Abstract
Direct calorimetric determinations of the rate of heat production along with simultaneous determinations of the rate of photon emission and the number of viable cells have provided insight into the growth of Beneckea harveyi and Photobacterium leiognathi. These experiments were performed with a Tronac isothermal microcalorimeter modified with a fiber optic light guide to allow in situ detection of light. Escherichia coli and a dark variant of P. leiognathi were also examined to provide points of reference. It is demonstrated that B. harveyi seems to pause in the rate of metabolic heat production at the same point in time that the enzyme luciferase begins to be synthesized. This effect is not removed if B. harveyi is grown in conditioned medium. The thermograms for all species are correlated with cell generation time. The heat production per cell indicates that uncrowded cultures produce more heat than older, more crowded cultures, supporting the original observation of Bayne-Jones and Rhees (1929). These observations reopen for examination the suggestion that living systems tend toward a state of minimum metabolism per unit mass.
Full text
PDF

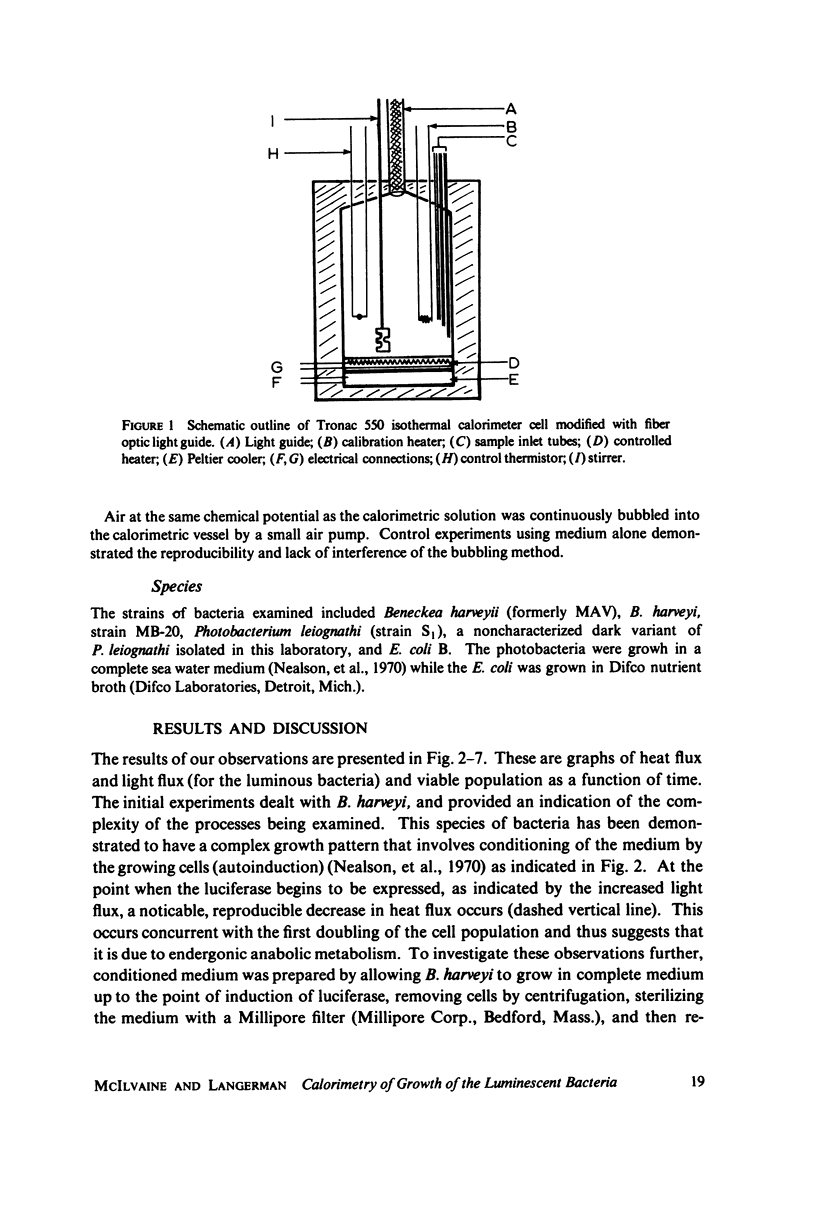
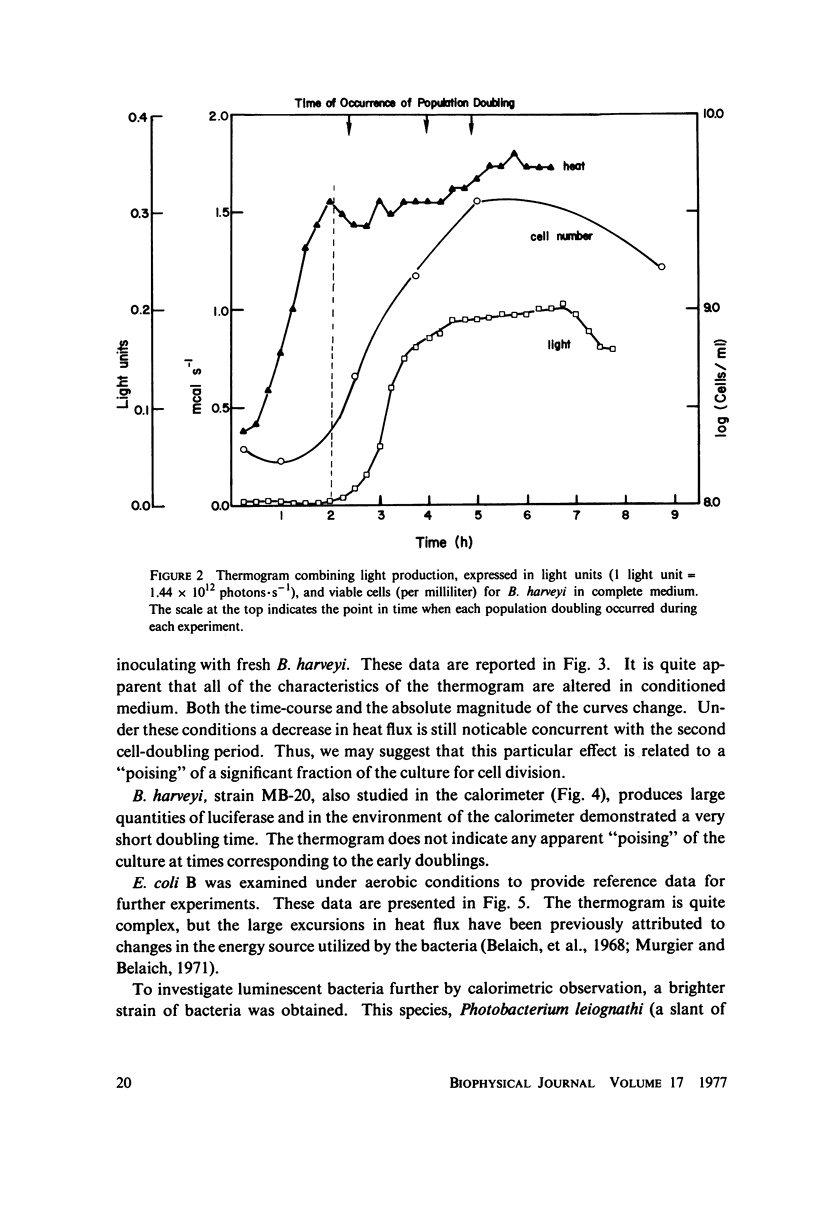
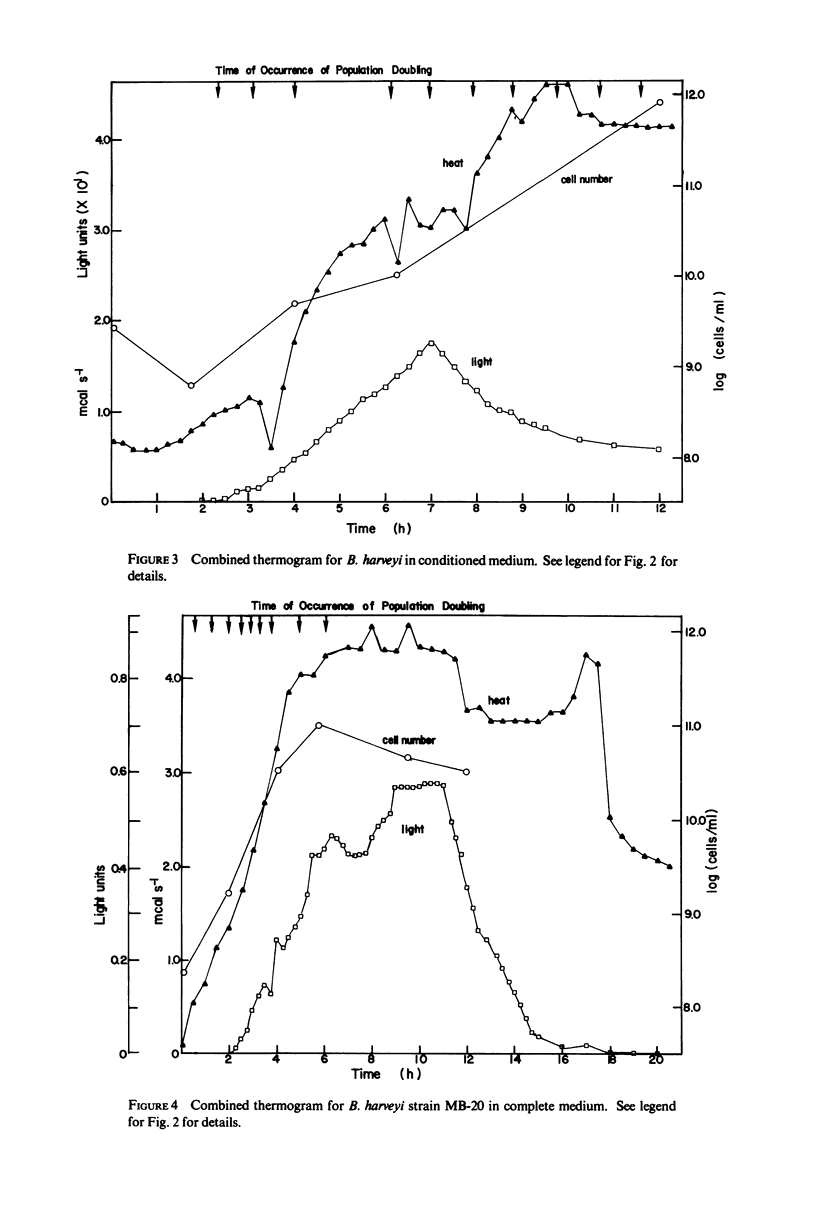
