Abstract
Human herpes simplex virus 1 (HSV-1) is a double-stranded DNA virus that causes facial, ocular, and encephalitic disease in humans. Previous work showed that the genome of HSV-1 is associated with acetylated and methylated histones during lytic infection. However, the physiological role of histone modifications in lytic infection of HSV-1 is unclear. We examined the role of protein methylation in lytic infection of HSV-1 using a protein methylation inhibitor, 5′-deoxy-5′-methylthioadenosine (MTA). We found that MTA strongly reduces the transcription and replication of HSV-1. Moreover, MTA treatment decreases the level of trimethylation of lysine 4 in histone H3 (H3K4me3) on the HSV-1 genome. These results suggest that protein methylation, and in particular, histone methylation, is involved in the lytic infection of HSV-1. To delineate the underlying mechanism, we investigated the role of two H3K4 methyltransferases, Set1 and Set7/9, in the lytic infection of HSV-1. Using small interference RNA, we found that the reduction of Set1, but not Set7/9, reduces the transcription and replication of HSV-1 and specifically decreases H3K4me3 on the virus genome. These results indicate that H3K4me3 mediated by Set1 is required for optimal gene expression and replication of HSV-1 during lytic infection and suggest that this pathway could be a potential point of pharmacological intervention during HSV-1 infection.
The nuclear genomes of eukaryotes ranging from the yeast Saccharomyces cerevisiae to mammals are incorporated into a protein structure composed of histones and nonhistone proteins. The histones associate with the DNA, forming nucleosomes, which constitute the lowest level of packaging for assembly of higher-ordered structures (13). These structures impart protection from damage and provide regulatory functions for genomic processes, including activity such as transcription and replication, as well as quiescence such as gene-specific repression and heterochromatic silencing (5).
One regulatory mechanism through histones and histone variants is the posttranslational attachment of covalent modifications (5, 35). These modifications include acetylation, phosphorylation, methylation, ubiquitylation, and sumoylation. Acetylation was the first modification to be characterized in detail, which led to the revelation that modification enzymes include transcriptional cofactors involved in activation (3, 7, 34). Lysine methylation is a particularly versatile modification, since it is involved in both activating and repressing the eukaryotic genome and is used to regulate cellular genes and promote or suppress other DNA-templated processes (19, 46). For example, methylation of histone H3 at Lys-4 (H3K4me) is characteristic of active genes, while H3K9me is commonly present in heterochromatic or silent regions (4, 6, 16, 33). An additional layer of potential regulation is provided by one, two, or three methyl groups on the lysine side chain (29, 30).
Mechanisms of regulation via H3K4me are well understood. The dimethyl form of H3K4me is found associated with gene open reading frames (6, 29) and may provide a molecular memory of transcription. Trimethylation is induced corresponding with transcriptional activity and occurs preferentially at the 5′ end of open reading frames (33). In general, H3K4 methylation appears to be involved in transcriptional elongation by RNA polymerase II (27).
Genomes of DNA viruses, like their host genomes, are subject to chromatin-based repression and regulation (47). DNA viruses appear to utilize host-encoded structural and functional chromatin proteins for their own gene regulation during both lytic and latent infections. During lytic infection, the genomes of papovaviruses, adenoviruses, and herpesviruses are assembled into chromatin of various degrees of regularity of the canonical nucleosome repeat structure (17, 24). Lytic genes of these viruses acquire histone modifications characteristic of active cellular genes, such as histone acetylation (11). Interestingly, it appears to be nearly uniformly the case that viruses use host-encoded enzymes rather than having acquired their own, or at least none have been identified to date.
Histone modifications during latent and lytic infections by herpes simplex virus 1 (HSV-1) have recently been described (1, 10, 11, 14, 40). Latency is characterized by high levels of H3K4me and low levels of H3K9me on the active latency-associated transcript gene promoter (14), while the opposite histone modification balance occurs on the repressed lytic gene promoters (40). Acute infection is accompanied by the presence of activity-associated histone modifications on the lytic genes, including H3K9/14ac and H3K4me (11). In addition, the HSV-1 coactivator VP16 recruits various histone modification enzymes, including histone acetyltransferases (HATs) and ATP-dependent remodeling enzymes (10), coordinately with the activation of the immediate-early (IE) genes during acute infection.
Thus, it appears that HSV-1 is at least partially in a nucleosomal state during acute infection and that alternative histone modifications decorate the HSV-1 genome during lytic and latent infection (10, 11, 14, 15, 40). However, it is not clear whether the histone modifications are important for regulation. In this study, we have investigated this question during acute infection. We focus on methylation, since we find that H3K4me levels increase during infection, correlating with transcriptional induction of representatives of each HSV-1 gene class. Moreover, in cellular extracts, VP16 is associated with a host protein complex that includes Set1, an H3K4 methyltransferase (45). In this study, we find that either blocking methylation or reducing the level of Set1 lowers HSV-1 transcription and replication during lytic infection. These results suggest that histone methylation is actively involved in regulating HSV-1 infection.
