Abstract
The movement protein (MP) of Tobacco mosaic virus mediates the cell-to-cell transport of viral RNA through plasmodesmata, cytoplasmic cell wall channels for direct cell-to-cell communication between adjacent cells. Previous in vivo studies demonstrated that the RNA transport function of the protein correlates with its association with microtubules, although the exact role of microtubules in the movement process remains unknown. Since the binding of MP to microtubules is conserved in transfected mammalian cells, we took advantage of available mammalian cell biology reagents and tools to further address the interaction in flat-growing and transparent COS-7 cells. We demonstrate that neither actin, nor endoplasmic reticulum (ER), nor dynein motor complexes are involved in the apparent alignment of MP with microtubules. Together with results of in vitro coprecipitation experiments, these findings indicate that MP binds microtubules directly. Unlike microtubules associated with neuronal MAP2c, MP-associated microtubules are resistant to disruption by microtubule-disrupting agents or cold, suggesting that MP is a specialized microtubule binding protein that forms unusually stable complexes with microtubules. MP-associated microtubules accumulate ER membranes, which is consistent with a proposed role for MP in the recruitment of membranes in infected plant cells and may suggest that microtubules are involved in this process. The ability of MP to interfere with centrosomal γ-tubulin is independent of microtubule association with MP, does not involve the removal of other tested centrosomal markers, and correlates with inhibition of centrosomal microtubule nucleation activity. These observations suggest that the function of MP in viral movement may involve interaction with the microtubule-nucleating machinery.
The intercellular spread of Tobacco mosaic virus (TMV) RNA (vRNA) through plasmodesmata (Pd), the cytoplasmic bridges that interconnect adjacent plant cells (29, 31), depends on virus-encoded movement protein (MP) (20). This protein modifies the size exclusion limit of Pd (70, 88, 95) and binds nucleic acids in vitro, which led to the suggestion that the protein may chaperone vRNA to form a viral ribonucleoprotein (vRNP) complex whose size and structure are compatible with transport through the modified Pd (16, 17). Studies to elucidate the pathway by which MP may target viral RNA to Pd have used MP in fusion with the green fluorescent protein (GFP) and shown that, in both plant cells and protoplasts, MP interacts with the cytoskeleton (32, 57) and with elements of the endoplasmic reticulum (ER) (33, 77). During infection, ER membranes transiently condense to form enlarging inclusion bodies (33, 77) that contain TMV replicase, vRNA, and MP and also produce viral capsid protein (2) and therefore likely function to provide a surface for viral factories in which virus protein translation occurs concomitantly with virus replication (33, 65).
Real-time imaging studies of infected epidermal cells demonstrated that MP-GFP-labeled inclusion bodies, recently also referred to as viral replication complexes (VRCs) (2), can exhibit rapid intracellular movements (41, 52). The movements are inhibited by antagonists of microfilaments but not by inhibitors of microtubule polymerization (41, 52), indicating that the intracellular movements are dependent on an intact actin cytoskeleton. This is in partial disagreement with earlier studies with infected protoplasts which indicated that the spatial distribution of inclusion bodies also depends on an intact microtubule cytoskeleton (33). Actin-dependent intracellular movements in epidermal cells have also been reported for transiently expressed GFP-fused 126-kDa replicase protein (52). Although it remains unclear whether the 126-kDa replicase-GFP fusion protein had retained its biological activity or whether the localization of 126-kDa replicase-GFP always reflects that of VRCs, the observations suggest that the observed VRC movements are mediated by the direct or indirect interaction of microfilaments with the 126-kDa replicase protein.
ER aggregates similar in size and shape to VRCs are also produced upon expression of MP-GFP in the absence of virus infection, indicating that ER aggregation is a function of MP (77). The number and structure of the VRCs are influenced by antagonists of the actin cytoskeleton (33, 52). However, since MP is present in VRCs and associates with microtubules (33), a role for MP-associated microtubules in the formation of ER-derived VRCs and inclusion bodies is conceivable.
The association of MP with inclusion bodies and microtubules suggested that microtubules may participate in the translocation of the vRNP from inclusion bodies to Pd (33). This hypothesis is supported by the finding that vRNA is localized to microtubules in a manner that depends on microtubule-associated MP and that TMV replicase also colocalizes with vRNA in BY-2 protoplasts (65, 66). Furthermore, several in vivo studies with infected plant tissues demonstrated a tight correlation between microtubule association of MP-GFP and the function of the protein in facilitating the spread of infection (9-12).
However, despite this correlative evidence and the well-documented function of microtubules in RNA transport in other systems (72, 87), the role of microtubules in the translocation of vRNPs from inclusion bodies to Pd remains unclear. One reason for this is that the fluorescent MP-GFP-microtubule complex is observed rather late during infection (33) and therefore is unlikely to account for the spread of infection between cells at the leading front of the radially expanding infection site. Moreover, recent studies have indicated that TMV movement is not inhibited in infected plant tissues infiltrated with biochemical inhibitors of microtubule polymerization (28, 41, 52). However, since an unequivocal demonstration of the total disruption of the endogenous microtubule cytoskeleton was not presented, the implications of these studies remain uncertain.
Recently, we reported that the association of MP with microtubules is conserved in mammalian cells and that expression of this protein has dramatic effects on the structure of the microtubule array (10). Given that mammalian cells retain the ability of MP to associate with microtubules, we decided to take advantage of this system to further investigate the MP-microtubule complex. Unlike microscopy of plant cells, microscopy of mammalian cells is greatly facilitated by the fact that the cells adhere to microscopic coverslips, are flat and transparent, and do not obscure fluorescence microscopy analysis by autofluorescence (in plants, e.g., because of chlorophyll). Moreover, mammalian cells have a rather planar microtubule array, allowing in toto observation within a limited focal distance, whereas in toto analysis of the barrel-shaped interphase array of plant cells is much harder to achieve, if not impossible, without the aid of confocal microscopy and computational reconstruction. Thus, many more animal cytoskeletons than plant cytoskeletons can be investigated within a given time. Moreover, whereas the microtubule array in plant cells is organized by the activity of dispersed microtubule-nucleating centers and consists of microtubules with opposite polarities, the array in mammalian cells is organized by the activity of one center, the centrosome, which forms a strictly defined interphase array consisting of microtubules attached to the centrosome by their minus ends and extending into the cell periphery with their plus ends. Thus, unlike in plant cells, effects on microtubule nucleation centers can be easily observed. Finally, and most importantly, research in mammalian systems has provided a plethora of reagents and tools for cell biology analyses that are as yet unavailable for analysis in plant cells.
Taking advantage of the mammalian system and by applying some of the available tools, new insight into MP function could be achieved. Our observations indicate that the association of MP with microtubules is direct, i.e., independent of association with ER, actin, or dynein motor. We demonstrate that the alignment of MP along microtubules leads to their stabilization and the recruitment of ER membranes. Reorganization of the microtubule array by MP is independent of microtubule association but correlates with the absence of centrosomal γ-tubulin and the inhibition of centrosomal microtubule nucleation activity. These findings indicate that MP is a structural microtubule-stabilizing microtubule binding protein (MAP) with the potential to affect the microtubule-nucleating machinery and the organization of microtubule and ER networks in mammalian cells. The implications of these findings in the context of plant cell architecture and infection are discussed.
MATERIALS AND METHODS
Cells.
African green monkey kidney COS-7 cells, human HeLa, JAR, and JEG-3 cells, Swiss mouse 3T3 cells, and Chinese hamster ovary (CHO) cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and supplemented with 2 μM l-glutamine.
Plasmids.
Plasmid pCMV-MP was generated by replacing the β-galactosidase gene of pCMVβ (Clontech) with the NdeI-AflII fragment of pT3′NA containing the MP gene of TMV. Subsequent replacement of the BglII-ClaI fragment of pCMV-MP with that of pTMV-Ls1:GFP (10) resulted in plasmid pCMV-Ls1. Mutant MPLs1 differs from the wild-type protein by a Pro-to-Ser substitution at position 154. Plasmid pMAP2c:EGFP (39) was kindly provided by A. Matus (Friedrich Miescher Institute for Biomedical Studies, Basel, Switzerland). Plasmid pCMVH50, encoding p50/dynamitin, and rabbit antibody raised against this protein were obtained from B. Sodeik (University of Hannover, Hannover, Germany) and R. Vallee (University of Massachusetts, Amherst).
Transfections.
Cells were seeded onto 12-mm round glass coverslips 6 to 7 h prior to transfection. Semiconfluent cell cultures were transfected by the calcium phosphate coprecipitation method by using 1 μg/cm2 pCMV-MP, pCMV-Ls1, pMAP2c:EGFP, or pCMVH50 to express MP, MPLs1, MAP2c-enhanced GFP (EGFP), or p50/dynamitin, respectively. About 24 h after transfection, cells were transferred to fresh medium, incubated for another 24 h, and harvested for immunoblot analysis or fixed for immunofluorescence analysis.
Cold and drug treatment.
For cold treatment, cells were placed on ice in a cold room (4°C) 3 h before fixation. If not indicated otherwise, the cytoskeleton-depolymerizing agents colchicine (United States Biochemical), nocodazole (Sigma), vinblastine (Sigma), cytochalasin D (Sigma), and latrunculin B (Sigma) were added to cell cultures 3 h before harvesting or fixation, at concentrations specified in the text and figure legends. The ER-disrupting ionophore ionomycin (Sigma) was applied for 7 min in fresh medium before fixation.
Immunofluorescence analysis.
Cells grown on coverslips were usually fixed for 20 min in phosphate-buffered saline (PBS), pH 7.0, containing 4% paraformaldehyde and 4% sucrose. After being washed for 10 min in PBS containing 5 mM EGTA, cells were usually permeabilized for 10 min with ice-cold acetone. In cases where cells were to be stained with antibody to detect pericentrin or pericentriolar material (PCM) antigen, cells were fixed and permeabilized by treatment with ice-cold methanol for 20 min. Paraformaldehyde- or methanol-fixed cells were washed twice for 10 min in PBS-5 mM EGTA-0.5% Tween 20 (PBST-E) before application of antibody.
