Abstract
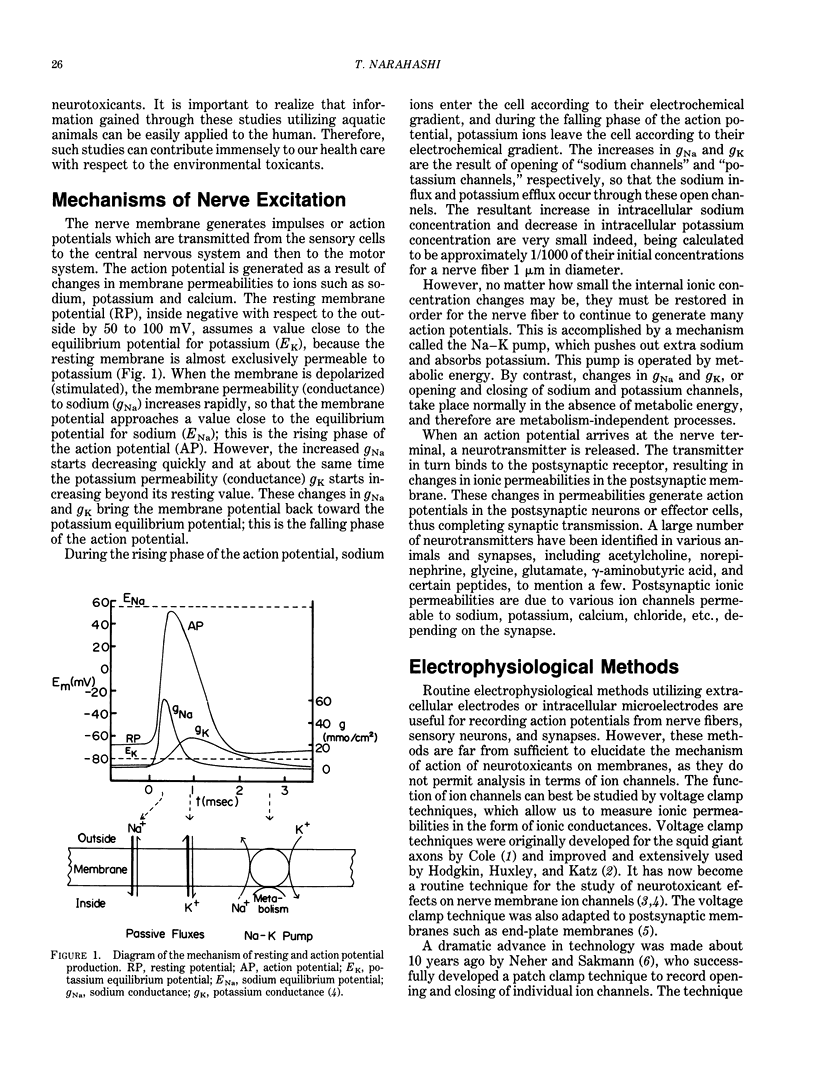
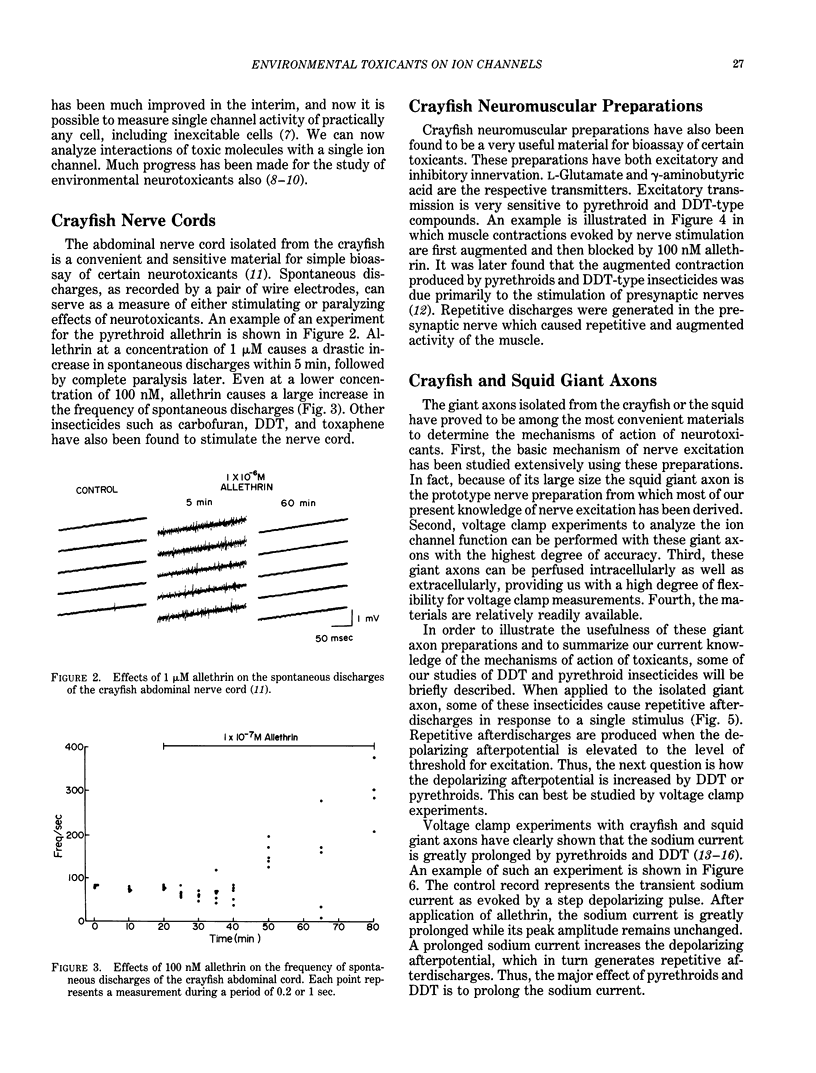
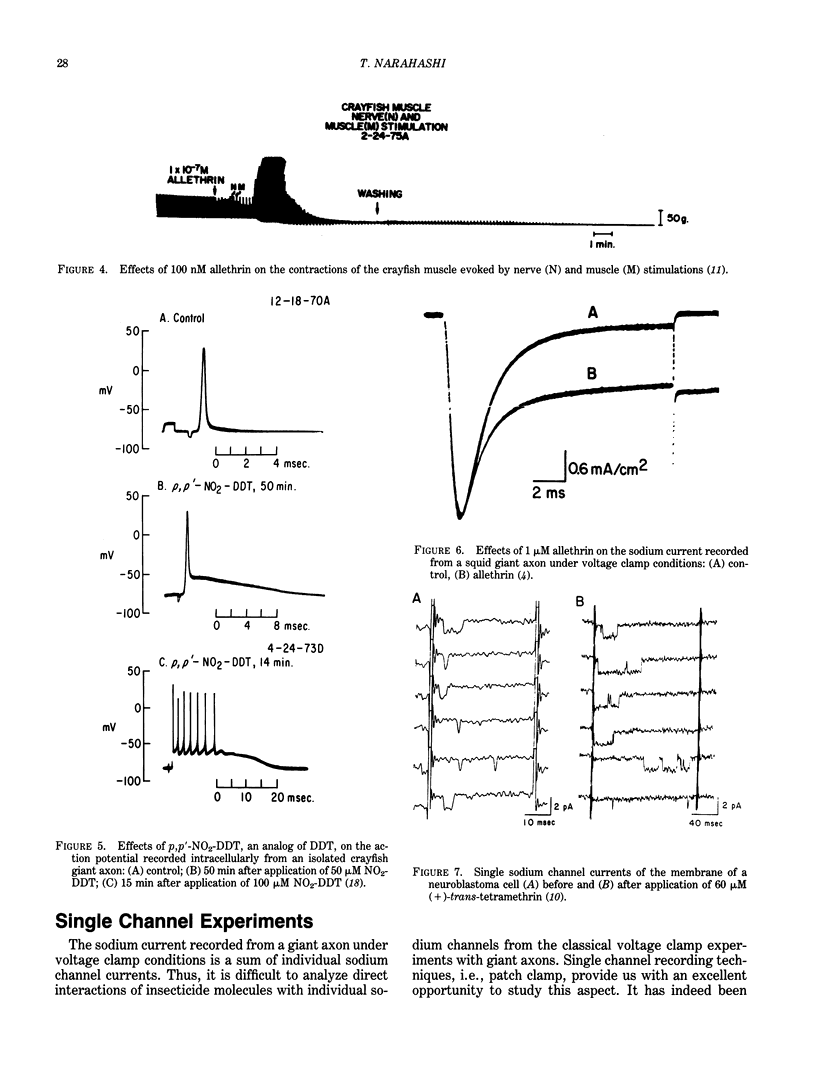
There are many environmentally important chemicals which exhibit potent effects on the nervous system. Examples include insecticides such as pyrethroids, DDT, cyclodienes, organophosphates and carbamates, and heavy metals such as mercury and lead. Since nerve excitation takes place in a fraction of a second, electrophysiological methods provide us with the most straightforward approach to the study of the mechanisms of action of environmental toxicants on the nervous system. Aquatic animals such as crayfish, lobster, squid, and marine snails represent extremely useful materials for such electrophysiological studies, because much of our knowledge of nerve excitation is derived from those animals. Nerve excitation takes place as a result of opening and closing of ion channels of the membrane. These functions are independent of metabolic energy, and can be measured most effectively by voltage clamp techniques as applied to the giant axons of the crayfish and the squid. Patch clamp techniques developed during the past 10 years have added a new dimension to the electrophysiological investigation. These techniques allow us to measure the activity of individual ion channels, thereby making it possible to analyze the interaction of toxic molecules directly with single ion channels. Examples are