Abstract
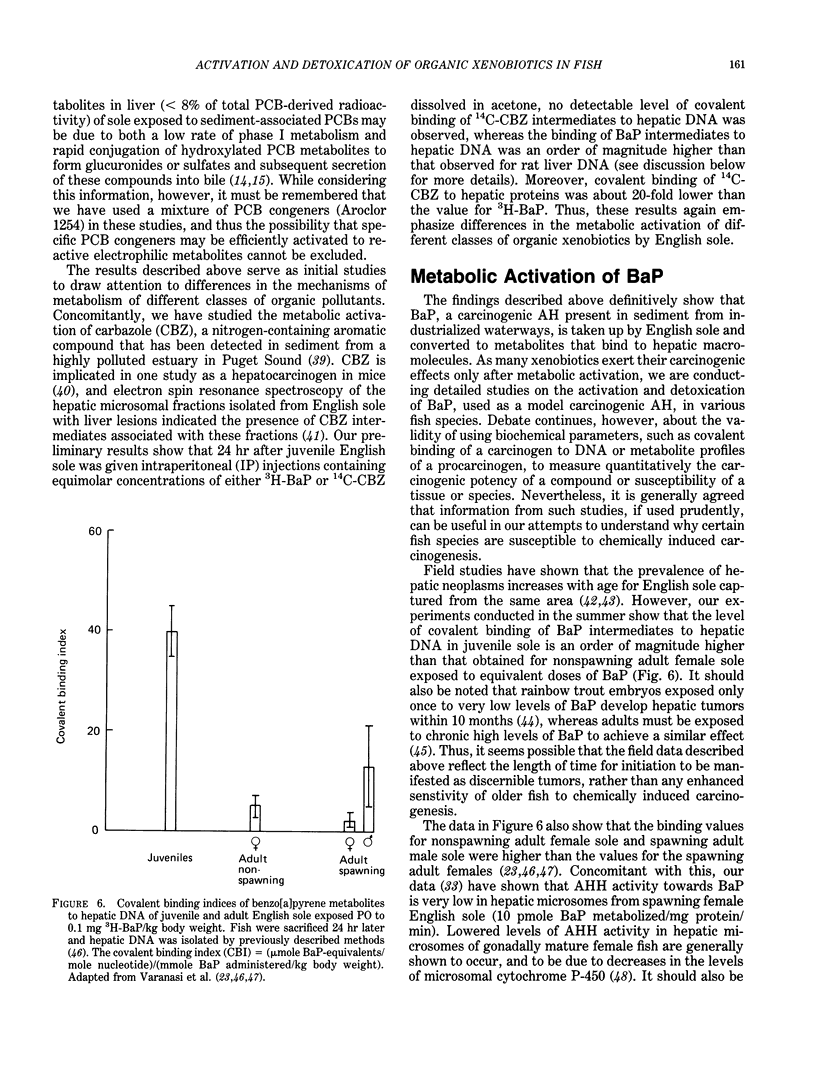
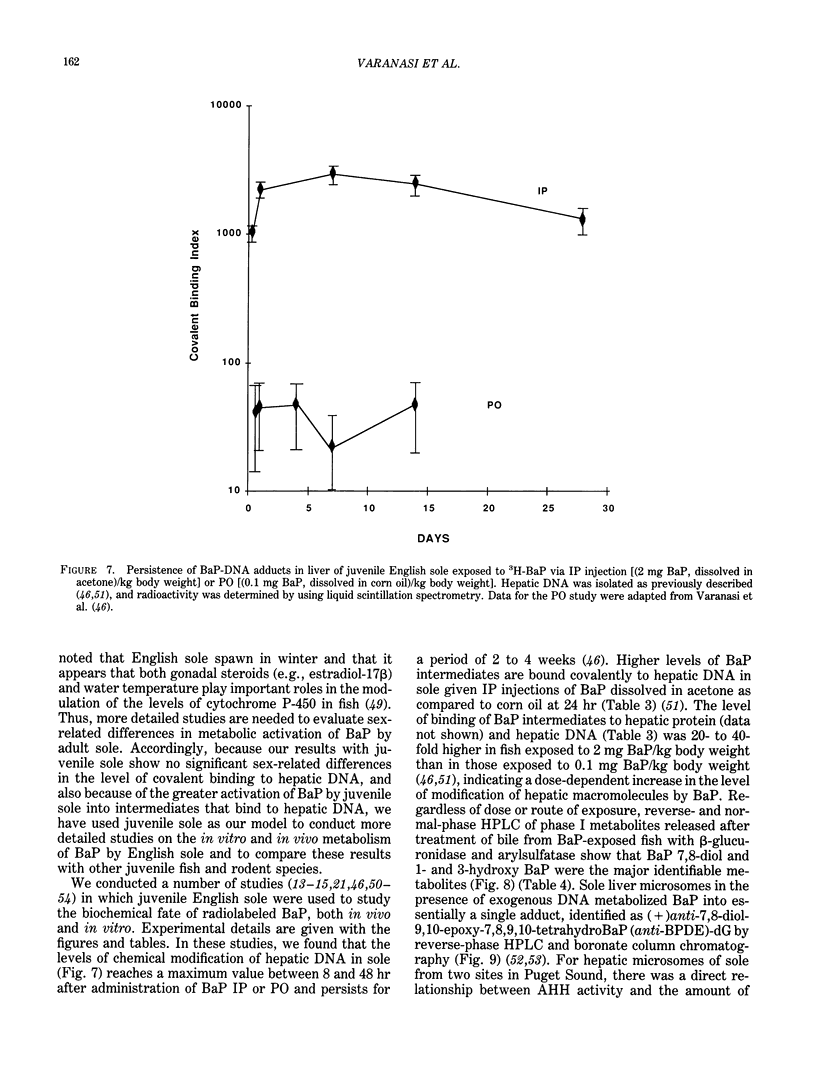
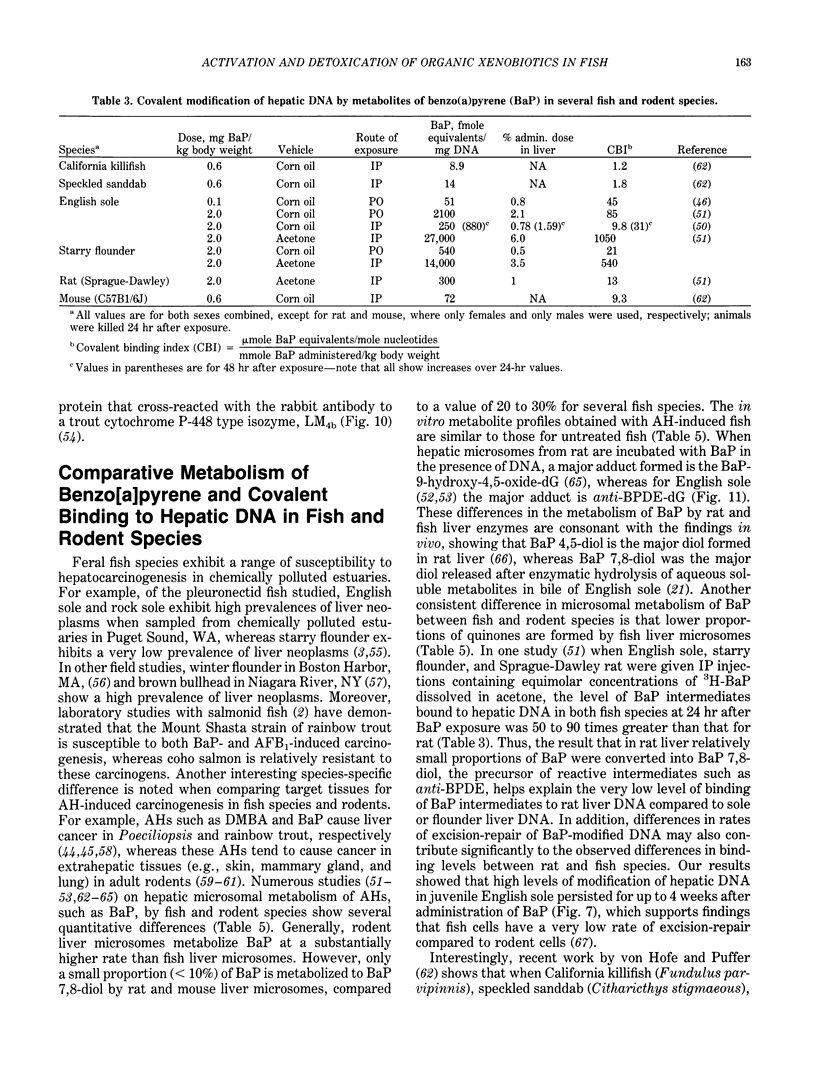
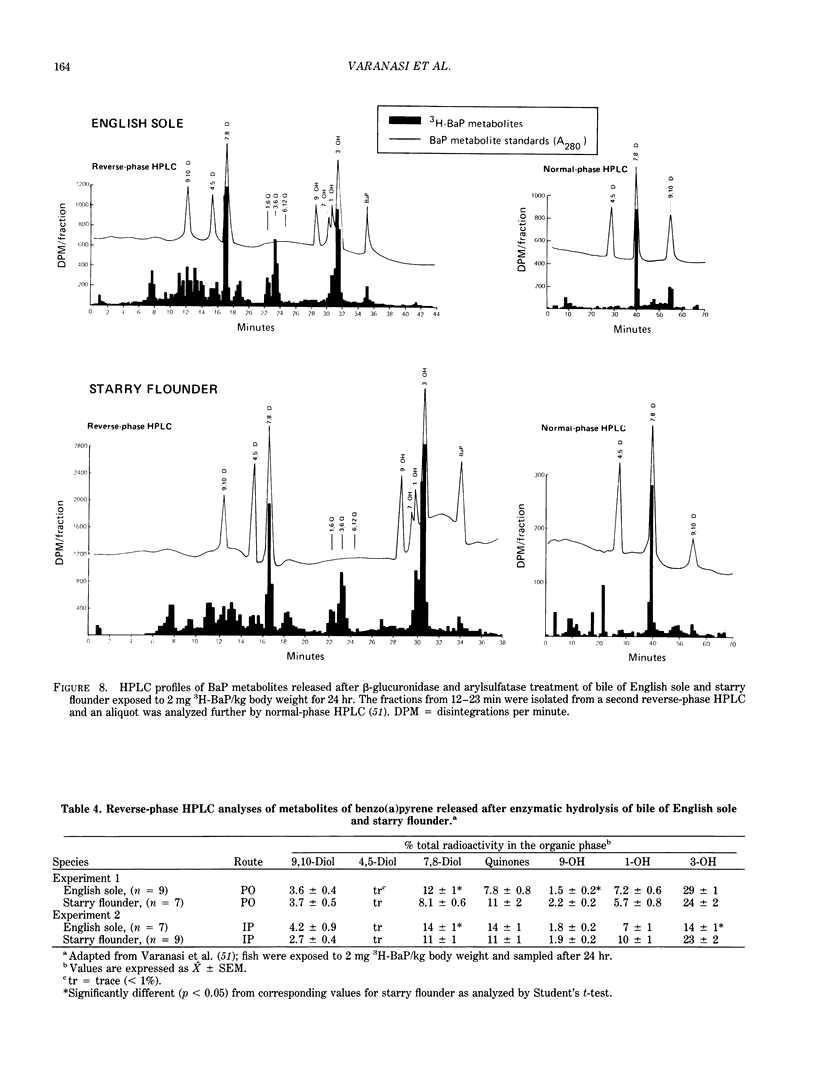
The high prevalence of liver neoplasms in English sole (Parophrys vetulus) and substantially lower prevalence of neoplasms in a closely related species, starry flounder (Platichthys stellatus) captured from industrialized waterways, provide a unique opportunity to compare biochemical processes involved in chemical carcinogenesis in feral fish species. Because levels of aromatic hydrocarbons (AHs) in urban sediments are correlated with prevalences of liver neoplasms in English sole, we have initiated detailed studies to evaluate the effects of endogenous and exogenous factors on uptake, activation and detoxication of carcinogenic AHs, such as benzo[a]pyrene (BaP), using spectroscopic, chromatographic, and radiometric techniques. The results obtained thus far show that sole readily takes up AHs associated with sediment from urban areas and that the presence of other xenobiotics, such as PCBs, in sediment increases tissue concentrations of BaP metabolites. Extensive metabolism of BaP occurred whether sole was exposed to this AH via sediment, per os, or intraperitoneally. Substantial modification of hepatic DNA occurred and persisted for a period of 2-4 weeks after a single exposure to BaP. The level of covalent binding of BaP intermediates to hepatic DNA was 10-fold higher in juvenile than adult sole and 90-fold higher in juvenile sole than in Sprague-Dawley rat, a species which is resistant to BaP-induced hepatocarcinogenesis. The level of chemical modification of hepatic DNA in juvenile flounder was 2-4 fold lower than that for juvenile sole and concentration of BaP 7,8-diol glucuronide in bile of sole was significantly higher than that in flounder bile, although the rate of formation of BaP 7,8-diol by hepatic microsomes was comparable for both species. Moreover, liver microsomes