be a requirement for the metastasis of other cell types (23). Therefore, it is possible that, for other tumor types and at other sites of distant metastasis, G12 signaling also plays a role in these later stages of metastasis. Nevertheless, given that we saw no difference in metastatic potential of the 4T1 cells expressing p115-RGS and control cells when they were seeded into the blood stream directly, the difference in the metastatic dissemination of these cells from the mammary fat pad must reflect a disparity in the rate that cells are shed from the primary tumors. Thus, G12 signaling appears to promote cancer metastasis by stimulating tumor cell invasion and entry into the blood stream.
The findings that blockade of G12 signaling dramatically impacts metastatic behavior of cancer cells prompted us to examine the expression the G12 proteins in tissue samples from patients with adenocarcinoma of the breast. We found that Gα12 expression was significantly higher in situ and in invasive carcinomas compared with normal epithelium. This finding is consistent with a previous report suggesting that Gα12 and Gα13 expression is elevated in cell lines derived from metastatic human breast, prostate, and colon cancers (33). This up-regulation of Gα12 in intraepithelial neoplasms also is similar to what was previously reported for receptors that couple to the G12 protein (16, 34) and the increased expression of the Rho proteins observed at this point or later in cancer development (16, 35). Thus, it appears that the G12 signaling pathway is up-regulated as an invasion-promoting signaling unit as breast cancer progresses to an invasive phenotype. Overall, the data presented here define G12 signaling as a key promoter of breast cancer invasion. Furthermore, from a clinical perspective, these studies suggest that targeted inhibition of G12 signaling may provide effective therapies to reduce local invasion, slow metastasis, and reduce cancer mortality.
Materials and Methods
Cell Lines, Reagents, and Antibodies.
All human cell lines were obtained from the American Type Culture Collection and grown under the recommended conditions. The 4T1 cell line (a generous gift from Fred Miller, Barbara Ann Karmanos Cancer Center, Detroit) was maintained in DMEM supplemented with 10% FCS. Recombinant human thrombin was from Enzyme Research Laboratories (South Bend, IN), and U46619 and tetanolysin were from Biomol (Plymouth Meeting, PA). Growth-factor-reduced Matrigel was from BD Biosciences (Bedford, MA), and the fibronectin from bovine plasma was from Sigma. GST-C3 expression construct was obtained from Judith Meinkoth (University of Pennsylvania, Philadelphia, PA). Antibodies to RhoA, Gαq, Gα12, and Gα13 and the blocking peptide for the Gα12 antibody were obtained from Santa Cruz Biotechnology, and monoclonal anti-hemagglutinin and anti-myc antibodies were obtained from Zymed Laboratories. Polyclonal anti-sera to Gα12 and Gα13 were also obtained from Stefan Offermanns (University of Heidelberg, Heidelberg, Germany). Polyclonal anti-serum to RGS2 was from David Siderovski (University of North Carolina, Chapel Hill). Polyclonal anti-serum to Gαq was from Tom Gettys (Pennington Biomedical Research Center, Baton Rouge, LA). Polyconal antiserum to E-cadherin was from Robert Brackenbury (University of Cincinnati College of Medicine, Cincinnati).
Adenoviral Infections.
Recombinant adenoviruses were constructed by subcloning human Gαq(Q209L), Gα12(Q231L), Gα13(Q226L), hemagglutinin-RGS2 (University of Missouri cDNA Resource, Rolla, Mo), myc-p115 (gift of Tohru Kosaza, University of Illinois, Chicago) and myc-p115(E29K) (generated by site-directed mutagenesis of the myc-p115) into the Adtrack-CMV vector (gift of Robert Weinberg, The Johns Hopkins University Medical Center) then recombining these with pAdEasy-1 in BJ5183 Escherichia coli (Stratagene). The resulting DNA was transfected into HEK 293 cells with Lipofectamine (Invitrogen), then the viruses were serially amplified and purified with Adeno-X Virus Purification kits (BD Biosciences). Cell lines were infected at a multiplicity of infection of 5–50, for 6–24 h at 37°C. Infection efficiencies ranged from 80–100%. For experiments using C3 toxin, 1 mg of purified C3 and 20 hemolytic units (as defined by Biomol) of tetanolysin were added to the cells for 1 h after the infection media was removed. The cells were then washed twice with PBS and then were incubated for 18 h in DMEM with 0.1% fatty-acid-free BSA.