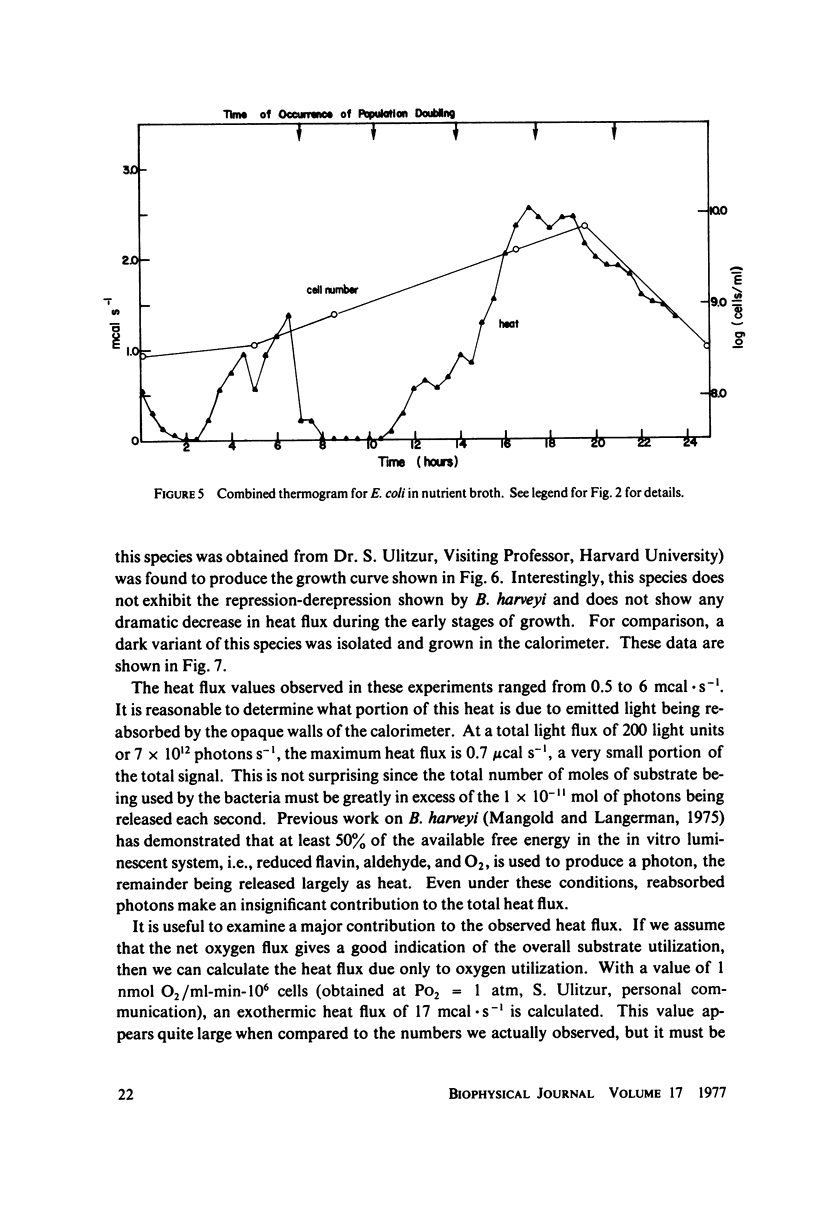


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayne-Jones S., Rhees H. S. BACTERIAL CALORIMETRY II. RELATIONSHIP OF HEAT PRODUCTION TO PHASES OF GROWTH OF BACTERIA. J Bacteriol. 1929 Feb;17(2):123–140. doi: 10.1128/jb.17.2.123-140.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaich A., Belaich J. P. Microcalorimetric study of the anaerobic growth of Escherichia coli: growth thermograms in a synthetic medium. J Bacteriol. 1976 Jan;125(1):14–18. doi: 10.1128/jb.125.1.14-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaich A., Belaich J. P. Microcalorimetric study of the anaerobic growth of Escherichia coli: measurements of the affinity of whole cells for various energy substrates. J Bacteriol. 1976 Jan;125(1):19–24. doi: 10.1128/jb.125.1.19-24.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaich J. P., Senez J. C., Murgier M. Microcalorimetric study of glucose permeation in microbial cells. J Bacteriol. 1968 May;95(5):1750–1757. doi: 10.1128/jb.95.5.1750-1757.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Bacterial bioluminescence in vivo: control and synthesis of aldehyde factor in temperature-conditional luminescence mutants. J Bacteriol. 