Abstract
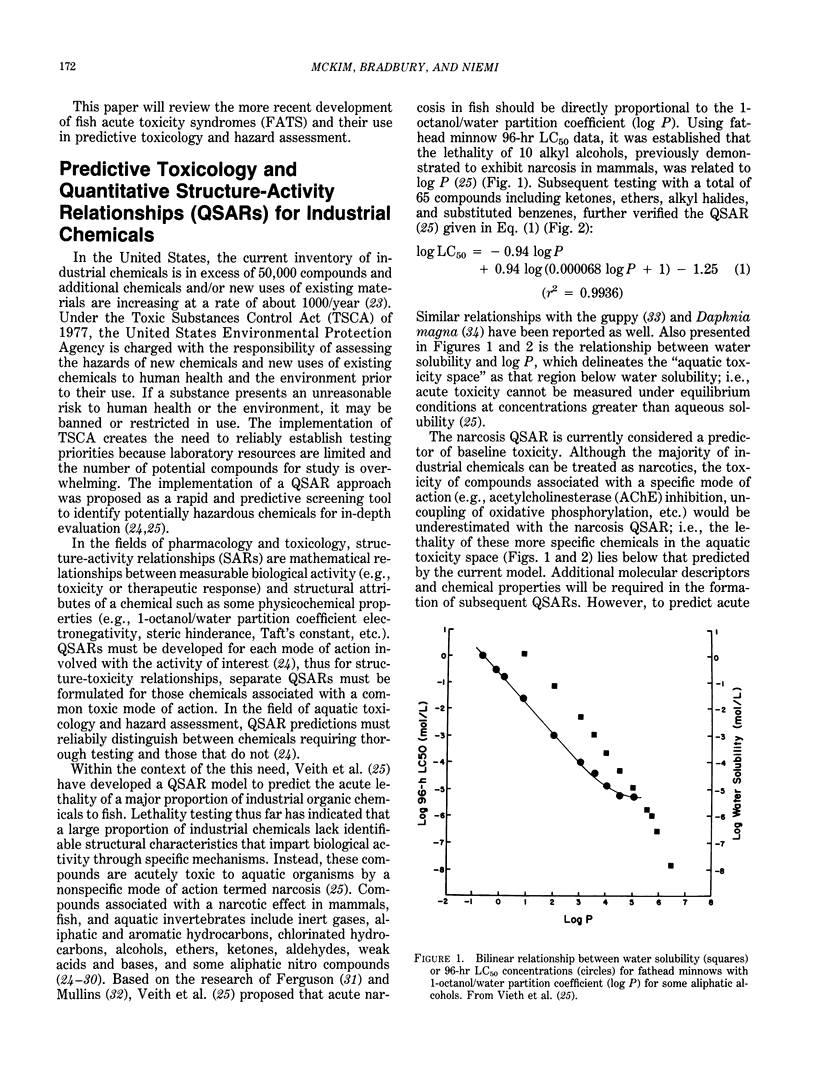
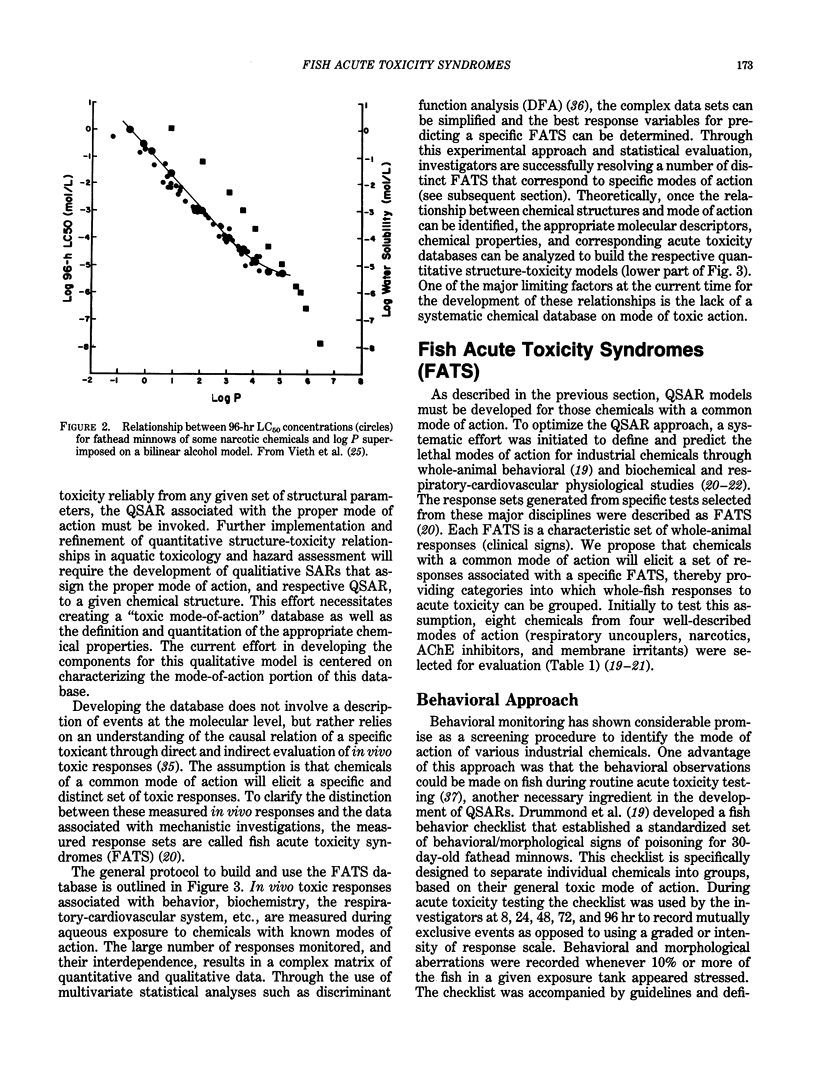
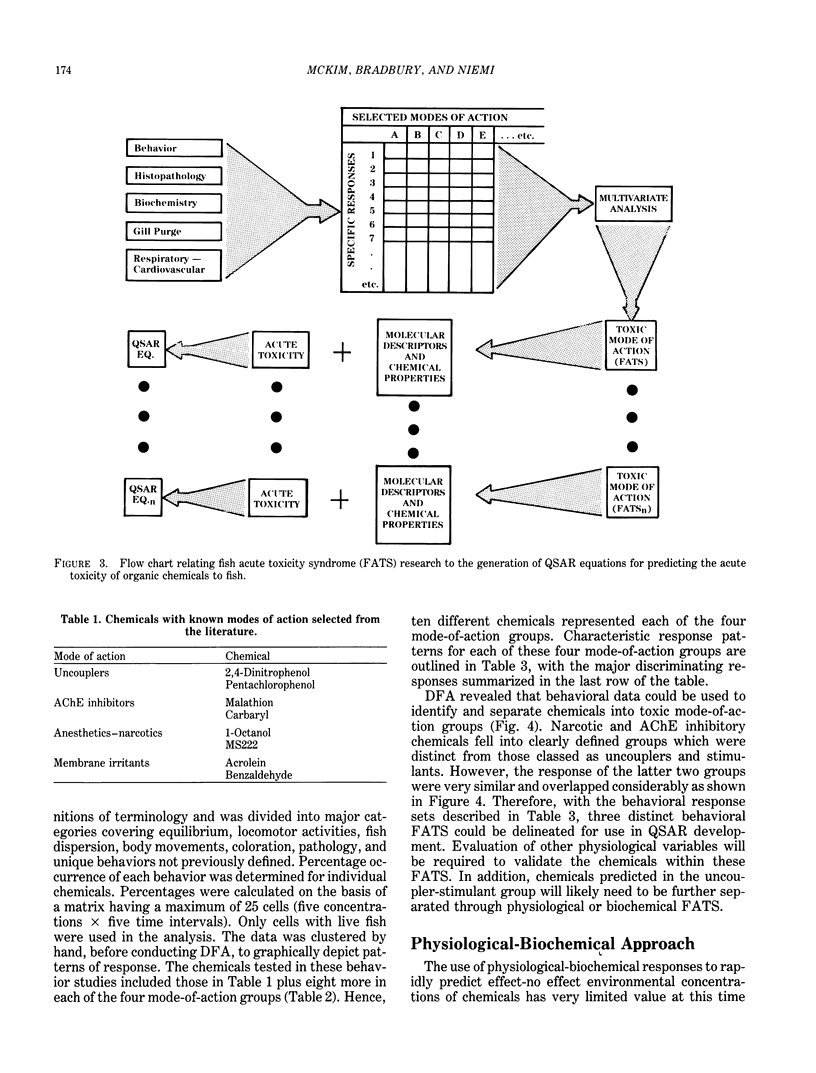
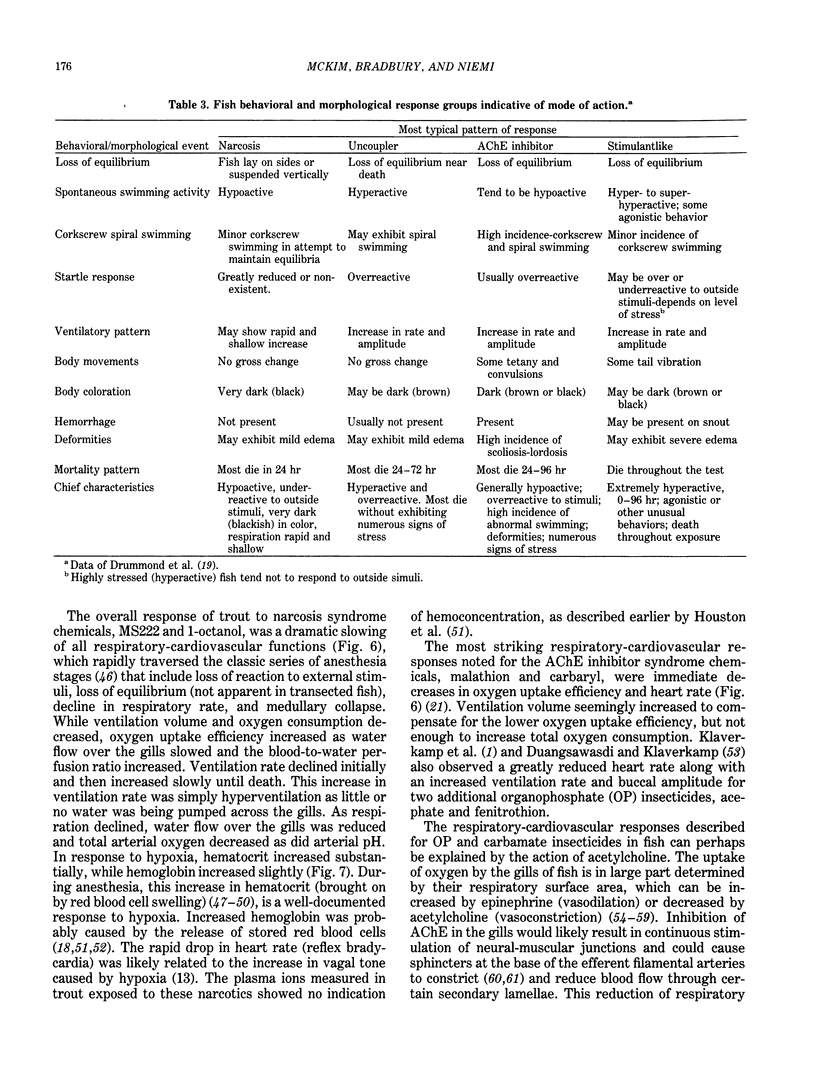
Implementation of the Toxic Substances Control Act of 1977 creates the need to reliably establish testing priorities because laboratory resources are limited and the number of industrial chemicals requiring evaluation is overwhelming. The use of quantitative structure activity relationship (QSAR) models as rapid and predictive screening tools to select more potentially hazardous chemicals for in-depth laboratory evaluation has been proposed. Further implementation and refinement of quantitative structure-toxicity relationships in aquatic toxicology and hazard assessment requires the development of a "mode-of-action" database. With such a database, a qualitative structure-activity relationship can be formulated to assign the proper mode of action, and respective QSAR, to a given chemical structure. In this review, the development of fish acute toxicity syndromes (FATS), which are toxic-response sets based on various behavioral and physiological-biochemical measurements, and their projected use in the mode-of-action database are outlined. Using behavioral parameters monitored in the fathead minnow during acute toxicity testing, FATS associated with acetylcholinesterase (AChE) inhibitors and narcotics could be reliably predicted. However, compounds classified as oxidative phosphorylation uncouplers or stimulants could not be resolved. Refinement of this approach by using respiratory-cardiovascular responses in the rainbow trout, enabled FATS associated with AChE inhibitors, convulsants, narcotics, respiratory blockers, respiratory membrane irritants, and uncouplers to be correctly predicted.