Abstract
ATP is a widely used extracellular signaling molecule. The mechanism of ATP release from cells is presently unresolved and may be either vesicular or channel-mediated. Erythrocytes release ATP in response to low oxygen or to shear stress. In the absence of vesicles, the release has to be through channels. Erythrocytes do not form gap junctions. Yet, here we show with immunohistochemical and electrophysiological data that erythrocytes express the gap junction protein pannexin 1. This protein, in addition to forming gap junction channels in paired oocytes, can also form a mechanosensitive and ATP-permeable channel in the nonjunctional plasma membrane. Consistent with a role of pannexin 1 as an ATP release channel, ATP release by erythrocytes was attenuated by the gap junction blocker carbenoxolone. Furthermore, under conditions of ATP release, erythrocytes took up fluorescent tracer molecules permeant to gap junction channels.
Keywords: ATP release, gap junction, hemichannel, dye uptake
Pannexins represent a recently discovered second family of gap junction proteins in vertebrates (1). Pannexins have no sequence homology with the well known connexin family of vertebrate gap junction proteins. Instead, they are related to innexins, which were originally considered to be exclusively invertebrate gap junction proteins. The functional role of pannexins is unknown. The existence of connexin-specific diseases, despite an overlap of connexin and pannexin expression, suggests a functional role of pannexins that is distinct from that of connexins. Pannexin 1, in addition to forming gap junctions in paired oocytes, also forms nonjunctional membrane channels that provide a passageway from the cytoplasm to the extracellular space for molecules in the size range of second messengers (2, 3). It can be hypothesized that the physiological role of pannexin 1 is formation of a nonjunctional membrane channel.
Although the role of ATP as an extracellular signaling molecule is well recognized (4), the release mechanism for ATP from cells to the extracellular space has remained enigmatic. Two general release modes have been proposed: (i) vesicular release akin to the exocytotic release of transmitters and (ii) channel-mediated release. Although vesicular ATP release is well documented (5, 6), it cannot account for all of the ATP release phenomena. In particular, ATP is released from erythrocytes, which, under physiological conditions, are vesicle-free (7). Various channels have been implicated in the process, including CFTR (cystic fibrosis transmembrane conductance regulator), connexin 43 (Cx43) hemichannels, a volume-regulated channel (VRAC), and the purinergic receptor P2X7 (5, 6, 8–11). However, the evidence for their involvement falls short because of questionable specificity of the pharmacological blockers used to determine channel identity.
Mechanical stress is a prime stimulus for ATP release in many cell types, including erythrocytes (12). An efficient release mechanism thus may involve a channel that is both mechanosensitive and permeable for ATP. Pannexin 1 fulfills these specifications; it forms a stress-sensitive, ATP-permeable channel when expressed in Xenopus oocytes (3). Furthermore, pannexin 1 channels can be activated by extracellular ATP when coexpressed with P2Y receptors (13). Thus, pannexin channels could mediate ATP-induced ATP release, which is observed in several cell types, including erythrocytes (14). Here, we show that erythrocytes express the gap junction protein pannexin 1, although these cells do not form gap junctions.
Results
Expression of Pannexin 1 in Erythrocytes.
To test whether pannexin 1 could be a physiological ATP release channel, we examined its expression and function in human erythrocytes. Custom-made chicken antibodies (Aves Labs, Tigard, OR) were used to test expression. Antibody 4512 was directed against a peptide sequence contained in the putative first extracellular loop of pannexin 1. Antibody 4515 was directed against carboxyl-terminal amino acids. When the “extracellular loop” antibody was applied to intact erythrocytes and reacted with a fluorescent secondary antibody, intense staining was visible on the erythrocyte surface, indicating an extracellular localization of the antigen (Fig. 1 A and B). To verify that the antibody interacts with pannexin 1, it was applied extracellularly to intact Xenopus oocytes. As shown in Fig. 1 C and D, fluorescent label was found on oocytes only when pannexin 1 was exogenously expressed. Application of the antibody to uninjected oocytes failed to induce a fluorescent signal. Similarly, no staining was detected in pannexin 1-expressing oocytes with preimmune serum, indicating a specific interaction of the antibody with the pannexin 1 epitope. The “COOH” antibody 4515 labeled oocytes only after cryosectioning, suggesting a cytoplasmic localization of the carboxyl terminus of pannexin 1 (Fig. 1E; see also Fig. 6, which is published as supporting information on the PNAS web site).