1974 Jun;118(3):1059–1066. doi: 10.1128/jb.118.3.1059-1066.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Mutated luciferases with altered bioluminescence emission spectra. J Biol Chem. 1974 Jul 25;249(14):4668–4669. [PubMed] [Google Scholar]
- Cline T., Hastings J. W. Temperature-sensitive mutants of bioluminescent bacteria. Proc Natl Acad Sci U S A. 1971 Feb;68(2):500–504. doi: 10.1073/pnas.68.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Hastings J. W. A postulated mechanism for the bioluminescent oxidation of reduced flavin mononucleotide. Biochem Biophys Res Commun. 1972 Apr 28;47(2):348–353. doi: 10.1016/0006-291x(72)90719-x. [DOI] [PubMed] [Google Scholar]
- FORREST W. W., WALKER D. J., HOPGOOD M. F. Enthalpy changes associated with the lactic fermentation of glucose. J Bacteriol. 1961 Nov;82:685–690. doi: 10.1128/jb.82.5.685-690.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold A., Langerman N. The enthalpy of oxidation of flavin mononucleotide. Temperature dependence of in vitro bacterial luciferase bioluminescence. Arch Biochem Biophys. 1975 Jul;169(1):126–133. doi: 10.1016/0003-9861(75)90324-0. [DOI] [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
