Abstract
Mercury exposure is widespread in the United States with methylmercury as the predominant chemical species and fish and shellfish as the source. Use of more advanced diagnostic techniques and application of population-based risk assessment methodologies have assisted in addressing the impact of mercury exposure on the United States population. Biomonitoring, particularly through analyses of blood mercury, provides both population-based data and exposure information that can be informative for physicians. Data from the National Health and Nutrition Examination Survey (NHANES) beginning in 1999 provide population-based exposure estimates for United States overall. Methylmercury exposures among women of childbearing age are of particular concern because of methylmercury's developmental neurotoxicity. Exposures of concern among women are estimated to occur in between ∼6% to 8% of the 16-to-49-year-old age group based on data from NHANES; and in ∼15% of this age and sex group if physiological factors such as the degree of transplacental transport of methylmercury are taken into consideration. Subgroups with high fish consumption (e.g., many island and coastal populations, some persons of Asian ethnicity, some individuals following “healthy” diets) can have methylmercury exposures substantially higher than those reported among the NHANES examinees. These subpopulations are not likely to be aware of their blood mercury concentrations or the possible health outcomes associated with such high blood mercury levels. The American Medical Association has adopted policies that express concerns about methylmercury exposure, and advise patient education. Non-neurological risks for adults associated with methylmercury, including the potential for adverse cardiac outcomes, have not yet been incorporated into risk assessments.
Introduction
Virtually everyone is exposed to mercury and their tissues contain mercury residues at some concentration. Typical exposures in the United States result from mercury in fish and shellfish, mercury released from dental amalgams and occasionally from exposures secondary to mercury in pharmaceutical products (1, 2). Some adults are exposed occupationally to mercury vapors. Occupational exposures to inorganic mercury in the felt hat industry in the United States were thoroughly described by the United States Public Health Service in the 1930s and 1940s. The renal and neurological effects and exposures associated with these effects among workers in the chloralkali industry were well described by Smith (3). Many physicians will remember seeing striking photographs of people with mercury poisoning in Japan (i.e., from Minamata and Niigata, Japan) in the 1960s (4). Others will remember the major outbreak in Iraq in the early 1970s (5) that resulted in the deaths of many hundreds of persons. The actual numbers are unknown because relatively few died in hospital. Through combined medical, public health, and legal actions the most extreme exposures to inorganic mercury, mercury vapors, and methylmercury have largely been brought under control. It is, nonetheless, important to recognize that fatalities following mercury exposure still occur in the United States (6). These high exposures represent the tip of the iceberg of a far broader, less intense, exposure pattern that is distributed across the United States population (7 ,8). The intensity of exposure is determined by food habits (fish consuming or not), presence of mercury-containing dental amalgams, occupation, and habits/hobbies (2). Fortunately fairly simple and relatively inexpensive methods are available to determine patients' exposure levels.
Medical concern and awareness of the need to reduce overall exposures to methylmercury was expressed in a policy statement issued by the American Medical Association (9) in 2004. The recommendations focused on improved monitoring patients' exposures to mercury through blood, hair, or urine mercury measurements; promoting testing fish for their mercury content; making data available on sources of mercury; and promoting patient education.
Fate and Transport of Mercury in the Environment
There is an extensive complex literature on the biogeochemistry describing the fate and transport of mercury in the environment. A brief description is provided. Mercury is widely distributed around the earth. One of the elements on the Periodic Table, mercury cannot be destroyed; the total amount on the planet will always be the same. Mercury cycles in the environment as a result of natural phenomena and human activities [for reviews see U.S. EPA's Mercury Study Report to Congress (10); Pirrone (11)]. Natural phenomena such as volcanoes cause mercury to be released in the air. Mercury has been widely used in industrial processes because of its chemical and physical properties (for example, it conducts electricity, it response to temperature and pressure changes, and it forms alloys with many metals). Industrial processes and combustion of mercury-containing wastes and fuels also release mercury.
Mercury that is released into the air is mercury vapor or inorganic mercury. Once in the atmosphere as a gas ultimately it is redeposited on the earth with precipitation. Once on the earth or in the waterways, it is incorporated into sludges or sediments, where it is methylated. The plant and sedimentary materials containing methylmercury are consumed by small fish that are consumed by progressively larger fish and finally by humans. During the course of this progression a great increase in concentration occurs—known as bioaccumulation. This increase can result in concentrations of methylmercury in fish tissues that are hundreds of thousands of times higher than the levels of inorganic mercury in the water (1).
Humans are exposed to methylmercury because they consume fish and shellfish. Predators at the top of the aquatic food web generally have higher mercury concentration than those lower in the food web. Fish such as shark, large tuna, sword fish, marlin, king mackerel contain methylmercury at concentrations that are 10-to-20 times higher than fish such as herring, cod, pollack, and shellfish such as shrimp or scallops (12). In the United States about 95% of ingested methylmercury comes from the consumption of fish and other seafood (12).
Chemical Species of Mercury Exposure in the United States
Before presenting our data on biomarkers of blood and hair mercury concentrations from the National Health and Nutrition Examination Survey (NHANES), a brief overview of the biomarkers of mercury exposure is provided. Chemical analyses of body fluids and tissues provide an indication of the patient's exposure profile including chemical species of mercury. Whole blood, urine, and hair (the tissues typically analyzed to determine their mercury concentration to provide biomarkers of exposure) provide different information regarding chemical form and duration of mercury exposure. For example, blood mercury concentration (typically used to indicate exposure to organic mercury) is always dominated by methylmercury. Urinary mercury level is an indicator of exposure to inorganic mercury. Hair mercury is dominated by exposure to methylmercury. Because mercury can deposit on hair from surface contamination, total mercury content of hair may not be a reflection of mercury excreted from the body, but may be dominated by mercury from surface contamination. In depth review articles on this topic include those by Risher et al. (13) and Mason et al. (14).
Organic Mercury
In the United States when total blood mercury exceeded ∼4 μg/L more than 90% of mercury present was organic mercury (i.e., methylmercury) based on data from children ages 1 through 6 years and adult women who participated during the years 1999 and 2000 in the National Health and Nutrition Examination Survey (NHANES) (8). This survey (NHANES) is a population-based survey which utilizes census data to identify subjects who undergo intense medical evaluation including biochemical assessment for exposure to a very large number of environmental contaminants not typically determined in routine medical evaluation. When appropriately treated statistically these data provide estimates for their subgroup of the United States population. In the years 1999 through 2001 NHANES determined mercury exposures only for children age 6 and younger and adult women ages 16 through 46 years (7). Males and females in other age groups did not have mercury analyses determined on their tissue samples during these years. The source of organic mercury for the general population is methylmercury from the consumption of fish and shellfish (8, 12).
Inorganic Mercury
Blood inorganic mercury concentrations have been used to detect acute, high dose exposures. For chronic, low-to-moderate inorganic mercury exposure, urinary mercury concentration is the preferred method for assessing inorganic mercury exposure (14). Health risks associated with various values are discussed elsewhere in this article. The half-life of inorganic mercury in blood is about three days based on experimental studies with radio-labelled mercury (15). A second, slower half-life of about two-to-three weeks has been indicated based on studies in chloralkali workers whose exposures were terminated (16). Urinary mercury levels are used to monitor sustained exposure to inorganic mercury.
Mercury Vapor
Exposure to mercury vapor is usually associated with occupational exposures, accidental exposures (6, 17), and some bizarre “recreational” exposure have been identified (18). These can be highly dangerous and life-threatening. Once inside the lungs mercury is oxidized forming Hg(II) complexes which are soluble in many body fluids. The half-time of Hg in blood absorbed as a vapor is 2-to-4 days after which 90% is excreted through urine and feces followed by a second phase with a half-time of 15–30 days (19). Between passage of elemental mercury through the alveolar membrane and complete oxidation, mercury accumulates in the central nervous system. During this process mercury can irreversibly damage the central nervous system. At exposures of moderate duration, the kidneys are also affected. Short-term exposure to high levels of mercury vapor produces chest pain, dyspnea, cough impaired pulmonary function, interstitial pneumonitis (19). Occupational exposures to mercury vapor have caused psychiatric symptoms, hallucinations, erethism (exaggerated emotional responses) insomnia, and muscular tremors (19).