Primary and secondary antibodies were diluted in PBST-E and applied to cells for 90 min at room temperature. Following incubation with a primary antibody and again also after incubation with a secondary antibody, cells were washed six times, for 10 min each time, with PBST-E. Finally, cells were rinsed with distilled water and mounted in Mowiol (Calbiochem) containing 2.5% 1,4-diazobicyclo[2.2.2]octane (DABCO). For detection of MP, we used affinity-purified polyclonal rabbit antibodies directed against amino acids 209 to 222 of MP (12). To label α-tubulin and γ-tubulin, we used monoclonal mouse (Amersham) and rat (Sigma) antibodies, respectively. Pericentrin antibody was commercially available (BAbCO), and monoclonal antibodies directed against PCM antigen (CTR453) were provided by Michel Bornens (Institut Curie, Paris, France). Antibody raised against human trans-Golgi network glycoprotein 51 (TGN51) was a gift from Renate Kain (Institute of Pathology, General Hospital, Vienna, Austria). The preparations were stained with fluorescein isothiocyanate-, tetramethyl rhodamine isothiocyanate-, and aminomethyl-coumarin-labeled secondary antibodies (Pierce and Jackson Immunoresearch).
Fluorescence microscopy was performed with a Nikon Eclipse E800 microscope equipped with CFI Plan Apochromat objectives (Nikon). For specific visualization of fluorescein isothiocyanate, tetramethyl rhodamine isothiocyanate, and aminomethyl-coumarin fluorescence, we used XF100 (Omega Optical), G-2A (Nikon), and XF03 (Omega Optical) filter sets, respectively. Images were acquired and processed with an ORCA-100 progressive-scan interline charge-coupled device camera (Hamamatsu Photonics) and Openlab 3 software (Improvision).
Immunoblot analysis.
Total cell extracts were prepared by lysing cells in sodium dodecyl sulfate (SDS)-containing sample buffer. Proteins were separated by 12% SDS-polyacrylamide gels and transferred to a Sequi-Blot polyvinylidene difluoride membrane (Bio-Rad) by standard procedures. Filters were blocked for 1 h with 5% nonfat milk powder in PBS-0.5% Tween 20. Specific primary antibody and peroxidase-conjugated secondary antibody (Pierce) were applied sequentially in the same buffer. Finally, the filters were developed with SuperSignal West Dura Extended Duration Substrate (Pierce).
Purification of MP and in vitro tubulin binding assay.
The purification of recombinant MP-H6 is described in detail elsewhere (9). For the tubulin binding experiments, MP (50 μg) was denatured with 1.4 ml of solubilization buffer (SB; 100 mM NaH2PO4, [pH 7.5], 1 M NaCl, 10 mM Tris base, 8 M urea, 10% [vol/vol] glycerol) and incubated overnight at 4°C. Ni2+-nitrilotriacetic acid (NTA) beads (30 μl of a 50% slurry; QIAGEN) were equilibrated with 500 μl of SB, sedimented briefly at 20,800 × g (room temperature), and resuspended in 200 μl (∼5 μg) of denatured MP solution. MP was allowed to bind the beads for 30 min at room temperature with gentle shaking before unbound protein was removed by sedimenting the beads and aspirating the resulting supernatants. MP was refolded on the beads by resuspending the pellets in 500 μl of pulldown buffer [PB; 40 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)-OH (pH 6.85), 0.5 mM MgCl2, 10% (vol/vol) glycerol, 0.01% (vol/vol) Tween 20, 5 mM imidazole] and incubating them for at least 1 h at 4°C, with gentle shaking. Beads were again pelleted and the supernatants discarded before binding reactions were set up. Under these conditions, we could confirm that MP had retained sequence-nonspecific RNA binding activity (not shown) and was therefore considered to be biochemically active.
To block nonspecific binding sites, the beads were resuspended in 40 μl of bovine serum albumin solution (8 μg in PB) and incubated for 10 min at 4°C. Tubulin (5 μg of tubulin dimers or in vitro-polymerized microtubules in PB containing 50 μM paclitaxel [Taxol]) was added to a final volume of 50 μl, and the mixture was incubated at 4°C for 15 min before the beads were pelleted by centrifugation at 20,800 × g for 1 min at room temperature. Supernatants containing unbound material were carefully transferred to fresh 1.5-ml Eppendorf tubes, and the pellets containing bound proteins were washed five times by repeatedly pelleting and resuspending the beads in fresh PB containing 10 μM paclitaxel (750 μl), with a 10-min incubation at 4°C between centrifugations. Bound proteins were eluted by incubating the beads for 30 min at 4°C in 50 μl of SB containing 500 mM imidazole before fractions containing bound and unbound proteins (10 μl) were separated by 12% SDS-PAGE and transferred to Sequi-Blot polyvinylidene difluoride membrane (Bio-Rad) by standard procedures. Membranes were probed with either anti-MP or anti-α-tubulin (DM1A), followed by peroxidase-conjugated secondary antibody (Pierce Biotechnology), and the presence of either protein was indirectly detected with Supersignal West Dura substrate (Pierce Biotechnology) by exposure to X-ray film. To control for nonspecific binding of tubulin to the Sepharose beads, the above-described procedure was also performed with beads that were not conjugated to MP.
Microtubules were polymerized in vitro at 37°C in 10-μl reaction mixtures containing 5 mg/ml purified bovine brain tubulin heterodimers (Cytoskeleton Inc.), 80 mM PIPES-OH (pH 6.9), 1 mM EGTA, 10% dimethyl sulfoxide, 1 mM MgCl2, and 1 mM GTP. To suppress microtubule dynamics, paclitaxel (Sigma) was added to a final concentration of 50 μM and microtubules were incubated for 12 h at room temperature. To remove unpolymerized tubulin, microtubules were twice collected by sedimentation at 20,800 × g for 5 min at room temperature and resuspended in 60 μl of microtubule binding buffer (12 mM PIPES-OH [pH 6.9], 0.5 mM MgCl2, 10% glycerol, 0.01% Tween 20, 10 μM paclitaxel). Aliquots of the microtubules were heat denatured and quantified with the bicinchoninic acid protein assay kit (Pierce Biotechnology). In order to reduce the likelihood of buffer-induced MP aggregation, 10 μl of bovine brain tubulin dimers (10 mg/ml; Cytoskeleton Inc.) and bovine serum albumin (2 mg/ml; Pierce Biotechnology) was dialyzed overnight at 4°C against 400 ml of PB.
RESULTS
MP aligns mammalian microtubules and alters their distribution.
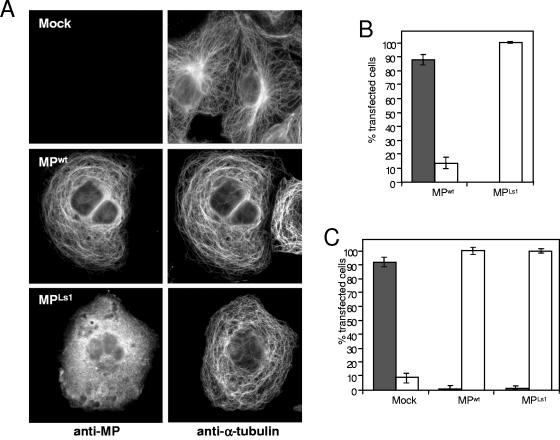
To further investigate the interaction of MP with microtubules in mammalian cells, we transiently transfected African green monkey kidney (COS-7) cells with plasmid pCMV-MP, which expresses full-length wild-type MP (MPwt) under the control of the cytomegalovirus promoter. Immunoblot analysis of cellular extracts prepared at various time points between 24 and 48 h posttransfection (hpt) demonstrated the presence of a single MP antibody-reactive protein of the expected molecular weight (data not shown). At about 48 hpt, cells were fixed and stained with antibodies against MP and α-tubulin. As previously noted (10), MPwt colocalizes with microtubules (Fig. 1A), indicating that microtubule association of MPwt does not depend on plant-specific or other viral factors. Moreover, in cells expressing MPwt, the microtubule cytoskeleton is dramatically reorganized. Whereas untransfected control cells display normal radial microtubule arrays consisting of microtubules that extend from the centrosomal region close to the nucleus toward the cell periphery, MPwt-expressing cells display abnormal arrays in which hoops of microtubules encircle the nucleus without converging to a centrosome (Fig. 1A). Similar observations were obtained when MPwt was expressed in human HeLa, JAR, and JEG-3 choriocarcinoma cells, Swiss mouse 3T3 cells, and CHO cells (data not shown). MP also localized to mammalian microtubules when fused to GFP (data not shown), but since the relative level of MP-GFP expression and subsequent association with microtubules was much lower than that found with unfused MPwt, we decided to use MPwt and immunodetection for further analysis.
FIG. 1.
MP binds and reorganizes microtubules in COS-7 cells. (A) Mock-transfected control cells and transiently transfected cells expressing either MPwt or MPLs1 were analyzed by indirect double-immunofluorescence assay to show the subcellular localization of MP (left) and α-tubulin (right). MPwt binds microtubules, whereas MPLs1 does not. However, expression of either protein changes the microtubule array. Thus, although MPLs1 is microtubule binding deficient, it still affects the organization of the microtubule cytoskeleton. Bar = 10 μm. (B) Percentages of transfected cells in which MP or MPLs1 is associated (gray bars) or not associated (white bars) with microtubules. (C) Percentages of mock-, MPwt-, or MPLs1-transfected cells showing either normal radial microtubule arrays (gray bars) or aberrant arrays (white bars).
Association of MP with microtubules is not required for their reorganization.
To test whether the microtubule-reorganizing function of MPwt depends on microtubule binding, we screened the population of MPwt-expressing cells for a correlation between microtubule-associated MPwt and microtubule reorganization. The association of MPwt with microtubules was regularly observed in 85% of the transfected COS-7 cells (Fig. 1B). In the remaining 15% of the transfected cells, MPwt appeared not to be localized to any discernible subcellular structure. Surprisingly, however, the microtubule cytoskeleton was also reorganized in these cells. To confirm that MPwt is capable of disrupting the radial microtubule array without aligning with microtubules, we transfected cells with MPLs1, a temperature-sensitive mutant form of MP that has a serine-to-proline substitution at position 154 (69) and fails to associate with microtubules or support intercellular transport in plants at the nonpermissive temperature (32°C) (10). In agreement with previous data obtained with plants, MPLs1 was found to be homogeneously distributed throughout the cytosol in all transfected cells cultured at the nonpermissive temperature of 37°C (Fig. 1A and B). Nevertheless, we found that in 99% of the transfected cells, MPLs1 induced a reorganization of the microtubule network to the same degree as MPwt (Fig. 1A and C). This confirms that MPwt expression can induce a dramatic reorganization of the microtubule cytoskeleton by an activity that is independent of microtubule association of the protein.
