Abstract
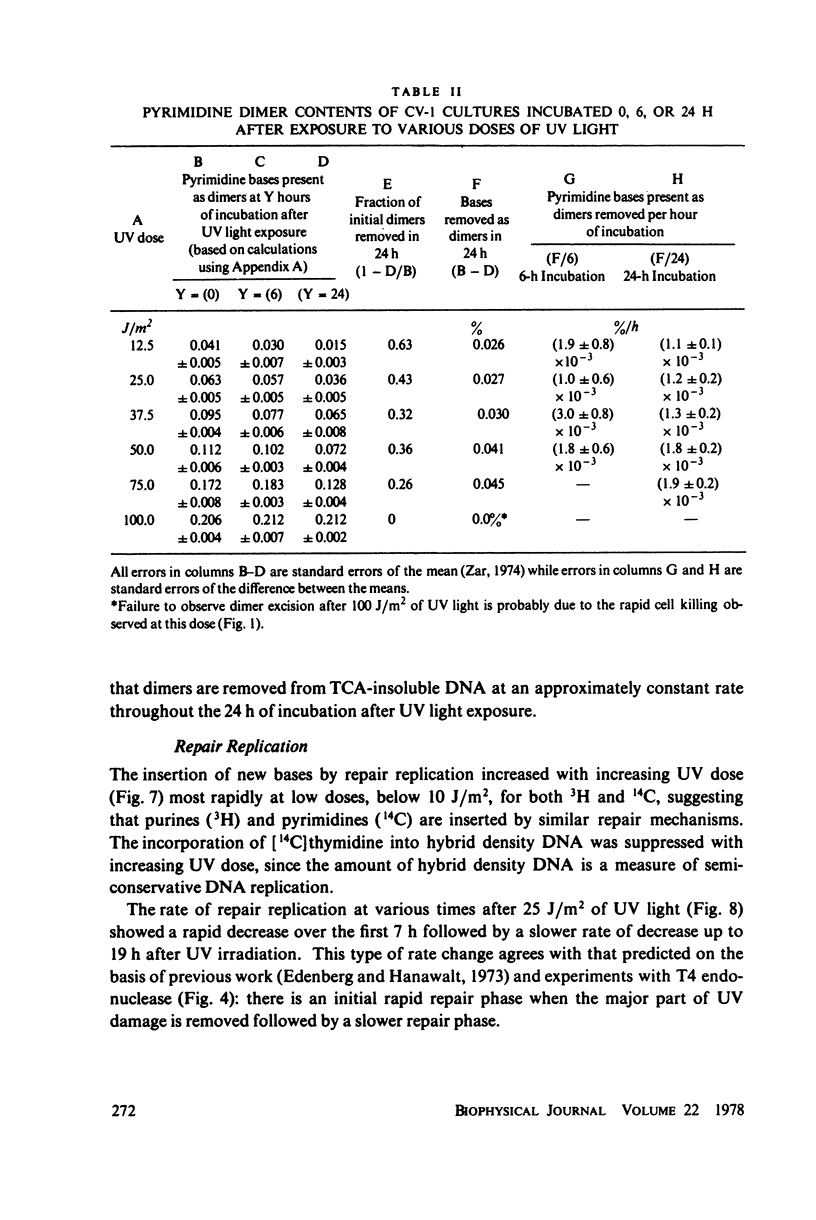
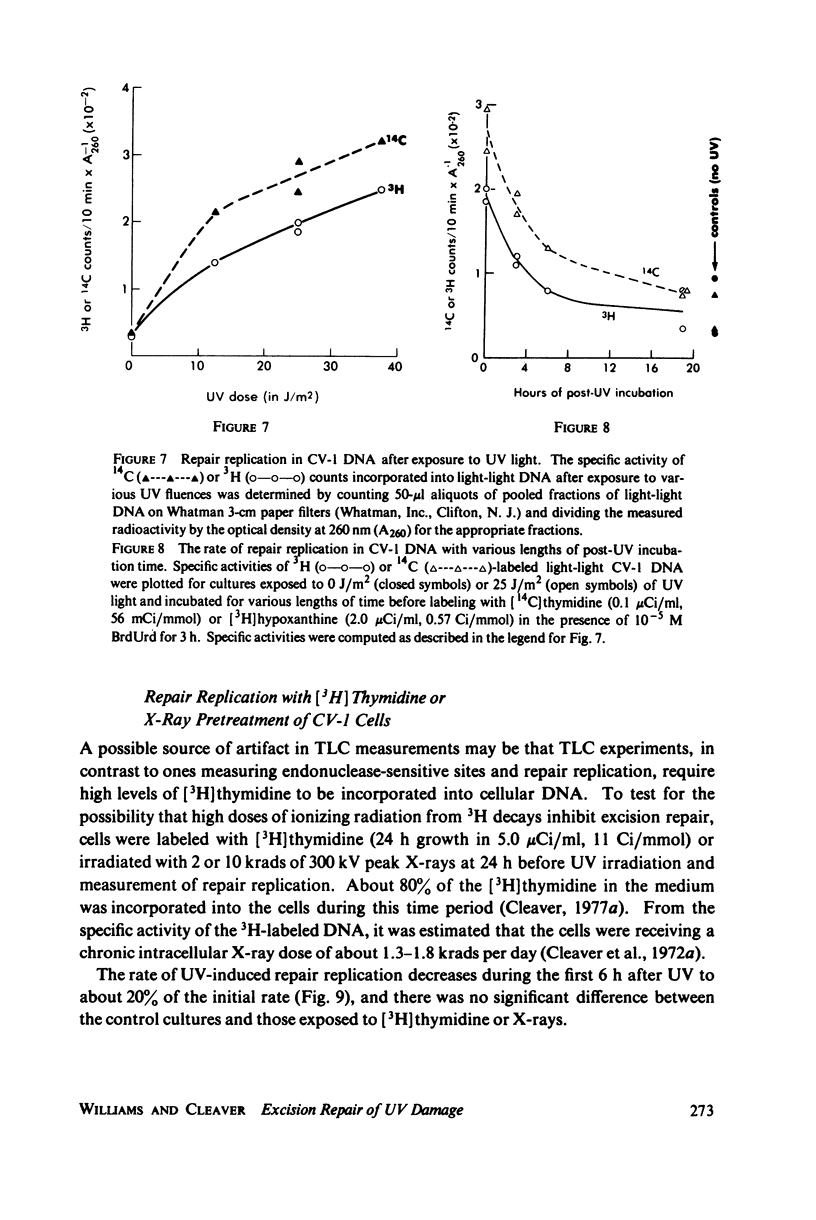
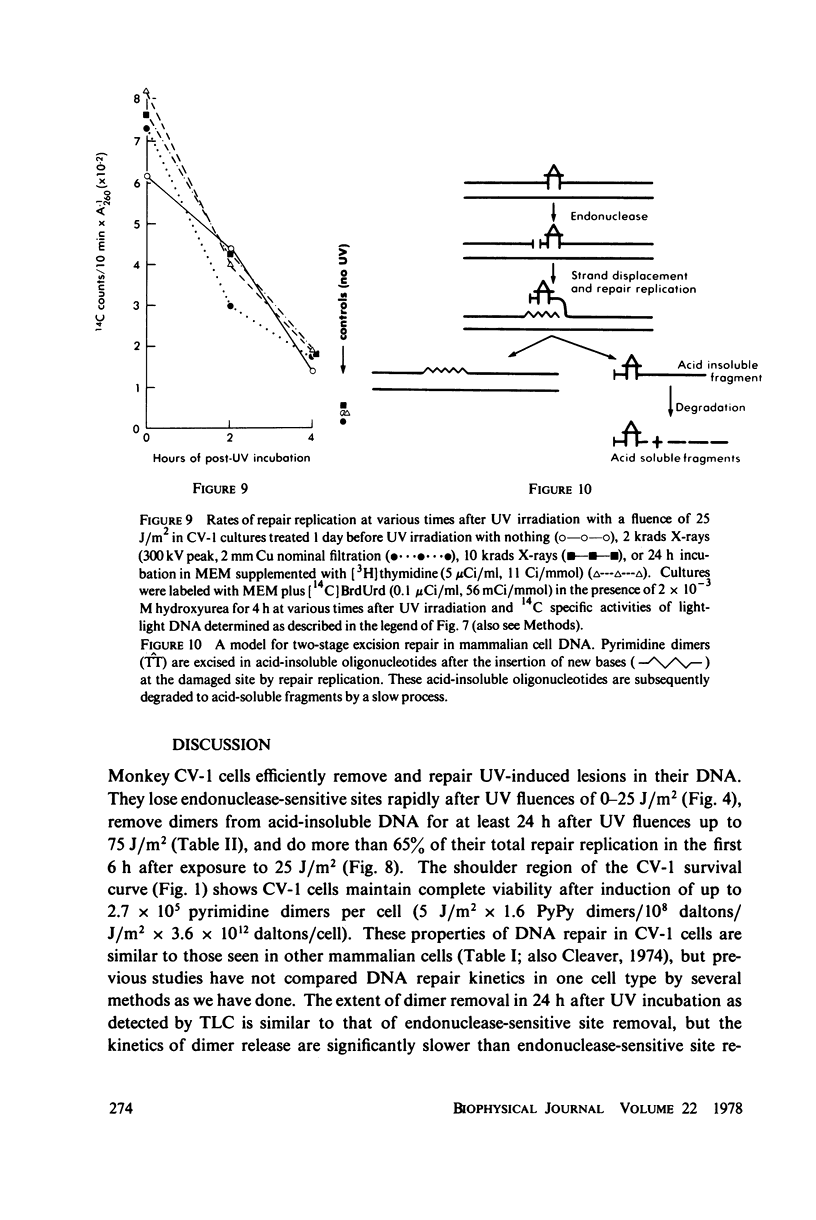
The incidence of pyrimidine dimer formation and the kinetics of DNA repair in African green monkey kidney CV-1 cells after ultraviolet (UV) irradiation were studied by measuring survival, T4 endonuclease V-sensitive sites, the fraction of pyrimidine dimers in acid-insoluble DNA as determined by thin layer chromatography (TLC), and repair replication. CV-1 cells exhibit a survival curve with extrapolation number n = 7.8 and Do = 2.5 J/m2. Pyrimidine dimers were lost from acid-insoluble DNA more slowly than endonuclease-sensitive sites were lost from or new bases were incorporated into high molecular weight DNA during the course of repair. Growth of CV-1 cultures in [3H]thymidine or X-irradiation (2 or 10 krads) 24 h before UV irradiation had no effect on repair replication induced by 25 J/m2 of UV. These results suggest that pyrimidine dimer excision measurements by TLC are probably unaffected by radiation from high levels of incorporated radionuclides. The endonuclease-sensitive site and TLC measurements can be reconciled by the assumption that pyrimidine dimers are excised from high molecular weight DNA in acid-insoluble oligonucleotides that are slowly degraded to acid-soluble fragments.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Lytle C. D. Decreased host cell reactivation of irradiated SV40 virus in xeroderma pigmentosum. Nature. 1970 Oct 24;228(5269):359–361. doi: 10.1038/228359a0. [DOI] [PubMed] [Google Scholar]
- Abrahams P. J., Van der Eb A. J. Host-cell reactivation of ultraviolet-irradiated SV40 DNA in five complementation groups of xeroderma pigmentosum. Mutat Res. 1976 Apr;35(1):13–22. doi: 10.1016/0027-5107(76)90164-0. [DOI] [PubMed] [Google Scholar]
- Abrahamson S., Bender M. A., Conger A. D., Wolff S. Uniformity of radiation-induced mutation rates among different species. Nature. 1973 Oct 26;245(5426):460–462. doi: 10.1038/245460a0. [DOI] [PubMed] [Google Scholar]
