Abstract
Transient electron paramagnetic resonance (EPR) methods are used to examine the spin populations of the light-induced radicals produced in spinach chloroplasts, photosystem I particles, and Chlorella pyrenoidosa. We observe both emission and enhanced absorption within the hyperfine structure of the EPR spectrum of P700+, the photooxidized reaction-center chlorophyll radical (Signal I). By using flow gradients or magnetic fields to orient the chloroplasts in the Zeeman field, we are able to influence both the magnitude and sign of the spin polarization. Identification of the polarized radical and P700+ is consistent with the effects of inhibitors, excitation light intensity and wavelength, redox potential, and fractionation of the membranes. The EPR signal of the polarized P700+ radical displays a 30% narrower line width than P700+ after spin relaxation. This suggests a magnetic interaction between P700+ and its reduced (paramagnetic) acceptor, which leads to a collapse of the P700+ hyperfine structure. Narrowing of the spectrum is evident only in the spectrum of polarized P700+, because prompt electron transfer rapidly separates the radical pair. Evidence of cross-relaxation between the adjacent radicals suggests the existence of an exchange interaction. The results indicate that polarization is produced by a radical pair mechanism between P700+ and the reduced primary acceptor of photosystem I. The orientation dependence of the spin polarization of P700+ is due to the g-tensor anisotropy of the acceptor radical to which it is exchange-coupled. The EPR spectrum of P700+ is virtually isotropic once the adjacent acceptor radical has passed the photoionized electron to a later, more remote acceptor molecule. This interpretation implies that the acceptor radical has g-tensor anisotropy significantly greater than the width of the hyperfine field on P700+ and that the acceptor is oriented with its smallest g-tensor axis along the normal to the thylakoid membranes. Both the ferredoxin-like iron-sulfur centers and the X- species observed directly by EPR at low temperatures have g-tensor anisotropy large enough to produce the observed spin polarization; however, studies on oriented chloroplasts show that the bound ferredoxin centers do not have this orientation of their g tensors. In contrast, X- is aligned with its smallest g-tensor axis predominantly normal to the plane of the thylakoid membranes. This is the same orientation predicted for the acceptor radical based on analysis of the spin polarization of P700+, and indicates that the species responsible for the anisotropy of the polarized P700+ spectrum is probably X-. The dark EPR Signal II is shown to possess anisotropic hyperfine structure (and possibly g-tensor anisotropy), which serves as a good indicator of the extent of membrane alignment.