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casida J. E., Gammon D. W., Glickman A. H., Lawrence L. J. Mechanisms of selective action of pyrethroid insecticides. Annu Rev Pharmacol Toxicol. 1983;23:413–438. doi: 10.1146/annurev.pa.23.040183.002213. [DOI] [PubMed] [Google Scholar]
- Cole L. M., Lawrence L. J., Casida J. E. Similar properties of [35S]t-butylbicyclophosphorothionate receptor and coupled components of the GABA receptor-ionophore complex in brains of human, cow, rat, chicken and fish. Life Sci. 1984 Oct 22;35(17):1755–1762. doi: 10.1016/0024-3205(84)90272-8. [DOI] [PubMed] [Google Scholar]
- Cremer J. E., Seville M. P. Comparative effects of two pyrethroids, deltamethrin and cismethrin, on plasma catecholamines and on blood glucose and lactate. Toxicol Appl Pharmacol. 1982 Oct;66(1):124–133. doi: 10.1016/0041-008x(82)90067-9. [DOI] [PubMed] [Google Scholar]
- Dunel S., Laurent P. L vascularisation branchiale chea l'Anguille: action de l'acétylcholine et de l'adrénaline sur la répartition d'une résine polymérisable dans les différents compartiments vasculaires. C R Acad Sci Hebd Seances Acad Sci D. 1977 May 23;284(20):2011–2014. [PubMed] [Google Scholar]
- Farrell A. P., Sobin S. S., Randall D. J., Crosby S. Intralamellar blood flow patterns in fish gills. Am J Physiol. 1980 Nov;239(5):R428–R436. doi: 10.1152/ajpregu.1980.239.5.R428. [DOI] [PubMed] [Google Scholar]
- Hesser C. M., Fagraeus L., Adolfson J. Roles of nitrogen, oxygen, and carbon dioxide in compressed-air narcosis. Undersea Biomed Res. 1978 Dec;5(4):391–400. [PubMed] [Google Scholar]