MATERIALS AND METHODS
Cell line and virus.
HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics. The F strain of HSV-1 was used to infect the cells at a multiplicity of infection (MOI) of 5.
Infection and MTA treatment.
HeLa cells were incubated with a concentration of 1 mM 5′-deoxy-5′-methylthioadenosine (MTA) (Sigma) for about 16 h or with dimethyl sulfoxide as a negative control. The cells were then infected with the F strain of HSV-1 at an MOI of 5 or left as uninfected controls. Samples were harvested for protein, DNA, and RNA analysis at 1, 3, 6, and 10 h postinfection. Cytotoxicity of the MTA treatment was measured using the CytoTox 96 nonradioactive cytotoxicity assay kit (Promega). In brief, HeLa cells were incubated with 1 mM MTA for about 24 h, and supernatants were then analyzed for lactate dehydrogenase release.
siRNA and transient transfection.
Small interference RNAs (siRNAs) against Set1 and Set7/9 were purchased from Dharmacon and transfected into HeLa cells at a final concentration of 100 nM using Lipofectamine 2000 (Invitrogen, Inc.). The siRNA against Set1 was a siGENOME SMARTpool from Dharmacon. The target sequences for these four duplexes are as follows: (i) GGAAAGAGCCAUCGGAAAUUU, (ii) GACAACAACGAAUGAAAUAUU, (iii) CAACGACUCAAAGUAUAUAUU, and (iv) GCGAUUCGUCUUCCAAAUGUU. The target sequences for the Set7/9 SMARTpool are as follows: (i) CCUGGACGAUGACGGAUUA, (ii) UGUAGACGGAGAGCUGAAC, (iii) UUUAUUGAUGGAGAGAUGA, and (iv) UCACUCCUUCACUCCAAAC. A control siRNA that targets the luciferase gene (Dharmacon) was used. Its target sequence is 5′-UAAGGCUAUGAAGAGAUAC-3′. After two rounds of transfections, cells were infected with HSV-1 at an MOI of 5.
Western analysis.
Histone proteins were isolated from infected and mock-infected HeLa cells by using previously described methods (22). In brief, cells were lysed, and proteins were acid extracted using 0.2 M hydrochloric acid. Protein (5 μg for H3K4me3 and H3K9me2 and 0.5 μg for total H3 antibody) was loaded onto a sodium dodecyl sulfate-polyacrylamide gel, and proteins were identified using the following antibodies: anti-histone H3, anti-H3K9me, and anti-H3K4me3 (Abcam).
Nucleic acid analysis.
DNA was isolated using the Wizard DNA purification kit (Promega), and RNA was isolated using the Absolutely RNA kit (Stratagene). SYBR green reagent (Applied Biosystems) was used to measure the relative amount of double-stranded DNA products with an ABI 7700 machine (Applied Biosystems). Data analysis was performed as described previously (11), except that the mRNA level of each virus gene was normalized to that of 28S RNA. Each experiment was done in triplicate, and standard deviations are presented. Primer sequences were described previously (11).
ChIP assay.
A chromatin immunoprecipitation (ChIP) assay was performed as described previously (11). Briefly, HeLa cells were fixed with 1% formaldehyde and lysed with lysis buffer (50 mM HEPES-KOH [pH 7.5] 140 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, 0.1% Na-deoxycholate with protease inhibitors). The cell lysate was sonicated to shear the DNA into 200 to 500 bp. After preclearing with protein A-agarose beads, antibody was added and incubated at 4°C overnight. The next day, protein A-agarose beads were added to a pull-down immunoprecipitated complex. The complex was washed twice with lysis buffer, once with high-salt buffer (50 mM HEPES-KOH [pH 7.5], 500 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, 0.1% Na-deoxycholate), twice with LiCl buffer (10 mM Tris-HCl [pH 8.0], 0.25 M LiCl, 0.5% NP-40, 0.5% Na-deoxycholate, 1 mM EDTA), and once with Tris-EDTA buffer and eluted in Tris-EDTA plus 1% sodium dodecyl sulfate. DNA was then reverse cross-linked and subjected to real-time PCR analysis.
RESULTS
MTA treatment reduces H3K4me3 and H3K9me2 levels.