Retrovirus Production.
Recombinant retroviruses were constructed by subcloning human Gαq(Q209L), Gα12(Q231L), Gα13(Q226L), hemagglutinin-RGS2 (University of Missouri cDNA Resource), myc-p115, and myc-p115RGS(E29K) into the pLXRN vector (Clontech) and pGL-2 firefly luciferase into pLPCX (Clontech). Constructs were then cotransfected into the GP2–293 packaging line (Clontech) with FuGene (Roche Applied Science). Viral supernatants were collected 48 h later, clarified by filtration, and concentrated by ultracentrifugation. The concentrated virus was used to infect 1 × 106 cells in a 60-mm dish with 8 μg/ml polybrene. 4T1 cells were selected with 2 μg/ml puromycin (Sigma) and 1.2 mg/ml Geneticin (GIBCO). MDA-MB-231 and PC3 cells were selected with 2 μg/ml puromycin (Sigma) and 800 mg/ml Geneticin (GIBCO).
Cell Invasion Assay.
For invasion assays, transwell chamber filters (8-μm pore size, polycarbonate filter, 6.5-mm diameter; Costar) were coated with 50 μg of growth-factor-reduced Matrigel (T47D, MDA-MB-231, and BT549) or 20 μg of collagen (4T1). After infection with adenovirus, cells were starved for 18 h in DMEM containing 0.1% BSA and detached, and 3 × 105 cells in 100 μl were placed to the upper chamber of the transwell with and without agonists. The insert was then transferred to a well containing 600 μl of 5 μg/ml of fibronectin in conditioned media from NIH 3T3. Cells were incubated for 30 h at 37°C in a humidified incubator. Cells in the top well were removed by swiping the top of the membrane with cotton swabs. The membranes were then stained (Hema3 staining kit, Fisher Scientific), and the remaining cells were counted. Five high-powered fields were counted for each membrane.
Rho Activity Assays.
The levels of activated Rho were determined by using pull-down assays with a GST fusion of the RhoA-binding domain of rhotekin as described in ref. 21.
In Vivo Metastasis.
Animal handling and procedures were approved by the Duke University Medical Center Institutional Animal Care and Use Committee. 4T1 cells expressing firefly luciferase (4T1-Luc) were generated by retroviral transduction. For mammary fat pad injections, 5 × 105 4T1-Luc cells were implanted in the right axillary mammary fat pad of 6-week-old female BALB/c mice. The volume of the primary tumors was quantified with caliper measurements in three dimensions. At day 21, the primary tumors were removed as described in ref. 26. Bioluminescence imaging was performed on all of the mice at ≈3-day intervals.
For direct metastasis to the lung, 1 × 105 4T1-Luc cells were injected into the lateral tail vein of 6-week-old female BALB/c mice. Bioluminescence imaging was performed on all of the mice at ≈3-day intervals.
Bioluminescence Imaging.
Mice were anesthetized and given 150 μg/g d-luciferin in PBS by i.p. injection. Fifteen minutes after injection, bioluminescence was imaged with a charge-coupled device camera (IVIS; Xenogen, Alameda, CA). Bioluminescence images were obtained with a 15-cm field of view, binning (resolution) factor of 8, 1/f stop, open filter, and with an imaging time of 30 sec. Bioluminescence from relative optical intensity was defined manually, and data were expressed as photon flux (photons/s per cm2/steradian). Background photon flux was defined from a relative optical intensity drawn over a mouse that was not given an injection of luciferin.
Immunohistochemistry.
