Abstract
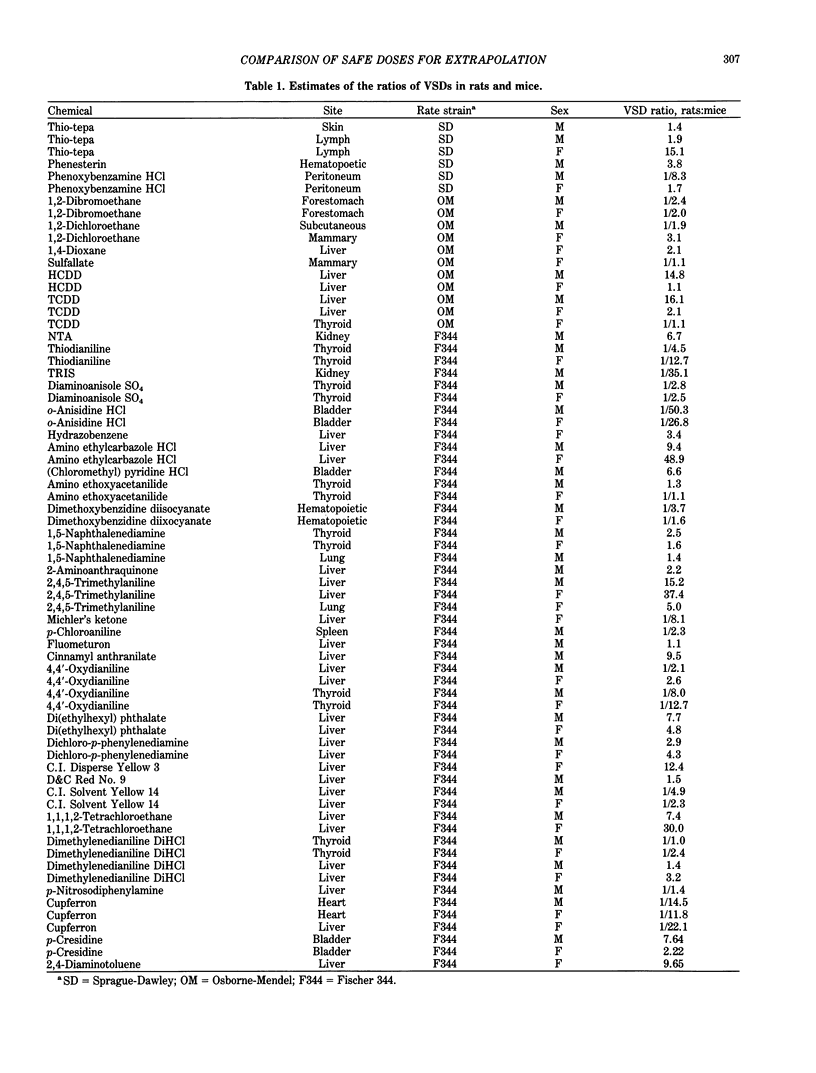
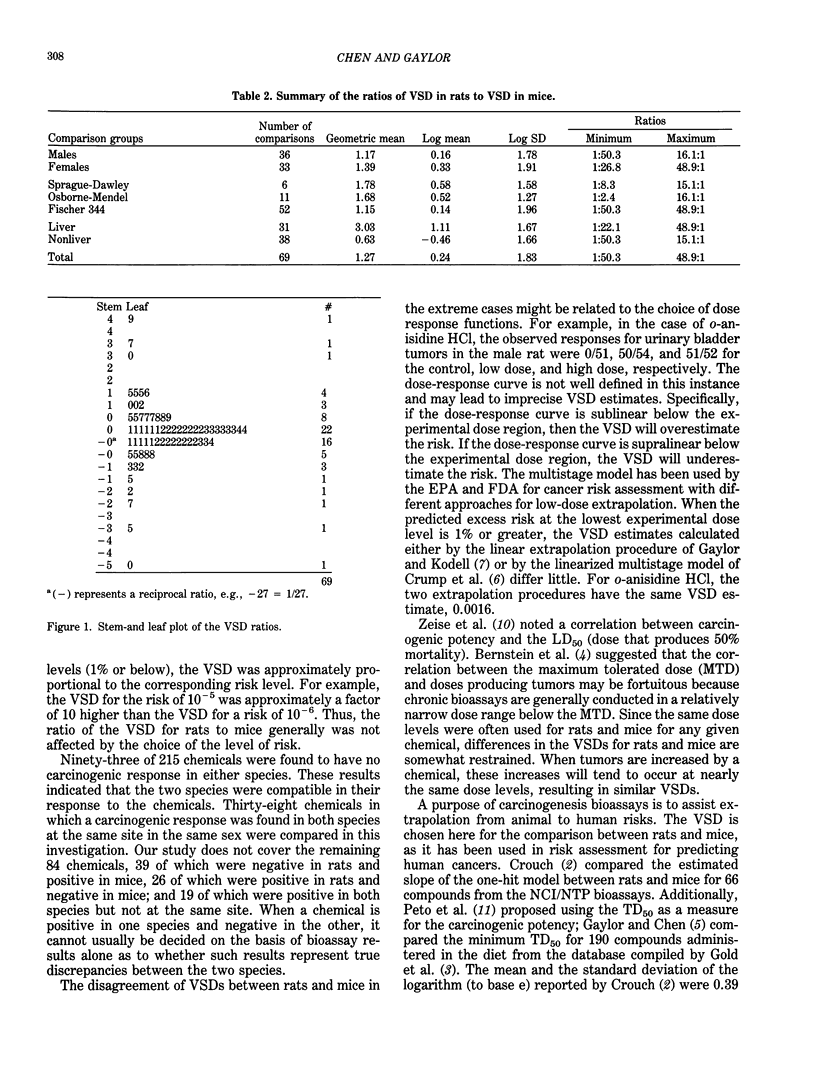
Data from the National Cancer Institute/National Toxicology Program (NCI/NTP) carcinogenesis bioassays were examined to compare cancer risks in rats and mice. Only those bioassays where chemicals were administered orally were used. The ratios for rats to mice of the virtually safe dose (VSD) levels associated with a risk of 10(-6) were compared. Comparisons of the ratios were made for those chemicals that NCI/NTP determined to be carcinogenic in at least one species and that showed a dose response trend in the same sex at the same tissue/organ site in the other species. In all, 69 comparisons from 38 carcinogens were performed. The overall geometric mean of the VSD ratios is 1.27 in terms of concentration (ppm); the mean and the standard deviation in logarithm are 0.24 and 1.83, respectively. The VSD ratios vary from 1:51 to 49:1. Without the restriction of the same sex and site, the geometric mean of the minimum VSDs is 1.38, and the standard deviation in logarithm is 1.79. By directly comparing the VSDs for rats and mice (as they are performed for risk assessment), this study showed a probability of 0.10 that the ratio of VSDs is greater than 10, and the ratio is greater than 20 with a probability of 0.05 when a chemical is carcinogenic in both species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crouch E. A. Uncertainties in interspecies extrapolations of carcinogenicity. Environ Health Perspect. 