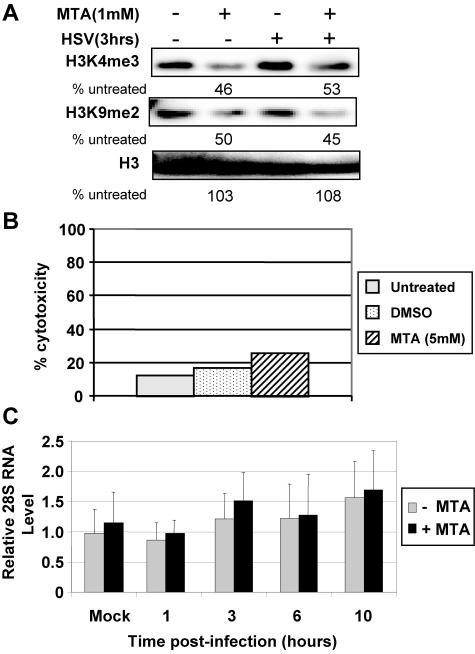
MTA is a pharmacological drug that inhibits protein methylation and was previously shown to modulate cell signaling and gene expression (2, 8, 23). We investigated whether MTA alters HSV-1 function via changes in chromatin and gene expression. As a first step, we determined whether MTA treatment changes the level of histone methylation in HeLa cells. Cells were treated with 1 mM MTA for 24 h, followed by HSV-1 infection for 3 h. Protein was extracted from cells, and equal amounts of protein were analyzed by Western blot analysis using antibodies that recognize histone methylation, H3K4me3, and H3K9me2. We found that MTA treatment reduces the global level of both modifications in bulk histones by approximately twofold (Fig. 1A). However, there was no apparent reduction of histone H3 levels (Fig. 1A). HSV-1 infection alone has little effect on global levels of H3K4me3 or H3K9me2 or on H3 levels (Fig. 1A), consistent with our previous observations (11).
FIG. 1.
MTA treatment reduces histone modifications in HeLa cells. (A) Cells were treated with 1 mM MTA for 24 h and then infected with HSV-1 for 3 h. Histones were extracted from cells, and Western blot analysis was performed with H3K4me3 and H3K9me2 antibodies. (B) After treatment with 1 mM MTA for 24 h, lactate dehydrogenase release was measured as the index for cytotoxicity. DMSO, dimethyl sulfoxide. (C) Cells were infected with HSV-1 for 1, 3, 6, and 10 h. Equal amounts of mRNA from each sample were reverse transcribed, and the level of 28S RNA was analyzed by quantitative real-time PCR.
We assessed the cytotoxicity of MTA treatment by measuring the release of lactate dehydrogenase (see Materials and Methods). Using this measure, there is a slight increase of cytotoxicity with 1 mM MTA treatment versus dimethyl sulfoxide or untreated controls (Fig. 1B). In addition, 1 mM MTA treatment combined with HSV-1 infection causes little change in 28S RNA levels assessed by quantitative PCR (Fig. 1C). Based on these assays, 1 mM MTA was used in further experiments.
MTA treatment reduces the H3K4me3 but not the H3K4me2 level on the HSV-1 genome.
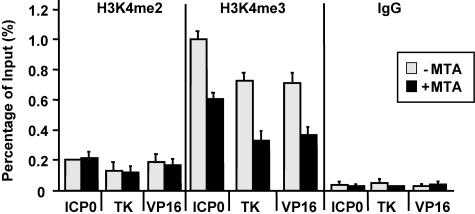
Previous work found that during lytic infection, the genome of HSV-1 is associated with nonregularly spaced nucleosomes (10, 11). Importantly, histones associated with the HSV-1 genome are methylated at sites involved in transcription (11). To determine whether MTA treatment reduces the level of histone modifications on the genome of HSV-1 during lytic infection, we used a ChIP assay to measure the level of H3K4me2 and H3K4me3 on the promoters and coding regions of ICP0, TK, and VP16. We found that MTA treatment decreases the level of H3K4me3 on the promoters of these three genes (Fig. 2), which specifically increases during transcriptional activation (6, 18, 33, 36). However, MTA treatment does not change the level of H3K4me2 (Fig. 2), which is not induced during gene activation (6, 18, 33, 36). We also investigated the level of H3K9me1, H3K9me2, and H3K9me3 and did not observe obvious recruitment of these modifications on the HSV-1 genome (data not shown).
FIG. 2.
MTA treatment decreases the recruitment of H3K4me3 on the genome of HSV-1. A ChIP assay was performed with cells treated with MTA (+MTA) (black bar) or without MTA (−MTA) (white bar) for 24 h, followed by HSV-1 infection for 1 h. The recruitment of H3K4me3 and H3K4me2 at the ICP0, TK, and VP16 promoters was measured by real-time PCR.
MTA treatment strongly inhibits transcription and replication of HSV-1.