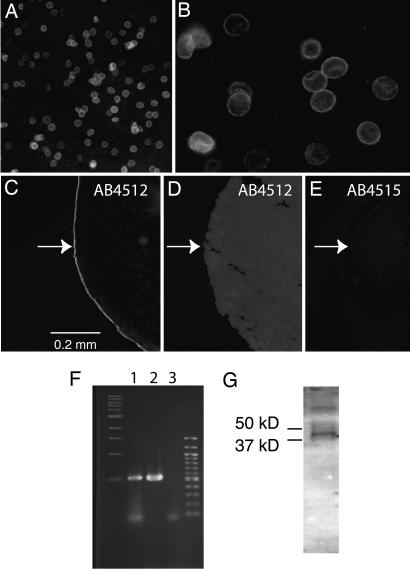
Fig. 1.
Expression of pannexin 1. (A) Human erythrocytes are stained by extracellular application of antibody 4512. (B) Antibody-reacted erythrocytes at higher magnification. (C and E) Extracellular application of antibody 4512 (C) but not 4515 (E) to intact oocytes expressing human pannexin 1 yielded immunostaining of the oocyte surface. Application of antibody 4515 to sectioned oocytes resulted in the same staining pattern as in C (Fig. 6). (D) Uninjected oocytes did not yield staining with either antibody, irrespective of the side of application. The arrows indicate the location of the oocyte surface. (F) PCR amplification of a human bone marrow cDNA library with pannexin 1-specific primers yielded the same size amplicon (lane 1) as seen with the pannexin 1 clone used for exogenous expression (lane 2); no amplicon was generated without addition of DNA (lane 3) to the PCR. Lanes 1–3 are flanked by size markers. (G) Western blot of human erythrocyte membranes with antibody 4512 yielded a band of expected size for pannexin 1. The higher-molecular-weight band may represent a dimer of the protein. Size markers were run on parallel lanes.
As an independent test for pannexin 1 expression in erythrocytes, a cDNA library of human bone marrow was tested for pannexin 1-specific sequences by PCR. Fig. 1F shows that, indeed, an amplicon of appropriate size was produced. Sequencing of the PCR product verified its pannexin 1 identity (Fig. 7, which is published as supporting information on the PNAS web site). Western blots of erythrocyte membranes with either antibody revealed a band of appropriate size for pannexin 1 monomer (Fig. 1G). In support of antibody specificity, Western blots of Xenopus oocyte lysates with antibody 4515 yielded unique bands in pannexin 1 mRNA-injected oocytes (Fig. 8, which is published as supporting information on the PNAS web site).
Cx43 has been shown to exhibit hemichannel activity, although only under extreme conditions (15). Cx43 is expressed during hematopoiesis (16). Because Cx43 hemichannels have been proposed to act as ATP release channels, we tested whether mature erythrocytes still contain Cx43 protein. Western blots of erythrocyte membranes did not show detectable levels of Cx43 (Fig. 8).
ATP Release.
ATP release from erythrocytes occurs physiologically in response to shear stress or at low oxygen content (7, 12). Experimentally, it can be induced by hypotonic stress. Pannexin 1 channels are mechanosensitive. However, they open and mediate release of ATP also with membrane depolarization (3). As an initial test for an involvement of pannexin 1 channels in ATP release from erythrocytes, we determined the effect of depolarization with high potassium. Fig. 2A shows that depolarization was slightly less effective in ATP release than osmotic stress. Carbenoxolone, a gap junction inhibitor, has been shown to close pannexin channels with higher efficiency than connexin channels (17). Fig. 2B shows that the drug inhibits the osmotic stress-induced release of ATP from erythrocytes.