Biological Media Used to Detect Mercury Exposure
Blood Mercury Concentrations
If blood mercury concentrations are under about 4 μg/L mercury exposures are like to combined inorganic mercury and methylmercury (8 ,20). The relative contribution of methylmercury from fish consumption or inorganic mercury from dental amalgams determines whether organic mercury or inorganic mercury dominates. If blood total mercury exceeds about 4 μg/L more than 90% of mercury present in the blood of adult women in a sample representative of the U.S. population was organic mercury (i.e., methylmercury) representing mercury from fish consumption.
After the reference range for blood mercury for the general population became available (based on publication (7 ,8) of data for children and adult women from the 1999–2000 National Health and Nutrition Examination Survey), the reference range that many commercial laboratories use for blood mercury data substantially decreased. Physicians may wish to consult their laboratories to verify the reference range used by a particular laboratory because some laboratories simply report results as elevated or not.
Under circumstances of high exposure to mercury vapor (e.g., occupational settings), blood inorganic mercury has been found to be elevated and correlates closely with urinary mercury [e.g., data on chlor-alkai workers—among others see Smith et al. (3)]. Blood mercury concentrations for subjects having this exposure pattern were far higher than generally observed in the general population of the United States; i.e., >20–30 μg/L whole blood. Exposure to mercury vapor may occur non-occupationally during mercury spills or following ritualistic use of mercury.
Urinary Mercury Concentrations
Urinary mercury concentration is usually expressed in μg/L. Frequently mercury concentrations are creatinine adjusted. The half-life of urinary excretion of mercury has been variably reported to range from as short as 20 days to as long as 90 days (14). Based on likely half-lives of 40-to-90 days, urinary mercury is an integrated marker of exposure over previous months (14). Urinary mercury is the preferred biomarker indicating exposure to inorganic mercury and over time increases in response to exposure to low levels of mercury vapor. Mercury concentrations in the urine will also increase with exposures to other mercurials including phenylmercury. Inorganic mercury can also arise from demethylation of methylmercury. An increase in urinary inorganic mercury can be shown in subjects with a high methylmercury intake (21 ,22). Kingman et al (23) in their study of more than 1100 former Vietnam era military personnel found more than 90% of urinary mercury was in the inorganic form.
Hair Mercury Concentrations
Growth, morphology and histochemistry of human hair have been reviewed in detail (24). As hair grows methylmercury is incorporated into hair. There is a general view that hair grows at the rate of about 1 cm per month, although there is evidently substantial variability around this value. Hair mercury analyses are complicated by the problem that mercury can be deposited onto the hair from external sources after it has been formed. Consequently data on hair mercury has to be constantly questioned because of the risk of external contamination.
Mercury that is actually incorporated into hair as it grows is predominantly methylmercury. The percent of total hair mercury that is methylmercury has been reported to be 80% (25) to values ranging up to 98% (26). Generally hair is thought to be 250 to 300 times more concentrated in mercury than is blood (25 ,27). A far wider range of individual values exists. For example, Dolbec et al. (26) reported hair-to-blood ratios ranging between 81 and 624. The extent to which this ratio is applicable across all age and ethnic/racial groups remains to be confirmed.
Seidel et al. (28) have noted many problems with commercial laboratories performing hair analyses for trace elements. Surface contamination of hair with mercury is a problem in interpreting hair mercury data. Consequently with the availability of a reference range for the United States population for blood mercury data many physicians are turning to blood mercury as the preferred biomarker to indicate exposure to organic mercury.
Inorganic mercury is not considered to be excreted in hair at typical exposure levels, although inorganic mercury can be a surface contaminant on hair. Hair is not considered a good indicator of exposure to mercury vapor (27) or to inorganic mercury (27).
Materials and Methods
Distribution of Blood Mercury and Hair Mercury in the United States.
In the United States the Centers for Disease Control conducts the NHANES which provides medical examinations, biochemistry assessments for exposure to environmental contaminants, dietary and medical histories, clinical chemistry profiles, and a large number of other specialized tests to approximately 8,000 persons per year. This survey is conducted in approximately 25 to 30 communities per year which are selected using complex statistical procedures, so that when statistically analyzed using appropriate population statistics, the data can provide a profile that is representative of the Untied States as a whole (7, 8).
Among adult women of childbearing age (the ages used in this survey were 16 years through 49 years) and young children (ages one through six years) beginning with survey years 1999 and 2000, biomarkers of mercury exposure were measured. These included hair mercury (total and inorganic), blood mercury (total and inorganic), and urinary mercury (total only and adult women only). Organic mercury was calculated by differences and chemical speciation of samples indicated that the predominant chemical species was methylmercury (7, 8). Although adult men, children older than six years, and women older than 49 years were included among the NHANES examinees, mercury measurements were not included for these age and gender groups during the 1999 and 2000 survey years. Mercury measurements were added for these groups beginning in 2003.
Results
Blood mercury concentration data for adult women who participated in NHANES during the years 1999 and 2000 are shown in Table 1 and Table 2 and organic blood mercury (i.e., methylmercury) data are shown in Table 3. Children in the 1-to-6-year age group have much lower blood mercury levels than did the adult women. Their data are not shown in this presentation. Hair mercury concentrations (29) are shown in Table 4 for the adult women and children who were examinees in the 1999 and 2000 NHANES.
TABLE 1.
Distribution of blood total mercury data for women 16 through 49 years, NHANES 1999–2000, by race/ethnic group [adapted from Mahaffey et al., (8)]
| Sample Persons | Geometric Mean | 95th CI | 5th | 50th | 95th | |
|---|---|---|---|---|---|---|
| Total | 1,709 | 1.02 | 0.85 –1.20 | ND | 0.94 | 7.13 |
| Race/Ethnicity | ||||||
| Mexican American | 579 | 0.82 | 0.68 –0.96 | 0.11 | 0.84 | 3.91 |
| Other Hispanic | 124 | 1.16 | 0.77 –1.55 | ND | 1.21 | 8.79 |
| Non-Hispanic white | 578 | 0.96 | 0.76 –1.16 | ND | 0.86 | 7.08 |
| Non-Hispanic black | 364 | 1.35 | 1.08 –1.61 | ND | 1.34 | 6.55 |
| Other | 64 | 1.37 | 0.35 –2.28 | ND | 1.20 | 10.04 |
TABLE 2.
Percentage of women 16–49 years of age with blood total mercury concentrations μg/L above selected thresholds by race/ethnic group, 1999–2000 NHANES [adapted from Mahaffey et al. (8)]
| Sample Persons | Percentage ≥15.0 μg/L | Percentage ≥5.8 μg/L | Percentage ≥5.0 μg/L | Percentage ≥3.5 μg/L | |
|---|---|---|---|---|---|
| Total | 1709 | 0.6 | 7.8 | 9.7 | 15.7 |
| Race/Ethnicity | |||||
| Mexican/American | 579 | 0.3 | 2.0 | 2.7 | 6.1 |
| Other Hispanic | 124 | 2.0 | 5.8 | 5.9 | 16.4 |
| Non-Hispanic white | 578 | 0.1 | 7.8 | 10.0 | 15.3 |
| Non-Hispanic black | 364 | 1.3 | 7.0 | 9.5 | 16.6 |
| Other | 64 | 3.5 | 21.7 | 24.8 | 31.5 |
TABLE 3.
Distribution of blood organic mercury (i.e. methylmercury) concentration, (μg/L) for women aged 16 through 49 years by race/ethnic group, NHANES 1999–2000 (adapted from Mahaffey et al. [(8)].
| Sample Persons | Geometric Mean | 95% CI | 5th | 50th | 95th | |
|---|---|---|---|---|---|---|
| Total | 1707 | 0.80 | ND | 0.60 | 6.73 | |
| Race/Ethnicity | ||||||
| Mexican American | 578 | 0.57 | 0.48 –0.67 | ND | 0.44 | 3.42 |
| Other Hispanic | 123 | 0.97 | 0.65 –1.29 | ND | 0.90 | 8.44 |
| Non-Hispanic White | 578 | 0.75 | 0.59 –0.90 | ND | 0.48 | 6.68 |
| Non-Hispanic black | 364 | 1.01 | 0.78 –1.24 | ND | 1.01 | 6.19 |
| Other | 64 | 1.06 | 0.18 –1.34 | ND | 0.80 | 9.64 |
TABLE 4.