MP expression disrupts centrosome function.
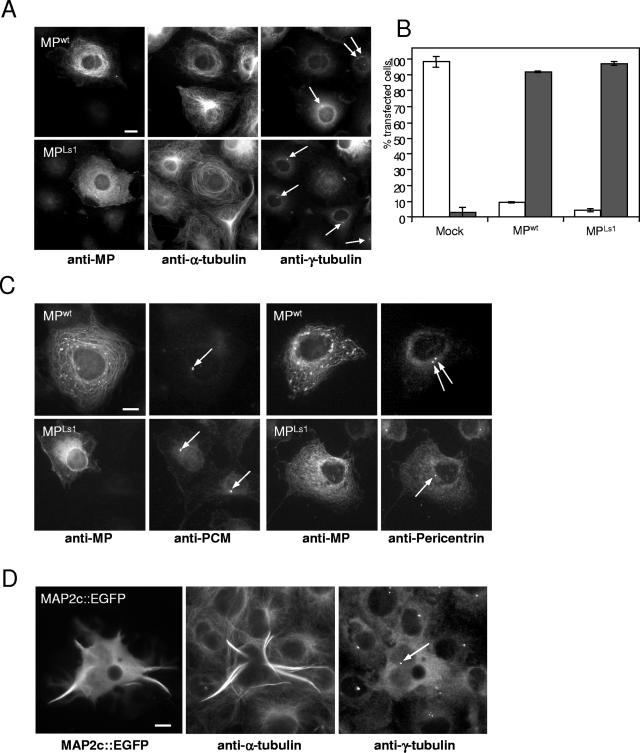
Since microtubules in cells expressing MPwt or MPLs1 did not show any attachment to the centrosomal region, we suspected that the microtubule-organizing center might be affected in these cells. Preliminary studies indicated that expression of MPwt leads to the displacement of centrosomal γ-tubulin (10), which, as part of a γ-tubulin-containing ring complex (96), is recruited to the centrosome and is essential for microtubule nucleation (38, 61). To further investigate this phenomenon, we stained MPwt- and MPLs1-transfected cells, as well as untransfected cells, with an antibody directed against γ-tubulin. As expected, untransfected cells contained one or two individual punctate γ-tubulin signals from which microtubules emerged (Fig. 2A). In contrast, the punctate γ-tubulin signals were absent in about 90% of MPwt-expressing cells and about 95% of MPLs1-expressing cells (Fig. 2A and B). To test whether MPwt and MPLs1 might inhibit the formation or maintenance of centrosomes, we stained transfected and untransfected cells with a monoclonal antibody directed against PCM antigen (6) or with an antibody against pericentrin (Fig. 2C), a centrosomal lattice component involved in the assembly and organization of γ-tubulin complexes (22, 24). These results demonstrated that centrosomes remain present in MPwt- and MPLs1-expressing cells (Fig. 2C), supporting the view that MP causes the removal or lack of recruitment of centrosomal γ-tubulin. Since MPwt and MPLs1 signals appear to be absent in centrosomes labeled with either antigen, it is likely that MP exerts its effect on centrosomal γ-tubulin from outside the centrosome.
FIG. 2.
Expression of MP causes displacement of γ-tubulin from the centrosome. (A) MPwt- and MPLs1-transfected COS-7 cells were triple immunostained for MP (left), α-tubulin (middle), and γ-tubulin (right). Cells expressing either MPwt or microtubule binding-deficient MPLs1 (left) display abnormal microtubule arrays (middle) and lack centrosomal γ-tubulin (right). Adjacent untransfected cells that are not labeled by MP antibody (left) display normal radial microtubule arrays (middle) which converge to centrosomes labeled with γ-tubulin antibody (right, arrows). Bar = 10 μm. (B) Percentages of mock-, MPwt-, and MPLs1-transfected cells in which a γ-tubulin-specific signal is present (white bars) or absent (gray bars). (C) Presence of centrosomes in MPwt- and MPLs1-expressing cells. MPwt- and MPLs1-transfected COS-7 cells were double immunostained for either MP and PCM antigen or for MP and pericentrin. MPwt- and MPLs1-expressing cells contain centrosomes (arrows) similar to adjacent untransfected cells. Bar = 10 μm. (D) MAP2c-EGFP expression causes the reorganization of microtubules without interfering with centrosomal γ-tubulin. Cells transfected with MAP2c-EGFP and surrounding untransfected cells were immunostained for α-tubulin and γ-tubulin. The MAP2c-EGFP-expressing cell exhibits a γ-tubulin signal (arrow), like surrounding cells. Bar = 10 μm.
To test whether the apparent MPwt-induced displacement of centrosomal γ-tubulin is a general feature of MAP overexpression, we transfected COS-7 cells with neuronal MAP2c fused to EGFP (39). Similar to previous reports (39), MAP2c-EGFP expression resulted in the formation of aberrant microtubule arrays consisting of centrosome-independent microtubule bundles that induce stiff cell processes (Fig. 2D). In contrast to MPwt-expressing cells, however, γ-tubulin remained present in distinct spots near the nucleus even though normal radial microtubule arrays were clearly disrupted (Fig. 2D).
In vertebrate somatic cells, the mechanism that recruits γ-tubulin to the centrosome is thought to be microtubule independent (44). To test if MP-induced displacement of γ-tubulin is a microtubule-dependent or -independent mechanism, we cultured MPwt- and MPLs1-transfected cells in the presence of colchicine, followed by antibody staining of γ-tubulin, α-tubulin, and MP at various time points. At 5 and 21 hpt, when MPwt or MPLs1 levels were too low for antibody detection, a γ-tubulin signal was present at perinuclear sites, although microtubules were depolymerized. However, at 48 hpt, when microtubules were still depolymerized but MPwt and MPLs1 were detectable, we found that γ-tubulin was displaced (data not shown). These observations demonstrate that the effect of MPwt or MPLs1expression on γ-tubulin displacement is indeed microtubule independent and also indicate that the effect does not occur as a consequence of microtubule reorganization induced by MP.
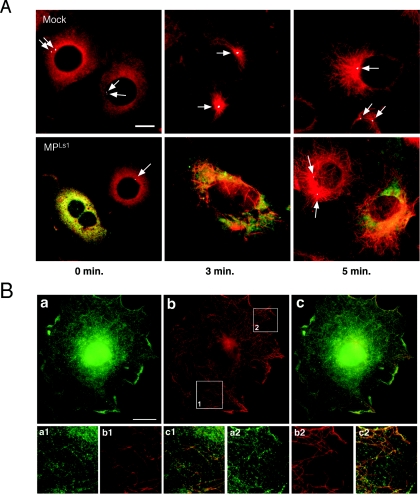
To test whether MP has an inhibitory effect on centrosomal microtubule nucleation activity, we assayed centrosomes in MPLs1-transfected and untransfected control cells for the capacity to renucleate microtubules following nocodazole-mediated disruption (Fig. 3A). In both untransfected and MPLs1-transfected cells, reassembly of microtubules started within 3 min after removal of nocodazole. However, whereas microtubules in untransfected control cells emerged from γ-tubulin-containing centrosomes (arrows), microtubules in MPLs1-transfected cells lacking centrosomal γ-tubulin emerged at random sites in the cytoplasm, indicating that centrosomal microtubule nucleation activity was inhibited.
FIG. 3.
MP disrupts centrosome function, resulting in noncentrosomal microtubule nucleation. (A) Untransfected cells and MPLs1-transfected cells fixed at 0 min, 3 min, and 5 min after removal of nocodazole and triple stained for MP (green), α-tubulin (red), and γ-tubulin (white, arrows). Untransfected cells nucleate microtubules at centrosomes, whereas (green fluorescence-labeled) MPLs1-transfected cells nucleate microtubules at random sites in the cytoplasm. Bar = 10 μm. (B) MP tends to associate with microtubules upon renucleation. MP-transfected cells cultured for 24 h in the presence of 1 μM colchicine, followed by 24 h of culture in the absence of the drug. Staining with MP antibody (a, a1, a2) reveals the presence of MP in filamentous structures that follow the pattern of microtubules (b, b1, b2). The yellow signal produced in part c upon merging of parts a and b indicates that renucleated microtubules are associated with MP (c, c1, c2). Lower parts show magnifications of the subcellular areas (1 and 2) indicated by white rectangles in part b. Bar = 10 μm.
In a similar experiment, MPwt-transfected and untransfected cells were treated with colchicine and stained for α-tubulin and MP following removal of the drug for 24 h. Successful removal of colchicine was characterized by the reestablishment of a normal radial microtubule array in untransfected cells (not shown). In contrast, cells expressing MPwt contained numerous short repolymerized microtubules that had no connection to the centrosome but were faintly associated with MPwt (Fig. 3B). Thus, these data collectively demonstrate that not only does MP induce microtubule-independent displacement of centrosomal γ-tubulin and inhibit centrosomal microtubule nucleation, but it also has the ability to associate with repolymerized, reorganized microtubules in a manner reminiscent of structural MAPs.
Since centrosomal γ-tubulin is essential for proper assembly of mitotic spindle microtubules (43, 48), we then investigated whether MPwt- and MPLs1-expressing cells were defective in producing mitotic spindles. Of about 500 MP-expressing cells analyzed (by α-tubulin staining), none contained spindle microtubules. In contrast, 12 to 15% of the untransfected control cells had mitotic spindles at the time of analysis (data not shown). Thus, in mammalian cells, MP expression interferes with mitosis, presumably via sequestration or displacement of centrosomal γ-tubulin.
MP-associated microtubules accumulate ER membranes.