Fig. 2.
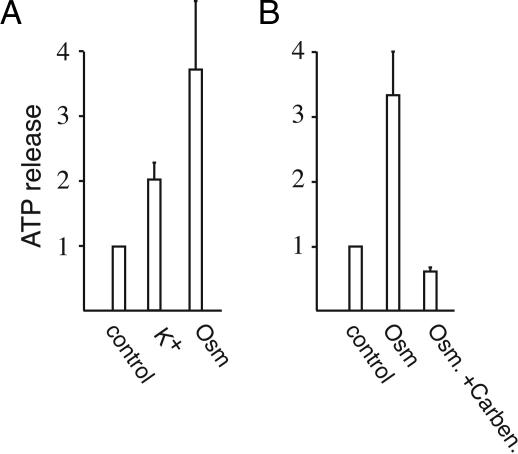
ATP release from human erythrocytes. ATP was measured with a luciferase assay in the supernatant after centrifugation. Data are normalized to basal release in unstimulated erythrocytes. (A) Release was increased by depolarization with potassium as well as by hypotonic stress with 1:1-diluted Krebs solution. (B) Inhibition of osmotically induced ATP release by 100 μM carbenoxolone. Means ± SEM are plotted (n = 5).
Uptake of Fluorescent Tracer Molecules.
For an independent verification that a gap junction-like channel is involved in ATP release, we tested the uptake of fluorescent tracer molecules by erythrocytes. Fig. 3 shows the uptake of carboxyfluorescein by erythrocytes depolarized by high K+ (K+ replacing Na+ in Krebs solution). All erythrocytes were fluorescent but with variable intensity. In comparison, control erythrocytes incubated with the dye dissolved in regular Krebs solution remained nonfluorescent, except for a few erythrocyte ghosts and debris (Fig. 3 A and B). Dye uptake also was observed when erythrocytes were incubated in Krebs solution with low oxygen content (Fig. 3 D and E). Quantitative analysis of dye uptake revealed significant uptake under conditions of ATP release (Fig. 3F). Like ATP release, dye uptake by erythrocytes was attenuated by carbenoxolone (data not shown).
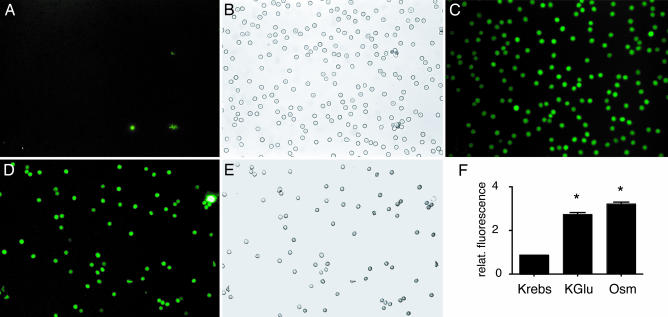
Fig. 3.
Uptake of the fluorescent tracer molecule carboxyfluorescein by human erythrocytes under conditions of ATP release. (A and B) In Krebs solution, fluorescence was only associated with cell debris or erythrocyte ghosts. (C) All erythrocytes in the field of view were fluorescent after stimulation of ATP release with high K+. (D and E) Low oxygen exposure yielded dye uptake in all erythrocytes in the field of view. (F) Quantitative analysis of tracer uptake induced by depolarization (KGlu) or osmotically (Osm) by incubating erythrocytes in 1:1-diluted Krebs solution. Means ± SEM are plotted (n = 5); P < 0.01 versus control (Krebs).
Pannexin 1-Like Channel Activity in Erythrocytes.