from both species, in the presence of exogenous DNA, metabolized BaP into essentially a single adduct, identified as (+)anti-7,8-diol-9,10-epoxy-7,8,9,10-tetrahydroBaP-dG. These results, along with our findings that hepatic GST activity in flounder was two times higher than in sole, demonstrate that microsomal metabolism of BaP does not accurately reflect the differences in the ability of these fish to form BaP-DNA adducts in vivo and also suggest that detoxication of reactive intermediates is an important factor in determining the levels of DNA modification by AHs and resulting toxic effects in feral fish.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Pesonen M., Johansson C. Differential induction of cytochrome P-450-dependent monooxygenase, epoxide hydrolase, glutathione transferase and UDP glucuronosyl transferase activities in the liver of the rainbow trout by beta-naphthoflavone or Clophen A50. Biochem Pharmacol. 1985 Sep 15;34(18):3309–3314. doi: 10.1016/0006-2952(85)90351-x. [DOI] [PubMed] [Google Scholar]
- Bailey G. S., Hendricks J. D., Nixon J. E., Pawlowski N. E. The sensitivity of rainbow trout and other fish to carcinogens. Drug Metab Rev. 1984;15(4):725–750. doi: 10.3109/03602538409041078. [DOI] [PubMed] [Google Scholar]
- Black J. J., Maccubbin A. E., Schiffert M. A reliable, efficient, microinjection apparatus and methodology for the in vivo exposure of rainbow trout and salmon embryos to chemical carcinogens. J Natl Cancer Inst. 1985 Dec;75(6):1123–1128. [PubMed] [Google Scholar]
- Chipman J. K., Frost G. S., Hirom P. C., Millburn P. Biliary excretion, systemic availability and reactivity of metabolites following intraportal infusion of [3H]benzo[a]pyrene in the rat. Carcinogenesis. 1981;2(8):741–745. doi: 10.1093/carcin/2.8.741. [DOI] [PubMed] [Google Scholar]
- Collier T. K., Gruger E. H., Jr, Varanasi U. Effect of Aroclor 1254 on the biological fate of 2,6-dimethylnaphthalene in coho salmon (Oncorhynchus kisutch). Bull Environ Contam Toxicol. 1985 Jan;34(1):114–120. doi: 10.1007/BF01609711. [DOI] [PubMed] [Google Scholar]