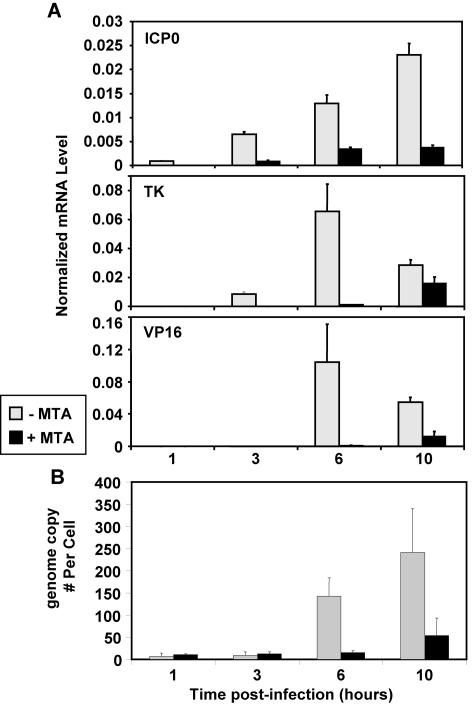
Histone modifications, and H3K4me in particular, are involved in the regulation of gene expression (6, 18, 33, 36). Since MTA treatment reduces the H3K4me3 level on the genome of HSV-1 (Fig. 2), it raises the possibility that MTA treatment may alter HSV-1 gene transcription during lytic infection. To test this hypothesis, we examined levels of HSV-1 transcripts with or without MTA treatment and analyzed the immediate-early gene ICP0, the early gene TK, and the late gene VP16. MTA treatment dramatically decreases the level of all these transcripts (Fig. 3A). Importantly, the levels of these transcripts are reduced at even 1 and 3 h before the onset of HSV-1 replication. Hence, MTA treatment strongly inhibits the transcription of HSV-1 during lytic infection.
FIG. 3.
MTA inhibits gene expression and replication of HSV-1. Cells were treated with MTA (+MTA) (black bar) or without MTA (−MTA) (white bar), followed by HSV-1 infection for 1, 3, 6, and 10 h. (A) mRNA levels for ICP0, TK, and VP16 were measured by real-time PCR and normalized to that of 28S RNA. (B) The genome copy number of HSV-1 was quantified by real-time PCR and normalized to cell numbers.
We then measured the genomic copy number of HSV-1 and found that MTA strongly inhibits HSV-1 replication (Fig. 3B). The effect on replication may be a direct effect of changes in the chromatin structure at viral origins of replication or could be an indirect effect due to decreasing transcription of IE and E genes (Fig. 3A), since many of these are involved in viral replication. Taken together, it appears that protein methylation events are critical for gene expression and replication of HSV-1 during the lytic infection and could in part be due to reduced histone methylation.
Set1 is involved in the regulation of HSV-1 lytic infection.
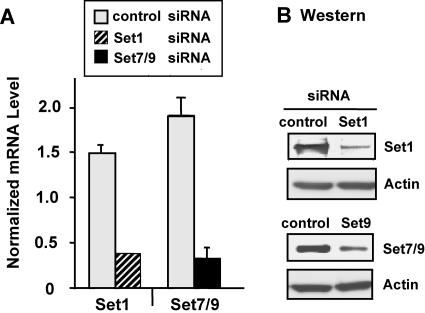
As described above, we observed a concomitant decrease during the lytic infection of H3K4me3 on the HSV-1 genome and HSV-1 gene expression. These observations led us to investigate a possible direct role of H3K4me3 in HSV-1 lytic infection. Importantly, previous studies revealed that host cell factor (HCF), a critical cellular factor for the initial gene expression of HSV-1, interacts with the cellular enzyme Set1 in a large complex (45). Set1 is a potential homologue of yeast Set1, a histone methyltransferase that trimethylates histone H3K4 (25). We examined a potential role of H3K4me3 in HSV-1 infection by reducing Set1 levels via lowering mRNA and protein using the siRNA technique. We compared the effects of reducing Set1 with those of lowering the amounts of a second cellular histone methyltransferase, Set7/9, which has been shown to monomethylate histone H3K4 in vitro but has not been tested in vivo (28). We found that siRNA against Set1 or Set7/9 reduces the mRNA (Fig. 4A) and protein (Fig. 4B) levels of each enzyme.
FIG. 4.
siRNA-mediated knockdown of Set1 and Set7/9. Cells were transfected with two rounds of siRNA against luciferase (control) (white bar), Set1 (shaded bar), or Set7/9 (black bar) for 48 h. (A) mRNA levels for Set1 and Set7/9 were measured and normalized to glyceraldehyde-3-phosphate dehydrogenase. (B) Western blot analysis was performed with Set1 or Set7/9 antibody.
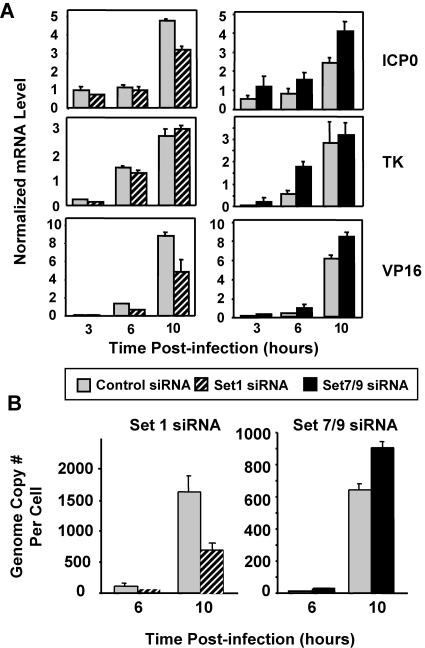
We examined whether the siRNA knockdown of Set1 or Set7/9 affects gene expression and replication of HSV-1 during lytic infection. The reduction of Set1 by siRNA reduces the transcription of HSV-1 genes (Fig. 5A), although the extent of reduction is gene specific. For example, VP16 gene expression is most strongly lowered, TK is reduced only slightly, and ICP0 is impaired to an intermediate degree (Fig. 5A). In contrast, the siRNA knockdown of Set7/9 increased the RNA levels of all three genes.