- Hughes G. M. Morphometrics of fish gills. Respir Physiol. 1972 Mar;14(1):1–25. doi: 10.1016/0034-5687(72)90014-x. [DOI] [PubMed] [Google Scholar]
- Könemann H. Quantitative structure-activity relationships in fish toxicity studies. Part 1: relationship for 50 industrial pollutants. Toxicology. 1981;19(3):209–221. doi: 10.1016/0300-483x(81)90130-x. [DOI] [PubMed] [Google Scholar]
- Lawrence L. J., Casida J. E. Stereospecific action of pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore complex. Science. 1983 Sep 30;221(4618):1399–1401. doi: 10.1126/science.6310756. [DOI] [PubMed] [Google Scholar]
- Mehrle P. M., Mayer F. L. Clinical tests in aquatic toxicology: state of the art. Environ Health Perspect. 1980 Feb;34:139–143. doi: 10.1289/ehp.8034139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. W., Conway M. W. Rotenone induced blood respiratory changes in the green sunfish, Lepomis cyanellus. Comp Biochem Physiol C. 1977;56(2):123–126. doi: 10.1016/0306-4492(77)90026-0. [DOI] [PubMed] [Google Scholar]
- Pfuderer P., Francis A. A. Phthalate esters: heartrate depressors in the goldfish. Bull Environ Contam Toxicol. 1975 Mar;13(3):275–279. doi: 10.1007/BF01685335. [DOI] [PubMed] [Google Scholar]
- Pfuderer P., Janzen S., Rainey W. T., Jr The identification of phthalic acid esters in the tissues of cyprinodont fish and their activity as heartrate depressors. Environ Res. 1975 Jun;9(3):215–223. doi: 10.1016/0013-9351(75)90002-x. [DOI] [PubMed] [Google Scholar]
