Abstract
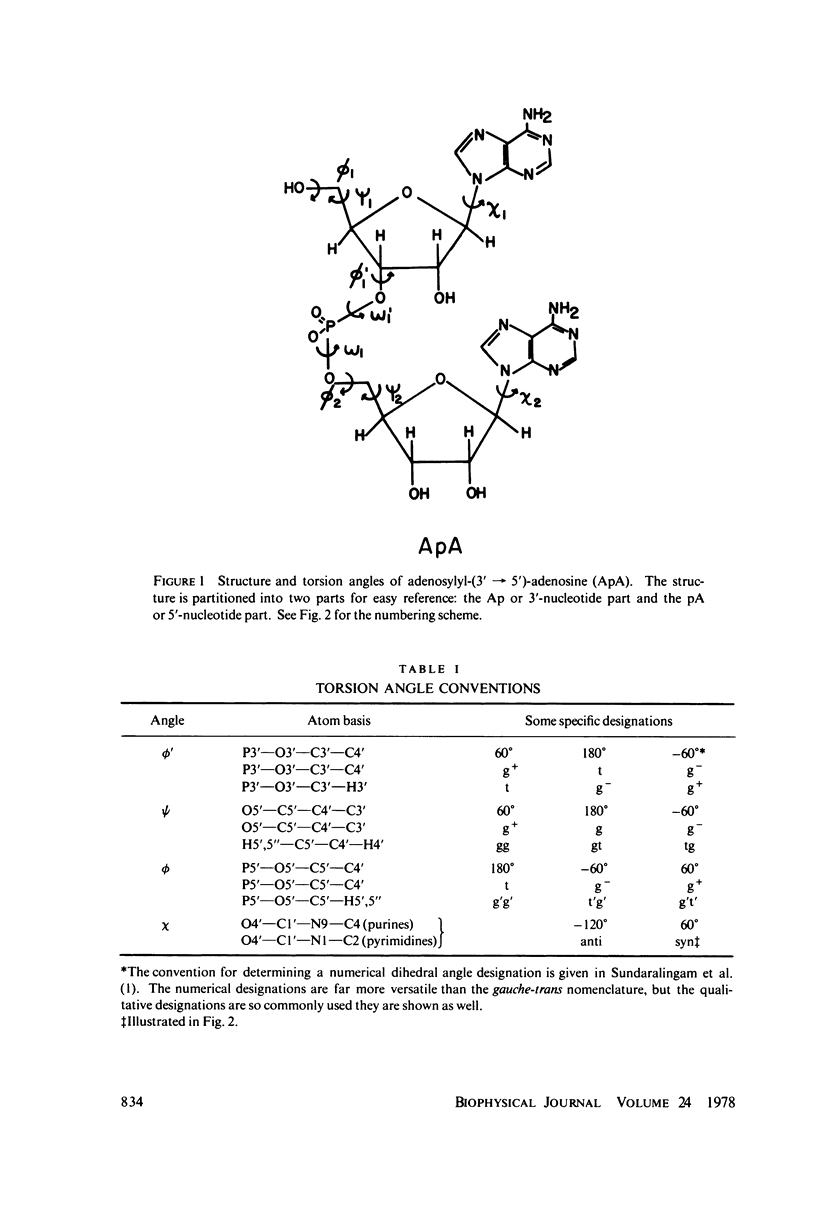
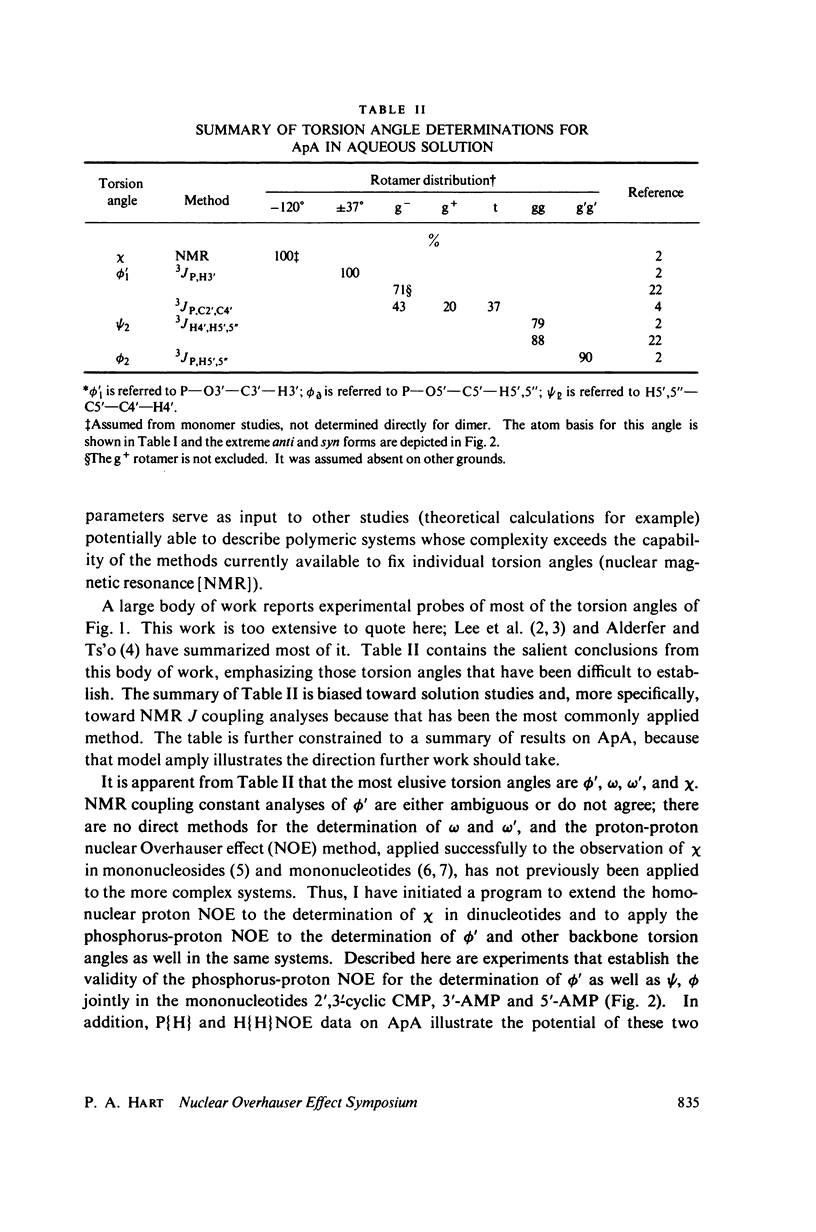
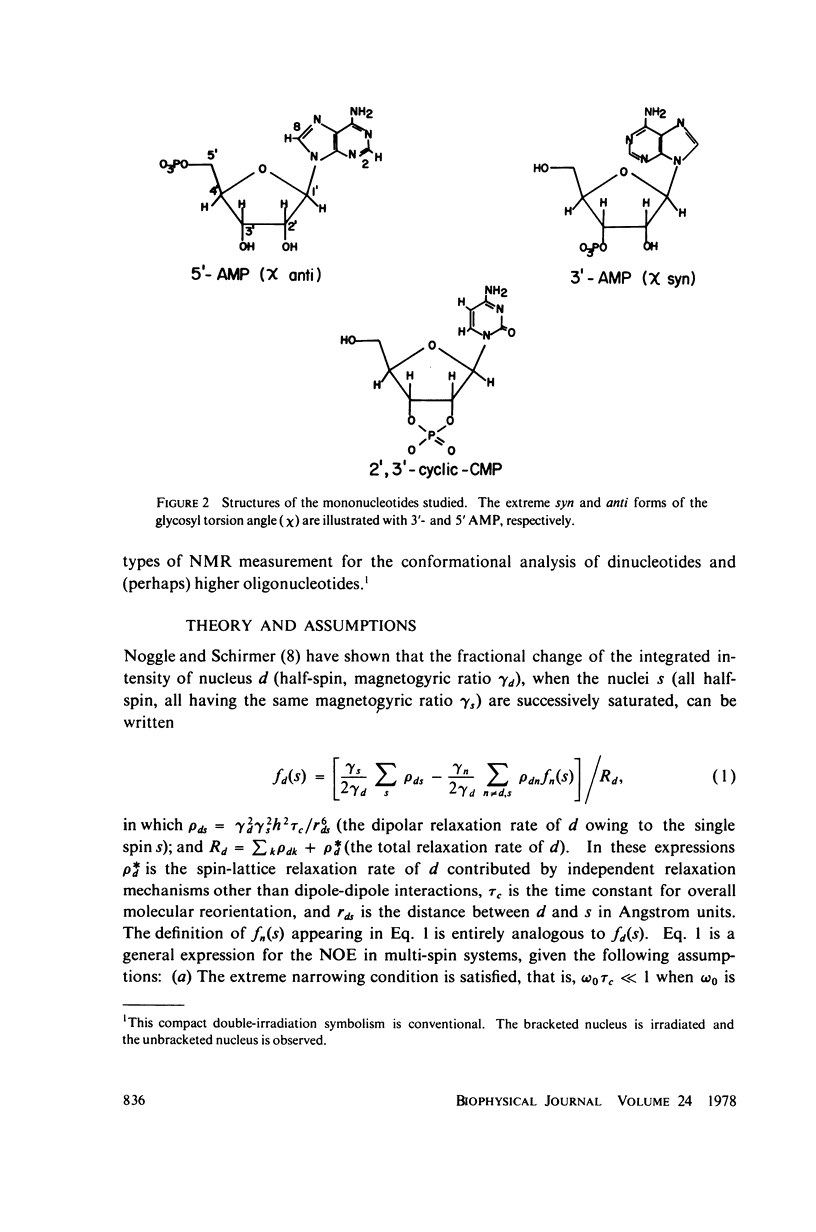
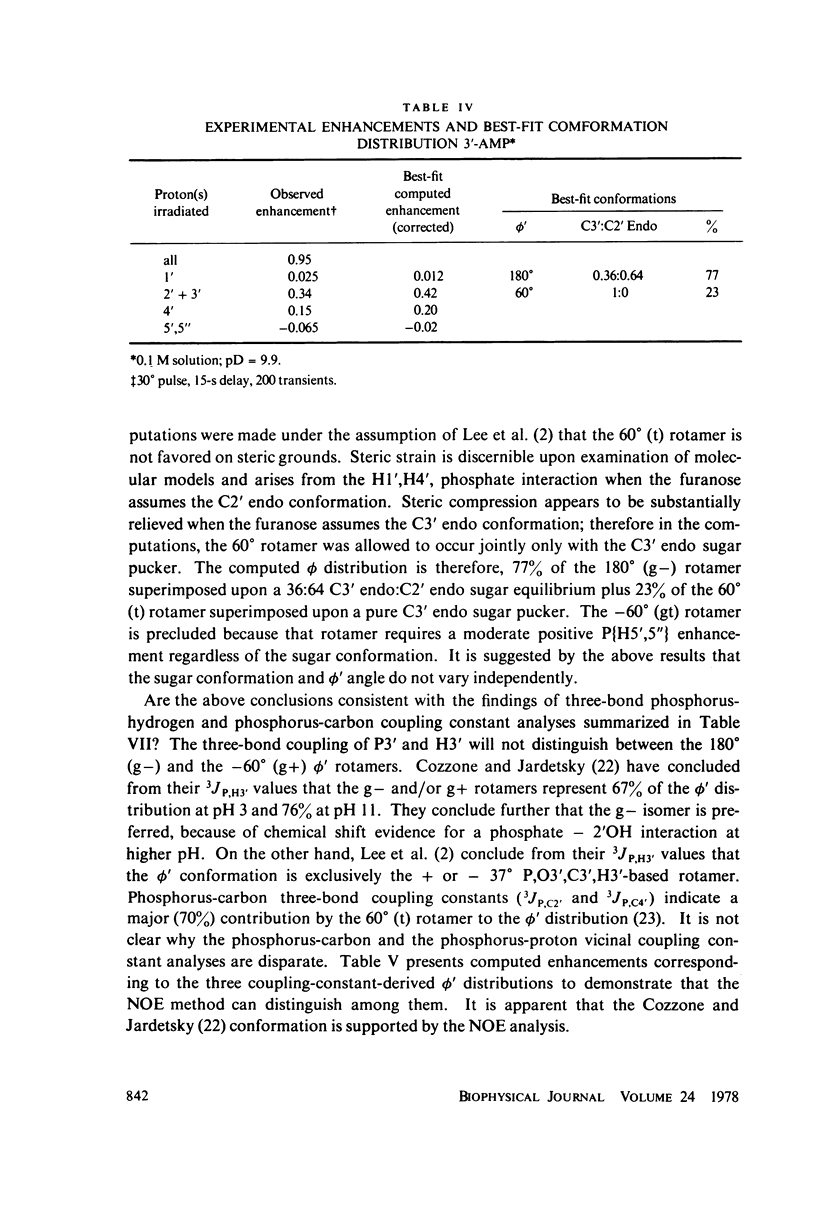
The phosphorus-proton nuclear Overhauser effect (NOE) was used to investigate the quantitative distribution of rotamers about the C3'--O3' bond (phi') of 3'-AMP and 2',3'-cyclic-CMP and the C4'--C5', C5'--O5' bonds (psi, phi) of 5'-AMP. Phosphorus-proton and proton-proton NOE's were used to provide a qualitative insight into the backbone conformation and the glycosyl angle torsions of adenosylyl-(3' leads to 5')-adenosine (ApA). The major psi rotamer in 5'-AMP is the 60 degree (gg) form, while the major phi rotamer is the 180 degrees (g'g') form. The constrained model, 2',3'-cyclic-CMP, manifests the C3'endo furanose pucker predominantly. The results from these two models are consistent with nuclear magnetic resonance (NMR) J coupling analyses. The phi; distribution of 3'-AMP is dominated (77%) by the 180 degrees g- rotamer. The 3'-AMP results are consistent with phosphorus-hydrogen coupling constant analyses, but do not accord with phosphorus-carbon coupling constant results. The phosphorus-proton NOE reveals that the phosphorus of ApA occupies a region of conformation space