FIG. 5.
Role of Set1 and Set7/9 in gene expression and replication of HSV-1. Cells were treated with siRNA against the control, Set1, or Set7/9 for 2 days, followed by HSV-1 infection for 3, 6, and 10 h. (A) mRNA levels of ICP0, TK, and VP16 were measured as described in the legend of Fig. 3. (B) The genome copy number of HSV-1 was measured as described in the legend of Fig. 3.
We then tested whether the differential transcriptional effects of the knockdown of Set1 and Set7/9 are also manifest on HSV-1 replication. Similar to the reduction in transcription, Set1 siRNA reduces the genome copy number (approximately 2.5-fold), but Set7/9 siRNA results in an increase in the HSV-1 copy number (Fig. 5B).
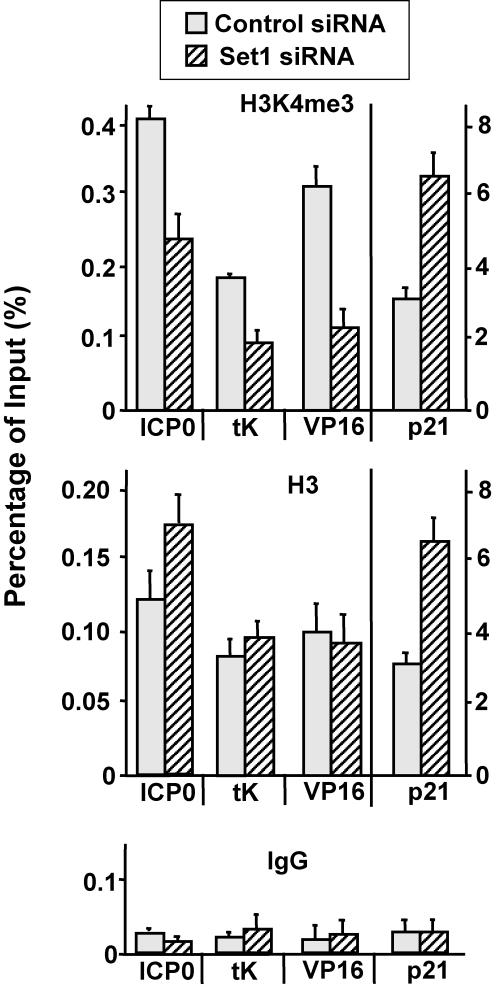
To further delineate the mechanism, we performed a ChIP assay to determine whether Set1 knockdown reduces the level of H3K4me3 on the HSV-1 genome. A cellular gene, p21/WAF1, was used as a control. Set1 knockdown decreases H3K4me3 on each of the viral genes tested (ICP0, TK, and VP16) but does not decrease H3K4me on the p21/WAF1 gene (Fig. 6). These results suggest that Set1 is involved in gene expression and replication of HSV-1 through regulating the active histone modification H3K4me3.
FIG. 6.
siRNA of Set1 reduces the recruitment of H3K4me3 on the genome of HSV-1. Cells were transfected with siRNA of the control, Set1, or Set7/9 for 2 days. A ChIP assay was performed to examine the recruitment of H3K4me3 or H3 on the genome of HSV-1 as described in the legend of Fig. 2. IgG, immunoglobulin G.
DISCUSSION
Current therapies for herpes simplex virus are largely designed to target virus proteins, including herpes DNA polymerase and HSV helicase-primase (12, 37, 38). However, there are many interactions between viruses and their hosts, which may constitute vulnerable points for successful drug intervention. Indeed, HSV-1 transcription involves many host-encoded transcription factors such as DNA-binding activators and components of the general transcription machinery, including RNA polymerase II (41). In addition, HSV-1 transcription has recently been shown to incorporate chromatin components and regulatory pathways (10). Our previous studies showed that during lytic infection, the genome of HSV-1 is associated with nonregularly spaced nucleosomes, and importantly, histones associated with HSV-1 lytic genes are acetylated and methylated, which are positively acting histone modifications (11). The dynamics of histone methylation on the HSV-1 genome correlate with the viral gene expression pattern, suggesting that the modifications contribute to viral gene regulation. In this study, we investigated the question of the physiological importance of histone H3K4 methylation using more direct approaches that reduce the level of the modification.