- Förlin L. Effects of clophen A50, 3-methylcholanthrene, pregnenolone-16 alpha-carbonitrile, and phenobarbital on the hepatic microsomal cytochrome P-450-dependent monooxygenase system in rainbow trout, Salmo gairdneri, of different age and sex. Toxicol Appl Pharmacol. 1980 Jul;54(3):420–430. doi: 10.1016/0041-008x(80)90169-6. [DOI] [PubMed] [Google Scholar]
- Gmur D. J., Varanasi U. Characterization of benzo[a]pyrene metabolites isolated from muscle, liver, and bile of a juvenile flatfish. Carcinogenesis. 1982;3(12):1397–1403. doi: 10.1093/carcin/3.12.1397. [DOI] [PubMed] [Google Scholar]
- Hendricks J. D., Meyers T. R., Shelton D. W., Casteel J. L., Bailey G. S. Hepatocarcinogenicity of benzo[a]pyrene to rainbow trout by dietary exposure and intraperitoneal injection. J Natl Cancer Inst. 1985 Apr;74(4):839–851. [PubMed] [Google Scholar]
- Hendricks J. D., Putnam T. P., Bills D. D., Sinnhuber R. O. Inhibitory effect of a polychlorinated biphenyl (Aroclor 1254) on aflatoxin B1 carcinogenesis in rainbow trout (Salmo gairdneri). J Natl Cancer Inst. 1977 Nov;59(5):1545–1551. doi: 10.1093/jnci/59.5.1545. [DOI] [PubMed] [Google Scholar]
- Ho D., Fahl W. E. Quantitative significance of glutathione and glutathione-S-transferase in regulating benzo[a]pyrene anti diol-epoxide level in reconstituted C3H/10T1/2 cell lysates, and comparison to rat liver. Carcinogenesis. 1984 Feb;5(2):143–148. doi: 10.1093/carcin/5.2.143. [DOI] [PubMed] [Google Scholar]
- Holder G. M., Yagi H., Jerina D. M., Levin W., Lu A. Y., Conney A. H. Metabolism of benzo[a]pyrene. Effect of substrate concentration and 3-methylcholanthrene pretreatment on hepatic metabolism by microsomes from rats and mice. Arch Biochem Biophys. 1975 Oct;170(2):557–566. doi: 10.1016/0003-9861(75)90151-4. [DOI] [PubMed] [Google Scholar]