The single channel properties of pannexin 1 channels are known (3). To test whether erythrocytes exhibit pannexin 1 channel activity, we patch-clamped the cells. Although a wide range of apertures of the patch pipettes was tested, no patch containing a single channel was obtained. Typically, a large amount of membrane slipped into the pipette upon application of suction to create the seal. Nevertheless, multichannel records were obtained from channels in erythrocyte membranes that shared properties with pannexin 1 channels (Fig. 4 and Table 1; see also Fig. 9, which is published as supporting information on the PNAS web site). Large conductance channels were observed that fall in the conductance range of pannexin 1 channels (475pS in KGluconate). Like pannexin 1 channels, the large conductance channels in erythrocytes were mostly closed at negative holding potentials (Fig. 4A) and exhibited high activity when held at positive potential (Fig. 4B). Application of salt gradients to membrane patches excised from erythrocytes and containing the large conductance channels resulted in reversal potentials as observed for pannexin 1 channels (3) (Fig. 4C). Furthermore, the large conductance channels in erythrocytes could be activated by mechanical stress, like pannexin 1 channels (Fig. 4D).
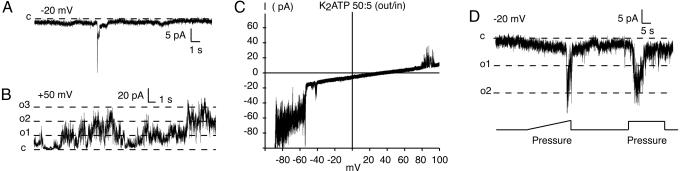
Fig. 4.
Channel currents in membrane patches excised from human erythrocytes with properties consistent with those of pannexin 1 channels. (A and B) Channel conductances of the magnitude of pannexin 1 channels were rarely seen at negative holding potentials (A) but were frequent at positive holding potentials (B). (C) The reversal potentials for membrane currents in a potassium ATP gradient (50 mM out/5 mM in) were of the same magnitude as those observed in pannexin 1 channels expressed in oocytes (3). (D) Like pannexin 1 channels, the large conductance channels in erythrocytes were mechanosensitive. The lines in B and D indicate current levels expected for full openings of pannexin 1 channels.
Table 1.
Comparison of large conductance channels in erythrocytes and pannexin 1 channels expressed in Xenopus oocytes
The assignment of the large conductance channel activity in erythrocytes to pannexin 1 is not trivial because of the pannexin channels’ preference for multiple subconductance states (3) (Fig. 9). With multiple channels in the pipette, this problem is compounded. Therefore, only by the fulfillment of several criteria, including voltage dependence of activity, reversal potential, and mechanosensitivity, is it likely that the large conductance channels indeed reflect pannexin 1 activity.
Discussion
There is no evidence that erythrocytes form intercellular gap junction channels. Therefore, the expression of a gap junction protein in these corpuscles seems counterintuitive. The findings of ATP efflux from erythrocytes being affected by carbenoxolone, the uptake of tracer molecules under conditions of ATP release, and the pannexin channel’s mechanosensitivity and high ATP permeability (3) suggest that pannexin 1 functions as an ATP release channel. Thus, a prime function of pannexins, rather than forming gap junctions redundant to those formed by connexins, may be to form a large, transient channel connecting the cytoplasm with the extracellular space. Because this channel is half of a gap junction channel, it is referred to as a “hemichannel.” Hemichannel function has been described also for some connexins under certain experimental conditions (18–22). In particular, Cx43 has been implicated as an ATP release channel (9, 23, 24), although calcium wave propagation was found to proceed in Cx43-deficient cells at normal rates (25, 26). Similarly, erythrocytes release ATP in the absence of detectable Cx43 expression (Fig. 8). Because of their gating properties, Cx43 channels are poor candidates for physiological ATP release channels. Channel activity that can unequivocally be attributed to Cx43 hemichannels has only been observed at membrane potentials in excess of +50 mV (15). Micromolar extracellular calcium concentrations may also open the channels (20). In contrast, pannexin 1 channels can be opened at the resting membrane potential by mechanical stress or by micromolar cytoplasmic calcium (3, 13). Furthermore, depolarization to potentials induced by high potassium is sufficient to open the channel (2). These gating properties of pannexin 1 channels are consistent with a physiological function as an ATP release channel. Given that pharmacological responses of pannexin 1 channels are similar to those of connexin channels, it is quite probable that some functions formerly attributed to connexin hemichannels actually are exerted by pannexin 1.