- Murgier M., Belaich J. P. Microcalorimetric determination of the affinity of Saccharomyces cerevisiae for some carbohydrate growth substrates. J Bacteriol. 1971 Feb;105(2):573–579. doi: 10.1128/jb.105.2.573-579.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Markovitz A. Mutant analysis and enzyme subunit complementation in bacterial bioluminescence in Photobacterium fischeri. J Bacteriol. 1970 Oct;104(1):300–312. doi: 10.1128/jb.104.1.300-312.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli M. Z., Hastings J. W. Bacterial luciferase. The hydrophobic environment of the reactive sulfhydryl. J Biol Chem. 1974 Apr 25;249(8):2393–2396. [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Microcalorimetric measurements of heat evolution and their correlation with oxygen uptake in Escherichia coli with genotypically- and phenotypically-modified electron transport chains. FEBS Lett. 1975 Oct 15;58(1):248–253. doi: 10.1016/0014-5793(75)80271-7. [DOI] [PubMed] [Google Scholar]
- STOWARD P. J. Thermodynamics of biological growth. Nature. 1962 Jun 9;194:977–978. doi: 10.1038/194977a0. [DOI] [PubMed] [Google Scholar]
- Ulitzur S., Yashphe J. An adenosine 3',5'-monophosphate-requiring mutant of the luminous bacteria Beneckea harveyi. Biochim Biophys Acta. 1975 Oct 9;404(2):321–328. doi: 10.1016/0304-4165(75)90339-6. [DOI] [PubMed] [Google Scholar]


