Abstract
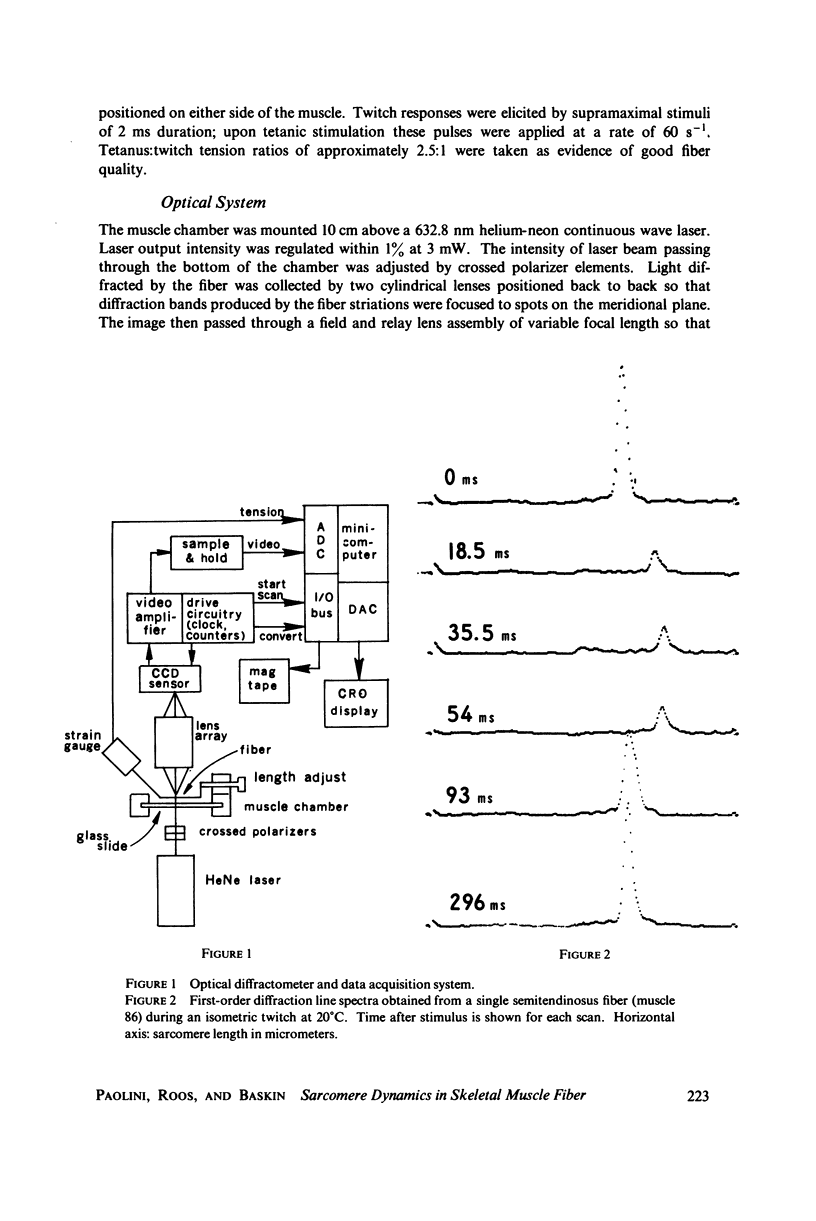
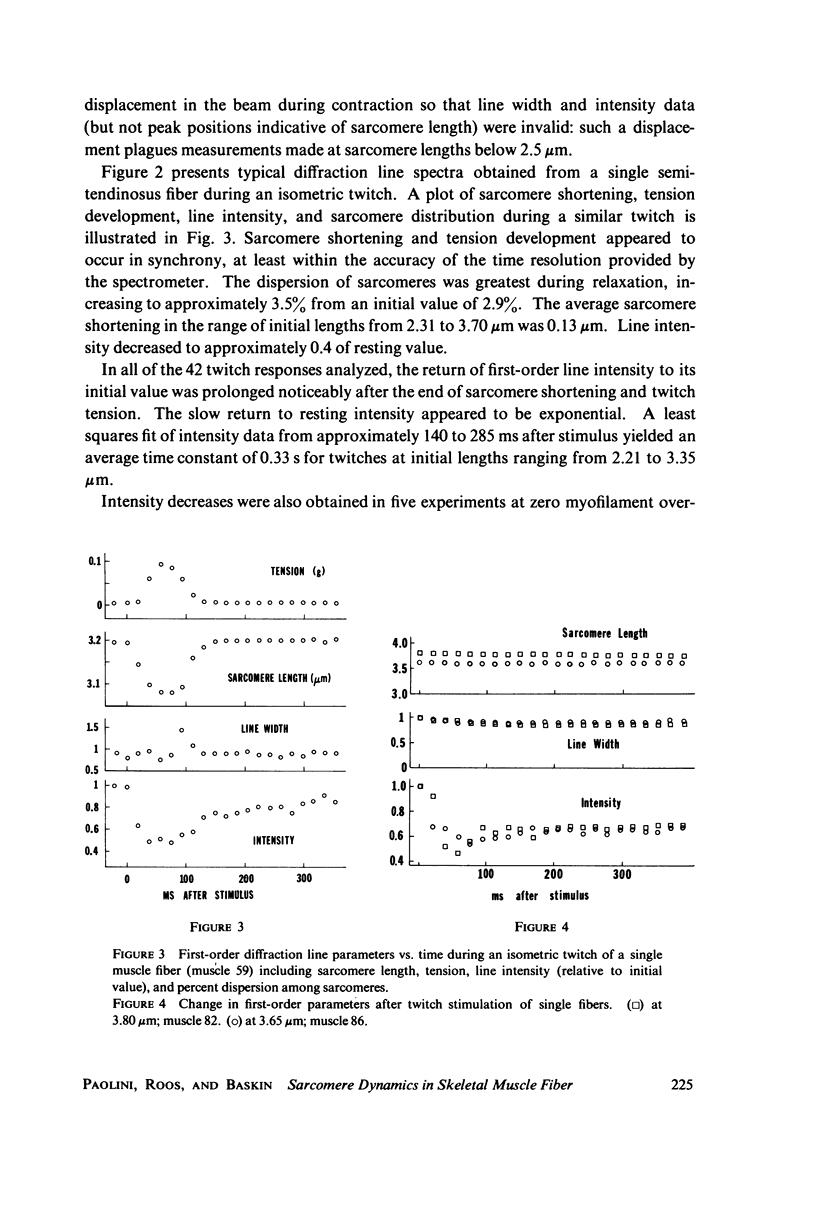
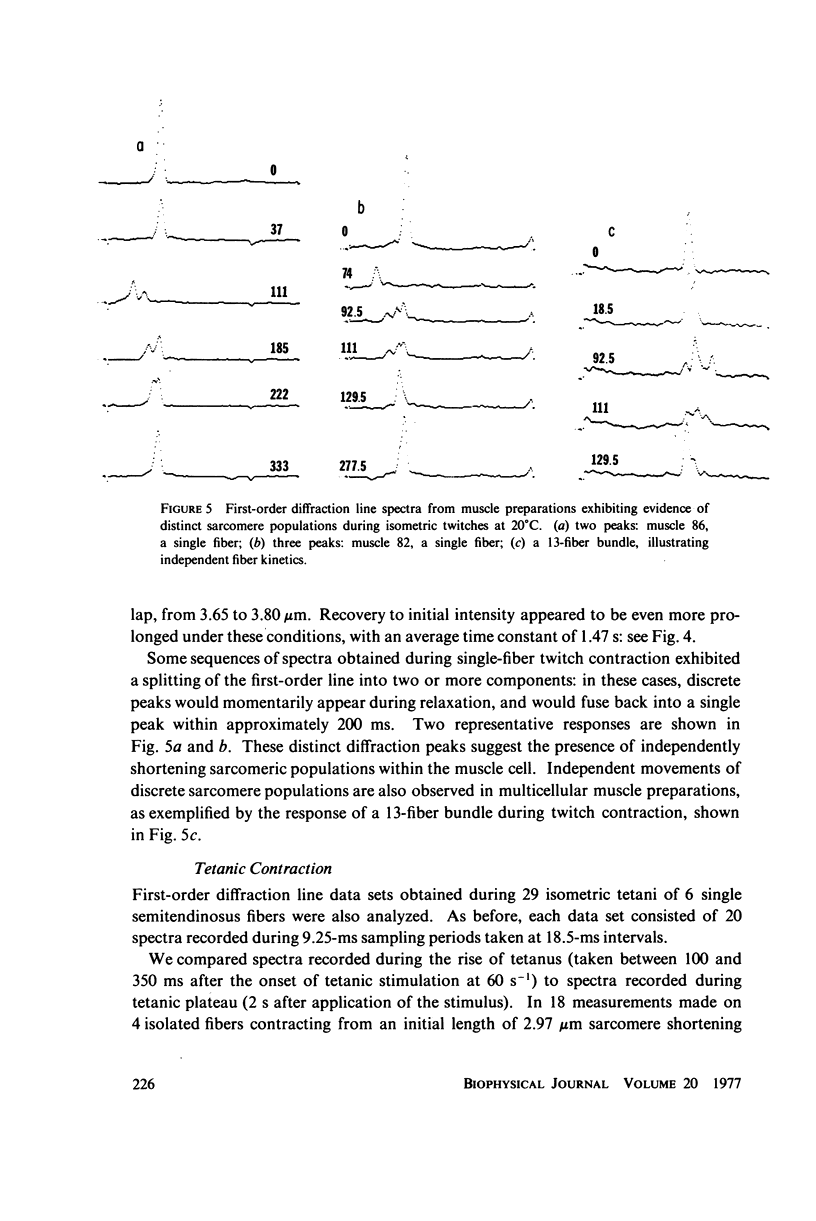
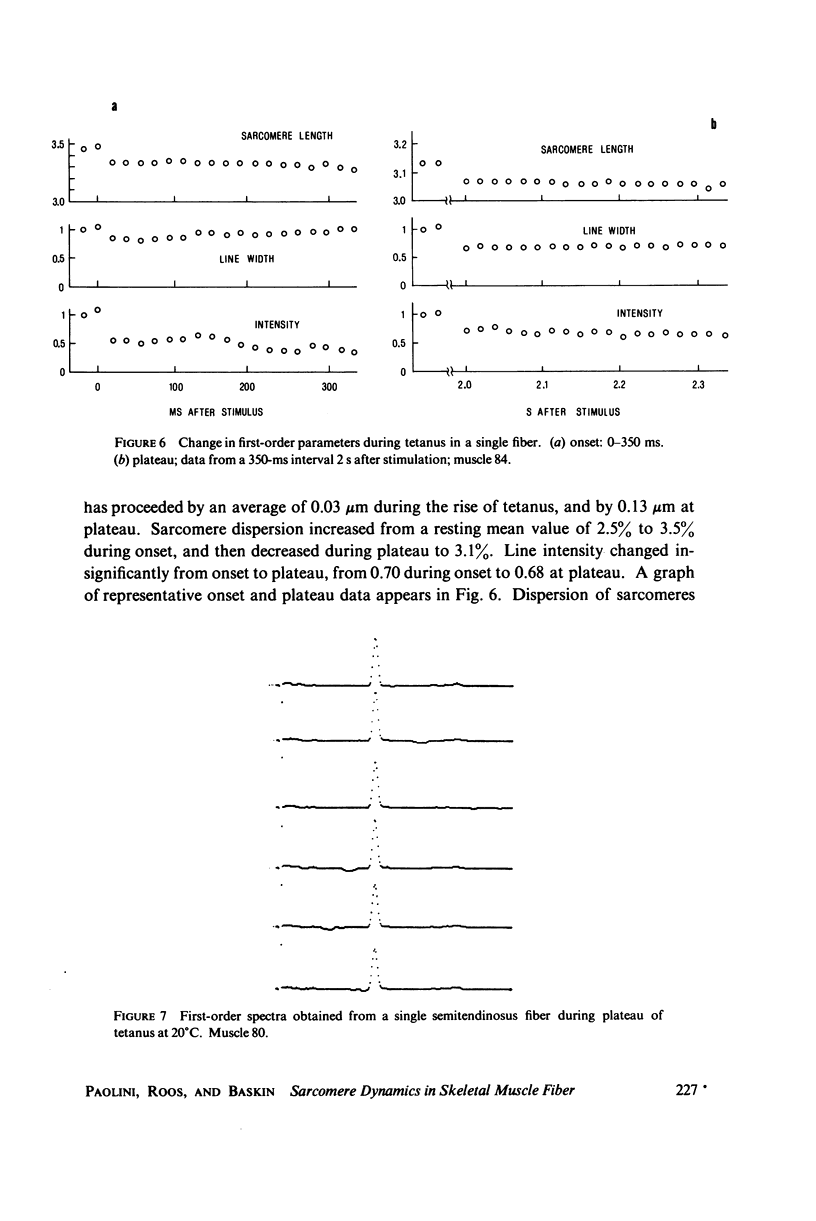
A position-sensitive optical diffractometer has been used to examine the diffraction spectra produced by single skeletal muscle fibers during twitch and tetanic contraction. First-order diffraction lines were computer-analyzed for mean sarcomere length, line intensity, and percent dispersion in sarcomere length. Line intensity was observed to decrease rapidly by about 60 percent during a twitch, with an exponential recovery to resting intensity persisting well beyond cessation of sarcomere shortening; recovery was particularly prolonged at zero myofilament overlap. A number of single fibers at initial lengths from 2.5 to 3.5 MICRON EXHIBITED a splitting of the first-order line into two or more components during relaxation, with components merging back into a single peak by 200 ms after stimulation. This splitting reflects the asynchronous nature of myofibrillar relaxation within a single fiber. During tetanus, the dispersion decreased by more than 10 percent from onset to plateau, implying a gradual stabilization of sarcomeres.