- James M. O., Little P. J. Polyhalogenated biphenyls and phenobarbital: evaluation as inducers of drug metabolizing enzymes in the sheepshead, Archosargus probatocephalus. Chem Biol Interact. 1981 Aug;36(2):229–248. doi: 10.1016/0009-2797(81)90022-3. [DOI] [PubMed] [Google Scholar]
- Jernström B., Babson J. R., Moldéus P., Holmgren A., Reed D. J. Glutathione conjugation and DNA-binding of (+/-)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene and (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in isolated rat hepatocytes. Carcinogenesis. 1982;3(8):861–866. doi: 10.1093/carcin/3.8.861. [DOI] [PubMed] [Google Scholar]
- Jernström B., Martinez M., Meyer D. J., Ketterer B. Glutathione conjugation of the carcinogenic and mutagenic electrophile (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-oxy-7,8,9,10-tetra hydrobenzo[a]pyrene catalyzed by purified rat liver glutathione transferases. Carcinogenesis. 1985 Jan;6(1):85–89. doi: 10.1093/carcin/6.1.85. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Myers M. S., Burrows D. G., Malins D. C. Determination of metabolites of xenobiotics in the bile of fish from polluted waterways. Xenobiotica. 1984 Aug;14(8):633–646. doi: 10.3109/00498258409151461. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Rhodes L. D., Myers M. S., Moore L. K., MacLeod W. D., Jr, Malins D. C. Associations between metabolites of aromatic compounds in bile and the occurrence of hepatic lesions in English sole (Parophrys vetulus) from Puget Sound, Washington. Arch Environ Contam Toxicol. 1986 Jan;15(1):61–67. doi: 10.1007/BF01055249. [DOI] [PubMed] [Google Scholar]
- Little P. J., James M. O., Pritchard J. B., Bend J. R. Benzo(a)pyrene metabolism in hepatic microsomes from feral and 3-methycholanthrene-treated southern flounder, Paralichthys lethostigma. J Environ Pathol Toxicol Oncol. 1984 Jul;5(4-5):309–320. [PubMed] [Google Scholar]
