Abstract
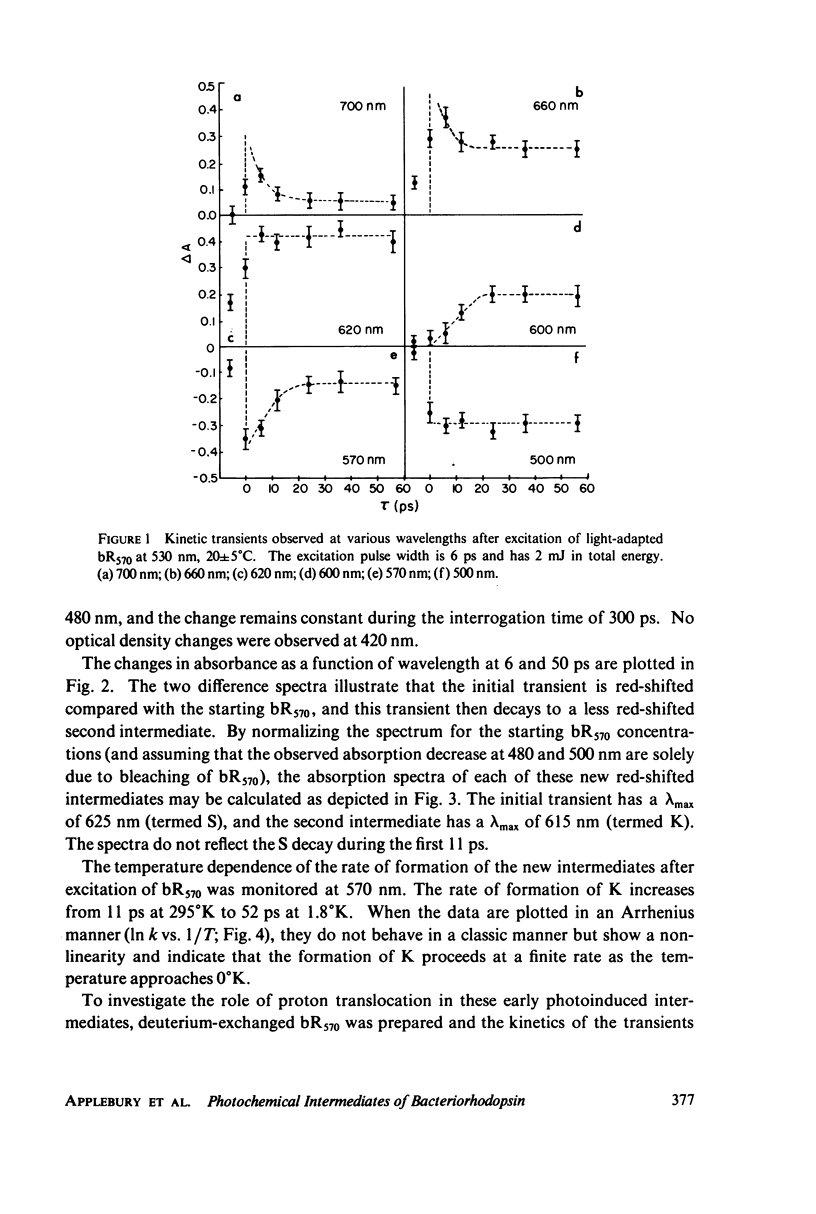
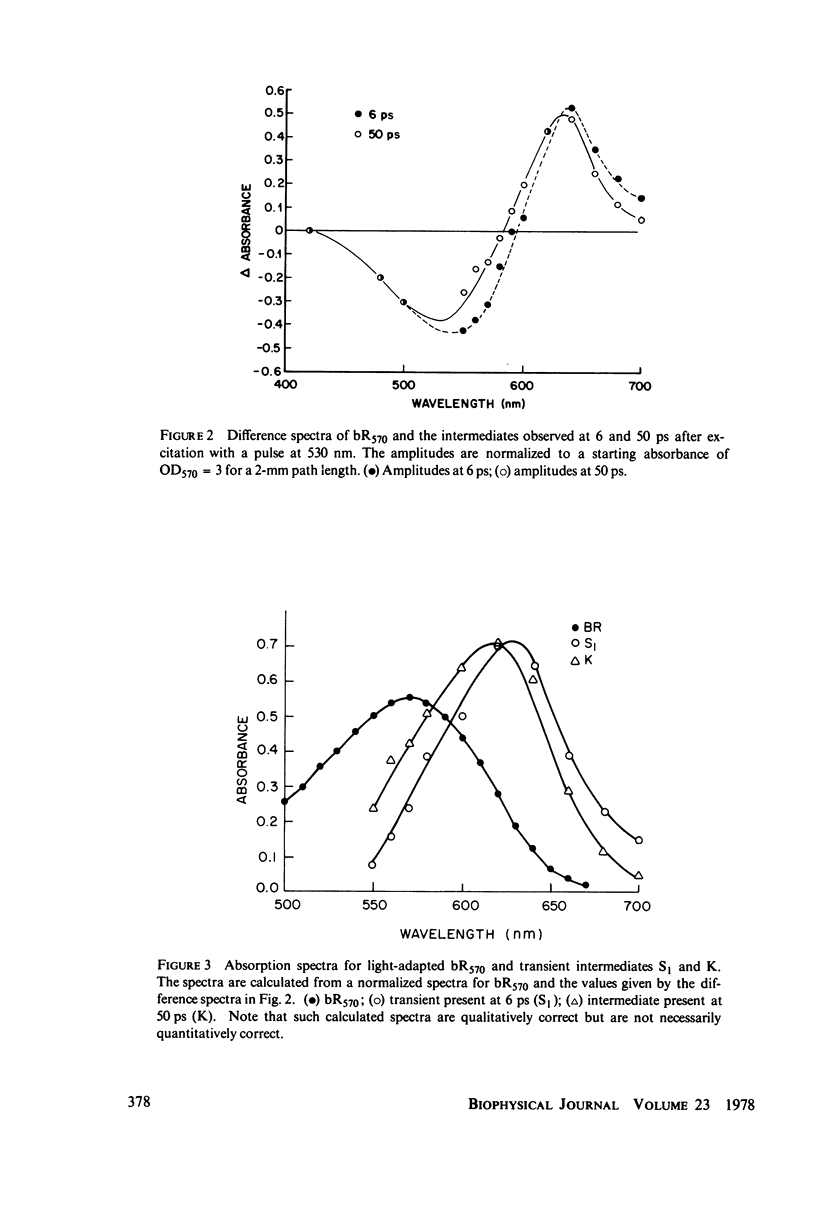
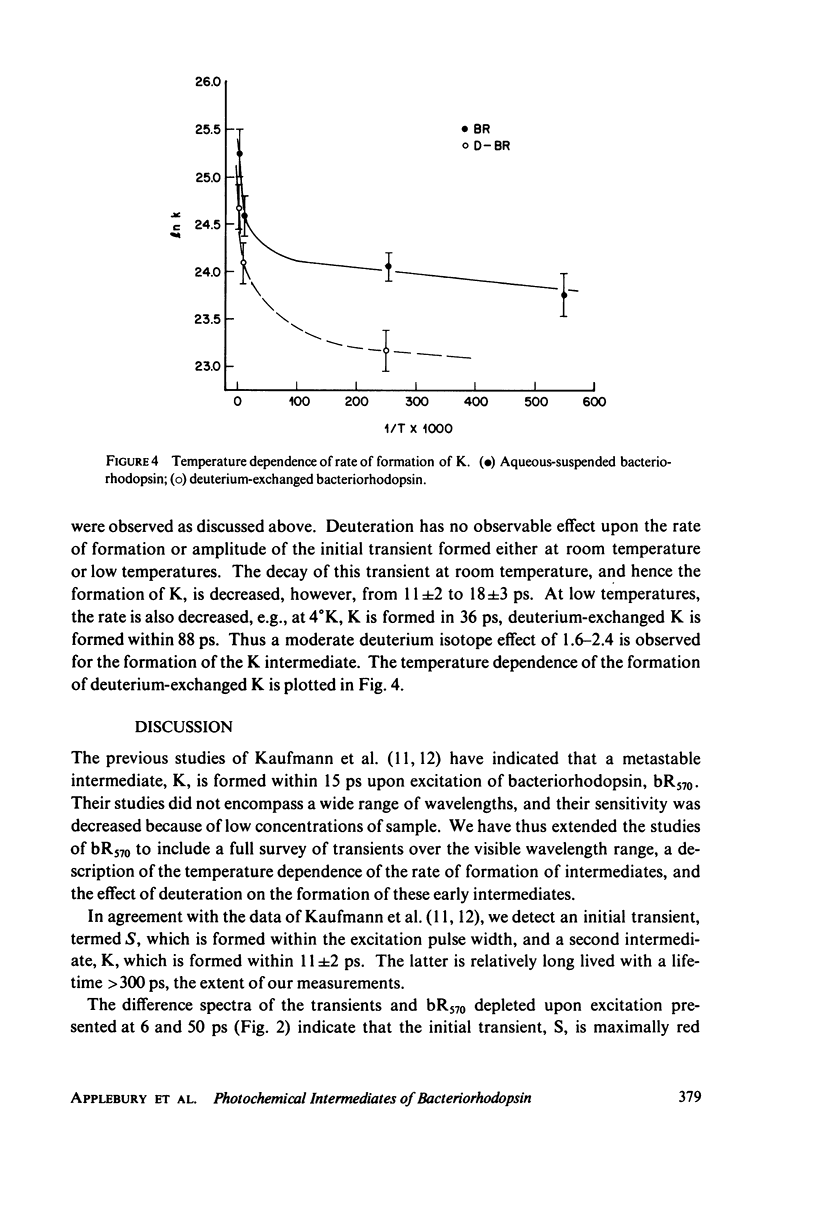
Picosecond studies of the primary photochemical events in the light-adapted bacteriorhodopsin, bR570, indicate that the first metastable intermediate K610 is formed with a rise time of 11 ps. Difference spectra obtained at 50 ps after excitation show that K610 is the same species as that trapped in low temperature glasses. A precursor species (S) of the K610 intermediate has been observed which is red shifted with respect to K610 and is formed within the 6-ps time width of the excitation pulse. The formation of the precursor has no observable thermal dependence between 298 degrees and 1.8 degrees K. The formation of K610 has a very low thermal barrier and at very low temperatures, the rate of formation becomes practically temperature independent which is characteristic of a tunneling process. The rate of formation becomes practically temperature independent which is characteristic of a tunneling process. The rate of formation of K610 has a moderate