In the first approach, we used a general protein methylation inhibitor, MTA. MTA has been used to inhibit both histone lysine and arginine methyltransferases (2, 8, 23), which in turn alter gene expression patterns. Indeed, we found that MTA strongly inhibits both gene expression and replication (Fig. 3) of HSV-1 during lytic infection. This functional inhibition correlates with decreased H3K4 methylation, providing a potential mechanism.
The strong decreases in HSV-1 transcription and replication resulting from MTA treatment occur with minimal host cell cytotoxicity. We used concentrations ranging up to 5 mM MTA in HeLa cells and did not observe obvious cytotoxicity (Fig. 1B and data not shown). MTA is a normal by-product of S-adenosyl-l-methionine biosynthesis and is generated in salvage pathways for adenosine and methionine. Normal concentrations in tissues are lower than 0.2 μmol/g. This suggests that the compound is either rapidly metabolized or excreted from cells (42), which may explain why MTA is well tolerated in cells. Thus, given the low cytotoxicity and the strong effect on HSV-1 infection, our results suggest that MTA could potentially serve as a drug to treat HSV-1 lytic infection.
To further investigate the importance of H3K4 methylation in HSV-1 infection, and to delineate the underlying mechanism, we used siRNA to knock down Set1, a histone H3K4 methyltransferase. We focused on Set1 because of previous observations that Set1 copurifies in a complex with VP16, one of the key transcriptional regulatory proteins in HSV-1 (44). VP16 is a virion structural protein and, upon infection, enters the host cell (32). A portion is transported to the nucleus and associates with two host cell factors, Oct1, a DNA-binding transcriptional activator, and HCF-1, a large protein functioning to promote cell proliferation (44). The VP16/Oct1/HCF-1 complex then binds to promoters of viral IE genes to begin a cascade of gene expression leading to viral replication and assembly into infectious viral particles (9, 43). In the absence of VP16, HCF-1 can be purified as part of a large complex with two classes of chromatin modification enzymes, histone deacetylase 1 (HDAC-1) and HDAC-2 and the histone methylase Set1 (45). However, in the presence of VP16, HCF-1 associates only with the Set1 subcomplex (45). Thus, the normal role of HCF-1 during cell proliferation appears to involve both negatively acting (HDAC) and positively acting (Set1 and H3K4me) histone modifications, whereas VP16 appears to capture only the subcomplex containing the positive enzyme. This is reasonable, given VP16's role as a transcriptional cofactor involved in gene activation.
Set1 complexes are highly homologous from yeast to human (20, 25, 31, 45). In yeast, Set1 is the only histone methyltransferase that is responsible for H3K4 methylation. However, there are other human methyltransferases that target H3K4, including ALL-1, MLL, and Set7/9 (21, 26, 28, 39). For example, Set7/9 is a second H3K4 methylase but is different from Set1 in that it apparently occurs as a monomer in vivo and monomethylates K4 in vitro (39). MLL and its enzymatic activity are critically involved in the regulation of Hox gene expression (21). ALL-1 exists as a large complex and is involved in acute leukemogenesis (26). Both MLL and ALL-1 are present in complexes that are similar to yeast Set1 (21, 26). We found that reducing the level of Set1 lowers HSV-1 replication, and this correlates with diminished gene expression and H3K4me. We analyzed the effect of reducing Set7/9 by siRNA and found that HSV-1 gene expression and replication are not lowered. In the future, it will be important to determine whether ALL and MLL (both able to trimethylate H3K4 in vivo) are involved in HSV-1 infection; however, neither of these proteins was found in physical association with HCF-1 (45). We also note that the lowering of Set1 has a relatively modest effect on HSV-1 compared to MTA. Since many histone sites are methylated by both arginine and lysine methyltransferases, it may be that there are additional histone methylation events involved in the upregulation of HSV-1 gene transcription. Future studies will address the full spectrum of histone modifications that regulate HSV-1.
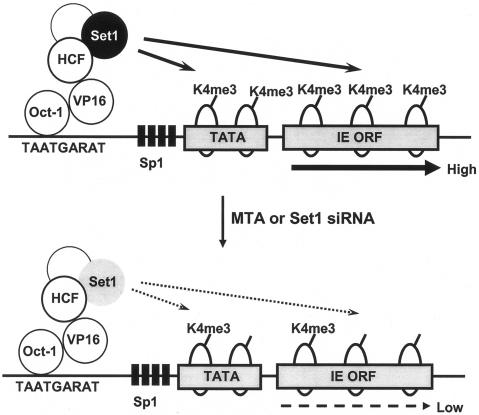
These results suggest a model for a role of Set1 during normal infection: to regulate HSV-1 transcription through promoting H3K4 methylation (Fig. 7, top). When methylation is inhibited, either via MTA or via a reduction of Set1 levels, H3K4 methylation is reduced, leading to lowered transcription (Fig. 7, bottom). Hence, our results suggest that histone methyltransferases, and specifically Set1, could serve as new cellular targets for the treatment of acute infection by HSV-1. It may be that MTA, although not specifically targeting Set1, could be an effective agent for HSV-1 infection. Future investigations will reveal whether chromatin structure and function are critical points of regulation for HSV-1 acute and latent infection and will help to reveal new possibilities for pharmacological intervention.