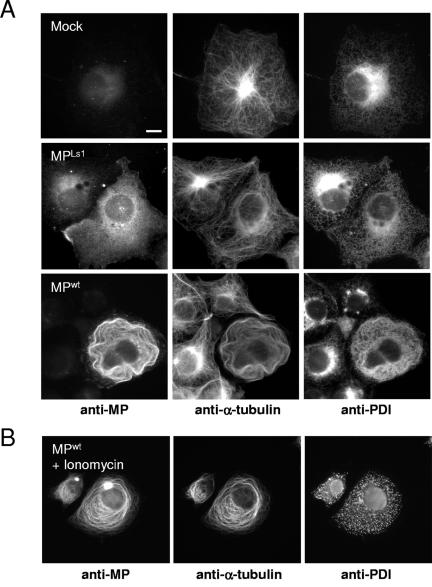
Infection of plant cells involves the recruitment of ER membranes into ER-derived inclusion bodies, which may serve to compartmentalize virus replication and protein synthesis (33, 65). To test if MP affects the distribution of ER in mammalian cells, we performed triple-staining experiments in which transfected and untransfected cells were simultaneously incubated with a set of antibodies recognizing MP, α-tubulin, and protein disulfide isomerase (PDI), a luminal ER protein (68). Although we did not observe colocalization of PDI with MPwt or MPLs1, ER distribution appeared to be affected by MP expression (Fig. 4A). In untransfected cells, ER membranes were concentrated in the centrosomal region and, following the radial array of microtubules, were evenly distributed to the periphery of the cells. In contrast, in cells expressing MPwt or MPLs1, the ER membranes followed the pattern of the reorganized microtubule network and encircled the nucleus without concentration in the centrosomal region, an observation consistent with the role of microtubules in ER membrane segregation (25, 50, 91, 93). Although such reorganization of the ER is likely to be a consequence of the reorganized microtubule network, additional observations suggest an active role for MP in ER membrane distribution.
FIG. 4.
Potential interactions of MP with ER membranes. (A) MP-associated microtubules accumulate ER membranes. Mock-, MPLs1-, and MPwt-transfected cells stained for MP, α-tubulin, and PDI, a luminal marker for ER. ER distribution is strongly affected by MP. Mock-transfected cells (top) display the normal radial array of microtubules and the normal network of ER segregating throughout the cell with some concentration in the centrosomal region. In MPLs1-expressing cells (middle), the ER follows the patterns of curved microtubules encircling the nucleus. In cells in which MPwt is aligned with microtubules (85% of transfected cells, bottom), membranes are enriched in areas exactly overlapping the MP-microtubule complex. MPLs1-transfected cells (middle) do not show enrichment of membranes, confirming that ER accumulation depends on microtubule association of MP. (B) MPwt-transfected cells treated with ionomycin and stained for MP (left), α-tubulin (center), and PDI (right). Vesiculation of the ER (right) does not disrupt the association of MPwt with microtubules (left and center). Bar = 10 μm.
In cells expressing microtubule-aligned MPwt, the ER was highly enriched in areas overlapping the MPwt-microtubule complex. Particularly densely packed ER elements were observed in cells in which MPwt-aligned microtubules were concentrated in hoops encircling the nucleus (Fig. 4A, MPwt-transfected cells). Such dense ER elements were present neither in cells containing non-microtubule-associated MPwt nor in cells transfected with microtubule binding-deficient MPLs1 (Fig. 4A, MPLs1-transfected cells), although microtubules were reorganized in both cases. These observations suggest that in this mammalian cell system microtubule-associated MPwt induces the aggregation of ER membranes near microtubules and that this activity of the protein depends on its microtubule association.
Microtubule association of MP is independent of ER, F-actin, and dynein.
Since MP has been shown to behave as an integral membrane protein in biochemical assays (15, 77), it is conceivable that the microtubule alignment of MP is caused by the alignment of specialized MP-associated ER tubules. To test this possibility, we treated MPwt-transfected cells with ionomycin, a Ca2+-ionophore that induces fragmentation and vesicularization of the ER membrane network (89). Subsequently, the cells were triple stained with antibodies against MP, α-tubulin, and PDI. As shown in Fig. 4B (MPwt-transfected cells plus ionomycin), ionomycin treatment disrupted the ER network but did not affect association of MPwt with microtubules. Thus, we conclude that the apparent association of MPwt with microtubules is not caused by alignment of MPwt-associated tubules of ER with microtubules.
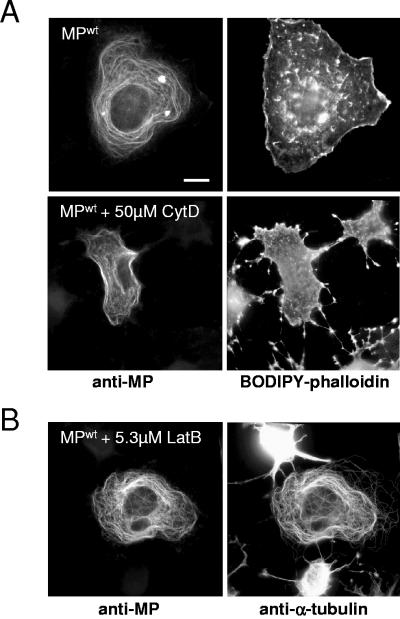
Evidence for an interaction of MP with plant actin has been reported (57). Since plant and animal actins are 83 to 88% identical in amino acid sequence (56), it is possible that at least some of the filamentous structures associated with MPwt in COS-7 cells consist of actin. To test this possibility, we double stained cells with antibodies against MP and with BODIPY-558/568-conjugated phalloidin, a cytological stain specific for actin. As shown in Fig. 5, we did not detect colocalization of actin- and MP-specific fluorescence signals. Disruption of the microfilament network by treatment of cells for 3 h with 50 μM cytochalasin D or 6.3 μM latrunculin B had no visible effect on the distribution of MPwt, although obvious changes in cell shape and actin distribution were apparent (Fig. 5).
FIG. 5.
MP does not colocalize with actin. (A) MPwt-transfected cells were stained with antibodies specific for MP and with BODIPY-558/568-conjugated phalloidin for actin. MP shows microtubule-specific distribution (top left) and no spatial overlap with actin (top right). Treatment of cells with cytochalasin D affects the pattern and function of actin (bottom right) without changing the circular-filamentous pattern of MP distribution (bottom left), indicating that this distribution is actin independent. (B) MPwt-transfected cells treated with latrunculin B and stained with antibodies for MP (left) and α-tubulin (right). Microfilament disruption is evident in untransfected cells, which show clear effects on cell shape (right, top and bottom cells). In MPwt-transfected cells, both cell shape and the association of MPwt with microtubules are not affected. Bar = 10 μm.
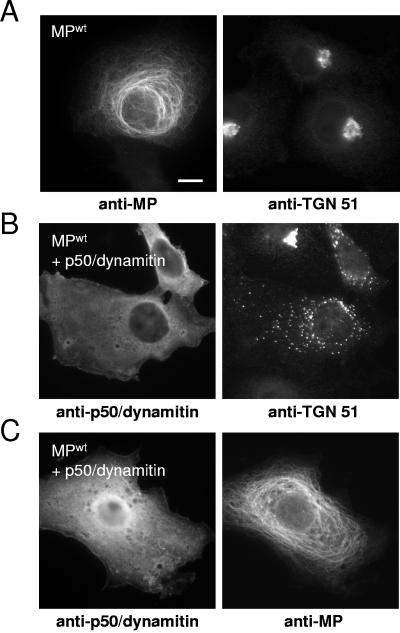
Many microtubule-dependent transport processes rely on motor proteins (5, 7, 46, 64, 86, 87). To examine the possibility that MPwt associates with microtubules in mammalian cells through binding to a dynein motor complex, we analyzed the subcellular localization of MPwt in transfected cells expressing p50/dynamitin, which acts as a dominant-negative inhibitor of dynein-dynactin function (26) (Fig. 6). The inhibitory effect of p50/dynamitin expression on dynein motor activity was verified by staining cells for TGN51 (40), which indicated the dispersal of the Golgi complex (Fig. 6B). Applying the same experimental conditions, we found that p50/dynamitin expression did not interfere with the association of MPwt with microtubules (Fig. 6C). Thus, we conclude that in COS-7 cells, the observed MPwt-associated filaments are indeed microtubules and that the alignment of MPwt with the microtubule is independent of ER membranes, actin filaments, and dynein motor protein.
FIG. 6.
MP binds to microtubules in p50/dynamitin-expressing cells. (A) MPwt-transfected cells stained with antibody for MP and antibody against TGN51. MPwt is associated with microtubules (left) and does not affect the normal localization and organization of the Golgi complex near the nucleus (right). (B) Cells transfected for coexpression of MPwt and p50/dynamitin and stained with specific antibodies for p50/dynamitin (left) and TGN51 (right). Expression of p50/dynamitin (left) interferes with dynein-dependent organization of the Golgi complex near the nucleus (right), indicating inhibition of dynein motor function. (C) Cells transfected for coexpression of MPwt and p50/dynamitin and stained with specific antibodies for p50/dynamitin (left) and MP (right). The association of MP with microtubules is not affected by expression of p50/dynamitin (left) and resulting inhibition of dynein motor function (B). Bar = 10 μm.
MP binds tubulin in vitro.