- Lutz W. K. In vivo covalent binding of organic chemicals to DNA as a quantitative indicator in the process of chemical carcinogenesis. Mutat Res. 1979 Dec;65(4):289–356. doi: 10.1016/0165-1110(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Malins D. C., Krahn M. M., Brown D. W., Rhodes L. D., Myers M. S., McCain B. B., Chan S. L. Toxic chemicals in marine sediment and biota from Mukilteo, Washington: relationships with hepatic neoplasms and other hepatic lesions in English sole (Parophrys vetulus). J Natl Cancer Inst. 1985 Feb;74(2):487–494. [PubMed] [Google Scholar]
- Malins D. C., Krahn M. M., Myers M. S., Rhodes L. D., Brown D. W., Krone C. A., McCain B. B., Chan S. L. Toxic chemicals in sediments and biota from a creosote-polluted harbor: relationships with hepatic neoplasms and other hepatic lesions in English sole (Parophrys vetulus). Carcinogenesis. 1985 Oct;6(10):1463–1469. doi: 10.1093/carcin/6.10.1463. [DOI] [PubMed] [Google Scholar]
- Malins D. C., McCain B. B., Myers M. S., Brown D. W., Krahn M. M., Roubal W. T., Schiewe M. H., Landahl J. T., Chan S. L. Field and laboratory studies of the etiology of liver neoplasms in marine fish from Puget Sound. Environ Health Perspect. 1987 Apr;71:5–16. doi: 10.1289/ehp.87715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchelano R. A., Wolke R. E. Epizootic Carcinoma in the Winter Flounder, Pseudopleuronectes americanus. Science. 1985 May 3;228(4699):587–589. doi: 10.1126/science.228.4699.587. [DOI] [PubMed] [Google Scholar]
- Niranjan B. G., Avadhani N. G., DiGiovanni J. Formation of benzo(alpha)pyrene metabolites and DNA adducts catalyzed by a rat liver mitochondrial monooxygenase system. Biochem Biophys Res Commun. 1985 Sep 16;131(2):935–942. doi: 10.1016/0006-291x(85)91329-4. [DOI] [PubMed] [Google Scholar]
- Nishimoto M., Varanasi U. Benzo[a]pyrene metabolism and DNA adduct formation mediated by English sole liver enzymes. Biochem Pharmacol. 1985 Jan 15;34(2):263–268. doi: 10.1016/0006-2952(85)90134-0. [DOI] [PubMed] [Google Scholar]
- Plummer J. L., Smith B. R., Ball L. M., Bend J. R. Metabolism and biliary excretion of benzo[a]pyrene 4,5-oxide in the rat. Drug Metab Dispos. 1980 Mar-Apr;8(2):68–72. [PubMed] [Google Scholar]