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry W. H., Carnay L. D. Changes in light scattered by striated muscle during excitation-contraction coupling. Am J Physiol. 1969 Nov;217(5):1425–1430. doi: 10.1152/ajplegacy.1969.217.5.1425. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J., Mason P. Sarcomere length changes during stimulation of frog semitendinosus muscle. J Mechanochem Cell Motil. 1976 Mar;3(3):155–161. [PubMed] [Google Scholar]
- Cleworth D., Edman K. A. Laser diffraction studies on single skeletal muscle fibers. Science. 1969 Jan 17;163(3864):296–298. doi: 10.1126/science.163.3864.296. [DOI] [PubMed] [Google Scholar]
- DELEZE J. B. The mechanical properties of the semitendinosus muscle at lengths greater than its length in the body. J Physiol. 1961 Sep;158:154–164. [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Fujime S. Optical diffraction study of muscle fibers. Biochim Biophys Acta. 1975 Jan 30;379(1):227–238. doi: 10.1016/0005-2795(75)90026-4. [DOI] [PubMed] [Google Scholar]
- HILL A. V. The mechanics of active muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):104–117. doi: 10.1098/rspb.1953.0027. [DOI] [PubMed] [Google Scholar]
- HILL D. K. The effect of stimulation on the diffraction of light by striated muscle. J Physiol. 1953 Mar;119(4):501–512. doi: 10.1113/jphysiol.1953.sp004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., GORDON A. M. Striation patterns in active and passive shortening of muscle. Nature. 1962 Jan 20;193:280–281. doi: 10.1038/193280b0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen P., Sten-Knudsen O. Sarcomere lengthening and tension drop in the latent period of isolated frog skeletal muscle fibers. J Gen Physiol. 1976 Sep;68(3):247–265. doi: 10.1085/jgp.68.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. Changes in transparency of muscle during a twitch. J Physiol. 1949 May 15;108(3):292–302. [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Kuntz I. D. Optical diffraction studies of muscle fibers. Biophys J. 1973 Sep;13(9):857–876. doi: 10.1016/S0006-3495(73)86031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. W., Pollack G. H. Myocardial sarcomere dynamics during isometric contraction. J Physiol. 