- Clarkson J. M., Hewitt R. R. Significance of dimers to the size of newly synthesized DNA in UV-irradiated Chinese hamster ovary cells. Biophys J. 1976 Oct;16(10):1155–1164. doi: 10.1016/S0006-3495(76)85764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Boyer H. W. Solubility and dialysis limits of DNA oligonucleotides. Biochim Biophys Acta. 1972 Mar 14;262(2):116–124. doi: 10.1016/0005-2787(72)90224-9. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and radiation sensitivity in human (xeroderma pigmentosum) cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(6):557–565. doi: 10.1080/09553007014551491. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Decline in mutation frequency in temperature-sensitive SV40 viruses before viral DNA synthesis. Mutat Res. 1977 Sep;44(3):291–298. doi: 10.1016/0027-5107(77)90088-4. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Excision repair: our current knowledge based on human (xeroderma pigmentosum) and cattle cells. Johns Hopkins Med J Suppl. 1972;(1):195–121. [PubMed] [Google Scholar]
- Cleaver J. E. Induction of thioguanine- and ouabain-resistant mutants and single-strand breaks in the DNA of Chinese hamster ovary cells by 3H-thymidine. Genetics. 1977 Sep;87(1):129–138. doi: 10.1093/genetics/87.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Burki H. J. Biological damage from intranuclear tritium: DNA strand breaks and their repair. Science. 1972 Sep 15;177(4053):996–998. doi: 10.1126/science.177.4053.996. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Trosko J. E., Lett J. T. Excision repair (dimer excision, strand breakage and repair replication) in primary cultures of eukaryotic (bovine) cells. Exp Cell Res. 1972 Sep;74(1):67–80. doi: 10.1016/0014-4827(72)90482-x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Weil S. UV-induced reversion of a temperature-sensitive late mutant of simian virus 40 to a wild-type phenotype. J Virol. 1975 Jul;16(1):214–216. doi: 10.1128/jvi.16.1.214-216.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. R., Painter R. B. Evidence for repair replication of HeLa cell DNA damaged by ultraviolet light. Biochim Biophys Acta. 1968 Jul 23;161(2):552–554. doi: 10.1016/0005-2787(68)90131-7. [DOI] [PubMed] [Google Scholar]