- Preston B. D., Miller J. A., Miller E. C. Non-arene oxide aromatic ring hydroxylation of 2,2',5,5'-tetrachlorobiphenyl as the major metabolic pathway catalyzed by phenobarbital-induced rat liver microsomes. J Biol Chem. 1983 Jul 10;258(13):8304–8311. [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action. Crit Rev Toxicol. 1984;13(4):319–395. doi: 10.3109/10408448409023762. [DOI] [PubMed] [Google Scholar]
- Schnell J. V., Gruger E. H., Jr, Malins D. C. Mono-oxygenase activities of coho salmon (Oncorhynchus kisutch) liver microsomes using three polycyclic aromatic hydrocarbon substrates. Xenobiotica. 1980 Mar;10(3):229–234. doi: 10.3109/00498258009033749. [DOI] [PubMed] [Google Scholar]
- Shen A. L., Fahl W. E., Jefcoate C. R. Metabolism of benzo(a)pyrene by isolated hepatocytes and factors affecting covalent binding of benzo(a)pyrene metabolites to DNA in hepatocyte and microsomal systems. Arch Biochem Biophys. 1980 Oct 15;204(2):511–523. doi: 10.1016/0003-9861(80)90063-6. [DOI] [PubMed] [Google Scholar]
- Smithgall T. E., Penning T. M. Inhibition of trans-dihydrodiol oxidation by the non-steroidal anti-inflammatory drugs. Carcinogenesis. 1986 Apr;7(4):583–588. doi: 10.1093/carcin/7.4.583. [DOI] [PubMed] [Google Scholar]
- Statham C. N., Elcombe C. R., Szyjka S. P., Lech J. J. Effect of polycyclic aromatic hydrocarbons on hepatic microsomal enzymes and disposition of methylnaphthalene in rainbow trout in vivo. Xenobiotica. 1978 Feb;8(2):65–71. doi: 10.3109/00498257809060385. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Hagiwara A., Shibata M., Ohshima M., Ito N. Carcinogenic effect of carbazole in the liver of (C57BL/6N x C3H/HeN)F1 mice. J Natl Cancer Inst. 1982 Dec;69(6):1383–1389. [PubMed] [Google Scholar]