Mean and selected percentiles of hair mercury (Hg) concentrations for children aged 1–5 years and women aged 16–49 years: National Health and Nutrition Examination survey, United States, 1999 [adapted from McDowell et al., (29)].
| Hair Hg | No. | Mean (95% CI) | Selected Percentiles | |||||
|---|---|---|---|---|---|---|---|---|
| 10th (95% CI) | 25th (95% CI) | 50th (95% CI) | 75th (95% CI) | 90th (95% CI) | 95th (95% CI) | |||
| Children | 838 | 0.22 (0.18, 0.25) | 0.03 (0.01, 0.05) | 0.06 (0.05, 0.07) | 0.11 (0.10, 0.13) | 0.21 (0.15, 0.27) | 0.41 (0.33, 0.49) | 0.64 (0.52, 0.76) |
| Women | 1726 | 0.47 (0.35, 0.58) | 0.04 (0.03, 0.05) | 0.09 (0.07, 0.11) | 0.19 (0.15, 0.23) | 0.42 (0.29, 0.55) | 1.11 (0.54, 1.68) | 1.73 (1.44, 2.02) |
Discussion
It is useful to place the results from the NHANES survey into a broader context. This includes the following:
Additional Surveys of Adult Men
The largest study of men from the general population is that of Kingman et al. (23) who analyzed urine and blood mercury concentrations among 1127 Vietnam-era United States Air Force pilots (all men, average age 53 years at the time of blood collection) for whom extensive dental records were available. Blood values were determined for total mercury, inorganic mercury and organic/methylmercury. The mean total blood mercury concentrations were 3.1 μg/L with a range of “zero” (i.e., detection limit of 0.2 μg/L) to 44 μg/L. Overall, 75% of total blood mercury was present as organic or methylmercury. Less than 1% of the variability in total blood mercury was attributable to variation in the number and size of silver-mercury amalgam dental restorations. Dietary data on the former pilots were very limited, so typical patterns of fish consumption were not reported.
Surveys from Individual States
Analyses of blood and hair samples for mercury indicate that some geographic areas have much higher exposure to methylmercury than have been identified (Table 5) to date in NHANES (23,30–37). The upper end of this exposure distribution extends into concentrations reported to be 50 μg/L to ∼90 μg/L for blood; hair values >10 ppm are also reported. Based on these reports from case series, community and state surveys, and medical practices, it is known that much higher exposures to methylmercury in the United States than were documented among the 1999–2000 NHANES examinees have been reported.
TABLE 5.
Mercury exposure among high fish consumers in United States and territories
| Report | Geographic Location | Social/Ethnic/Cultural Group | Summary of Data |
|---|---|---|---|
| Burge and Evans (30) | Arkansas | Gamefish consumers. | Blood [Hg]. Arithmetic mean 10.5 μg/L. 5% of men >30 μg/L. maximum 75 μg/L. |
| Knobeloch et al. (31) | Madison, WI | Professional family | Adults' blood [Hg] >30 μg/L |
| Kingman et al., (23) | National | Varied. Former Vietnam-era retired military. All male sample. | Blood [Hg]. Arithmetic mean total 2.55 (2.01 organic Hg). Geometric mean 1.75 (1.11 organic Hg). Values as high as 44 μg/L total Hg reported. |
| Bellanger et al. (32) | Louisiana | State screening program for persons concerned that they may have elevated mercury exposures. | Blood [Hg] ranged from <0.3 μg/L to 35 μg/L. 1.9% of 313 subjects >20 μg/L. Higher values reported among commercial fishermen and their families. |
| Stern et al. (33) | New Jersey | Pregnant women. | Hair [Hg]. 1% to 2% had hair [Hg] >4 ppm. |
| Kales and Goldman (34) | Boston, MA | Urbanites. | Blood [Hg]. Fish consumption associated with blood [Hg] >15 μg/L (19 to 53 μg/L). |
| Hightower and Moore (35) | San Francisco, CA | Affluent, professional urbanites. | Blood [Hg] ranged 2 to 89.5 μg/L. Mean for 66 women was 15 μg/L. Mean for 23 men was 13 μg/L. |
| Ortiz-Roque and Lopez-Rivera (36) | Vieques (Puerto Rico) | Island group. | Hair [Hg]: 90th percentile 9 ppm. 3 women had concentrations of 15, 25, and 101 ppm. |
| Saint-Phard et al. (37) | New York City | Professional urbanites. | Blood [Hg] 27–96 μg/L. |
As physicians have become more aware of mercury as a health concern for the general population, many more are ordering mercury measurements on patients' blood, hair and/or urine samples. As these data are reported in the medical literature the magnitude of mercury exposures among groups not typically considered at risk of elevated exposure to methylmercury and/or inorganic will be better understood. Groups having especially high fish consumption include persons living in particular geographic locations (e.g., coastal and island populations), ethnic groups with food habits which prefer fish (e.g., Asian populations), and life styles (e.g., affluent patients who have been consuming a high-fish diet for promotion of cardiovascular health or “fitness”). Table 5 summarizes reports describing groups more highly exposed to methylmercury. High exposure to inorganic mercury is usually either occupational (e.g., dentists and dental technicians) or result from some unusual practice. Subcultures within the United States that may experience high inorganic mercury exposure include persons with ritualistic use of mercury (38), persons using ayurvedic herbal medicine products (39), or Asian traditional remedies (40), cosmetics including skin-lightening creams (41), and persons with “hobbies” that include heating inorganic mercury which forms mercury vapor (6, 18).
Where does the mercury exposure come from? A Brief Description of Various Chemical Forms of Mercury and Their Sources
Methylmercury
Methylmercury is bound to the amino acids in fish muscle and cannot be removed by food preparation (e.g., skinning the fish) or cooking techniques [including removing the visible fat, among other reports see (42)]. Fish and shellfish are the dietary source of methylmercury, although trace amounts of total mercury may be detected in other dietary components [e.g., eggs, organ meats such as kidney (43), or offal (44)]. The methylmercury concentration in fish is determined by the feeding habits of the fish, the mercury concentration in the tissues of its prey, the fish's age, and place in the food chain. The concentration of methylmercury in fish and shellfish species ranges from <0.1 ppm for shellfish species to >1 ppm for high-end predatory fish including ocean fish [such as tuna (45), marlin (46), and sharks (47)] and certain freshwater fish [e.g., walleye and northern pike (48, 49)]. Consequently a person's mercury intake depends on the species of fish consumed, as well as the quantity of fish eaten.
Association between Blood Mercury Concentration and Frequency of Fish Consumption
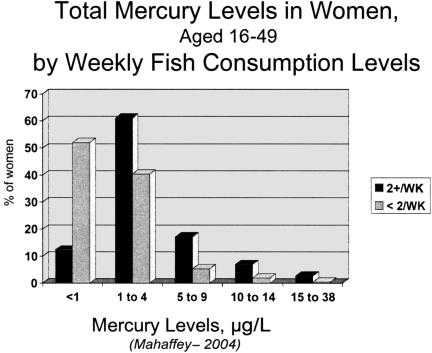
Dietary intakes of fish and shellfish vary enormously within the population of the United States (8). Based on NHANES data 9% of the women of childbearing age consume fish or shellfish on a weekly basis (8), and 3% of women consume fish daily (8). As part of the dietary history questionnaire women who participated in NHANES were asked about their past 24-hour and their monthly patterns of fish consumption. When compared with their blood mercury concentrations, there is an association between the frequency of fish consumption and whether they consumed fish two or more times per week (Figure 2).
Fig. 2.
Blood mercury (total in μg/L) for adult women aged 16 to 49 years examinees who participated in NHANES 1999/2000 by weekly fish consumption.
Variation by Geographic Location
Some health departments in the United States (e.g., the states of North Carolina, Louisiana, Florida) have begun to offer screening for blood mercury to people who may be concerned that their mercury exposures are elevated. For example, in Louisiana the State Office of Public Health began offering to measure blood mercury levels and reported screening values for 313 participants (32) whose blood values ranged from <0.3 μg/L to 35 μg/L with 1.9% >20 μg/L. Higher values were reported among commercial fishermen and their families. Blood mercury values were twice as high among people who ate fish at least once a week compared with people who ate fish twice a month or less. Elevated blood mercury concentrations have been found among gamefish consumer in Arkansas (30).