- Cook K. H., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. II. The use of one-dimensional systems. Anal Biochem. 1976 Jun;73(2):411–418. doi: 10.1016/0003-2697(76)90188-3. [DOI] [PubMed] [Google Scholar]
- Coppey J. Common precursor pathways of Herpes DNA and of repair synthesis in ultraviolet irradiated cells. Nature. 1977 Jan 20;265(5591):260–261. doi: 10.1038/265260a0. [DOI] [PubMed] [Google Scholar]
- Coppey J., Nocentini S. Herpes virus and viral DNA synthesis in ultraviolet light-irradiated cells. J Gen Virol. 1976 Jul;32(1):1–15. doi: 10.1099/0022-1317-32-1-1. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd Cellular reactivation of ultraviolet-irradiated human adenovirus 2 in normal and xeroderma pigmentosum fibroblasts. Photochem Photobiol. 1974 Jan;19(1):9–13. doi: 10.1111/j.1751-1097.1974.tb06467.x. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Hanawalt P. C. The timecourse of DNA repair replication in ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):206–217. doi: 10.1016/0005-2787(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Ehmann U. K., Cook K. H., Friedberg E. C. The kinetics of thymine dimer excision in ultraviolet-irradiated human cells. Biophys J. 1978 May;22(2):249–264. doi: 10.1016/S0006-3495(78)85487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann U. K., Lett J. T. Review and evaluation of molecular weight calculations from the sedimentation profiles of irradiated DNA. Radiat Res. 1973 Apr;54(1):152–162. [PubMed] [Google Scholar]
- Friedberg E. C., Clayton D. A. Electron microscopic studies on substrate specificity of T4 excision repair endonuclease. Nature. 1972 May 12;237(5350):99–100. doi: 10.1038/237099a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth R., Cleaver J. E. Metabolism of caffeine to nucleic acid precursors in mammalian cells. Mutat Res. 1976 Jul;36(1):105–114. doi: 10.1016/0027-5107(76)90025-7. [DOI] [PubMed] [Google Scholar]