Full text
PDF


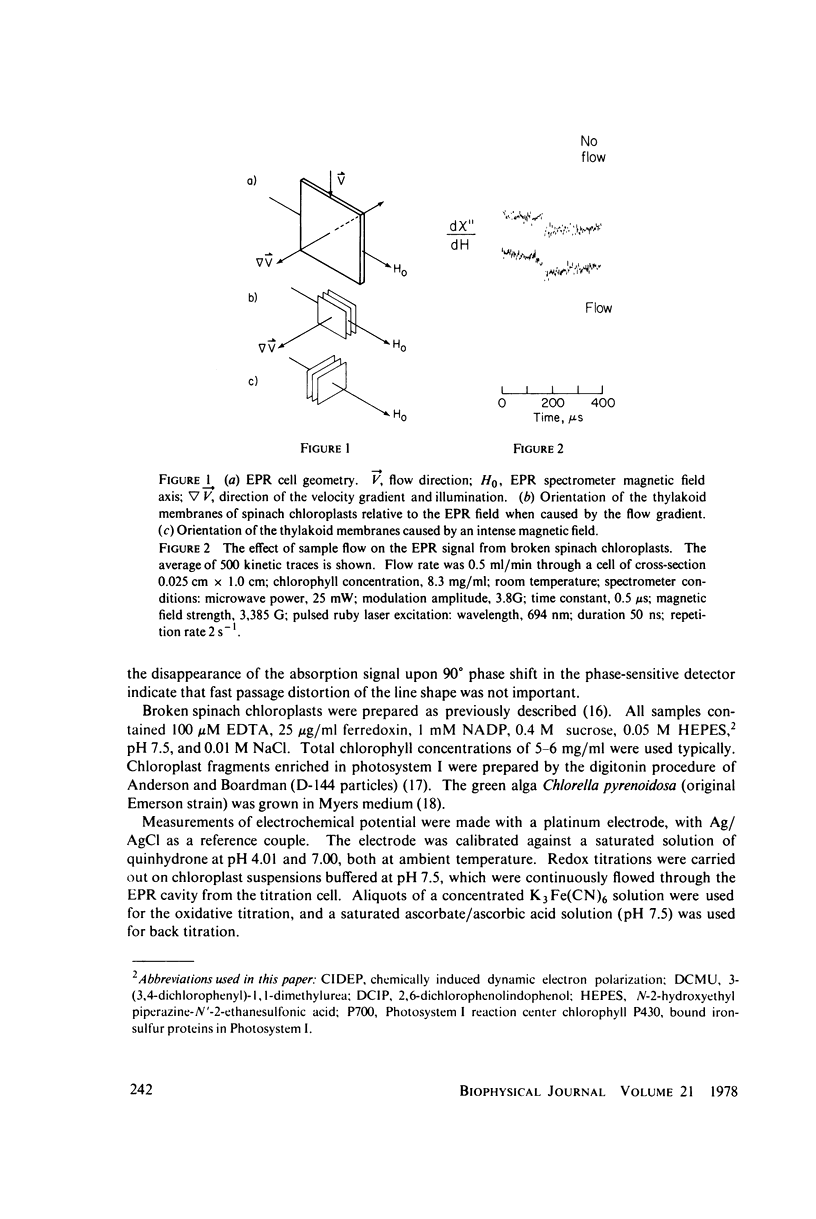

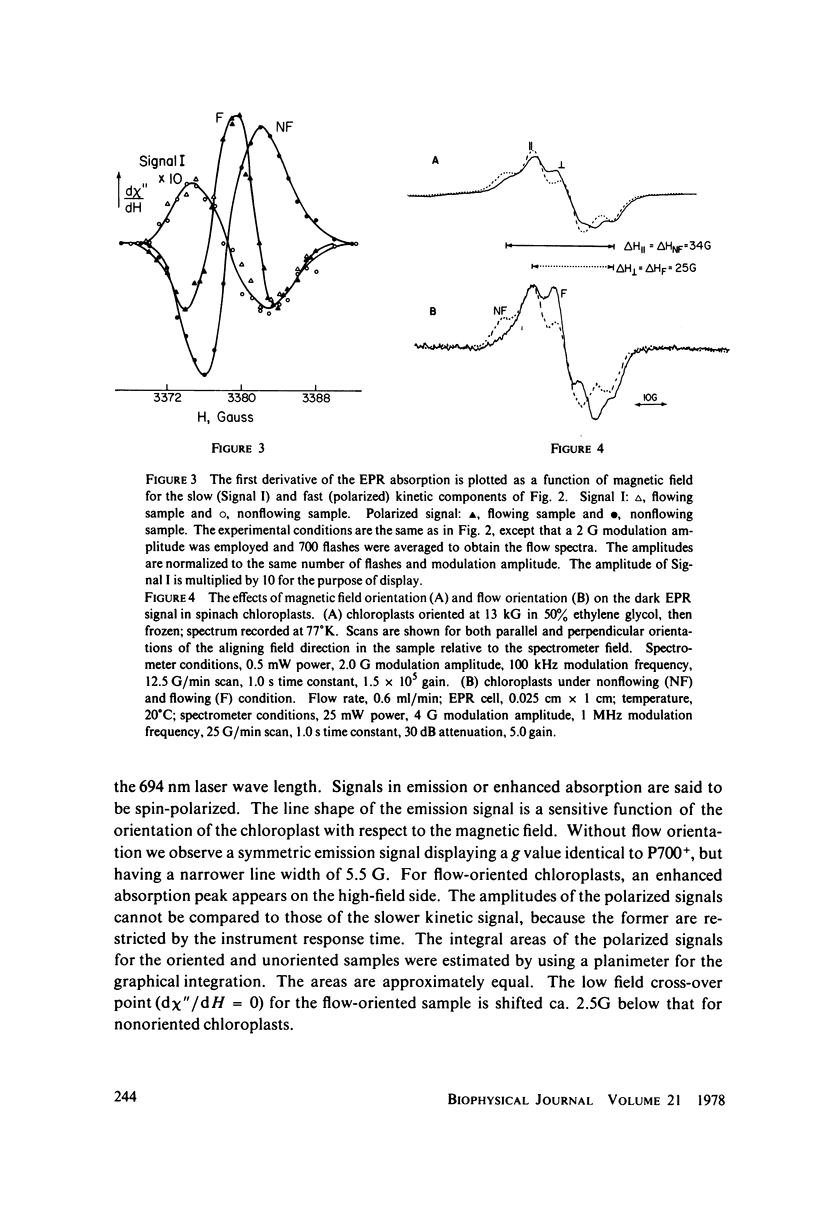

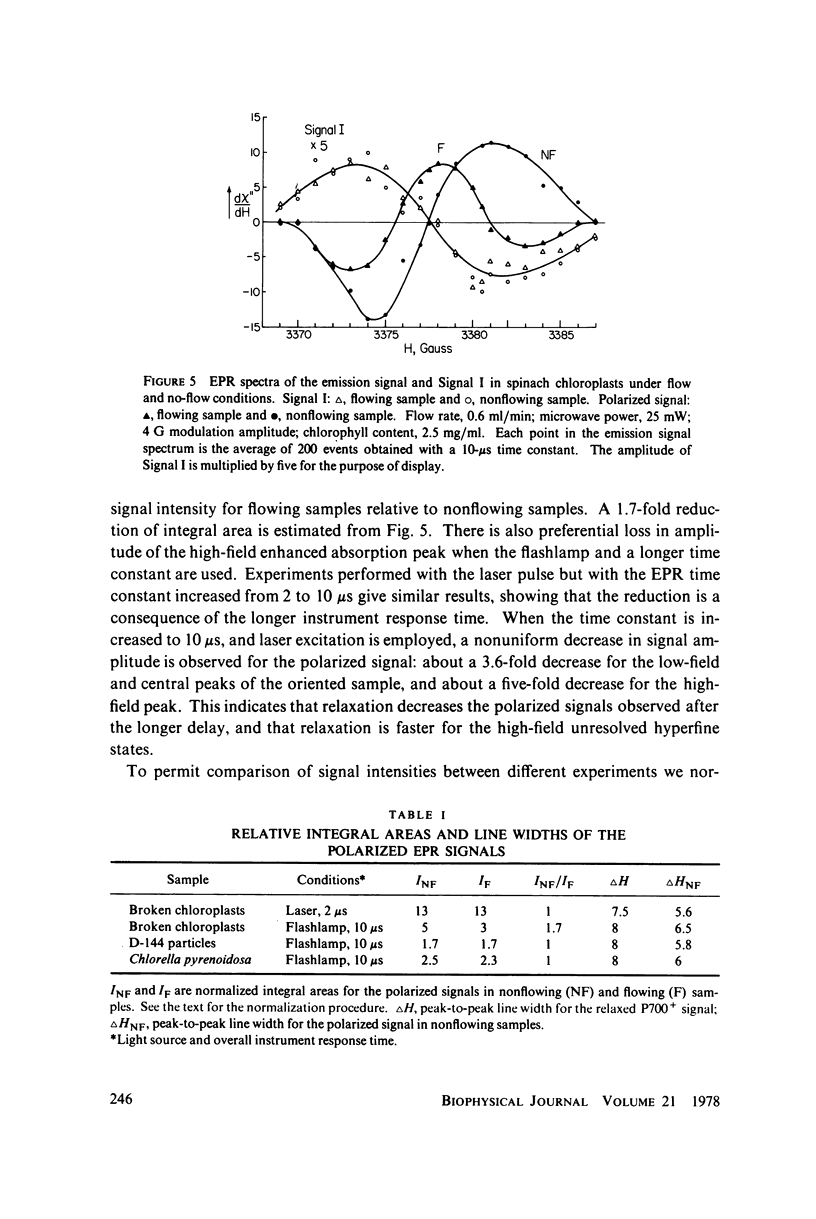
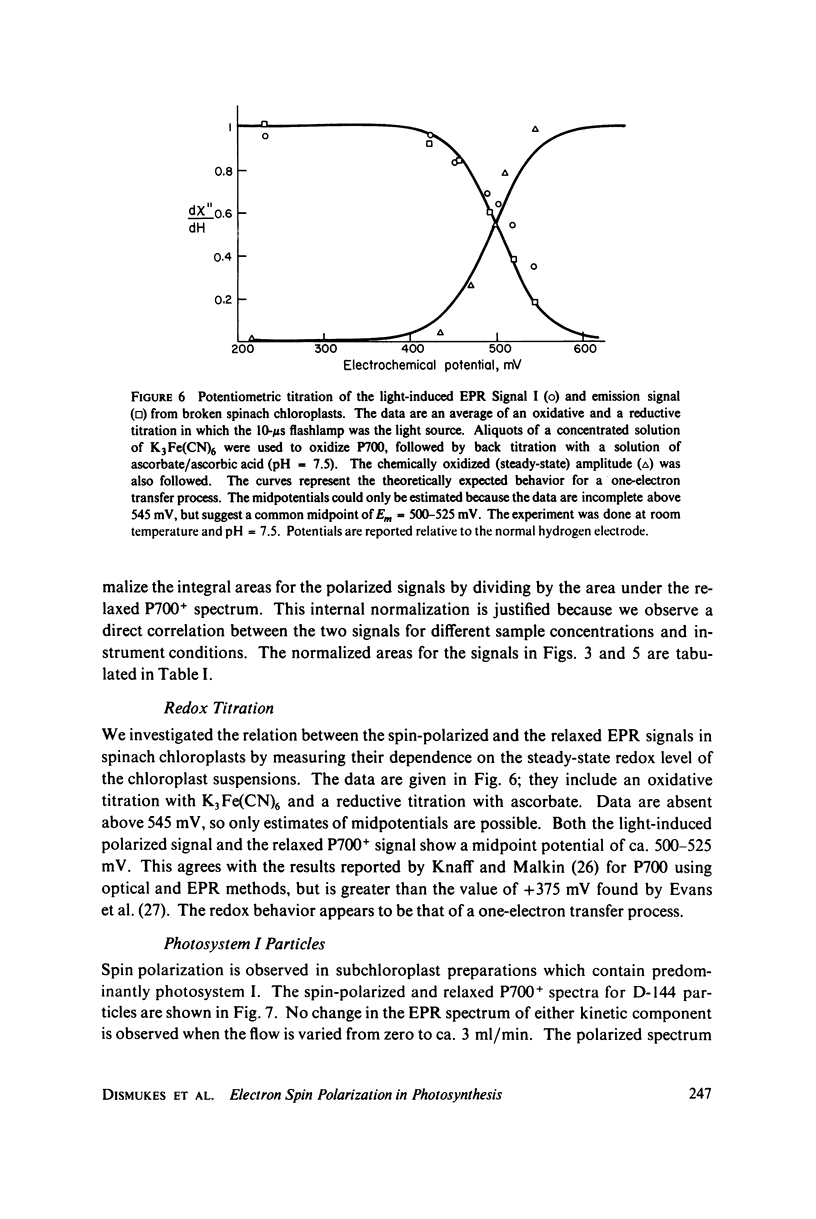








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Sauer K. Electron paramagnetic resonance signal II in spinach chloroplasts. II. Alternative spectral forms and inhibitor effects on kinetics of signal II in flashing light. Biochim Biophys Acta. 1973 Dec 14;325(3):504–519. doi: 10.1016/0005-2728(73)90210-7. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Primary photochemical reactions in chloroplast photosynthesis. Q Rev Biophys. 1974 May;7(2):131–177. doi: 10.1017/s0033583500001396. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., Sauer K. Manganese in photosynthetic oxygen evolution. I. Electron paramagnetic resonance study of the environment of manganese in Tris-washed chloroplasts. Biochim Biophys Acta. 1974 Aug 23;357(2):252–266. doi: 10.1016/0005-2728(74)90065-6. [DOI] [PubMed] [Google Scholar]
- Blankenship R., McGuire A., Sauer K. Chemically induced dynamic electron polarization in chloroplasts at room temperature: evidence for triplet state participation in photosynthesis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4943–4947. doi: 10.1073/pnas.72.12.