1983 Apr;50:321–327. doi: 10.1289/ehp.8350321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Wilson R. Interspecies comparison of carcionogenic potency. J Toxicol Environ Health. 1979 Nov;5(6):1095–1118. doi: 10.1080/15287397909529817. [DOI] [PubMed] [Google Scholar]
- Crump K. S., Guess H. A., Deal K. L. Confidence intervals and test of hypotheses concerning dose response relations inferred from animal carcinogenicity data. Biometrics. 1977 Sep;33(3):437–451. [PubMed] [Google Scholar]
- Gaylor D. W., Chen J. J. Relative potency of chemical carcinogens in rodents. Risk Anal. 1986 Sep;6(3):283–290. doi: 10.1111/j.1539-6924.1986.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Gaylor D. W., Kodell R. L. Linear interpolation algorithm for low dose risk assessment of toxic substances. J Environ Pathol Toxicol. 1980 Nov;4(5-6):305–312. [PubMed] [Google Scholar]
- Gold L. S., Sawyer C. B., Magaw R., Backman G. M., de Veciana M., Levinson R., Hooper N. K., Havender W. R., Bernstein L., Peto R. A carcinogenic potency database of the standardized results of animal bioassays. Environ Health Perspect. 1984 Dec;58:9–319. doi: 10.1289/ehp.84589. [DOI] [PMC free article] [PubMed] [Google Scholar]