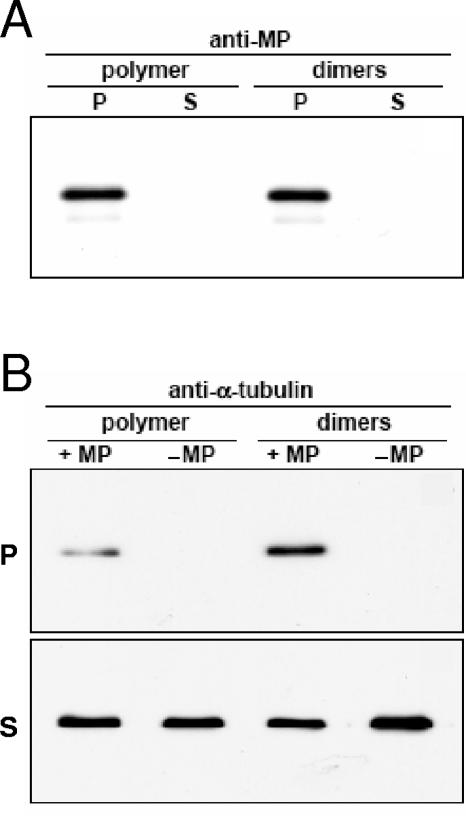
Structural MAPs participate in the organization of microtubule arrays through binding to the outside surface of growing microtubules, thereby promoting their polymerization and stabilization. In order to determine whether MP also binds microtubules directly, purified recombinant MPwt was immobilized on Ni2+-NTA Sepharose beads and used as affinity bait for the coprecipitation of either in vitro-polymerized microtubules or tubulin dimers. Pellet (bound) and supernatant (unbound) fractions were separated by centrifugation and analyzed by SDS-PAGE and Western blotting. To control for nonspecific binding of tubulin to Sepharose, the same procedure was followed in the absence of MPwt. As shown in Fig. 7A, blots probed with anti-MP antibody showed that throughout the course of the binding reaction, MPwt remained coupled to the affinity matrix in amounts that were comparable between reactions. Probing equivalent Western blots with anti-α-tubulin antibody (Fig. 7B) revealed that, even after extensive washing, detectable levels of tubulin could be found in pellet fractions containing MPwt. In control pellet fractions without MPwt, no tubulin was detected, indicating that precipitation of tubulin had occurred via an interaction with MPwt and not by virtue of nonspecific contacts with the Sepharose beads. Repeating the experiment confirmed that, under the conditions used, MPwt interacted with both tubulin dimers and microtubules. The fact that soluble tubulin dimers were able to bind MPwt per se suggests that polymer-specific structural forms of tubulin are not a prerequisite for MPwt binding. Nevertheless, these observations demonstrate that, similar to other MAPs, MPwt can associate with purified tubulin via a direct interaction, suggesting that association of MP with microtubules in vivo may not require the involvement of other factors.
FIG. 7.
In vitro coprecipitation of microtubules and tubulin heterodimers with MP. Ni2+-NTA beads conjugated with MP (+ MP) or without MP (− MP) were incubated with in vitro-polymerized microtubules (polymer) or tubulin dimers (dimers). Pellet (P) and supernatant (S) fractions were separated by centrifugation and analyzed by SDS-PAGE and Western blotting with antibodies specific for MP and α-tubulin, respectively. (A) Immunodetection of MP in pellet but not in supernatant fractions, indicating that MP remained coupled to the Ni2+-NTA beads throughout the course of the binding reaction. (B) Immunodetection of α-tubulin in pellet and supernatant fractions. In pellet fractions containing MP, detectable amounts of both tubulin polymer and tubulin dimers were observed. In the absence of MP, no tubulin was detected in the pellets, indicating that tubulin cosedimentation with Ni2+-NTA beads occurred because of an interaction with MP.
MP stabilizes microtubules.
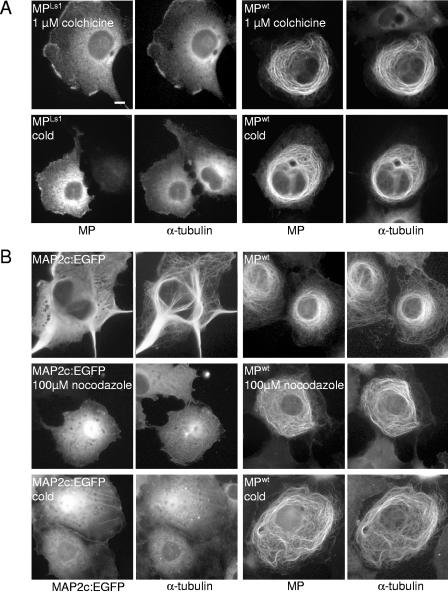
A general feature of structural MAPs is the ability to stabilize microtubules against microtubule-depolymerizing agents (35, 90, 92, 94). Since MP is a microtubule-associating protein, we suspected that it may be similar to MAPs in conferring stability on microtubules. To test this hypothesis, we treated cells with colchicine or cold (Fig. 8A). As expected, in untransfected cells (not shown), as well as in MPLs1-expressing cells, treatment with 1 μM colchicine or cold led to the depolymerization of microtubules, resulting in a diffuse pattern of α-tubulin staining in the cytoplasm. In contrast, microtubules aligned with MPwt in MPwt-expressing cells were resistant to disruption by either treatment. Similar results were obtained when cells were treated with 1.65 μM nocodazole or with 1 μM vinblastine (data not shown) where, again, we observed that MPwt-interacting microtubules were stable and essentially unaffected by these compounds, whereas microtubules in untransfected control cells or in MPLs1-expressing cells were depolymerized.
FIG. 8.
MP-interacting microtubules are resistant to disruption. (A) MPLs1- and MPwt-transfected cells treated for 3 h with either 1 μM colchicine (top) or cold (bottom) and subsequently stained for MP and α-tubulin. Microtubules in cells expressing microtubule binding-deficient MPLs1 are completely depolymerized, whereas MPwt-associated microtubules are resistant to this treatment. (B) MAP2c-EGFP- and MPwt-transfected cells either left untreated (top) or treated for 3 h with either 100 μM nocodazole (middle) or cold (bottom) and subsequently stained for MP (in the case of MPwt-transfected cells) and α-tubulin. MAP2c-EGFP was visualized by GFP fluorescence. MAP2c-EGFP-associated microtubules are bundled that extend into stiff cell processes (top left), whereas MPwt-associated microtubules encircle the nuclear region (top right). MAP2c-EGFP-associated microtubules are disrupted after 3 h of treatment with either 100 μM nocodazole (middle left) or low temperature (bottom left), whereas MPwt-associated microtubules are resistant to such treatment (middle right and bottom right). Bar = 10 μm.
To further test the stability of MPwt-associated microtubules in mammalian cells, we directly compared the stability of MP-associated microtubules with that of microtubules associated with a stabilizing MAP. Therefore, we treated MPwt-, MPLs1-, and MAP2c-EGFP-expressing cells with increasing concentrations of nocodazole. As expected, incubation of cells with 10 μM nocodazole for 30 min led to the disruption of microtubules in both MPLs1-expressing cells and untransfected control cells. In contrast, microtubules in cells that expressed either MAP2c-EGFP or MPwt remained intact after treatment (data not shown), confirming that both MAP2c and MPwt stabilize microtubules. However, treatment of the cells for longer times and with higher concentrations of nocodazole revealed that MP conferred a much higher degree of stabilization of microtubules than did MAP2c-EGFP (Fig. 8B). For example, upon treatment with 100 μM nocodazole for 3 h, most of the microtubules in MAP2c-GFP-transfected cells were disrupted whereas the MPwt-associated microtubule cytoskeleton in MPwt-expressing cells was unaffected. Surprisingly, MP-interacting microtubules were stable even in the presence of a 100-fold higher concentration of the inhibitor (10 mM; data not shown). The difference between the degrees of microtubule stability induced by MPwt and MAP2c-EGFP was also observed at low temperature. After 3 h on ice, microtubules in MAP2c-GFP-transfected cells were depolymerized whereas MPwt-interacting microtubules remained stable (Fig. 8B). These results demonstrate that MP, unlike neuronal MAP2c, produces microtubules that are resistant to cold and depolymerizing agents. This finding is consistent with the high stability of MP-associated microtubule complexes occurring during TMV infection in plant cells (10) and supports the conclusion that MP binding protects microtubules against various assaults.
DISCUSSION
The experiments described here were undertaken to further characterize the nature of the interaction between MP and the cytoskeleton by extending the previous observation that the association of MP with microtubules is conserved in mammalian cells (10), allowing us to investigate this interaction in the absence of virus infection and plant-specific factors. The association of MPwt with mammalian cell microtubules is robust and occurs with high frequency, although we found that, unlike in plant cells, the association is strongly affected if MP is fused to GFP. Concentrating on experiments with unfused MPwt, we found that the alignment of MPwt with microtubules is independent of ER, actin, or dynein motor, supporting the hypothesis that MP acts as a structural MAP and associates with microtubules through direct interactions with tubulin. The latter is confirmed by coprecipitation experiments showing that MPwt is able to bind bovine tubulin dimers and polymerized microtubules in vitro. Since plant and animal tubulins are highly conserved (85), our findings strongly support the hypothesis that the association of MP with microtubules during infection in plant cells is mediated by direct MP-tubulin interactions (10). In addition, we observed that MP affects the organization of mammalian microtubules through activities that are independent of microtubule association and are correlated with the absence of centrosomal γ-tubulin and the loss of centrosomal microtubule nucleation activity. Although microtubule nucleation in plants is dispersed rather than concentrated at a centrosome, the observations imply a potential role for microtubule-organizing complexes during infection in plants.
MP association stabilizes microtubules.
The finding that MP stabilizes microtubules upon binding is consistent with other known and well-characterized MAPs. MAPs such as MAP1, MAP2, tau, and MAP4 and also several plant MAPs are thought to stabilize microtubules by suppressing their disassembly, thereby supporting an overall elongation of microtubules (13, 76, 79). However, despite this overall stabilizing effect, MAP-associated microtubules usually remain susceptible to low temperature (4, 54, 74) and microtubule-disrupting agents (67, 90). MP seems to differ from these MAPs since MP-associated microtubules, in contrast to MAP2c-associated microtubules, withstand prolonged treatments with high concentrations of depolymerizing agents and cold. This observation is in agreement with our previous observation that MP-associated microtubules isolated from TMV-infected plant protoplasts are resistant to disruption by cold and millimolar amounts of salt (10). Obviously, MP forms specialized complexes with microtubules that differ from the salt-sensitive complexes formed by other MAPs (36, 81). Exceptional MAPs that are similar to MPwt with respect to providing outstanding stability to microtubules have been described (8). However, whether there is any functional similarity between MPwt and this class of MAPs remains to be seen. The ability of MPwt to provide stability to microtubules may contribute to the spread of TMV in plant tissues treated with microtubule-disrupting agents (28, 41). The abilities of MP to stabilize microtubules and to cause interference with mitosis in mammalian cells may appear to be in disagreement with the normal development of transgenic MP-expressing plants (20). However, although transgenic plants express considerable amounts of MP (21), interference with microtubule dynamics in these plants appears unlikely since no clear localization of the protein to sites other than Pd has been reported (3, 23, 78, 80).