- Pfuderer P., Williams P., Francis A. A. Partial purification of the crowding factor from Carassius auratus and Cyprinus carpio. J Exp Zool. 1974 Mar;187(3):375–382. doi: 10.1002/jez.1401870306. [DOI] [PubMed] [Google Scholar]
- RANDALL D. J., SHELTON G. THE EFFECTS OF CHANGES IN ENVIRONMENTAL GAS CONCENTRATIONS ON THE BREATHING AND HEART RATE OF A TELEOST FISH. Comp Biochem Physiol. 1963 Jul;9:229–239. doi: 10.1016/0010-406x(63)90046-x. [DOI] [PubMed] [Google Scholar]
- Ray D. E. An EEG investigation of decamethrin-induced choreoathetosis in the rat. Exp Brain Res. 1980 Jan;38(2):221–227. doi: 10.1007/BF00236743. [DOI] [PubMed] [Google Scholar]
- Roth S. H. Membrane and cellular actions of anesthetic agents. Fed Proc. 1980 Apr;39(5):1595–1599. [PubMed] [Google Scholar]
- STEEN J. B., KRUYSSE A. THE RESPIRATORY FUNCTION OF TELEOSTEAN GILLS. Comp Biochem Physiol. 1964 Jun;12:127–142. doi: 10.1016/0010-406x(64)90168-9. [DOI] [PubMed] [Google Scholar]
- Sastry K. V., Siddiqui A. A. Chronic toxic effects of the carbamate pesticide sevin on carbohydrate metabolism in a freshwater snakehead fish, Channa punctatus. Toxicol Lett. 1982 Nov;14(1-2):123–130. doi: 10.1016/0378-4274(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Veith G. D., De Foe D., Knuth M. Structure-activity relationships for screening organic chemicals for potential ecotoxicity effects. Drug Metab Rev. 1984;15(7):1295–1303. doi: 10.3109/03602538409029961. [DOI] [PubMed] [Google Scholar]
- Wood C. M. A pharmacological analysis of the adrenergic and cholinergic mechanisms regulating branchial vascular resistance in the rainbow trout (Salmo gairdneri). Can J Zool. 1975 Nov;53(11):1569–1577. doi: 10.1139/z75-191. [DOI] [PubMed] [Google Scholar]