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Cammack R. Determination of the oxidation-reduction potential of the bound iron-sulphur proteins of the primary electron acceptor complex of photosystem I in spinach chloroplasts. FEBS Lett. 1974 Dec 1;49(1):111–114. doi: 10.1016/0014-5793(74)80644-7. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Cammack R. The properties of the primary electron acceptor in the Photosystem I reaction centre of spinach chloroplasts and its interaction with P700 and the bound ferredoxin in various oxidation-reduction states. Biochem J. 1976 Jul 15;158(1):71–77. doi: 10.1042/bj1580071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Slabas A. R. The oxidation-reduction potential of the reaction-centre chlorophyll (P700) in Photosystem I. Evidence for multiple components in electron-paramagnetic-resonance signal 1 at low temperature. Biochem J. 1977 Jan 15;162(1):75–85. doi: 10.1042/bj1620075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geacintov N. E., Van Norstrand F., Becker J. F., Tinkel J. B. Magnetic field induced orientation of photosynthetic systems. Biochim Biophys Acta. 1972 Apr 20;267(1):65–79. doi: 10.1016/0005-2728(72)90138-7. [DOI] [PubMed] [Google Scholar]
- Hoff A. J., Gast P., Romijn J. C. Time-resolved ESR and chemically induced dynamic electron polarisation of the primary reaction in a reaction center particle of Rhodopseudomonas sphaeroides wild type at low