MP expression triggers the formation of centrosome-independent microtubules.
MPwt induces the reorganization of the microtubule cytoskeleton by an activity that is retained by microtubule binding-deficient mutant MPLs1 and thus is independent of microtubule binding and stabilization. This is again in contrast to “classical” MAPs (such as MAP2c), for which the microtubule-reorganizing effect has been proposed to be a consequence of the microtubule stabilization conferred by these proteins (51). Our findings correlate the reorganization of the microtubule array by MP with the lack of centrosomal γ-tubulin and with the loss of centrosomal microtubule nucleation activity rather than with microtubule stabilization. Although it remains to be shown whether these events are indeed causally related, it appears worthwhile to consider the potential mechanism by which MP may produce noncentrosomal microtubules, as well as the potential implications for a microtubule nucleation-modifying function of MP during infection in plant cells.
A priori, there are four potential mechanisms that generate noncentrosomal microtubules: self-assembly of microtubules in the cytoplasm, nucleation of microtubules at noncentrosomal sites, breakage or severing of centrosomal microtubules along their length, and release of microtubules from the centrosome (42). It seems unlikely that MP causes the formation of noncentrosomal microtubules by katanin-mediated severing (58) since, in the presence of MP, the centrosome is inactivated and no centrosome-associated microtubule fragments were observed. Cytoplasmic self-assembly of microtubules occurs if the critical concentration for microtubule assembly is reduced. This mechanism accounts for the formation of noncentrosomal microtubules induced by MAP2 (Fig. 8) and paclitaxel (19), which bind and stabilize microtubules. However, since MP leads to the formation of noncentrosomal microtubules independently of microtubule binding and stabilization, this mechanism seems not to apply to this protein. Thus, the most likely mechanisms by which MP might form centrosome-independent microtubules are centrosomal release and noncentrosomal nucleation. Centrosomal release is proposed to be the primary mechanism for the production of noncentrosomal microtubules in cultured cells (42). Since the noncentrosomal microtubules induced by MP expression are correlated with the lack of centrosomal γ-tubulin, MP may induce cleavage between the centrosome and the microtubule-nucleating γ-tubulin ring complex (γTuRC) and thus the release of “capped” microtubules. Alternatively, MP may directly or indirectly interfere with the recruitment of γTuRC to the centrosome. Since we could not detect MPwt and MPLs1 at the centrosome, the protein likely acts from the cytoplasm. In the extreme case, the protein may by itself bind and sequester centrosomal and cytoplasmic γTuRCs for noncentrosomal nucleation. Such titration of γ-tubulin by MP would be consistent with the lack of mitotic spindles in MP- and MPLs1-expressing cells, as the onset of mitosis involves the sudden recruitment of γ-tubulin to centrosomes (44). Direct interaction between MP and γ-tubulin may also be conceivable given that point mutations within a short region of MP that has sequence similarity to the tubulin M loop confer a lack of microtubule association and function during infection in plant cells (10) and therefore may identify γ-tubulin as a specific target of MP.
With respect to potential implications of the centrosome-inactivating activity of MP for a microtubule nucleation-modifying function of the protein during infection in plant cells, one has to acknowledge the fundamental differences in the organization of microtubules between animal and plant cells. Importantly, microtubule nucleation in plants is dispersed rather than localized to a centrosome (18, 53, 62). However, despite this fundamental difference, the general mechanism of microtubule nucleation is nonetheless conserved (53, 82). As in mammalian cells, microtubules in plants are nucleated by γ-tubulin, and the γTuRC components known from yeast have been detected in plants (27, 63, 83, 84). Moreover, as in mammalian cells, microtubule nucleation depends on the recruitment of cytoplasmic γ-tubulin to microtubule nucleation sites. According to a recent study (62), such microtubule nucleation sites are formed at the sides of previously formed cortical microtubules. Apparently, γ-tubulin complexes shuttle between cytosol and the side of a cortical microtubule, being active for nucleation while bound to the microtubule and being released to the cytosol when the original microtubule depolymerizes (62). Our findings obtained with mammalian cells may suggest that MP may interfere with such mechanisms in order to manipulate microtubules during infection. Although MP is targeted to Pd by cytoskeleton-independent mechanisms (75), Pd-associated MP may manipulate the cytoskeleton near the channel, as is suggested by the presence of MP-associated filamentous structures across plasmodesmal cell junctions in MP-transgenic plants (23, 49, 60) and multicellular cyanobacteria (30, 34). Related precedents for the involvement of microtubule nucleation sites in intercellular virus movement are human T-cell leukemia virus type 1 and human immunodeficiency virus, which move between lymphocytes by mechanisms that involve virus-induced reorientation of the microtubule-organizing center and polarization of the cytoskeleton to cell junctions (37, 73).
MP causes aggregation of the ER.
The observations suggesting an ability of microtubule-associated MPwt to cause accumulation of ER membranes in COS7 cells may be consistent with the role of MP in the formation of ER aggregates observed in TMV-infected plant cells (33, 77). In planta studies with ER-localized GFP provided evidence that the aggregates are formed by transient recruitment and accumulation of ER membranes and that the formation of the aggregates and the subsequent reversion to normal tubular ER parallel the time course of MP accumulation and degradation (77). ER aggregates similar in size and shape are also produced upon expression of MP-GFP in the absence of virus infection, indicating that ER aggregation is a function of MP (77). The number and structure of the aggregates are influenced by antagonists of the actin cytoskeleton (33, 52). However, treatment of infected protoplasts with oryzalin, which disrupts microtubules, leads to collapse and fusion of the aggregates, which indicates that microtubules are involved in their anchoring and spatial separation within the cell (33). Although the dynamic behavior of ER membranes in plant cells is predominantly actin based, as opposed to microtubule based as in mammalian cells, evidence of a role for microtubules in the general architecture of plant endomembranes has been reported (45, 55). Furthermore, since MP has characteristics of a transmembrane protein (14, 15, 60) and behaves like an integral membrane protein in biochemical fractionation experiments (77), our observation that microtubule-associated MPwt induces ER accumulation in mammalian cells may be consistent with a model in which MP interacts simultaneously with both the ER and microtubules in plant cells, thereby leading to the formation of an ER-to-microtubule bridge that might not usually exist in uninfected cells. By forming this bridge, MP could place ER architecture under the control of microtubules and/or microtubule-based motility. It should be noted, however, that MP is dispensable for TMV replication (59) and that accumulation of MP in ER aggregates is not required for movement (12). Thus, although TMV replication occurs in association with membranes (71) and although the ER aggregates have been proposed to function as virus factories (2, 33, 65) and as infectious entities that move from initially mechanically inoculated cells into adjacent cells (41), further studies are needed to investigate whether ER aggregation is indeed a requirement for successful infection.
MP does not interact with actin in mammalian cells.
Although evidence for an interaction of MP with plant microfilaments has been reported (57), we could not find evidence for such an interaction in transfected COS-7 cells. Since plant and animal actins are well conserved in amino acid sequence (56), our observations do not support a direct interaction between MP and actin and suggest that MP may require additional plant factors for stable interaction with microfilaments. However, further studies are needed to verify a functional interaction of MP with microfilaments in plants. Although the presence of actin and myosin within or near Pd (reviewed in reference 1) suggested a role for microfilaments in the Pd targeting and function of MP, the accumulation of MP in Pd seems to be neither microtubule nor actin dependent (10, 75). Moreover, although the stabilization, anchorage, and intracellular trafficking of ER aggregates (or VRCs) are sensitive to the presence of actin antagonists (41, 52, 65), the exact role of microfilaments in vRNA transport is not clear. Indeed, as in COS-7 cells, actin antagonists had little effect on the overall distribution of MP in infected plant protoplasts (33). These observations may suggest that actin-dependent intracellular movements of VRCs are independent of MP, despite the fact that MP is present in these complexes. This proposal is consistent with the dispensability of VRC-associated MP for virus movement (12), as well as with recent evidence suggesting that the intracellular movements of the VRCs are mediated by the 126-kDa replicase (52).
Acknowledgments
We thank Roger N. Beachy for support and guidance in initial studies performed in his former laboratory at The Scripps Research Institute (La Jolla, CA). We also thank Angray Kang, Stephanie Schmid, Ulrich Kruse, and Peter K. Vogt for initial support in animal cell culture and transfection and Michael Frese for testing the expression of MP-GFP. We are also grateful to Andrew Matus, Beate Sodeik, Ron Vallee, Michel Bornens, and Renate Kain for providing DNA and antibody reagents. Moreover, we thank Barbara Hohn, Andrew Matus, Helen Rothnie, and Gertraud Orend for critical suggestions during the preparation of the manuscript.
This work was performed with financial support from the Novartis Research Foundation, the Swiss National Research Foundation (SNF), the Human Frontier Science Program Organization (HFSPO), and the Centre National de la Recherche Scientifique (CNRS).