- Hiss E. A., Preston R. J. The effect of cytosine arabinoside on the frequency of single-strand breaks in DNA of mammalian cells following irradiation or chemical treatment. Biochim Biophys Acta. 1977 Sep 6;478(1):1–8. doi: 10.1016/0005-2787(77)90238-6. [DOI] [PubMed] [Google Scholar]
- Horikawa M., Nikaido O., Sugahara T. Dark reactivation of damage induced by ultraviolet light in mammalian cells in vitro. Nature. 1968 May 4;218(5140):489–491. doi: 10.1038/218489b0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Kleinman L. F., Black P. H. Cell cycle dependence of simian virus 40 induction from transformed hamster cells by ultraviolet irradiation. Virology. 1975 Nov;68(1):215–220. doi: 10.1016/0042-6822(75)90162-2. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Wilbert S. M., Collins J. J., Rakusanova T., Zamansky G. B., Black P. H. Isolation of simian virus 40-transformed inbred hamster cell lines heterogeneous for virus induction by chemicals or radiation. Virology. 1975 Nov;68(1):200–214. doi: 10.1016/0042-6822(75)90161-0. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping the genes of simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):53–60. doi: 10.1101/sqb.1974.039.01.010. [DOI] [PubMed] [Google Scholar]
- Lucas C. J. Immunological demonstration of the disappearance of pyrimidine dimers from nuclei of cultured human cells. Exp Cell Res. 1972 Oct;74(2):480–486. doi: 10.1016/0014-4827(72)90404-1. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Benane S. G., Bockstahler L. E. Ultraviolet-enhanced reactivation of Herpes virus in human tumor cells. Photochem Photobiol. 1974 Aug;20(2):91–94. doi: 10.1111/j.1751-1097.1974.tb06554.x. [DOI] [PubMed] [Google Scholar]
- Meneghini R., Hanawalt P. T4-endonuclease V-sensitive sites in DNA from ultraviolet-irradiated human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):428–437. doi: 10.1016/0005-2787(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Cleaver J. E. Repair replication, unscheduled DNA synthesis, and the repair of mammalian DNA. Radiat Res. 1969 Mar;37(3):451–466. [PubMed] [Google Scholar]