People who live in states and territories of the United States (among others: Florida, Hawaii, Puerto Rico) who have geographic proximity to a steady supply of fresh fish have a greater intake of methylmercury than more inland populations. Based on year-long records of fish consumption, residents of Florida were found to consume substantially higher intakes of fish and shellfish than national averages (50). The average intake of fish and shellfish reported for the Florida households (50) was approximately equal to the 90th percentile intake for women identified from 24-hour recall dietary intake data reported by women who participated in the 1999–2000 NHANES (8). These differences are also seen in their blood mercury and hair mercury levels. For example, Ortiz-Roque and Lopez-Rivera (36) investigated blood mercury concentrations and seafood consumption frequency among reproductive-age women in two areas of Puerto-Rico. Overall-United States data from NHANES indicated 30-day consumption of fish and shellfish at the 50th percentile was 1.54 meals (95% CI: 1.25–1.82) and at the 90th percentile 10.81 meals (95% CI: 7.15–14.47) (7). Average Puerto Rico total seafood consumption was 12.1 meals/30 days in NE Puerto Rico and 21.2 meals/30 days in Vierques, an island municipality. That is to say that average Puerto Rican fish and shellfish consumption was higher than 90th percentile intake for the overall United States. The percent of women whose mercury intake exceeded US EPA's Reference Dose (discussed below) was 6.6% in NE Puerto Rico and 26.8% in Vieques. Among the Viequenses, three of the 41 women had hair mercury concentrations >12 ppm which is the lower bound of the benchmark dose for mercury, an effect level for adverse neurological outcomes to the developing fetus (36).
Variation by Socio-Economic and Ethnic Characteristics
Other groups reported with high blood mercury concentrations include well-educated, affluent professionals who have been consuming diets high in fish in the view that such diets will be advantageous to their health. Some of the highest blood mercury concentrations (e.g., > 80 μg/L) have been reported among this group of patients (35, 37). For example, Hightower and Moore (35) reported blood mercury concentrations from a private practice patient population in San Francisco. From a total of 116 patients evaluated, 89% had blood mercury concentrations ≥5 μg/L, and 16% had concentrations ≥20 μg/L. Following the 2003 publication, Hightower tested an additional 107 patients whose average blood mercury concentration was 21 μg/L. Saint-Phard et al. (37) reported a case series of patients from the New York City area having elevated blood mercury (range 27 μg/L to 96 μg/L) associated with fish consumption who also had neurological symptoms (i.e., including paresthesias of the extremities and/or electro-diagnostic evidence of sensori-motor peripheral neuropathy). Additional clinic reporting elevated blood mercury concentrations associated with fish consumption include Kales and Goldman (34) from New Haven.
In general, members of some minority groups eat fish more often than the general population and eat larger amounts of fish. For example, U.S. residents of Asian/Pacific Islander (51) and Caribbean Islander (36) ethnicity and some Native American groups consume fish at higher levels than other subpopulations in the US.
Exposures to Inorganic Mercury and non-Methyl-Organo-Mercurials
An alloy of silver, copper, tin, and 50% inorganic mercury has been used in dental practices as a restorative material to fill teeth. Mercury released from these amalgam fillings occurs in multiple forms: elemental mercury vapor, metallic ions, and/or fine particles. Results indicate that placement of mercury-containing amalgams in teeth result in an increased body burden of mercury in body tissues (52, 53). Barregard et al. (54) and Francis et al., (55) indicate that individual variation in habits can influence the amount of mercury released from mercury amalgams including bruxism and gum chewing.
Other sources of inorganic mercury are numerous. Ingestion of small quantities of elemental mercury is fairly common. For example, nearly 3,000 cases of mercury exposure were reported to the American Association of Poison Control Centers Toxic Exposure Surveillance System in 1996 (56). Elemental mercury may be deliberately ingested, as well as accidentally ingested. Elemental mercury is part of various folk remedies, particularly for gastroenteritis (57), and may produce medical complications depending on the quantity ingested (58). Garvey et al. (40) estimate that 30% of the U.S. population uses some form of homeopathic or alternative therapy. Mercury is a frequent contaminant of Asian traditional remedies (40) and ayurvedic herbal medicine products (39).
In various areas in the United States ethnic and folk uses of mercury are associated with cultural practices known as “Santeria”, “Espiritismo” and “voodoo”. Mercury in the form of metallic mercury (59) is sold in botanicas, stores that specialize in selling “religious” items used in Espiritismo, voodoo, and Santeria (60). A cluster of cases in New York has been described in which vapors of metallic mercury from mercury to be used in mercury-filled ampulets prepared for practitioners of Santeria were the source of elevated urinary mercury levels (59, 61). The extent of this practice is not known.
Mercury is an ingredient in “beauty” and skin-lightening creams or lotions manufactured worldwide. One product that has been well described was found to contain between 6% and 10% (6,000 and 10,000 ppm) mercury by weight (41), was distributed across the Mexican-US border, and was associated with an increased in urinary excretion of inorganic mercury (62) to a level >100 μgL (reference range: 0 to 20 μg/L) among users of this product which contained “calomel” or mercurous chloride (63). Although in the United States mercury compounds can only be legally used as preservatives in eye-area cosmetics at concentrations not exceeding 65 ppm (63), standards for production and regulation of cosmetic products vary worldwide (41). Ingredients that are restricted in one country may be entirely legal in another.
Occupational exposures can result in significant exposures to inorganic mercury and mercury vapors. Occupations include dentists, dental technicians, workers in chlor-alkali industries, miners, manufacturers of measuring devices, fluorescent lamp recyclers, and rarely chemists and chemical laboratory technicians. Campbell et al. (64) estimated that within the United States about 70,000 workers are exposed annually to mercury.
Ethylmercury under the trade name thimerosal has been used as a preservative in vaccines since the 1930s (65) and in biological products (ophthalmic solutions, optic suspensions, creams) for at least a century. Many vaccines contained thimerosal as a preservative. Thimerosal has been removed from a large number of vaccines (66). Mercury-containing ingredients currently used in biologicals sold in the United States include thimerosal, phenylmercuric acetate, phenylmercuric nitrate, mercuric acetate, mercuric nitrate, merbromin, and mercuric oxide (<http://www.fda.gov/cder/fdama/mercury300.htm> accessed 5-15-2004). This information was derived from submissions made in response to the Food and Drug Administration's Modernization Act of 1997 which required US FDA to review the risks of all mercury-containing food and drugs. These products include vaccines, ophthalmic solutions, nasal sprays, and immune globulins. Phenylmercury compounds have been used in the past as sanitizers and antifungal agents.
Reports of Elevated Biomarkers of Inorganic Mercury Exposure
Within the NHANES data a few subjects were identified who had unusually high levels of blood inorganic mercury and urinary mercury. Kingman et al. (23) reported mean total mercury in urine was 3.1 μg/L ranging from non-detectible to a maximum value of 35 μg/L. Forty-seven percent of subjects had [Hg] <2 μg/L and 1.3% had values >15 μg/L. Most (93%) of the urinary mercury was inorganic.
Adverse Health Effects Produced by Mercury Exposures
Methylmercury at high exposures is extremely well documented as a human neurotoxin with effects mainly on the motor and sensory systems, especially in the area of sensory-motor integration. During the 1950s and 1960s, major epidemics of methylmercury poisoning in Japan resulted in deaths and severe neurological damage, were caused by consumption of seafood in Minamata, and freshwater fish in Niigata (4). Domestic animals such as cats that consumed fish also developed neurological problems. Epidemics of methylmercury poisoning resulting from consumption of methylmercury used as a fungicide on grain occurred in Iraq in the 1960s and 1970s (5). These epidemics, and a number of case reports, including one from the United States provide the strongest possible evidence linking exposure to methylmercury with human fatalities and neurological disease.
Neurological Effects in Adults
Methlymercury's effects of the nervous system follow a sharp dose-response curve. Adult neurological effects have been used in establishing limits aimed at protecting the public's health. The development of paresthesia has been considered to be the most sensitive neurological effect of methylmercury exposure among adults (25). Blood mercury concentrations of 200 μg/L were judged to WHO (25) to be associated with a 5% prevalence of paresthesias in the adult population based on data from the Iraqi poisoning outbreak (5). Until the past few years, such changes were thought to occur when mercury concentrations in hair were ≥50 ppm. More recently adverse effects on neuromotor function and visual contrast sensitivity have been reported among adults whose hair mercury concentrations were lower than 50 ppm (67–69). Saint-Phard et al. (37) identified paresthesias among adults whose methylmercury exposure produced blood mercury concentrations in the range of 34 to 97 μg/L, however, the diagnosis was based on far more sophisticated diagnostic methods than were available in Iraq in the 1960s. Cree subjects from Northern Quebec with chronic exposure to methylmercury have been found to have altered eye movements (pursuit, fixation, and dynamic saccades) and altered accuracy and sharpness of prompted saccades (68). Among adults living in fishing villages, hair mercury exposures over the range of 0.56 to 13.6 ppm (mean 4.2 ± 2.4 ppm) were associated with detectable alterations in performance on tests of fine motor speed and dexterity, and concentration (69). Verbal learning and memory were also disrupted by mercury exposures suggesting that adults exposed to methylmercury in this range may be at risk for deficit in neurocognitive function (69). These newer data suggest effects below that previously considered the threshold for clinical effects among adults.