FIG. 7.
Model for the role of Set1 in the lytic infection by HSV-1. See Discussion for a description.
Acknowledgments
S.L.B. and N.W.F. acknowledge research support from a PO1 grant (NS33768) from the NIH. J.R.K. acknowledges support from an NIH training grant, Training in Virology (T32 AI-07324), at the University of Pennsylvania.
We thank members of the Berger and Fraser laboratories for valuable discussions.
REFERENCES
- 1.Arthur, J. L., C. G. Scarpini, V. Connor, R. H. Lachmann, A. M. Tolkovsky, and S. Efstathiou. 2001. Herpes simplex virus type 1 promoter activity during latency establishment, maintenance, and reactivation in primary dorsal root neurons in vitro. J. Virol. 75:3885-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avila, M. A., E. R. Garcia-Trevijano, S. C. Lu, F. J. Corrales, and J. M. Mato. Methylthioadenosine. Int. J. Biochem. Cell Biol. 36:2125-2130. [DOI] [PubMed]
- 3.Bannister, A. J., and T. Kouzarides. 1996. The CBP co-activator is a histone acetyltransferase. Nature 384:641-643. [DOI] [PubMed] [Google Scholar]
- 4.Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120. [DOI] [PubMed] [Google Scholar]
- 5.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein, B. E., E. L. Humphrey, R. L. Erlich, R. Schneider, P. Bouman, J. S. Liu, T. Kouzarides, and S. L. Schreiber. 2002. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc. Natl. Acad. Sci. USA 99:8695-8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843. [DOI] [PubMed] [Google Scholar]
- 8.Chau, C. M., and P. M. Lieberman. 2004. Dynamic chromatin boundaries delineate a latency control region of Epstein-Barr virus. J. Virol. 78:12308-12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleary, M. A., S. Stern, M. Tanaka, and W. Herr. 1993. Differential positive control by Oct-1 and Oct-2: activation of a transcriptionally silent motif through Oct-1 and VP16 corecruitment. Genes Dev. 7:72-83. [DOI] [PubMed] [Google Scholar]
- 10.Herrera, F. J., and S. J. Triezenberg. 2004. VP16-dependent association of chromatin-modifying coactivators and underrepresentation of histones at immediate-early gene promoters during herpes simplex virus infection. J. Virol. 78:9689-9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent, J. R., P.-Y. Zeng, D. Atanasiu, J. Gardner, N. W. Fraser, and S. L. Berger. 2004. During lytic infection herpes simplex virus type 1 is associated with histones bearing modifications that correlate with active transcription. J. Virol. 78:10178-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleymann, G. 2004. Helicase primase: targeting the Achilles heel of herpes simplex viruses. Antivir. Chem. Chemother. 15:135-140. [DOI] [PubMed] [Google Scholar]
- 13.Kornberg, R. D., and Y. Lorch. 1999. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98:285-294. [DOI] [PubMed] [Google Scholar]
- 14.Kubat, N. J., A. L. Amelio, N. V. Giordani, and D. C. Bloom. 2004. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J. Virol. 78:12508-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubat, N. J., R. K. Tran, P. McAnany, and D. C. Bloom. 2004. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J. Virol. 78:1139-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lachner, M., D. O'Carroll, S. Rea, K. Mechtler, and T. Jenuwein. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116. [DOI] [PubMed] [Google Scholar]
- 17.Leinbach, S. S., and W. C. Summers. 1980. The structure of herpes simplex virus type 1 DNA as probed by micrococcal nuclease digestion. J. Gen. Virol. 51:45-59. [DOI] [PubMed] [Google Scholar]
- 18.Litt, M. D., M. Simpson, M. Gaszner, C. D. Allis, and G. Felsenfeld. 2001. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science 293:2453-2455. [DOI] [PubMed] [Google Scholar]
- 19.Martin, C., and Y. Zhang. 2005. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6:838. [DOI] [PubMed] [Google Scholar]
- 20.Miller, T., N. J. Krogan, J. Dover, H. Erdjument-Bromage, P. Tempst, M. Johnston, J. F. Greenblatt, and A. Shilatifard. 2001. COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. USA 98:12902-12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milne, T. A., S. D. Briggs, H. W. Brock, M. E. Martin, D. Gibbs, C. D. Allis, and J. L. Hess. 2002. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10:1107-1117. [DOI] [PubMed] [Google Scholar]
- 22.Mizzen, C. A., J. E. Brownell, R. G. Cook, and C. D. Allis. 1999. Histone acetyltransferases: preparation of substrates and assay procedures. Methods Enzymol. 304:675-696. [DOI] [PubMed] [Google Scholar]
- 23.Mowen, K. A., J. Tang, W. Zhu, B. T. Schurter, K. Shuai, H. R. Herschman, and M. David. 2001. Arginine methylation of STAT1 modulates IFNalpha/beta-induced transcription. Cell 104:731-741. [DOI] [PubMed] [Google Scholar]