REFERENCES
- 1.Aaziz, R., S. Dinant, and B. L. Epel. 2001. Plasmodesmata and plant cytoskeleton. Trends Plant Sci. 6:326-330. [DOI] [PubMed] [Google Scholar]
- 2.Asurmendi, S., R. H. Berg, J. C. Koo, and R. N. Beachy. 2004. Coat protein regulates formation of replication complexes during tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 101:1415-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins, D., R. Hull, B. Wells, K. Roberts, P. Moore, and R. N. Beachy. 1991. The tobacco mosaic virus 30K movement protein in transgenic tobacco plants is localized to plasmodesmata. J. Gen. Virol. 72:209-211. [DOI] [PubMed] [Google Scholar]
- 4.Baas, P. W., T. P. Pienkowski, K. A. Cimbalnik, K. Toyama, S. Bakalis, F. J. Ahmad, and K. S. Kosik. 1994. Tau confers drug stability but not cold stability to microtubules in living cells. J. Cell Sci. 107:135-143. [DOI] [PubMed] [Google Scholar]
- 5.Baas, P. W. 2002. Microtubule transport in the axon. Int. Rev. Cytol. 212:41-62. [DOI] [PubMed] [Google Scholar]
- 6.Bailly, E., M. Dorée, P. Nurse, and M. Bornens. 1989. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 8:3985-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr, F. A., and J. Egerer. 2005. Golgi positioning: are we looking at the right MAP? J. Cell Biol. 168:993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosc, C., A. Andrieux, and D. Job. 2003. STOP proteins. Biochemistry 42:12125-12132. [DOI] [PubMed] [Google Scholar]
- 9.Boyko, V., J. A. Ashby, E. Suslova, J. Ferralli, O. Sterthaus, C. M. Deom, and M. Heinlein. 2002. Intramolecular complementing mutations in Tobacco mosaic virus movement protein confirm a role for microtubule association in viral RNA transport. J. Virol. 76:3974-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyko, V., J. Ferralli, J. Ashby, P. Schellenbaum, and M. Heinlein. 2000. Function of microtubules in intercellular transport of plant virus RNA. Nat. Cell Biol. 2:826-832. [DOI] [PubMed] [Google Scholar]
- 11.Boyko, V., J. Ferralli, and M. Heinlein. 2000. Cell-to-cell movement of TMV RNA is temperature-dependent and corresponds to the association of movement protein with microtubules. Plant J. 22:315-325. [DOI] [PubMed] [Google Scholar]
- 12.Boyko, V., J. van der Laak, J. Ferralli, E. Suslova, M.-O. Kwon, and M. Heinlein. 2000. Cellular targets of functional and dysfunctional mutants of tobacco mosaic virus movement protein fused to green fluorescent protein. J. Virol. 74:11339-11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bre, M. H., and E. Karsenti. 1990. Effects of brain microtubule-associated proteins on microtubule dynamics and the nucleating activity of centrosomes. Cell Motil. Cytoskelet. 15:88-98. [DOI] [PubMed] [Google Scholar]
- 14.Brill, L. M., S. Dechongkit, B. DeLaBarre, J. Stroebel, R. N. Beachy, and M. Yeager. 2004. Dimerization of recombinant tobacco mosaic virus movement protein. J. Virol. 78:3372-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brill, L. M., R. S. Nunn, T. W. Kahn, M. Yeager, and R. N. Beachy. 2000. Recombinant tobacco mosaic virus movement protein is an RNA-binding, α-helical membrane protein. Proc. Natl. Acad. Sci. USA 97:7112-7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citovsky, V., D. Knorr, G. Schuster, and P. Zambryski. 1990. The P30 movement protein of tobacco mosaic virus is a single-stranded nucleic acid binding protein. Cell 60:637-647. [DOI] [PubMed] [Google Scholar]
- 17.Citovsky, V., M. L. Wong, A. L. Shaw, B. V. Prasad, and P. Zambryski. 1992. Visualization and characterization of tobacco mosaic virus movement protein binding to single-stranded nucleic acids. Plant Cell 4:397-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cyr, R. J., and B. A. Palevitz. 1995. Organization of cortical microtubules in plant cells. Curr. Opin. Cell Biol. 7:65-71. [DOI] [PubMed] [Google Scholar]
- 19.De Brabander, M., G. Geuens, R. Nuydens, R. Willebrords, and J. De Mey. 1981. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc. Natl. Acad. Sci. USA 78:5608-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deom, C. M., M. J. Oliver, and R. N. Beachy. 1987. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science 237:384-389. [DOI] [PubMed] [Google Scholar]
- 21.Deom, C. M., K. R. Schubert, S. Wolf, C. A. Holt, W. J. Lucas, and R. N. Beachy. 1990. Molecular characterization and biological function of the movement protein of tobacco mosaic virus in transgenic plants. Proc. Natl. Acad. Sci. USA 87:3284-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dictenberg, J. B., W. Zimmermann, C. A. Sparks, A. Young, C. Vidair, Y. Zheng, W. Carrington, F. S. Fay, and J. Doxsey. 1998. Pericentrin and γ-tubulin form a protein complex and are organised into a novel lattice at the centrosome. J. Cell Biol. 141:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding, B., J. S. Haudenshield, R. J. Hull, S. Wolf, R. N. Beachy, and W. J. Lucas. 1992. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell 4:915-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doxsey, S. J., P. Stein, L. Evans, P. D. Calarco, and M. Kirschner. 1994. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell 76:639-650. [DOI] [PubMed] [Google Scholar]
- 25.Du, Y., S. Ferro-Novick, and P. Novick. 2004. Dynamics and inheritance of the endoplasmic reticulum. J. Cell Sci. 117:2871-2878. [DOI] [PubMed] [Google Scholar]
- 26.Echeverri, C. J., B. M. Paschal, K. T. Vaughan, and R. B. Vallee. 1996. Molecular characterization of the 50-kDa subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132:617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erhardt, M., V. Stoppin-Mellet, S. Campagne, J. Canaday, J. Mutterer, T. Fabian, M. Sauter, T. Muller, C. Peter, A. M. Lambert, and A. C. Schmit. 2002. The plant Spc98p homologue colocalizes with gamma-tubulin at microtubule nucleation sites and is required for microtubule nucleation. J. Cell Sci. 115:2423-2431. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie, T., P. Boevink, S. Haupt, A. G. Roberts, R. Toth, T. Vantine, S. Chapman, and K. J. Oparka. 2002. Functional analysis of a DNA shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of tobacco mosaic virus. Plant Cell 14:1207-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinlein, M. 2002. Plasmodesmata: dynamic regulation and role in macromolecular cell-to-cell signalling. Curr. Opin. Plant Biol. 5:543-552. [DOI] [PubMed] [Google Scholar]
- 30.Heinlein, M. TMV movement protein targets cell-cell channels in plants and prokaryotes: possible roles of tubulin- and FtsZ-based cytoskeletons, in press. In F. Baluska, D. Volkmann, and P. W. Barlow (ed.), Cell-cell channels. Landes Bioscience, Georgetown, Tex. www.eurekah.com/abstract.php?chapid=2711&bookid=192&catid=16.
- 31.Heinlein, M., and B. L. Epel. 2004. Macromolecular transport and signaling through plasmodesmata. Int. Rev. Cytol. 235:93-164. [DOI] [PubMed] [Google Scholar]
- 32.Heinlein, M., B. L. Epel, H. S. Padgett, and R. N. Beachy. 1995. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science 270:1983-1985. [DOI] [PubMed] [Google Scholar]
- 33.Heinlein, M., H. S. Padgett, J. S. Gens, B. G. Pickard, S. J. Casper, B. L. Epel, and R. N. Beachy. 1998. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 10:1107-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinlein, M., M. R. Wood, T. Thiel, and R. N. Beachy. 1998. Targeting and modification of prokaryotic cell-cell junctions by tobacco mosaic virus cell-to-cell movement protein. Plant J. 14:345-351. [DOI] [PubMed] [Google Scholar]
- 35.Hirokawa, N. 1994. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr. Opin. Cell Biol. 6:74-81. [DOI] [PubMed] [Google Scholar]
- 36.Hugdahl, J. D., C. L. Bokros, V. R. Hanesworth, G. R. Aalund, and L. C. Morejohn. 1993. Unique functional characteristics of the polymerization and MAP binding regulatory domains of plant tubulin. Plant Cell 5:1063-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-1 between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 38.Job, D., O. Valiron, and B. Oakley. 2003. Microtubule nucleation. Curr. Opin. Cell Biol. 15:111-117. [DOI] [PubMed] [Google Scholar]
- 39.Kaech, S., B. Ludin, and A. Matus. 1996. Cytoskeletal plasticity in cells expressing neuronal microtubule-associated proteins. Neuron 17:1189-1199. [DOI] [PubMed] [Google Scholar]
- 40.Kain, R., K. Angata, D. Kerjaschki, and M. Fukuda. 1998. Molecular cloning and expression of a novel human trans-Golgi network glycoprotein, TGN51, that contains multiple tyrosine-containing motifs. J. Biol. Chem. 273:981-988. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami, S., Y. Watanabe, and R. N. Beachy. 2004. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc. Natl. Acad. Sci. USA 101:6291-6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keating, T. J., J. G. Peloquin, V. I. Rodionov, D. Momcilovic, and G. G. Borisy. 1997. Microtubule release from the centrosome. Proc. Natl. Acad. Sci. USA 94:5078-5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellogg, D. R., M. Moritz, and B. M. Alberts. 1994. The centrosome and cellular organization. Annu. Rev. Biochem. 63:639-674. [DOI] [PubMed] [Google Scholar]
- 44.Khodjakov, A., and C. L. Rieder. 1999. The sudden recruitment of γ-tubulin to the centrosome at the outset of mitosis and its dynamic exchange throughout the cell cycle do not require microtubules. J. Cell Biol. 146:585-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knebel, W., H. Quader, and E. Schnepf. 1990. Mobile and immobile endoplasmic reticulum in onion bulb epidermis cells: short- and long-term observations with a confocal laser scanning microscope. Eur. J. Cell Biol. 52:328-340. [PubMed] [Google Scholar]
- 46.Kneussel, M. 2005. Postsynaptic scaffold proteins at non-synaptic sites. The role of postsynaptic scaffold proteins in motor-protein-receptor complexes. EMBO Rep. 6:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotlizky, G., A. Katz, J. van der Laak, V. Boyko, M. Lapidot, R. N. Beachy, M. Heinlein, and B. L. Epel. 2001. A dysfunctional movement protein of Tobacco mosaic virus interferes with targeting of wild type movement protein to microtubules. Mol. Plant-Microbe Interact. 7:895-904. [DOI] [PubMed] [Google Scholar]