given summarizing electrophysiological studies of environmental neurotoxicants. The abdominal nerve cords and neuromuscular preparations isolated from the crayfish are convenient materials for bioassay of certain environmental toxicants such as pyrethroids, chlorinated hydrocarbons, and other insecticides. Detailed voltage clamp and patch clamp analyses have revealed that pyrethroids and DDT modify the sodium channel to remain open for an extended period of time. This change causes an increase in depolarizing afterpotential which reaches the threshold for repetitive discharges to be produced.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinn K., Narahashi T. Stabilization of sodium channel states by deltamethrin in mouse neuroblastoma cells. J Physiol. 1986 Nov;380:191–207. doi: 10.1113/jphysiol.1986.sp016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A. E., Narahashi T. Dose-dependent interaction of the pyrethroid isomers with sodium channels of squid axon membranes. Neurotoxicology. 1982 Jul;3(1):11–24. [PubMed] [Google Scholar]
- Narahashi T. Nerve membrane ionic channels as the primary target of pyrethroids. Neurotoxicology. 1985 Summer;6(2):3–22. [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Quandt F. N., Narahashi T. Modification of single sodium channels by the insecticide tetramethrin. Brain Res. 1983 Sep 12;274(2):344–349. doi: 10.1016/0006-8993(83)90716-3. [DOI] [PubMed] [Google Scholar]