1975 Oct;251(3):627–643. doi: 10.1113/jphysiol.1975.sp011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini P. J., Baskin R. J., Roos K. P., Cline J. W. Dual-channel diffractometer utilizing linear image sensor charge-coupled devices. Rev Sci Instrum. 1976 Jun;47(6):698–702. doi: 10.1063/1.1134711. [DOI] [PubMed] [Google Scholar]
- Paolini P. J., Roos K. P. Length-dependent optical diffraction pattern changes in frog sartorius muscle. Physiol Chem Phys. 1975;7(3):235–254. [PubMed] [Google Scholar]
- Paolini P. J., Sabbadini R., Roos K. P., Baskin R. J. Sarcomere length dispersion in single skeletal muscle fibers and fiber bundles. Biophys J. 1976 Aug;16(8):919–930. doi: 10.1016/S0006-3495(76)85742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. H., Krueger J. W. Sarcomere dynamics in intact cardiac muscle. Eur J Cardiol. 1976 May;4 (Suppl):53–65. [PubMed] [Google Scholar]
- Rüdel R., Taylor S. R. The influence of stimulus parameters on contractions of isolated frog muscle fibres. J Physiol. 1969 Nov;205(2):499–513. doi: 10.1113/jphysiol.1969.sp008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W. Chemistry of muscle contraction. Annu Rev Biochem. 1972;41(10):577–616. doi: 10.1146/annurev.bi.41.070172.003045. [DOI] [PubMed] [Google Scholar]