Developmental Neurological Effects
Following the birth in Japan of severely damaged infants to mothers who themselves had minimal symptoms of methylmercury poisoning, the increased sensitivity of the fetus was recognized (25 ,70). The fetal nervous system is currently considered to be the organ system most vulnerable to the effects of methylmercury. Epidemiology data associated changes in children's blood pressure (72) with maternal hair mercury levels <10 ppm suggest health outcomes in addition to delays in neurological development may be used in setting standards aimed at protecting public health (such as EPA's Reference Dose). The developing fetal nervous system had been judged to be five to ten times more sensitive to methylmercury than the adult nervous system (25, 71). Government recommendations on methylmercury exposure (e.g., guidance on fish consumption, regulations regarding release of mercury into water, power plant mercury emissions) are based on protection of the fetal nervous system. Although the dose of methylmercury regarded as “safe” varies with specific recommendations, there is a consensus that at high exposures the developing nervous system can be disastrously damaged and that fetuses are more sensitive to methylmercury than adults. Currently it is thought that adverse effects can be identified in the child when the pregnant woman's exposures result in maternal hair concentrations between approximately 5 ppm for subtle developmental changes (72) to a range of 10 ppm to 20 ppm for clinically obvious changes such as delayed walking (25).
The basis for US EPA's recommendations on health consequences of exposure to methylmercury begins with the evaluation by the Committee on Toxicology of Methylmercury (73) of the National Research Council of the National Academy of Sciences and by the United States Environmental Protection Agency (74). In 2000, this NAS Committee recommended a benchmark dose level of 58 μg/L in cord blood based on adverse developmental effects in young children following in utero methylmercury exposure and an Uncertainty Factor (UF) of 10. The benchmark dose is the lower 95% confidence internal (CI) on an estimated dose that doubles the prevalence of children with scores on a test of intellectual development that would fall into the clinically subnormal range (73). The U.S. EPA subsequently adopted these recommendations and expanded consideration to multiple studies on the impact of in utero methylmercury exposure to multiple tests of children from the Faroese, New Zealand, and Seychelles cohorts (74). The NRC Committee recommended and the U.S. EPA adopted the use of a UF of 10 to calculate an RfD corresponding to a concentration of 5.8 μg/L Hg in cord blood. At the time of these recommendations, the UF of 10 had been based on the assumption of a 1:1 ratio of cord blood mercury to maternal blood mercury.
More recent evaluations (75), as well as additional data from subsequent studies of mother-newborn pairs (20, 76) indicate that cord blood is, on average approximately 70% higher in mercury concentration than is maternal blood. Assuming the ratio of 1.7:1.0 and calculating the average blood total mercury concentration that is associated with the benchmark dose lower limit and RfD (using the same UF of 10) suggests that a blood total mercury level ≥3.5 μg/L may be associated with increased risk to the developing fetal nervous system. The RfD established in 2000 was based on use of a UF of 10 and the assumption that the cord to maternal blood ratio was 1:1 (74, 77).
Recognizing that methylmercury is concentrated across the placenta suggests that mercury concentrations expressing risk to the fetus based on cord blood mercury cannot be directly applied maternal blood mercury concentrations. Expressing this as a simple ratio of 1.7:1.0 a BMDL based on cord blood of 58 μg/L is associated with a maternal blood mercury concentration of 35 μg/L. The pregnant subjects within the NHANES survey showed a 10% difference in hemoglobin and hematocrit compared with nonpregnant participants indicating that the ratio of cord blood to adult nonpregnant women's blood may be more likely to be 1.7:1.1 than 1.7:1.0 and associated with blood total mercury concentration ratio of 5.8 μg/L to 3.8 μg/L, rather than 5.8 μg/L to 3.5 μg/L. These blood mercury concentrations are associated with exposure to mercury at the RFD.
Estimating Newborn Mercury Exposures
Based on National Vital Statistics Reports (78), in 2000 the number of births in the U.S. population was just over four million (specifically, 4,058,814). Applying the overall population estimate for adult women of 7.8% (95% CI, 5.0–10.5) of women 16–49 years had blood total mercury at 5.8 μg/L or higher resulted in more than 300,000 newborns per year with in utero mercury exposures associated with increased risk of adverse neurodevelopmental effects. If the value 3.5 μg/L is used which reflects the adult women's blood mercury associated with exposure to methylmercury at or above the RfD of 0.1 μg/kg-bw/day, overall 15.7% of women had total blood mercury concentrations at or higher than this concentration. Using this estimate more than 600,000 newborns per year experience in utero mercury exposures associated with increased risk of adverse neurodevelopmental effects.
Subsequent years of NHANES data (2001 and 2002) have suggested somewhat lower blood mercury concentrations than were reported in 1999 and 2000 (79). As additional years of data are obtained there will be greater certainty in the national estimates. All in all these data indicate, nonetheless, that several hundred thousand infants are born each year with in utero exposures to methylmercury exceeding those considered to be free of risk from adverse neurodevelopmental effects.
As indicated above NHANES data did not identify groups with the highest mercury exposures within the United States based on either hair or blood mercury concentrations. Within the case series data from affluent populations in New York and San Francisco consuming fish frequently for “health” reasons, blood mercury concentrations in excess of the benchmark dose have been identified (35, 37). Dietary exposures to mercury at the BMDL, if expressed in terms of the adult woman's blood, are associated with a concentration of approximately 35 μg/L whole blood. Such mercury exposures have also been reported in island populations as shown by blood mercury and hair mercury levels [Puerto Rico (36); and Bermuda (80)]. Asian populations are also at increased risk because of higher than average fish and shellfish consumption (51). Within the NHANES data for 1999–2000 the group classified as “Other race/ethnicity group” which includes Asians, Native Americans/persons of Caribbean and Pacific Island ancestry rose to 31.5% compared with 15.7% having blood total mercury greater than 3.5 μg/L (8).
Non-Neurological Risks of Methylmercury Exposure for Adults
In addition to neurological damage produced by methylmercury exposure, other organ systems appear to be adversely affected by exposure to methylmercury: cardiac, endocrine, and immune. Cardiac changes identified initially in an longitudinal prospective epidemiological study among Eastern Finnish men include increases in carotid atherosclerosis, myocardial infarction, and death in men as a function of increased hair mercury (81–83). Similar findings have also been observed in a multi-center study carried out in Europe and Israel in which Guallar et al. (84) reported a significant association between mercury body burden and the risk of myocardial infarction in men after controlling for levels of fatty acids in fish. What initially appeared to be contrasting results were reported by Yoshizawa et al. (85) in a population of male health professionals that included a large portion of dentists who experienced exposure to inorganic mercury as well as to methylmercury. Initially adverse effects were not identified based on total mercury levels in this population, however, when the predominately methylmercury-exposed group was assessed separate from the dentists, a trend toward adverse effects of mercury on cardiovascular endpoints was identified. This trend was, however, nonsignificant because of the reduced number of subjects.
Cardiac effects in men and women (separate from the maternal-fetal pair) have not been included in risk assessments for methylmercury. This is an area of active interest, as are other organ systems including endocrine, immune, and reproductive effects. The recommendations that fish and shellfish be consumed two or more times per week has been related to the omega-3 fatty acid content of some species of fish (12). There is little connection between the concentration of mercury in a particular fish species and the level of omega-3 fatty acids in the fish species (12). For example, shark and swordfish frequently contain more than 1 ppm of methylmercury and are low in omega-3 fatty acids compared with other fish species. Fish species including herring, salmon, and trout, as well as shellfish such as shrimp, are comparatively low in mercury (i.e., ∼0.1 ppm or less) and far higher in omega-3 fatty acids than are shark and swordfish (12). Consequently careful choice of fish and shellfish species can provide fish and shellfish selections that are good sources of omega-3 fatty acids while limiting exposures to methylmercury.