Recently, it has been shown that P2X7 receptors are involved in the release of ATP from astrocytes in low divalent cation solution (27). Human erythrocytes express several purinergic receptors, apparently also including the P2X7 receptor (28). Uptake of carboxyfluorescein by erythrocytes was not stimulated by the P2X7 agonist BzATP, nor was the potassium-induced uptake inhibited by the antagonist brilliant blue G (Fig. 10, which is published as supporting information on the PNAS web site). Furthermore, ATP release and dye uptake by erythrocytes takes place at regular extracellular calcium concentration. Thus, P2X7 is unlikely to represent the release pathway for ATP from erythrocytes.
With such a high level of a gap junction protein expressed, the obvious question arises: Why are there no gap junctions between erythrocytes? There are at least two possible reasons. Most likely, the rich glycoprotein content of erythrocyte membranes represents a steric hindrance for the docking of hemichannels. The formation rate of gap junction channels is slowed by steric hindrance (29, 30). In addition, pannexins may have low affinity for each other, making docking of hemichannels a rare event. There is a precedent for low affinity of a gap junction protein. Connexin 38 is inefficient in forming homotypic gap junction channels but readily forms heterotypic junctional channels with Cx43 (29).
Connexins are typically recognized by their punctate appearance under the light microscope. We have not observed punctate staining of pannexin in any tissue analyzed so far. Although functional gap junction channels are formed by pannexin 1 in the paired oocyte system (2), one has to keep in mind that paired oocytes represent a forced system that may exaggerate weak tendencies. Thus, it remains to be determined whether pannexins form gap junction channels under physiological conditions.
The physiological stimuli for ATP release by erythrocytes are shear stress and low oxygen content (7, 12). Extracellular ATP can induce further ATP release by means of P2Y receptors on the erythrocyte membrane (14). This ATP-induced ATP release may also be mediated by pannexin 1 channels, because they can be activated by extracellular ATP when coexpressed with P2Y receptors (13). ATP in blood vessels also interacts with P2Y receptors on endothelial cells, initiating a propagated calcium wave that ultimately leads to the release of nitric oxide onto vascular smooth muscle (31, 32). Relaxation of the muscle leads to increased perfusion. Thus, ATP is a key factor in a local control loop of oxygen delivery (33, 34). The role of pannexin 1 channels in this control loop may not be restricted to erythrocytes but may also extend to the calcium wave propagation in endothelial cells. The pannexin protein is expressed at high levels in these cells as determined immunohistochemically (Fig. 11, which is published as supporting information on the PNAS web site). The expression levels are highest in the vasculature of heart and skeletal muscle, consistent with the prominence of the local perfusion control by ATP in these tissues. Fig. 5 illustrates how pannexin 1 channels could be involved in the local control of blood perfusion.
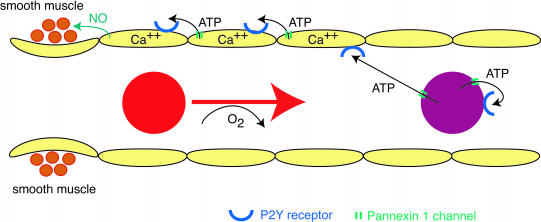
Fig. 5.
Scheme depicting a possible involvement of pannexin 1 channels in local blood-flow regulation. Oxygen-deprived or shear-stressed erythrocytes release ATP, which binds to P2Y receptors on erythrocytes and on endothelial cells. In erythrocytes, activation of the receptor leads to further release of ATP through pannexin 1 channels (ATP-induced ATP release). In endothelial cells, binding of ATP to P2Y receptors or shear stress initiates a calcium wave by opening pannexin 1 channels. The wave propagates retrogradely and reaches the precapillary sphincter region. Endothelial NO synthase is activated, and NO released from the endothelial cell relaxes the smooth muscle. In arterioles, the smooth muscle would be covering all endothelial cells and could be relaxed by NO without the need of a propagated wave.