- Painter R. B. DNA damage and repair in eukaryotic cells. Genetics. 1974 Sep;78(1):139–148. doi: 10.1093/genetics/78.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Lohman P. H., Sluyter M. L. Use of UV endonuclease from Micrococcus luteus to monitor the progress of DNA repair in UV-irradiated human cells. Mutat Res. 1973 Aug;19(2):245–256. doi: 10.1016/0027-5107(73)90083-3. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN R. E., PAINTER R. B. EVIDENCE FOR REPAIR OF ULTRA-VIOLET DAMAGED DEOXYRIBONUCLEIC ACID IN CULTURED MAMMALIAN CELLS. Nature. 1964 Sep 26;203:1360–1362. doi: 10.1038/2031360a0. [DOI] [PubMed] [Google Scholar]
- Rabson A. S., Tyrrell S. A., Legallais F. Y. Growth of ultraviolet-damaged herpesvirus in xeroderma pigemntosum. Proc Soc Exp Biol Med. 1969 Nov;132(2):802–806. [PubMed] [Google Scholar]
- Rasmussen R. E., Painter R. B. Radiation-stimulated DNA synthesis in cultured mammalian cells. J Cell Biol. 1966 Apr;29(1):11–19. doi: 10.1083/jcb.29.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINCLAIR W. K., MORTON R. A. X-RAY AND ULTRAVIOLET SENSITIVITY OF SYNCHRONIZED CHINESE HAMSTER CELLS AT VARIOUS STAGES OF THE CELL CYCLE. Biophys J. 1965 Jan;5:1–25. doi: 10.1016/s0006-3495(65)86700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinton D. C., Hanawalt P. C. The fate of pyrimidine dimers in ultraviolet-irradiated Chlamydomonas. Photochem Photobiol. 1973 Jun;17(6):361–375. doi: 10.1111/j.1751-1097.1973.tb06370.x. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Rice M., Sutherland B. M. Photoreactivation of herpes simplex virus in human fibroblasts. Nature. 1975 Apr 17;254(5501):627–628. doi: 10.1038/254627a0. [DOI] [PubMed] [Google Scholar]
- Wilkins R. J., Hart R. W. Preferential DNA repair in human cells. Nature. 1974 Jan 4;247(5435):35–36. doi: 10.1038/247035a0. [DOI] [PubMed] [Google Scholar]
- Wilkins R. J. Letter: DNA repair: a simple enzymatic assay for human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Dec;24(6):609–613. doi: 10.1080/09553007314551531. [DOI] [PubMed] [Google Scholar]