Institutional Review Board-approved breast samples were from Ardais (Lexington, MA). Tissue microarray (#BR801) was from U.S. Biomax (Rockville, MD). After paraffin removal and quenching of endogenous peroxidase, 5-μm sections were steamed in 10 mM citrate, pH 6.0, for 15 min in a steamer (model no. HS900, Black & Decker), then incubated with Background Buster (Innovex Biosci, Richmond, CA) for 30 min. Sections were then incubated with G12 antisera (Santa Cruz Biotechnology) diluted 1:100 in PBS for 1 h, followed by biotinylated goat anti-rabbit antisera (Vector Laboratories, Burlingame, CA) diluted 1:200 in PBS for 30 min, followed by horseradish peroxidase-labeled streptavidin (Jackson ImmunoResearch) for 30 min, all at room temperature. Bound immune complex was visualized with DAB (Innovex Biosci, Richmond, CA); hematoxylin counterstain (Fisher, Pittsburgh, PA) was used. The G12 staining was graded 0–3+ based on intensity, and data were analyzed by using one-way ANOVA and Dunn’s multiple comparison test in prism, Version 4.0c (GraphPad, San Diego).
Supporting Information.
Additional details can be found in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank C. DeMarco (Duke University Medical Center) for generating the myc-p115-RGS(E29K) mutant, T. Meigs and B. Berwin for advice and suggestions, K. Young for technical assistance, and D. Kaplan and L. Stemmle for their critical review of this manuscript. Supported by National Institutes of Health Grants CA100869 (to P.J.C.); 5P50 CA68438 (to Duke University Medical Center); CA92240 and CA063071 (to C.J.D.); DK62833 (to T.A.F.); and AG17952 and DK 60917 (to Y.D.) and Department of Defense Grant DAMD17-03-1-0691 (to T.A.F.). J.J. was supported by a Human Frontiers Science Program fellowship. P.K. was supported by the Duke University Medical School Alumni Scholarship.
Abbreviation
- RGS
regulator of G protein signaling.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pierce K. L., Premont R. T., Lefkowitz R. J. Nat. Rev. Mol. Cell. Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Rohrer D. K., Kobilka B. K. Physiol. Rev. 1998;78:35–52. doi: 10.1152/physrev.1998.78.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Offermanns S. Oncogene. 2001;20:1635–1642. doi: 10.1038/sj.onc.1204189. [DOI] [PubMed] [Google Scholar]
- 4.Strathmann M. P., Simon M. I. Proc. Natl. Acad. Sci. USA. 1991;88:5582–5586. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan A. M., Fleming T. P., McGovern E. S., Chedid M., Miki T., Aaronson S. A. Mol. Cell. Biol. 1993;13:762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu N., Bradley L., Ambdukar I., Gutkind J. S. Proc. Natl. Acad. Sci. USA. 1993;90:6741–6745. doi: 10.1073/pnas.90.14.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhika V., Dhanasekaran N. Oncogene. 2001;20:1607–1614. doi: 10.1038/sj.onc.1204274. [DOI] [PubMed] [Google Scholar]
- 8.Riobo N. A., Manning D. R. Trends Pharmacol. Sci. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Shi C. S., Sinnarajah S., Cho H., Kozasa T., Kehrl J. H. J. Biol. Chem. 2000;275:24470–24476. doi: 10.1074/jbc.M908449199. [DOI] [PubMed] [Google Scholar]
- 10.Martin C. B., Mahon G. M., Klinger M. B., Kay R. J., Symons M., Der C. J., Whitehead I. P. Oncogene. 2001;20:1953–1963. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- 11.Rumenapp U., Asmus M., Schablowski H., Woznicki M., Han L., Jakobs K. H., Fahimi-Vahid M., Michalek C., Wieland T., Schmidt M. J. Biol. Chem. 2001;276:2474–2479. doi: 10.1074/jbc.M004957200. [DOI] [PubMed] [Google Scholar]