deuterium isotope effect of kH/kD approximately 1.6 at 298 degrees K and 2.4 at 4 degrees K. The mechanism for formation of K610 is found to involve a rate-limiting proton transfer which occurs by tunneling at low temperatures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. The quantum efficiency for the photochemical conversion of the purple membrane protein. Biophys J. 1977 Feb;17(2):185–191. doi: 10.1016/S0006-3495(77)85636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownds D. Site of attachment of retinal in rhodopsin. Nature. 1967 Dec 23;216(5121):1178–1181. doi: 10.1038/2161178a0. [DOI] [PubMed] [Google Scholar]
- Briggs G. M. Why study nutrition history? Fed Proc. 1977 May;36(6):1905–1905. [PubMed] [Google Scholar]
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C. R., Kalisky O., Rosenfeld T., Ottolenghi M. The quantum efficiency of the bacteriorhodopsin photocycle. Biophys J. 1977 Feb;17(2):179–183. doi: 10.1016/S0006-3495(77)85635-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C. R., Ottolenghi M., Korenstein R. On the primary quantum yields in the bacteriorhodopsin photocycle. Biophys J. 1976 Jul;16(7):839–843. doi: 10.1016/S0006-3495(76)85732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Rentzepis P. M., Stoeckenius W., Lewis A. Primary photochemical processes in bacteriorhodopsin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1109–1115. doi: 10.1016/0006-291x(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Sundstrom V., Yamane T., Rentzepis P. M. Kinetics of the 580-nm ultrafast bacteriorhodopsin transient. Biophys J. 1978 Apr;22(1):121–124. doi: 10.1016/S0006-3495(78)85476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenstein R., Sherman W. V., Caplan S. R. Kinetic isotope effects in the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1976 Dec 22;2(3):267–276. doi: 10.1007/BF00535372. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom V., Rentzepis P. M., Peters K., Applebury M. L. Kinetics of rhodopsin at room temperature measured by picosecond spectroscopy. Nature. 1977 Jun 16;267(5612):645–646. doi: 10.1038/267645a0. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]