- 48.Kuriyama, R., and G. G. Borisy. 1981. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J. Cell Biol. 91:822-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lapidot, M., R. Gafny, B. Ding, S. Wolf, W. J. Lucas, and R. N. Beachy. 1993. A dysfunctional movement protein of tobacco mosaic virus that partially modifies the plasmodesmata and limits spread in transgenic plants. Plant J. 4:959-970. [Google Scholar]
- 50.Lee, C., M. Ferguson, and L. B. Chen. 1989. Construction of the endoplasmic reticulum. J. Cell Biol. 109:2045-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee, G., and R. Brandt. 1992. Microtubule-bundling studies revisited: is there a role for MAPs? Trends Cell Biol. 2:286-289. [DOI] [PubMed] [Google Scholar]
- 52.Liu, J.-Z., E. B. Blancaflor, and R. S. Nelson. 2005. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 138:1877-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lloyd, C., and J. Chan. 2004. Microtubules and the shape of plants to come. Nat. Rev. Mol. Cell Biol. 5:13-22. [DOI] [PubMed] [Google Scholar]
- 54.Masson, D., and T. E. Kreis. 1993. Identification and molecular characterization of E-MAP-115, a novel microtubule-associated protein predominantly expressed in epithelial cells. J. Cell Biol. 123:357-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathur, J., N. Mathur, B. Kernebeck, B. P. Srinivas, and M. Hülskamp. 2003. A novel localization pattern for an EB1-like protein links microtubule dynamics to endomembrane organization. Curr. Biol. 13:1991-1997. [DOI] [PubMed] [Google Scholar]
- 56.McDowell, J. M., S. Huang, E. C. McKinney, Y.-Q. An, and R. B. Meagher. 1996. Structure and evolution of the actin gene family in Arabidopsis thaliana. Genetics 142:587-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLean, B. G., J. Zupan, and P. C. Zambryski. 1995. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco plants. Plant Cell 7:2101-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNally, F. J., K. Okawa, A. Iwamatsu, and R. D. Vale. 1996. Katanin, the microtubule-severing ATPase, is concentrated at centrosomes. J. Cell Sci. 109(Pt. 3):561-567. [DOI] [PubMed] [Google Scholar]
- 59.Meshi, T., Y. Watanabe, T. Saito, A. Sugimoto, T. Maeda, and Y. Okada. 1987. Function of the 30 kd protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J. 6:2557-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore, P., C. A. Frenczik, C. M. Deom, and R. N. Beachy. 1992. Developmental changes in plasmodesmata in transgenic tobacco expressing the movement protein of tobacco mosaic virus. Protoplasma 170:115-127. [Google Scholar]
- 61.Moritz, M., and D. A. Agard. 2001. Gamma-tubulin complexes and microtubule nucleation. Curr. Opin. Struct. Biol. 11:174-181. [DOI] [PubMed] [Google Scholar]
- 62.Murata, T., S. Sonobe, T. I. Baskin, S. Hyodo, S. Hasezawa, T. Nagata, T. Horio, and M. Hasebe. 2005. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat. Cell Biol. 7:961-968. [DOI] [PubMed] [Google Scholar]
- 63.Murphy, S. M., A. M. Preble, U. K. Patel, K. L. O'Connell, D. P. Dias, M. Moritz, D. Agard, J. T. Stults, and T. Stearns. 2001. GCP5 and GCP6: two new members of the human gamma-tubulin complex. Mol. Biol. Cell 12:3340-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murshid, A., and J. F. Presley. 2004. ER-to-Golgi transport and cytoskeletal interactions in animal cells. Cell Mol. Life Sci. 61:133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Más, P., and R. N. Beachy. 1999. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement in intracellular distribution of viral RNA. J. Cell Biol. 147:945-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Más, P., and R. N. Beachy. 2000. Role of microtubules in the intracellular distribution of tobacco mosaic virus movement protein. Proc. Natl. Acad. Sci. USA 97:12345-12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen, H.-L., S. Chari, D. Gruber, C.-M. Lue, S. J. Chapin, and J. C. Bulinski. 1997. Overexpression of full- or partial-length MAP4 stabilizes microtubules and alters cell growth. J. Cell Sci. 110:281-294. [DOI] [PubMed] [Google Scholar]
- 68.Noiva, R., and W. J. Lennarz. 1992. Protein disulphide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J. Biol. Chem. 267:3553-3556. [PubMed] [Google Scholar]
- 69.Ohno, T., N. Takamatsu, T. Meshi, Y. Okada, M. Nishigushi, and Y. Kiho. 1983. Single amino acid substitution in 30k protein of TMV defective in virus transport function. Virology 131:255-258. [DOI] [PubMed] [Google Scholar]
- 70.Oparka, K. J., D. A. M. Prior, S. Santa Cruz, H. S. Padgett, and R. N. Beachy. 1997. Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus. Plant J. 12:781-789. [DOI] [PubMed] [Google Scholar]
- 71.Osman, T. A., and K. W. Buck. 1996. Complete replication in vitro of tobacco mosaic virus RNA by a template-dependent, membrane-bound RNA polymerase. J. Virol. 70:6227-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palacios, I. M., and D. St. Johnston. 2001. Getting the message across: the intracellular localization of mRNAs in higher eukaryotes. Annu. Rev. Cell Dev. Biol. 17:569-614. [DOI] [PubMed] [Google Scholar]
- 73.Piguet, V., and Q. Sattentau. 2004. Dangerous liaisons at the virological synapse. J. Clin. Investig. 114:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pirollet, F., D. Job, E. H. Fischer, and R. L. Margolis. 1983. Purification and characterization of sheep brain cold-stable microtubules. Proc. Natl. Acad. Sci. USA 80:1560-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prokhnevsky, A. I., V. V. Peremyslov, and V. V. Dolja. 2005. Actin cytoskeleton is involved in targeting of a viral Hsp70 homolog to the cell periphery. J. Virol. 79:14421-14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pryer, N. K., R. A. Walker, V. P. Skeen, B. D. Bourns, M. F. Soboeiro, and E. D. Salmon. 1992. Brain microtubule-associated proteins modulate microtubule dynamic instability in vitro. Real-time observations using video microscopy. J. Cell Sci. 103:965-976. [DOI] [PubMed] [Google Scholar]
- 77.Reichel, C., and R. N. Beachy. 1998. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmatic reticulum. Proc. Natl. Acad. Sci. USA 95:11169-11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reichel, C., P. Más, and R. N. Beachy. 1999. The role of the ER and cytoskeleton in plant viral trafficking. Trends Plant Sci. 4:458-463. [DOI] [PubMed] [Google Scholar]
- 79.Rutten, T., J. Chan, and C. W. Lloyd. 1997. A 60-kDa plant microtubule-associated protein promotes the growth and stabilization of neurotubules in vitro. Proc. Natl. Acad. Sci. USA 94:4469-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sagi, G., A. Katz, D. Guenoune-Gelbart, and B. L. Epel. 2005. Class 1 reversibly glycosylated polypeptides are plasmodesmal-associated proteins delivered to plasmodesmata via the Golgi apparatus. Plant Cell 17:1788-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schellenbaum, P., M. Vantard, C. Peter, A. Felous, and A. M. Lambert. 1993. Co-assembly properties of higher plant microtubule-associated proteins with purified brain and plant tubulins. Plant J. 32:253-260. [Google Scholar]
- 82.Schmit, A. C. 2002. Acentrosomal microtubule nucleation in higher plants. Int. Rev. Cytol. 220:257-289. [DOI] [PubMed] [Google Scholar]
- 83.Seltzer, V., T. Pawlowski, S. Campagne, J. Canaday, M. Erhardt, J. L. Evrard, E. Herzog, and A. C. Schmit. 2003. Multiple microtubule nucleation sites in higher plants. Cell Biol. Int. 27:267-269. [DOI] [PubMed] [Google Scholar]
- 84.Seltzer-Tichanné, V. 2005. Etude des protéines d'Arabidopsis thaliana impliquées dans la nucléation des microtubules. Ph.D. thesis. Université Louis Pasteur, Strasbourg, France.
- 85.Silflow, C. D., D. G. Oppenheimer, S. D. Kopczak, S. E. Ploense, S. R. Ludwig, N. Haas, and D. P. Snustad. 1987. Plant tubulin genes: structure and differential expression during development. Dev. Genet. 8:435-460. [Google Scholar]
- 86.Smith, G. A., and L. W. Enquist. 2002. Break ins and break outs: viral interactions with the cytoskeleton of mammalian cells. Annu. Rev. Cell Dev. Biol. 18:135-161. [DOI] [PubMed] [Google Scholar]
- 87.St. Johnston, D. 2005. Moving messages: the intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 6:363-375. [DOI] [PubMed] [Google Scholar]
- 88.Storms, M. M. H., C. van der Schoot, M. Prins, R. Kormelink, J. W. M. van Lent, and R. W. Goldbach. 1998. A comparison of two methods of microinjection for assessing altered plasmodesmat gating in tissues expressing viral movement proteins. Plant J. 13:131-140. [Google Scholar]
- 89.Subramanian, K., and T. Meyer. 1997. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell 89:963-971. [DOI] [PubMed] [Google Scholar]
- 90.Takemura, R., S. Okabe, T. Umeyama, Y. Kanai, N. J. Cowan, and N. Hirokawa. 1992. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J. Cell Sci. 103:953-964. [DOI] [PubMed] [Google Scholar]
- 91.Terasaki, M., L. B. Chen, and K. Fujiwara. 1986. Microtubules and the endoplasmic reticulum are highly interdependent structures. J. Cell Biol. 103:1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Damme, D., K. Van Poucke, E. Boutant, C. Ritzenthaler, D. Inze, and D. Geelen. 2004. In vivo dynamics and differential microtubule-binding activities of MAP65 proteins. Plant Physiol. 136:3956-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waterman-Storer, C., and E. D. Salmon. 1998. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr. Biol. 8:798-806. [DOI] [PubMed] [Google Scholar]
- 94.Wiche, G., C. Oberkanins, and A. Himmler. 1991. Molecular structure and function of microtubule-associated proteins. Int. Rev. Cytol. 124:217-273. [DOI] [PubMed] [Google Scholar]
- 95.Wolf, S., C. M. Deom, R. N. Beachy, and W. J. Lucas. 1989. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 246:377-379. [DOI] [PubMed] [Google Scholar]
- 96.Zheng, Y., M. L. Wong, B. Alberts, and T. Mitchison. 1995. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature 378:578-583. [DOI] [PubMed] [Google Scholar]