not seen in 5'-AMP. The proton-proton NOE on APA shows a significant portion of syn rotamer in both X distributions and detects a cross-purine ring interaction consistent with base stacking known to exist in this system.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderfer J. L., Ts'o P. O. Conformational properties of the furanose phosphate backbone in nucleic acids. A carbon-13 nuclear magnetic resonance study. Biochemistry. 1977 May 31;16(11):2410–2416. doi: 10.1021/bi00630a016. [DOI] [PubMed] [Google Scholar]
- Coulter C. L. Structural chemistry of cyclic nucleotides. II. Crystal and molecular structure of sodium -cytidine 2',3'-cyclic phosphate. J Am Chem Soc. 1973 Jan 24;95(2):570–575. doi: 10.1021/ja00783a042. [DOI] [PubMed] [Google Scholar]
- Cozzone P. J., Jardetzky O. Phosphorus-31 Fourier transform nuclear magnetic resonance study of mononucleotides and dinucleotides. 2. Coupling constants. Biochemistry. 1976 Nov 2;15(22):4860–4865. doi: 10.1021/bi00667a017. [DOI] [PubMed] [Google Scholar]
- Evans F. E., Sarma R. H. The intramolecular conformation of adenosine 5'-monophosphate in aqueous solution as studied by fast Fourier transform 1H and 1H-(31P) nuclear magnetic resonance spectroscopy. J Biol Chem. 1974 Aug 10;249(15):4754–4759. [PubMed] [Google Scholar]