Methylmercury exposure also adversely affects other organ systems. In the human mercury exposure is reported to adversely affect the endocrine system, interferes with reproduction, and alters the immune system.
Summary
Environmental fate and transport of mercury and its incorporation through the food web to bioconcentrate as methylmercury in fish and shellfish is well recognized and understood. Data from the National Health and Nutrition Examination Survey providing population estimates for the United States as a whole is altering the reference range for blood, hair, and urinary mercury concentrations. Estimates on the number of newborns at risk of in utero exposures to methylmercury above levels regarded as “safe” number in the several hundred thousand each year in the United States. Case reports and screening among highly exposed subpopulations have identified persons with exposures in a range associated with adverse clinical effects. The source of methylmercury producing these effects is consumption of fish and shellfish. Monitoring exposures through measurement of patients' blood, hair, and urinary mercury concentrations allows physicians to determine what steps need to be taken to reduce the patient's risk. The American Medical Association adopted a policy statement (9) providing guidance on this issue in 2004.
Fig. 1.
Organic/Methylmercury as a percent of total blood mercury vs. total blood mercury [from Mahaffey et al. (8)].
BENCHMARK DOSE LOWER LIMIT FOR METHYLMERCURY (BMDL) (8)
BMDL for methylmercury is the dose that doubles the prevalence of scores in the clinically subnormal range on standardized tests of neuropsychological functioning.
The BMDL is associated with blood mercury concentrations.
| • | BMDL [Hg] associated with 58 μg/L in cord blood. Approximately equal to 35 μg/L to 38 μg/L in maternal blood. |
| • | BMDL divided by Uncertainty Factor (UF) equals the Reference Dose (RfD). |
| • | UF of 10 deals with human variability in kinetics and tissue sensitivity to methylmercury, target organs other than the nervous system (e.g., cardiac, immune, endocrine). |
| • | RfD associated with hair mercury of 1 ppm and cord blood [Hg] of 5.8 μg/L. |
| • | Cord blood [Hg] is on average 70% higher than adult woman's blood [Hg]. Adult women's blood [Hg] of ∼3.5 μg/L. |
| • | RfD associated with maternal or adult women's blood [Hg] of ∼3.5 |
Footnotes
The views presented in this article are the professional views of Dr. Mahaffey and do not necessarily represent the policies of the United States Environmental Protection Agency.
DISCUSSION
Limacher, Gainesville: Thanks for very much for this information. I wonder why there's so much species variation in the content of mercury. If they're all fished from the same area, what determines the mercury content in the fish?
Mahaffey, Washington: Basically it's the eating habits of the fish and the concentration of mercury in the food the fish eats. There are piscivorous fish that consume other fish as part or all of their diet. Each time they consume another fish, all of the methylmercury that was present in the fish that the larger fish consumes is retained by the larger fish. This is referred to as bioaccumulation. Fish species that are very high in the food chain such as sharks, swordfish, tuna, the king mackerel are among the highest in methylmercury concentration. There are usually four or five layers in the food chain, so that all the mercury that was in the fish that came before them has been accumulated into these high pelagic fish.
Stevenson, Stanford: Kate, that was really wonderful presentation. You have reinforced the same effect as my daughter, a marine biologist, had on my eating behavior. I now eat less fish and more beef. However, this is very complex. I have one question that I want to ask you. There were questions before this about the differences in the fish and their eating habits. Why is it that we have an accumulation of mercury in the fetus? Do we understand why that happens?
Mahaffey: We actually think the reason for this is the mercury is bound to carrier proteins and transported on the amino acid carriers that transport the amino acids across the placenta to the fetus. For this reason, the blood reaching the fetus contains (at the mean) a 70% higher concentration of methylmercury than did the maternal blood. The 70% higher value is the mean of the distribution. The cord blood:maternal blood mercury concentration is approximately 300% higher (i.e., 3:1) at the 95th percentile and drops down to just under 1:1 at around the 5th percentile. This means that for about 5 in 100 newborns their in utero exposures to methylmercury are approximately three-times higher than that shown by their mother's blood mercury concentrations. Currently we have no way of knowing which women are the ones who will transport methylmercury across the placenta at this accelerated rate.
Stevenson: Can you speculate about how we might block that phenomenon once we identify a woman with such a problem?
Mahaffey: No, because the essential amino acids are needed. The real way to deal with this is environmental controls over the amount of mercury that reaches the fish, and the only way really to reduce this in the interim by selection of the kinds of fish people eat. There is a major effort in advisory work, but at the same time for populations who have a relatively limited choice in the kinds of fish that they have accessed to; these advisories are not particularly helpful solutions.
Billings, Baton Rouge: I read recently in an article by Murray Carpenter that loons have one the higher levels of mercury. I wonder if that has anything to do with “crazy as a loon,” as it used to have to do with “mad as a hatter.”
Mahaffey: I don't know the source of the “crazy as a loon” expression, but one of the things that we've come to realize; years ago when mercury was added as a fungicide to seed grains, when they would distribute this grain the birds would eat some of the grains and die. And when we were working on the United States Environmental Protection Agency's Mercury Study Report to Congress back in the mid-1990s, one of the questions became ‘why aren't we seeing dead birds?' If the fish are contaminated and the birds are eating the fish, then the question was ‘why aren't there more dead birds?' Well, the fish don't seem to get quite the high level that actually kills the birds, but at these lower levels because of endocrine effects, mercury impairs reproduction of the birds. More recently we are seeing data that show that mercury impairs reproduction of the fish. Taken together, these data show that there are substantial effects of mercury on those fish and birds and are these effects are only beginning to be described. So this is an area where we will see a lot more and as a group with Climatological interests certainly, I think this is one that will be of increasing interest.
REFERENCES
- 1.Mahaffey KR. Methylmercury: A new look at the risks. Public Health Repts. 1999;114:397–413. [PMC free article] [PubMed] [Google Scholar]
- 2.Mahaffey KR. “Exposures to Mercury in the Americas.”. In: Pirrone N, Mahaffey KR, editors. Dynamics of Mercury Pollution on Regional and Global Scales. Kluwer Springer Press; 2005. [Google Scholar]
- 3.Smith RG, Vorwald AJ, Patil LS, Mooney TF., Jr . Am Ind Hyg Assoc J. Vol. 31. 1970. Effects of exposure to mercury in the manufacture of chlorine; pp. 687–700. [DOI] [PubMed] [Google Scholar]
- 4.Tsubaki T, Irukyama K, editors. Minamata Disease. Amsterdam: Elsevier; 1977. [Google Scholar]
- 5.Bakir F, Damluji SF, Amin-Zaki L. Methylmercury poisoning in Iraq. Science. 1973;180:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 6.Solis MT, Yuen E, Cortez PS, Goebel PJ. Family poisoned by mercury vapor inhalation. Am J Emerg Med. 2000;5:599–602. doi: 10.1053/ajem.2000.4006. [DOI] [PubMed] [Google Scholar]
- 7.Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, Garrett ES, Canady RA, Dillon CF, Sun Y, Joseph CB, Mahaffey KR. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA. 2003;289:1667–1674. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- 8.Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Medical Association. [Accessed 2-6-2005]; < http://www.ama-assn.org/ama/pub/category/13619.html>.