The observation that antibody 4512 interacted with the protein when applied extracellularly indicates that the epitope is located on the cell surface. As with connexins, hydrophobicity plots for the pannexin sequence suggest four transmembrane segments. The epitope recognized by the antibody would be part of the carboxyl-terminal portion of the first extracellular loop. Because antibody 4515 only reacted with sectioned cells, it can be assumed that the epitope recognized by this antibody has a cytoplasmic localization. These findings are consistent with an overall topology of pannexins similar to that of connexins.
Materials and Methods
Two anti-pannexin 1 antibodies were custom-made by Aves Labs in chickens and are directed against the sequences VQQKSSLQSESGN (4512) and EKNSRQRLLNPS (4515). Secondary goat antibodies against chicken IgY coupled to horseradish peroxidase or fluorescein (Aves Labs) were used for Western blots and immunohistochemistry, respectively. For detection of Cx43, a commercial Cx43 antibody (Zymed) was used.
Oocyte expression of pannexin 1 was performed as described in ref. 3. Antibodies were applied either to intact oocytes after mechanical removal of the vitelline membrane or to cryosections of oocytes.
Human erythrocytes (G.D.’s) were washed three times in Krebs solution by low-speed centrifugation. Erythrocytes were suspended at 25% hematocrit, and aliquots of 100 μl were used for experiments. ATP levels were determined in the supernatant of a 960 × g spin with a luciferase assay (Promega). For determination of dye uptake, erythrocytes were incubated in Krebs solution containing 2 mM 5,6-carboxyfluoresceine for 10 min. Erythrocytes were viewed after five washes by epifluorescence in a microscope, and the images were digitally recorded. Low oxygen conditions were achieved by keeping the erythrocytes in a nitrogen atmosphere. For quantitative analysis, erythrocytes were pelleted after the washes and taken up in 200 μl of distilled water for lysis. After centrifugation, 50 μl of the supernatants was analyzed in a fluorometer. Data were normalized to the control condition (incubation in Krebs solution).
Human bone marrow cDNA was obtained from BioChain Institute (Hayward, CA). For PCR amplification, the primers gaggtatctgaaagcaccacttcaagtaccc and gaccactgctcttaatattctccagtacc were used. Human pannexin 1 (MRS1) was obtained from Graeme Bolger (University of Alabama, Birmingham, AL).
Patch clamp of erythrocytes was performed with borosilicate glass pipettes with 20- to 30-megaohm resistance. Currents through excised erythrocyte membrane patches were recorded with a WPC-100 amplifier (E.S.F. Electronic, Goettingen, Germany). Currents were filtered at 5 kHz, digitized by using a VR-10B digital data recorder, and stored on videotape. The recordings were transferred to a Power Macintosh computer (Apple) by using an ITC-18 computer interface (Instrutech, Mineola, NY) and analyzed. Acquisition and analysis were done with the acquire and tac programs (both from Bruxton, Seattle).
Supplementary Material
Acknowledgments
We thank Drs. N. Chaudhari, R. Keane, and K. Magleby for valuable discussions. This work was supported by National Institutes of Health Grant GM48610. S.L. was supported by a predoctoral fellowship from the American Heart Association.