- Lapper R. D., Smith I. C. A 13 C and 1 H nuclear magnetic resonance study of the conformations of 2',3'-cyclic nucleotides. J Am Chem Soc. 1973 May 2;95(9):2878–2880. doi: 10.1021/ja00790a024. [DOI] [PubMed] [Google Scholar]
- Lavallee D. K., Coulter C. L. Structural chemistry of cyclic nucleotides. 3. Proton magnetic resonance studies of -pyrimidine nucleotides. J Am Chem Soc. 1973 Jan 24;95(2):576–581. doi: 10.1021/ja00783a043. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Ezra F. S., Kondo N. S., Sarma R. H., Danyluk S. S. Conformational properties of dinucleoside monophosphates in solution: dipurines and dipyrimidines. Biochemistry. 1976 Aug 10;15(16):3627–3639. doi: 10.1021/bi00661a034. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Tinoco I., Jr Studies of the conformation of modified dinucleoside phosphates containing 1,N6-ethenoadenosine and 2'-O-methylcytidine by 360-MHz 1H nuclear magnetic resonance spectroscopy. Investigation of the solution conformations of dinucleoside phosphates. Biochemistry. 1977 Dec 13;16(25):5403–5414. doi: 10.1021/bi00644a001. [DOI] [PubMed] [Google Scholar]