- 10.US EPA. Mercury Study Report to Congress. Washington: EPA; 1997. Summary. Vol 1. In: Environmental Protection Agency (US) [Google Scholar]
- 11.Pirrone N. In: Dynamics of Mercury Pollution on Regional and Global Scales. Pirrone N, Mahaffey KR, editors. Kluwer Springer Press; 2005. [Google Scholar]
- 12.Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the omega-3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: risks and benefits. Environ Res. 2004;95:414–28. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Risher JF, Murray HE, Prince GR. Organic mercury compounds: human exposure and its relevance to public health. Toxicol Ind Health. 2002;18:109–60. doi: 10.1191/0748233702th138oa. [DOI] [PubMed] [Google Scholar]
- 14.Mason HJ, Hindell P, Williams NR. Biological monitoring and exposure to mercury. Occup Med (Lond) 2001;51:2–11. doi: 10.1093/occmed/51.1.2. [DOI] [PubMed] [Google Scholar]
- 15.Cherian MG, Hursh JR, Clarkson TW, Allen J. Radioactive mercury distribution in biological fluids and excretion in human subjects after inhalation of mercury vapor. Arch Environ Health. 1978;33:109–114. doi: 10.1080/00039896.1978.10667318. [DOI] [PubMed] [Google Scholar]
- 16.Barregard L, Hultberg B, Schutz A, Attewell R, Skerfving S, Jarvholm B. Kinetics of mercury in blood and urine after brief occupational exposure. Arch Environ Health. 1992;47:176–84. doi: 10.1080/00039896.1992.9938347. [DOI] [PubMed] [Google Scholar]
- 17.Zeitz P, Orr MF, Kaye WE. Public health consequences of mercury spills: Hazardous Substances Emergency Events Surveillance System, 1993–1998. Environ Health Perspect. 2002;110:129–132. doi: 10.1289/ehp.02110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry LK, Routree PP, Levin JL, Collins S, Anger WK. The Texarkana mercury incident. Tex Med. 1999;95:65–70. [PubMed] [Google Scholar]
- 19.World Health Organization. Environmental Health Criteria 100: Mercury. Geneva: WHO; 1991. [Google Scholar]
- 20.Morrisette J, Takser L, St Amour G, Smargiassi A, Lafond J, Mergler D. Temporal variation of blood and hair mercury levels in pregnancy in relation to fish consumption history in a population living along the St. Lawrence risk. Environ Res. 2004;95:363–374. doi: 10.1016/j.envres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Carta P, Flore C, Alinovi R, Ibba A, Tocco MG, Aru G, Carta R, Girei I, Mutti A, Lucchini R, Randaccio FS. Sub-clinical neurobehavioral abnormalities associated with low level of mercury exposure through fish consumption. Neurotoxicology. 2003;24:617–623. doi: 10.1016/S0161-813X(03)00080-9. [DOI] [PubMed] [Google Scholar]
- 22.Johnsson C, Sallsten G, Schutz A, Sjors A, Barregard L. Hair mercury levels versus freshwater fish consumption in house hold members of Swedish angling societies. Environ Res. 2004;96:257–63. doi: 10.1016/j.envres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Kingman A, Albertini T, Brown LJ. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. J Dent Res. 1998;77:461–471. doi: 10.1177/00220345980770030501. [DOI] [PubMed] [Google Scholar]
- 24.Swift JA. Morphology and histochemistry of human hair. EXC. 1997;78:49–175. doi: 10.1007/978-3-0348-9223-0_4. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Environmental Health Criteria 101: Methylmercury. Geneva: WHO; 1990. [Google Scholar]
- 26.Dolbec J, Mergler D, Souse Passos C-J, Sousa de Morais S, Lebel J. Methylmercury exposure affects motor performance of a riverine population of the Tapajos River, Brazilian Amazon. Int. Arch Occup Environ Health. 2000;73:195–203. doi: 10.1007/s004200050027. [DOI] [PubMed] [Google Scholar]
- 27.Veiga MM. UNIDO/UBC/CETEM. Introducing new technologies for abatement of global mercury pollution in Latin America. Ed. UNIDO/UBC/CETEM Rio de Janeiro. p. 94.
- 28.Seidel S, Kreutzer R, Smith D, McNeel S, Gilliss D. Assessment of commercial laboratories performing hair mineral analysis. JAMA. 2001;285:67–72. doi: 10.1001/jama.285.1.67. [DOI] [PubMed] [Google Scholar]
- 29.McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizari E, Fernando R, Montes de Oca R, Schober SE, Sinks T, Jones RL, Mahaffey KR. Hair mercury levels in US children and women of childbearing age. Reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burge P, Evans S. Mercury contamination in Arkansas gamefish. A public health perspective. J Ark Med Soc. 1994;90:542–544. [PubMed] [Google Scholar]
- 31.Knobeloch LM, Ziarnik M, Anderson HA, Dodson VN. Imported seabass as a source of mercury exposure: A Wisconsin case study. Environ Health Perspect. 1995;103:604–606. doi: 10.1289/ehp.95103604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellanger TM, Caesar EM, Trachtman L. Blood mercury levels and fish consumption in Louisiana. J La State Med Soc. 2000;152:64–73. [PubMed] [Google Scholar]
- 33.Stern AH, Gochfeld M, Weisel C, Burger J. Mercury and methylmercury exposure in the New Jersey pregnant population. Arch Environ Health. 2001;56:4–10. doi: 10.1080/00039890109604048. [DOI] [PubMed] [Google Scholar]
- 34.Kales SN, Goldman RH. Mercury exposure: Current concepts, controversies, and a clinic's experience. J Occup Environ Med. 2002;44:143–154. doi: 10.1097/00043764-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortiz-Roque C, Lopez-Rivera Y. Mercury contamination in reproductive age women in a Caribbean island: Vieques. J Epidemiol Community Health. 2004;58:756–757. doi: 10.1136/jech.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saint-Phard D, Gonzalez PG, Sherman P. Poster 88. Unsuspected mercury toxicity linked to neurologic symptoms: A case series. Arch Phys Med Rehabil. 2004;85:E25. [Google Scholar]
- 38.Riley DM, Newby CA, Leal-Almeraz TO, Thomas VM. Assessing elemental mercury vapor exposure from cultural and religious practices. Environ Health Perspect. 2001;109:779–784. doi: 10.1289/ehp.01109779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RH, Phillips RS. Heavy metal content of ayurvedic herbal medicine products. JAMA. 2004;292:2868–2873. doi: 10.1001/jama.292.23.2868. [DOI] [PubMed] [Google Scholar]
- 40.Garvey GJ, Hahn G, Lee RV, Harbison RD. Heavy metal hazards of Asian traditional remedies. Int J Environ Health Res. 2001;11:63–71. doi: 10.1080/09603120020019656. [DOI] [PubMed] [Google Scholar]
- 41.Balluz LS, Philen RM, Sewell CM, Voorhees RE, Falter KH, Paschal D. Mercury toxicity associated with a beauty lotion. New Mexico. Intern J Epidemiol. 1997;26:1131–1132. doi: 10.1093/ije/26.5.1131. [DOI] [PubMed] [Google Scholar]
- 42.Morgan JN, Berry MR, Jr, Graves RL. Effects of commonly used cooking practices on total mercury concentration in fish and their impacts on exposure assessments. J Expo Anal Environ Epidemiol. 1997;7:119–133. [PubMed] [Google Scholar]
- 43.Larsen EH, Andersen NL, Moller A, Petersen A, Mortensen GH, Petersen J. Monitoring the content and intake of trace elements from food in Denmark. Food Addit Contam. 2002;19:33–46. doi: 10.1080/02652030110087447. [DOI] [PubMed] [Google Scholar]
- 44.Ysert G, Miller P, Croasdate M, Crews H, Robb P, Baxter M. 1997. UK Total diet Study—dietary exposures to aluminum, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin, and zinc. Food Addit. Contam. 2000;17:775–786. doi: 10.1080/026520300415327. [DOI] [PubMed] [Google Scholar]
- 45.Storelli MM, Stuffler RG, Marcotrigiano GO. Total and methylmercury residues in tuna fish from the Mediterranean sea. Food Addit Contam. 2002;19:715–720. doi: 10.1080/02652030210153569. [DOI] [PubMed] [Google Scholar]
- 46.Schultz CD, Crer D, Pearson JE, Rivera JE, Hylin JW. Total and organic mercury in the Pacific blue marlin. Bull Environ Contam Toxicol. 1976;15:230–234. doi: 10.1007/BF01685166. [DOI] [PubMed] [Google Scholar]
- 47.Penedo de Pinho A, Daves Guinaraes, Martins AS, Costa RA, Olavo G, Valentin J. Total mercury in muscle tissue of five shark species from Brazilian off-shore waters: effects of feeding habit, sex, and length. Environ. Res. 2002;89:250–58. doi: 10.1006/enrs.2002.4365. [DOI] [PubMed] [Google Scholar]
- 48.Gilmour CC, Riedel GS. A survey of size-specific mercury concentrations in game fish from Maryland fresh and estaurine waters. Arch. Environ Contam Toxicol. 2000;39:53–59. doi: 10.1007/s002440010079. [DOI] [PubMed] [Google Scholar]