- 24.Muggeridge, M. I., and N. W. Fraser. 1986. Chromosomal organization of the herpes simplex virus genome during acute infection of the mouse central nervous system. J. Virol. 59:764-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy, P. L., J. Griesenbeck, R. D. Kornberg, and M. L. Cleary. 2002. A trithorax-group complex purified from Saccharomyces cerevisiae is required for methylation of histone H3. Proc. Natl. Acad. Sci. USA 99:90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura, T., T. Mori, S. Tada, W. Krajewski, T. Rozovskaia, R. Wassell, G. Dubois, A. Mazo, C. M. Croce, and E. Canaani. 2002. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol. Cell 10:1119-1128. [DOI] [PubMed] [Google Scholar]
- 27.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2003. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11:709-719. [DOI] [PubMed] [Google Scholar]
- 28.Nishioka, K., S. Chuikov, K. Sarma, H. Erdjument-Bromage, C. D. Allis, P. Tempst, and D. Reinberg. 2002. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 16:479-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pokholok, D. K., C. T. Harbison, S. Levine, M. Cole, N. M. Hannett, T. I. Lee, G. W. Bell, K. Walker, P. A. Rolfe, and E. Herbolsheimer. 2005. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122:517. [DOI] [PubMed] [Google Scholar]
- 30.Rice, J. C., S. D. Briggs, B. Ueberheide, C. M. Barber, J. Shabanowitz, D. F. Hunt, Y. Shinkai, and C. D. Allis. 2003. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol. Cell 12:1591-1598. [DOI] [PubMed] [Google Scholar]
- 31.Roguev, A., D. Schaft, A. Shevchenko, W. W. Pijnappel, M. Wilm, R. Aasland, and A. F. Stewart. 2001. The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J. 20:7137-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe et al. (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 33.Santos-Rosa, H., R. Schneider, A. J. Bannister, J. Sherriff, B. E. Bernstein, N. C. T. Emre, S. L. Schreiber, J. Mellor, and T. Kouzarides. 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419:407. [DOI] [PubMed] [Google Scholar]
- 34.Spencer, T. E., G. Jenster, M. M. Burcin, C. D. Allis, J. Zhou, C. A. Mizzen, N. J. McKenna, S. A. Onate, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1997. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194-198. [DOI] [PubMed] [Google Scholar]
- 35.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41. [DOI] [PubMed] [Google Scholar]
- 36.Strahl, B. D., R. Ohba, R. G. Cook, and C. D. Allis. 1999. Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in Tetrahymena. Proc. Natl. Acad. Sci. USA 96:14967-14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villarreal, E. C. 2003. Current and potential therapies for the treatment of herpes-virus infections. Prog. Drug Res. 60:263-307. [DOI] [PubMed] [Google Scholar]
- 38.Visalli, R. J., and M. van Zeijl. 2003. DNA encapsidation as a target for anti-herpesvirus drug therapy. Antivir. Res. 59:73-87. [DOI] [PubMed] [Google Scholar]
- 39.Wang, H., R. Cao, L. Xia, H. Erdjument-Bromage, C. Borchers, P. Tempst, and Y. Zhang. 2001. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol. Cell 8:1207-1217. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Q. Y., C. Zhou, K. E. Johnson, R. C. Colgrove, D. M. Coen, and D. M. Knipe. 2005. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc. Natl. Acad. Sci. USA 102:16055-16059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weir, J. P. 2001. Regulation of herpes simplex virus gene expression. Gene 271:117-130. [DOI] [PubMed] [Google Scholar]
- 42.Williams-Ashman, H. G., J. Seidenfeld, and P. Galletti. 1982. Trends in the biochemical pharmacology of 5′-deoxy-5′-methylthioadenosine. Biochem. Pharmacol. 31:277-288. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, A. C., K. LaMarco, M. G. Peterson, and W. Herr. 1993. The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell 74:115-125. [DOI] [PubMed] [Google Scholar]
- 44.Wysocka, J., and W. Herr. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 28:294-304. [DOI] [PubMed] [Google Scholar]
- 45.Wysocka, J., M. P. Myers, C. D. Laherty, R. N. Eisenman, and W. Herr. 2003. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 17:896-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, Y., and D. Reinberg. 2001. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15:2343-2360. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, J., C. Chau, Z. Deng, W. Stedman, and P. M. Lieberman. 2005. Epigenetic control of replication origins. Cell Cycle 4:889-892. [DOI] [PubMed] [Google Scholar]