Abstract
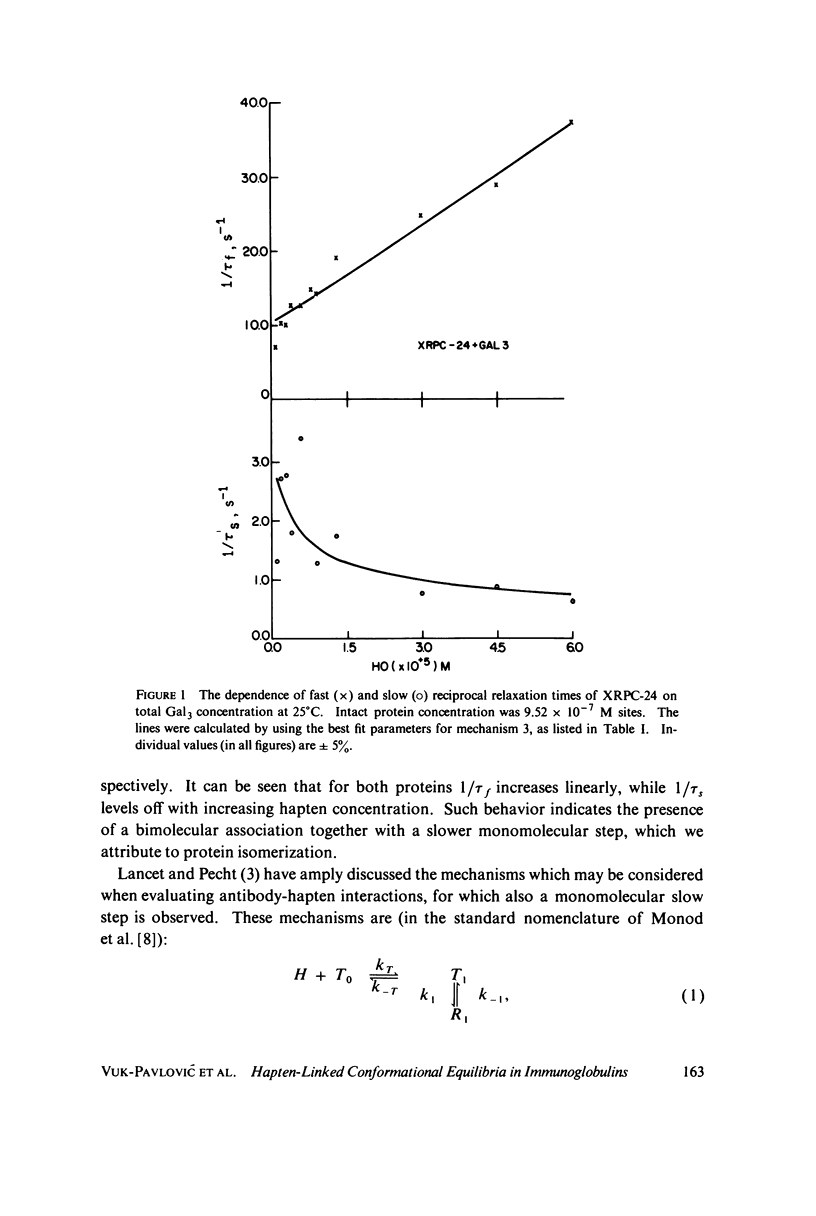
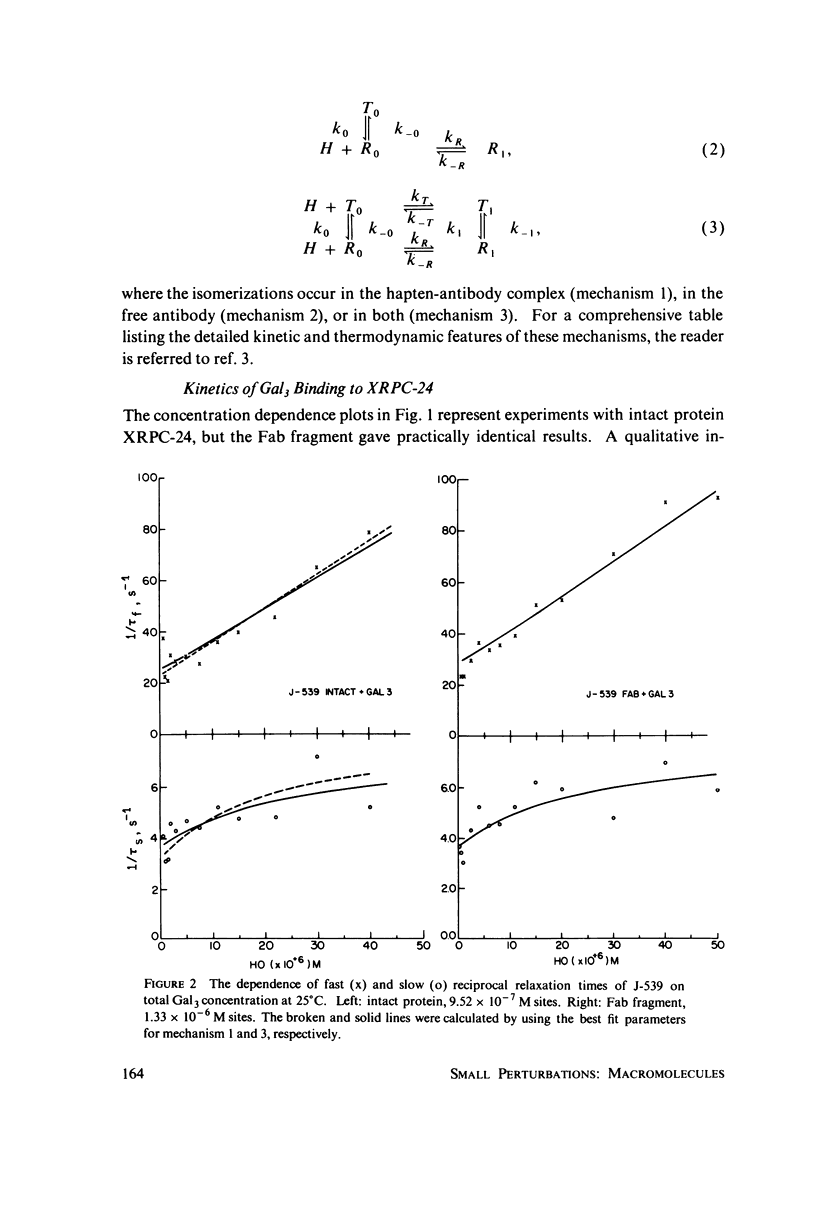
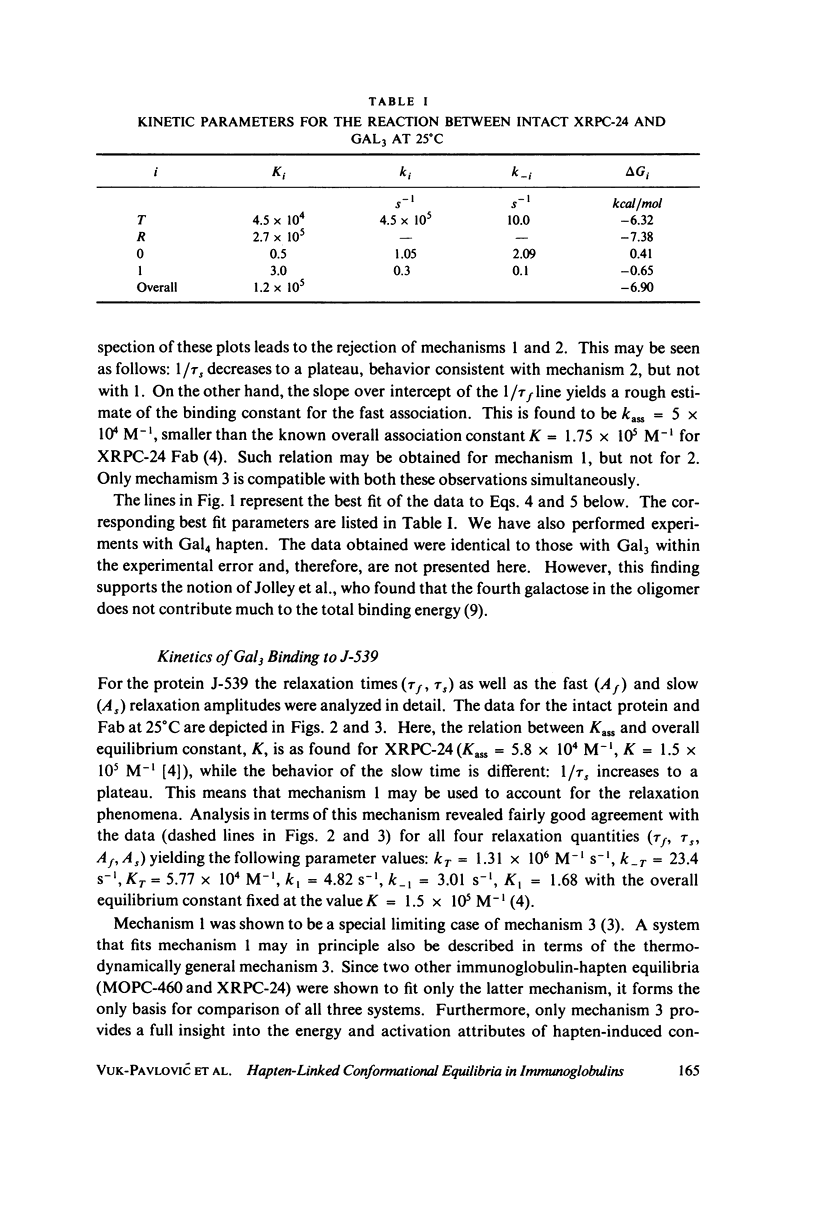
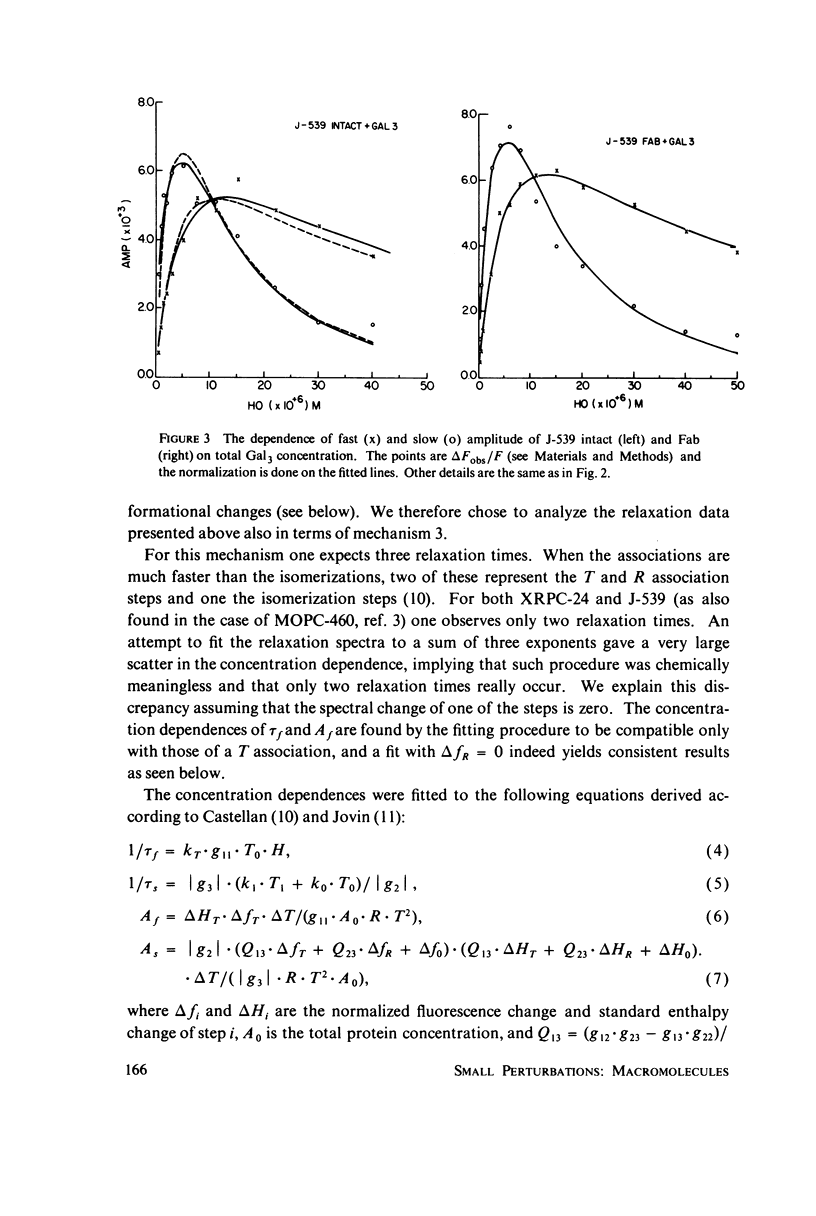
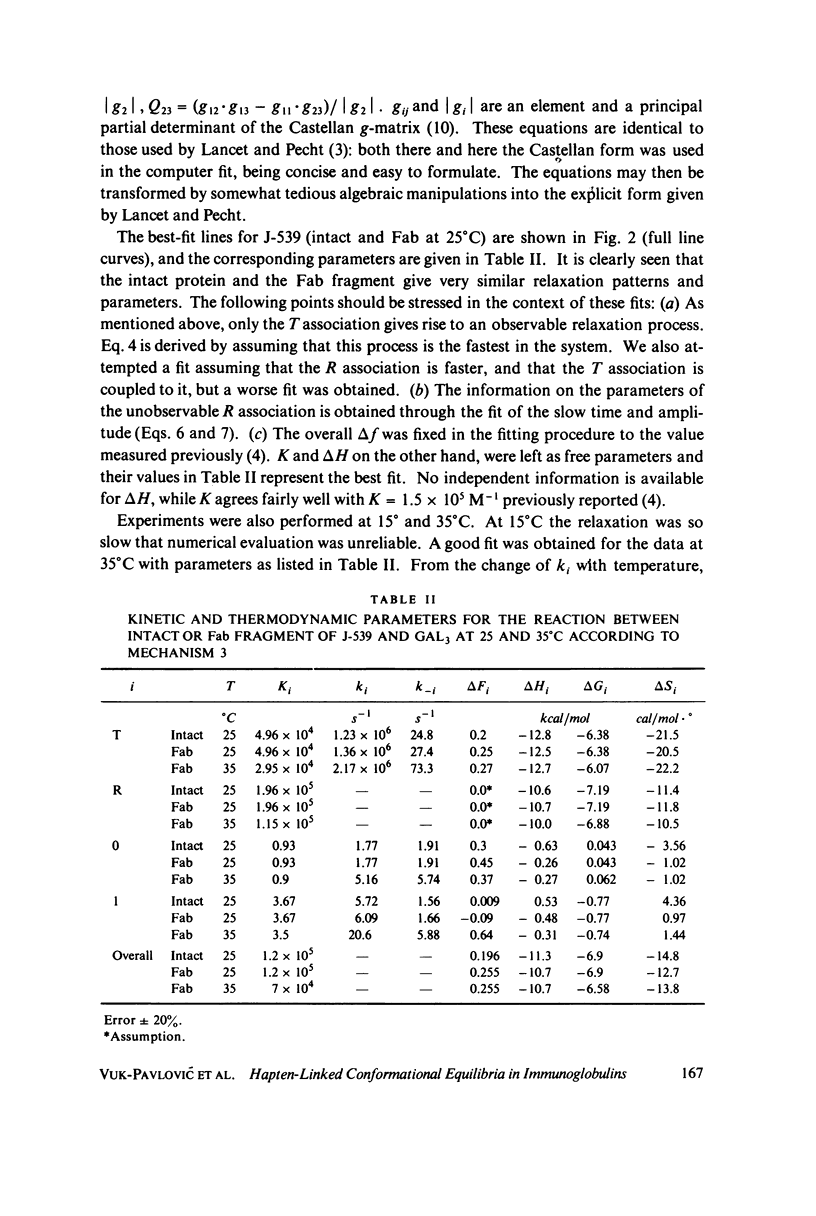
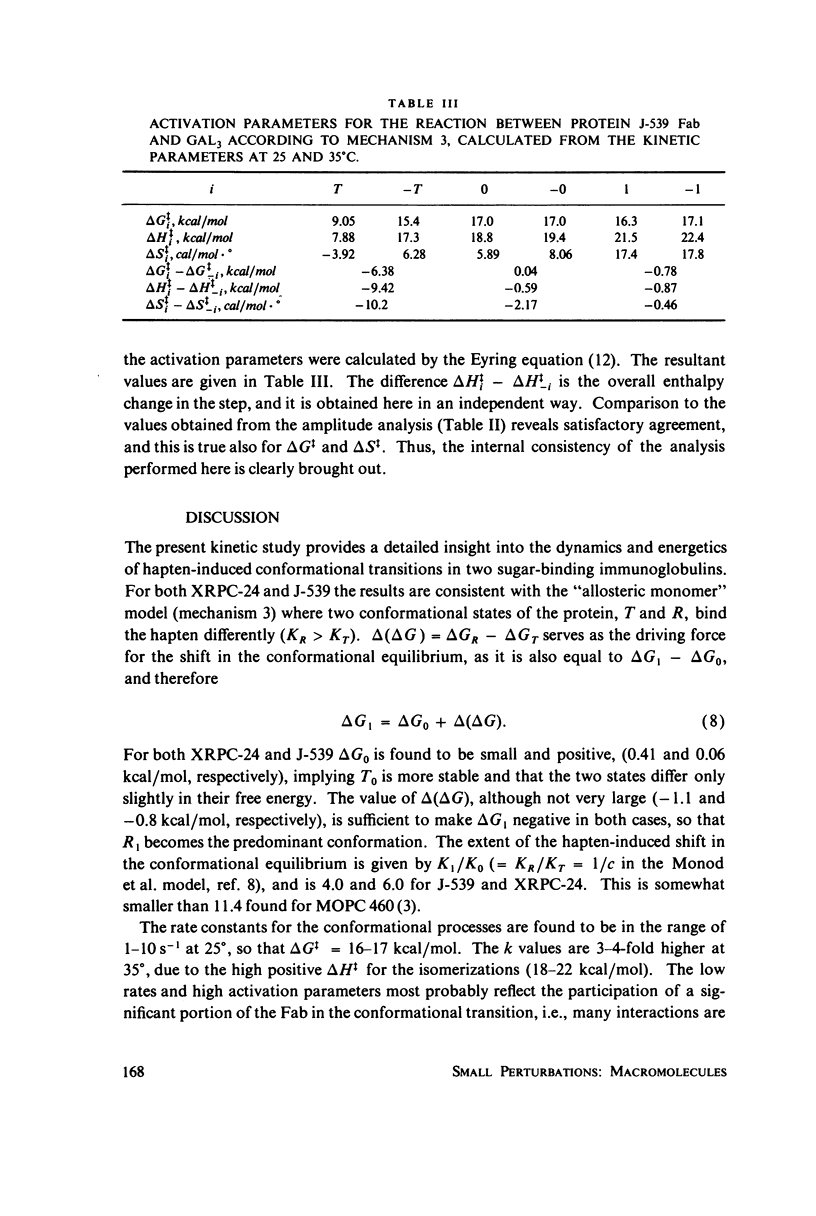
The interaction of oligogalactan haptens with the murine myeloma proteins XRPC-24 and J-539 has been investigated by the fluorescence temperature-jump method. The relaxation spectrum is composed of two processes, the faster representing hapten assocaition and the slower a protein isomerization. In both cases the concentration dependence