- 49.Jewett SC, Zhang X, Naidu AS, Kelley JJ, Dasher D, Duffy LK. Comparison of mercury and methylmercury in northern pike and arctic grayling from western Alaska rivers. Chemosphere. 2003;50:383–92. doi: 10.1016/s0045-6535(02)00421-6. [DOI] [PubMed] [Google Scholar]
- 50.Denger R, Adams C, Moss S, Mack S. Per capita fish and shellfish consumption in Florida. Gainesville, FL: Florida Agriculture Market Research Center. University of Florida; 1994. [Google Scholar]
- 51.Sechena R, Liao S, Lorenzana R, Nakano C, Polissar N, Fenske R. Asian American and Pacific Islander seafood consumption—a community based study in King County, Washington. J Expo Anal Environ Epidemiol. 2003;13:256–266. doi: 10.1038/sj.jea.7500274. [DOI] [PubMed] [Google Scholar]
- 52.Henderson B. Dental amalgam: scientific consensus and CDA policy. J Can Dent Assoc. 1995;61:429–31. [PubMed] [Google Scholar]
- 53.Weiner JA, Nylander M. The relationship between mercury concentration in human organs and different predictor variables. Sci Total Environ. 1993;138:101–115. doi: 10.1016/0048-9697(93)90408-x. [DOI] [PubMed] [Google Scholar]
- 54.Barregard L, Sallsten G, Jarholm B. People with high mercury intake from their own dental amalgam fillings. Occup Med. 1995;52:123–128. doi: 10.1136/oem.52.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francis PC, Birge WJ, Roberts BL, Black JA. Mercury content of human hair: a survey of dental personnel. J Toxicol Environ Health. 1982;10:667–72. doi: 10.1080/15287398209530285. [DOI] [PubMed] [Google Scholar]
- 56.Litovitz TL, Smilkstein M, Felberg L, Klein-Felberg L, Klein-Schwartz W, Berlin R, Morgan JL. 1996. Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerge Med. 1997;15:447–500. doi: 10.1016/s0735-6757(97)90193-5. [DOI] [PubMed] [Google Scholar]
- 57.Geffner ME, Sandler A. Oral metallic mercury. A folk medicine remedy for gastroenteritis. Clin Pediatr (Phila) 1980;19:435–437. doi: 10.1177/000992288001900611. [DOI] [PubMed] [Google Scholar]
- 58.McKinney PE. Elemental mercury in the appendix: an unusual complication of Mexican-American folk remedy. J Toxicol Clin Toxicol. 1999;37:103–7. doi: 10.1081/clt-100102415. [DOI] [PubMed] [Google Scholar]
- 59.Forman J, Moline J, Cernichiari E, Sayegh S, Torres JC, Landrigan MM, Hudson J, Adel HN, Landrigan PJ. A cluster of pediatric metallic mercury exposure cases treated with meso-2,3-dimercaptosuccinic acid (DMSA) Environ Health Perspect. 2000;108:575–7. doi: 10.1289/ehp.00108575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zagas LH, Ozuah O. Mercury use in Espiritismo: a survey of botanicas. Am J Public Hlth. 1996;86:111–112. doi: 10.2105/ajph.86.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.US EPA. Task Force on Ritualistic Use of Mercury Report. Mercury is used to attract luck, love, or money. It is also used to protect against evil. 2002 URL: < http://www.epa.gov/superfund/action/community/mercury.pdf>. [Google Scholar]
- 62.Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, Ballus LS, Dutton RJ. Mercury poisoning associated with a Mexican beauty cream. West J Med. 2000;173:15–18. doi: 10.1136/ewjm.173.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.CDC. Centers for Disease Control. Mercury poisoning associated with beauty cream—Texas, New Mexico, and California, 1995–1996. Morb Mortal Wkly Rep. 1996;45:400. [PubMed] [Google Scholar]
- 64.Campbell D, Gonzales M, Sullivan JM. “Mercury”. In: Sullivan JB, Rigger G, editors. Hazardous Materials Toxicology. Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins; 1992. [Google Scholar]
- 65.Midthun K. Thimerosal as a preservative in vaccines: an FDA perspective. Presented 2004 in “Mercury: Medical and Public Health Issues.” Tampa, Florida. April 25–28, 2004.
- 66.CDC. Centers for Disease Control. Thimerosal in vaccines: a joint statement of the American Academy of Pediatrics and the Public Health Service. MMWR. Morb Mortal Wkly Rep. 1999;48:563–565. [PubMed] [Google Scholar]
- 67.Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, Dolbec J. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- 68.Beuter A, Edwards R. Effect of chronic exposure to methylmercury on eye movements in Cree subjects. Int Arch Occup Environ Health. 2004;77:97–107. doi: 10.1007/s00420-003-0480-3. [DOI] [PubMed] [Google Scholar]
- 69.Yokoo EM, Valente JG, Grattan L, Schmidt SL, Platt I, Silbergeld EK. Low level methylmercury exposure affects neuropsychological function in adults. Environ Health. 2003;2:8. doi: 10.1186/1476-069X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harada Y. “Congenital Minamata Disease”. In: Tsubaki T, Irukyama K, editors. Minamata Disease. Amsterdam: Elsevier; 1977. pp. 209–239. [Google Scholar]
- 71.Kommission “Human-Biomonitoring” des Umwelbundesamtes. Stoffmonographic quecksilber-referenz-und human-biomonitoring-werte graphie. Quecksilber-referenz-und-human biomonitoring Werte Berlin: Kommission “Human-Biomonitoring-Werte. Berlin: Kommission” Human-Biomonitoring des umweltbundesamtes; 1999. [Google Scholar]
- 72.Sorensen N, Murata K, Budtz-Jorgensson E, Weihe P, Grandjean P. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiol. 1999;10:370–375. [PubMed] [Google Scholar]
- 73.National Research Council/National Academy of Sciences. Committee on Toxicology of Methylmercury. Toxicology of Methylmercury. Washington DC: National Academy of Sciences Press; 2000. [Google Scholar]
- 74.Rice DC, Schoeny R, Mahaffey KR. Methods and rationale for derivation of a reference dose for methylmercury by U.S. EPA. Risk Anal. 2003;23:107–115. doi: 10.1111/1539-6924.00294. [DOI] [PubMed] [Google Scholar]
- 75.Stern AH, Smith AE. An assessment of the cord blood-maternal blood methylmercury ratio: implication for risk assessment. Environ. Health Perspect. 2003;111:1465–1470. doi: 10.1289/ehp.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakamoto M, Kubota M, Liu XJ, Murata K, Nakai K, Satoh H. Maternal and fetal mercury and omega-3 polyunsaturated fatty acids as a risk and benefit of fish consumption to the fetus. Environ Sci Technol. 2004;38:3860–63. doi: 10.1021/es034983m. [DOI] [PubMed] [Google Scholar]
- 77.Rice DC. The US EPA reference dose for methylmercury: sources of uncertainty. Environ Res. 2004;95:406–413. doi: 10.1016/j.envres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 78.Ventura SJ, Hamilton BE, Sutton PD. Revised Birth and Fertility Rates for the United States, 2000 and 2001. [accessed 4 November 2003];National Vital Statistics Reports 51(4) Available: http://www.cdc.gov/nchs/data/nvs/nvs51_04.pdf. [PubMed]
- 79.CDC. Centers for Disease Control and Prevention. Blood mercury levels in young children and childbearing-aged women—United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2004;53:1018–1020. [PubMed] [Google Scholar]
- 80.Anonymous. The Royal Gazette. Hamilton. Bermuda: [Accessed 11/18/2004]. Study: High mercury levels in babies. < www.theroyalgazette.com>. [Google Scholar]
- 81.Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid perioxidation and the risk of myocardial infarction and coronary, cardiovascular and any death in Eastern Finnish men. Circulation. 1995;91:645–655. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- 82.Salonen JT, Seppanen K, Lakka TA, Salonen R, Kaplan GA. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population based prospective 4-year follow-up study in men in eastern Finish. Atherosclerosis. 148:265–273. doi: 10.1016/s0021-9150(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 83.Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Toumainen TP, Korhonen MJ, Valkonen VP, Seppanen K, Laukkanen JA, Salonen JT. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005;25:228–33. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- 84.Guallar E, Sanz-Gallardo MI, van't Veer P, Bode P, Aro A, Gomez-Aracena J, Kark JD, Riemersa RA, Martin-Morento JM, Kok FJ. Mercury, fish, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 85.Yoshizawa K, Rimm EB, Morris SS, Spate VL, Hsiah CC, Spiegelman D, Stampfer MJ, Willett WC. Mercury and the risk of coronary heart disease in men. N Engl J Med. 347:1735–1736. doi: 10.1056/NEJMoa021437. [DOI] [PubMed] [Google Scholar]