temperature. FEBS Lett. 1977 Feb 1;73(2):185–190. doi: 10.1016/0014-5793(77)80977-0. [DOI] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Malkin R. The oxidation-reduction potentials of electron carriers in chloroplast photosystem I fragments. Arch Biochem Biophys. 1973 Nov;159(1):555–562. doi: 10.1016/0003-9861(73)90488-8. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcintosh A. R., Bolton J. R. Electron spin resonance spectrum of species "X" which may function as the primary electron acceptor in photosystem I of green plant photosynthesis. Biochim Biophys Acta. 1976 Jun 8;430(3):555–559. [PubMed] [Google Scholar]
- Sauer K. Molecular Orientation in Quantasomes: III. A Flow Dichroism Apparatus and Its Application to the Study of the Structure of Spinach Quantasomes. Biophys J. 1965 May;5(3):337–348. doi: 10.1016/s0006-3495(65)86720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Blankenship R. E., Klein M. P. Conversion of an E-3 ESR spectrometer to 1 MHz field modulation. Rev Sci Instrum. 1977 Mar;48(3):282–286. doi: 10.1063/1.1135007. [DOI] [PubMed] [Google Scholar]
- Warden J. T., Jr, Mohanty P., Bolton J. R. Flash photolysis--electron spin resonance studies of the dynamics of photosystem I. 3. Temperature dependence of the decay of signal I. Biochem Biophys Res Commun. 1974 Aug 5;59(3):872–878. doi: 10.1016/s0006-291x(74)80060-4. [DOI] [PubMed] [Google Scholar]