of relaxation times and amplitudes was consistent with the general mechanism formulated by Lancet and Pecht (1976, Proc. Natl. Acad. Sci. U.S.A. 73:3549), in which the equilibrium between two conformations of the protein is shifted by hapten binding. The intact proteins and their Fab fragment had identical kinetic behavior, indicating that the conformational changes are located in the Fab region. Temperature dependence analysis for protein J-539 permitted the calculation of activation parameters and led to a consistent energy profile for all the elementary steps. The conformational states are separated by large activation barriers, but have similar free energies. The results suggest that hapten-induced conformational changes in immunoglobulins are more general phenomena than was previously thought.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Haselkorn D., Friedman S., Givol D., Pecht I. Kinetic mapping of the antibody combining site by chemical relaxation spectrometry. Biochemistry. 1974 May 7;13(10):2210–2222. doi: 10.1021/bi00707a030. [DOI] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Glaudemans C. P., Rudikoff S., Potter M. Structural requirements for the binding of derivatives of D-galactose to two homogeneous murine immunoglobulins. Biochemistry. 1974 Jul 16;13(15):3179–3184. doi: 10.1021/bi00712a028. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Rudikoff S., Potter M., Glaudemans C. P. Spectral changes on binding of oligosaccharides to murine immunoglobulin A myeloma proteins. Biochemistry. 1973 Jul 31;12(16):3039–3044. doi: 10.1021/bi00740a015. [DOI] [PubMed] [Google Scholar]
- Lancet D., Pecht I. Kinetic evidence for hapten-induced conformational transition in immunoglobin MOPC 460. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3549–3553. doi: 10.1073/pnas.73.10.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maeda H., Schmidt-Kessen A., Engel J., Jaton J. C. Kinetics of binding of oligosaccharides to a homogeneous pneumococcal antibody: dependence on antigen chain length suggests a labile intermediate complex. Biochemistry. 1977 Sep 6;16(18):4086–4089. doi: 10.1021/bi00637a023. [DOI] [PubMed] [Google Scholar]
- Metzger H. Effect of antigen binding on the properties of antibody. Adv Immunol. 1974;18:169–207. doi: 10.1016/s0065-2776(08)60310-7. [DOI] [PubMed] [Google Scholar]