- Van Cantfort J., Gielen J. E., Nebert D. W. Benzo[a]pyrene metabolism in mouse liver. Association of both 7,8-epoxidation and covalent binding of a metabolite of the 7,8-diol with the Ah locus. Biochem Pharmacol. 1985 May 15;34(10):1821–1826. doi: 10.1016/0006-2952(85)90655-0. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Collier T. K., Williams D. E., Buhler D. R. Hepatic cytochrome P-450 isozymes and aryl hydrocarbon hydroxylase in English sole (Parophrys vetulus). Biochem Pharmacol. 1986 Sep 1;35(17):2967–2971. doi: 10.1016/0006-2952(86)90494-6. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Nishimoto M., Reichert W. L., Le Eberhart B. T. Comparative metabolism of benzo(a)pyrene and covalent binding to hepatic DNA in English sole, starry flounder, and rat. Cancer Res. 1986 Aug;46(8):3817–3824. [PubMed] [Google Scholar]
- Varanasi U., Nishimoto M., Reichert W. L., Stein J. E. Metabolism and subsequent covalent binding of benzo[a]pyrene to macromolecules in gonads and liver of ripe english sole (Parophrys vetulus). Xenobiotica. 1982 Jul;12(7):417–425. doi: 10.3109/00498258209052483. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Stein J. E., Hom T. Covalent binding of benzo[a]pyrene to dna in fish liver. Biochem Biophys Res Commun. 1981 Nov 30;103(2):780–787. doi: 10.1016/0006-291x(81)90517-9. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Uhler M., Stranahan S. I. Uptake and release of naphthalene and its metabolites in skin and epidermal mucus of salmonids. Toxicol Appl Pharmacol. 1978 May;44(2):277–289. doi: 10.1016/0041-008x(78)90190-4. [DOI] [PubMed] [Google Scholar]
- Wogan G. N., Gorelick N. J. Chemical and biochemical dosimetry of exposure to genotoxic chemicals. Environ Health Perspect. 1985 Oct;62:5–18. doi: 10.1289/ehp.85625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofe E., Puffer H. W. In vitro metabolism and in vivo binding of benzo(a)pyrene in the California killifish (Fundulus parvipinnis) and speckled sanddab (Citharicthys stigmaeous). Arch Environ Contam Toxicol. 1986 May;15(3):251–256. doi: 10.1007/BF01061101. [DOI] [PubMed] [Google Scholar]