Abbreviation
- Cx43
connexin 43.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Panchin Y., Kelmanson I., Matz M., Lukyanov K., Usman N., Lukyanov S. Curr. Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 2.Bruzzone R., Hormuzdi S. G., Barbe M. T., Herb A., Monyer H. Proc. Natl. Acad. Sci. USA. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao L., Locovei S., Dahl G. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G., Knight G. E. Int. Rev. Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 5.Bal-Price A., Moneer Z., Brown G. C. Glia. 2002;40:312–323. doi: 10.1002/glia.10124. [DOI] [PubMed] [Google Scholar]
- 6.Maroto R., Hamill O. P. J. Biol. Chem. 2001;276:23867–23872. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- 7.Bergfeld G. R., Forrester T. Cardiovasc. Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Reisin I. L., Prat A. G., Abraham E. H., Amara J. F., Gregory R. J., Ausiello D. A., Cantiello H. F. J. Biol. Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- 9.Cotrina M. L., Lin J. H., Alves-Rodrigues A., Liu S., Li J., Azmi-Ghadimi H., Kang J., Naus C. C., Nedergaard M. Proc. Natl. Acad. Sci. USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisadome K., Koyama T., Kimura C., Droogmans G., Ito Y., Oike M. J. Gen. Physiol. 2002;119:511–520. doi: 10.1085/jgp.20028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parpura V., Scemes E., Spray D. C. Neurochem. Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Sprague R. S., Ellsworth M. L., Stephenson A. H., Kleinhenz M. E., Lonigro A. J. Am. J. Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 13.Locovei S., Wang J., Dahl G. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Di Virgilio F., Chiozzi P., Ferrari D., Falzoni S., Sanz J. M., Morelli A., Torboli M., Bolognesi G., Baricordi O. R. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 15.Contreras J. E., Saez J. C., Bukauskas F. F., Bennett M. V. Proc. Natl. Acad. Sci. USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montecino-Rodriguez E., Leathers H., Dorshkind K. Blood. 2000;96:917–924. [PubMed] [Google Scholar]
- 17.Bruzzone R., Barbe M. T., Jakob N. J., Monyer H. J. Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 18.Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I., Goodenough D. A. J. Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebihara L. Biophys. J. 1996;71:742–748. doi: 10.1016/S0006-3495(96)79273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Liu T. F., Lazrak A., Peracchia C., Goldberg G. S., Lampe P. D., Johnson R. G. J. Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfahnl A., Zhou X. W., Werner R., Dahl G. Pflügers Arch. 1997;433:773–779. doi: 10.1007/s004240050344. [DOI] [PubMed] [Google Scholar]
- 22.Kamermans M., Fahrenfort I., Schultz K., Janssen-Bienhold U., Sjoerdsma T., Weiler R. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 23.Leybaert L., Braet K., Vandamme W., Cabooter L., Martin P. E., Evans W. H. Cell Commun. Adhes. 2003;10:251–257. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- 24.Stout C. E., Costantin J. L., Naus C. C., Charles A. C. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 25.Scemes E., Suadicani S. O., Spray D. C. J. Neurosci. 2000;20:1435–1445. doi: 10.1523/JNEUROSCI.20-04-01435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suadicani S. O., De Pina-Benabou M. H., Urban-Maldonado M., Spray D. C., Scemes E. Glia. 2003;42:160–171. doi: 10.1002/glia.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suadicani S. O., Brosnan C. F., Scemes E. J. Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sluyter R., Shemon A. N., Barden J. A., Wiley J. S. J. Biol. Chem. 2004;279:44749–44755. doi: 10.1074/jbc.M405631200. [DOI] [PubMed] [Google Scholar]
- 29.Dahl G., Werner R., Levine E., Rabadan-Diehl C. Biophys. J. 1992;62:172–180. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine E., Werner R., Dahl G. Am. J. Physiol. 1991;261:C1025–C1032. doi: 10.1152/ajpcell.1991.261.6.C1025. [DOI] [PubMed] [Google Scholar]
- 31.Moerenhout M., Himpens B., Vereecke J. Cell Calcium. 2001;29:125–136. doi: 10.1054/ceca.2000.0165. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K., Korenaga R., Kamiya A., Qi Z., Sokabe M., Ando J. Am. J. Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- 33.Ellsworth M. L., Forrester T., Ellis C. G., Dietrich H. H. Am. J. Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Alonso J., Olsen D. B., Saltin B. Circ. Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.