- Sarma R. H., Mynott R. T., Wood D. J., Hruska F. E. Determination of the preferred conformations constrained along the C-4'-C-5' and C-5'-O-5' bonds of beta-5'-nucleotides in solution. Four-bond 31P-1H coupling. J Am Chem Soc. 1973 Sep 19;95(19):6457–6459. doi: 10.1021/ja00800a055. [DOI] [PubMed] [Google Scholar]
- Schirmer R. E., Davis J. P., Noggle J. H., Hart P. A. Conformational analysis of nucleosides in solution by quantitative application of the nuclear Overhauser effect. J Am Chem Soc. 1972 Apr 19;94(8):2561–2572. doi: 10.1021/ja00763a001. [DOI] [PubMed] [Google Scholar]
- Schleich T., Cross B. P., Smith I. C. A conformational study of adenylyl-(3',5')-adenosine and adenylyl-(2',5')-adenosine in aqueous solution by carbon-13 magnetic resonance spectroscopy. Nucleic Acids Res. 1976 Feb;3(2):355–370. doi: 10.1093/nar/3.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son T. D., Guschlbauer W., Guéron M. Flexibility and conformations of guanosine monophosphates by the Overhauser effect. J Am Chem Soc. 1972 Nov 1;94(22):7903–7911. doi: 10.1021/ja00777a038. [DOI] [PubMed] [Google Scholar]
- Sundaralingam M. Stereochemistry of nucleic acid constituents. 3. Crystal and molecular structure of adenosine 3'-phosphate dihydrate (adenylic acid b). Acta Crystallogr. 1966 Oct 10;21(4):495–506. doi: 10.1107/s0365110x66003372. [DOI] [PubMed] [Google Scholar]