- 12.Jiang H., Wu D., Simon M. I. FEBS Lett. 1993;330:319–322. doi: 10.1016/0014-5793(93)80896-3. [DOI] [PubMed] [Google Scholar]
- 13.Voyno-Yasenetskaya T. A., Pace A. M., Bourne H. R. Oncogene. 1994;9:2559–2565. [PubMed] [Google Scholar]
- 14.Kurose H. Life Sci. 2003;74:155–161. doi: 10.1016/j.lfs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Repesh L. A. Invasion Metastasis. 1989;9:192–208. [PubMed] [Google Scholar]
- 16.Even-Ram S., Uziely B., Cohen P., Grisaru-Granovsky S., Maoz M., Ginzburg Y., Reich R., Vlodavsky I., Bar-Shavit R. Nat. Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 17.Booden M. A., Eckert L. B., Der C. J., Trejo J. Mol. Cell. Biol. 2004;24:1990–1999. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z., Singer W. D., Wells C. D., Sprang S. R., Sternweis P. C. J. Biol. Chem. 2003;278:9912–9919. doi: 10.1074/jbc.M212695200. [DOI] [PubMed] [Google Scholar]
- 19.Sahai E., Marshall C. J. Nat. Rev. Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 20.Chikumi H., Vazquez-Prado J., Servitja J. M., Miyazaki H., Gutkind J. S. J. Biol. Chem. 2002;277:27130–27134. doi: 10.1074/jbc.M204715200. [DOI] [PubMed] [Google Scholar]
- 21.Meigs T. E., Juneja J., DeMarco C. T., Stemmle L. N., Kaplan D. D., Casey P. J. J. Biol. Chem. 2005;280:18049–18055. doi: 10.1074/jbc.M500445200. [DOI] [PubMed] [Google Scholar]
- 22.Meigs T. E., Fedor-Chaiken M., Kaplan D. D., Brackenbury R., Casey P. J. J. Biol. Chem. 2002;277:24594–24600. doi: 10.1074/jbc.M201984200. [DOI] [PubMed] [Google Scholar]
- 23.Chambers A. F., Groom A. C., MacDonald I. C. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 24.Aslakson C. J., Miller F. R. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 25.Yang J., Mani S. A., Donaher J. L., Ramaswamy S., Itzykson R. A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R. A. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Smith M. C., Luker K. E., Garbow J. R., Prior J. L., Jackson E., Piwnica-Worms D., Luker G. D. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney T. J., Mailander V., Tucker A. A., Olomu A. B., Zhang W., Cao Y., Negrin R. S., Contag C. H. Proc. Natl. Acad. Sci. USA. 1999;96:12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J., Wang F., Van Keymeulen A., Herzmark P., Straight A., Kelly K., Takuwa Y., Sugimoto N., Mitchison T., Bourne H. R. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 29.Parks S., Wieschaus E. Cell. 1991;64:447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- 30.Lin F., Sepich D. S., Chen S., Topczewski J., Yin C., Solnica-Krezel L., Hamm H. J. Cell Biol. 2005;169:777–787. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Offermanns S., Mancino V., Revel J. P., Simon M. I. Science. 1997;275:533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- 32.Wong C. W., Song C., Grimes M. M., Fu W., Dewhirst M. W., Muschel R. J., Al-Mehdi A. B. Am. J. Pathol. 2002;161:749–753. doi: 10.1016/S0002-9440(10)64233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutkind J. S., Coso O. A., Xu N. In: G proteins, Receptors, and Disease. Spiegel A. M., editor. Totowa, NJ: Humana; 1998. pp. 101–117. [Google Scholar]
- 34.Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M. E., McClanahan T., Murphy E., Yuan W., Wagner S. N., et al. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 35.Kleer C. G., Griffith K. A., Sabel M. S., Gallagher G., van Golen K. L., Wu Z. F., Merajver S. D. Breast Cancer Res. Treat. 2005;93:101–110. doi: 10.1007/s10549-005-4170-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.