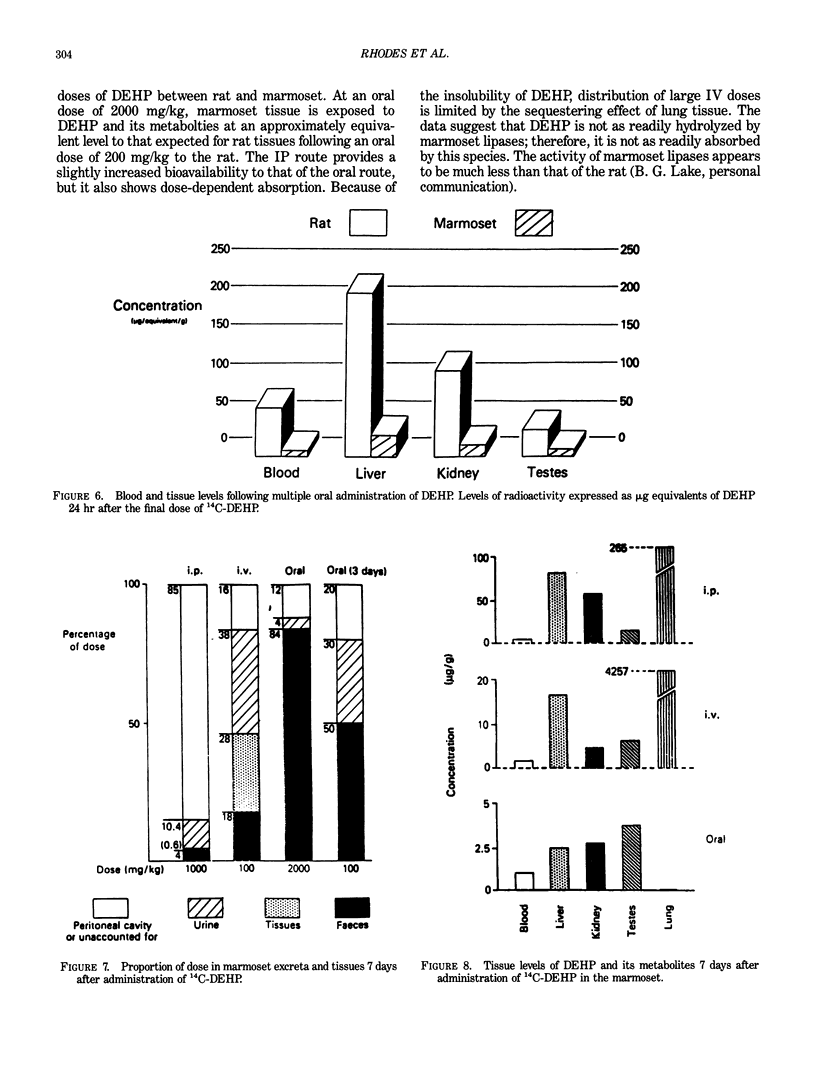
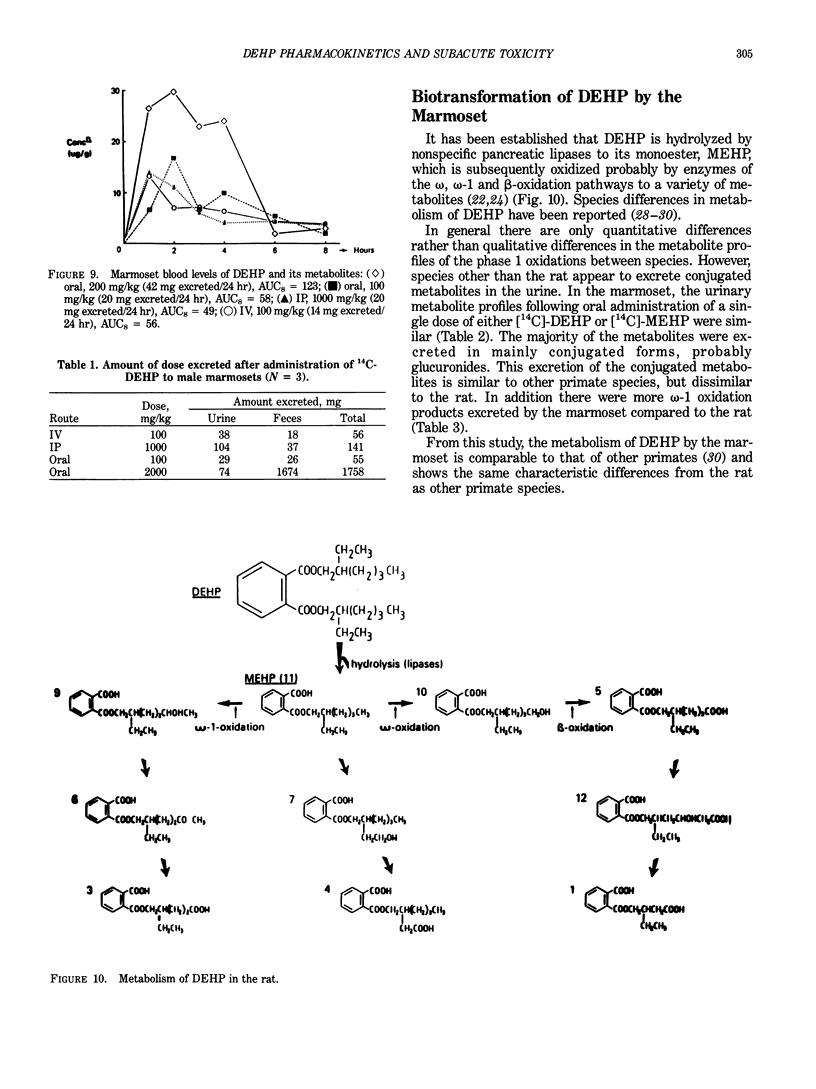
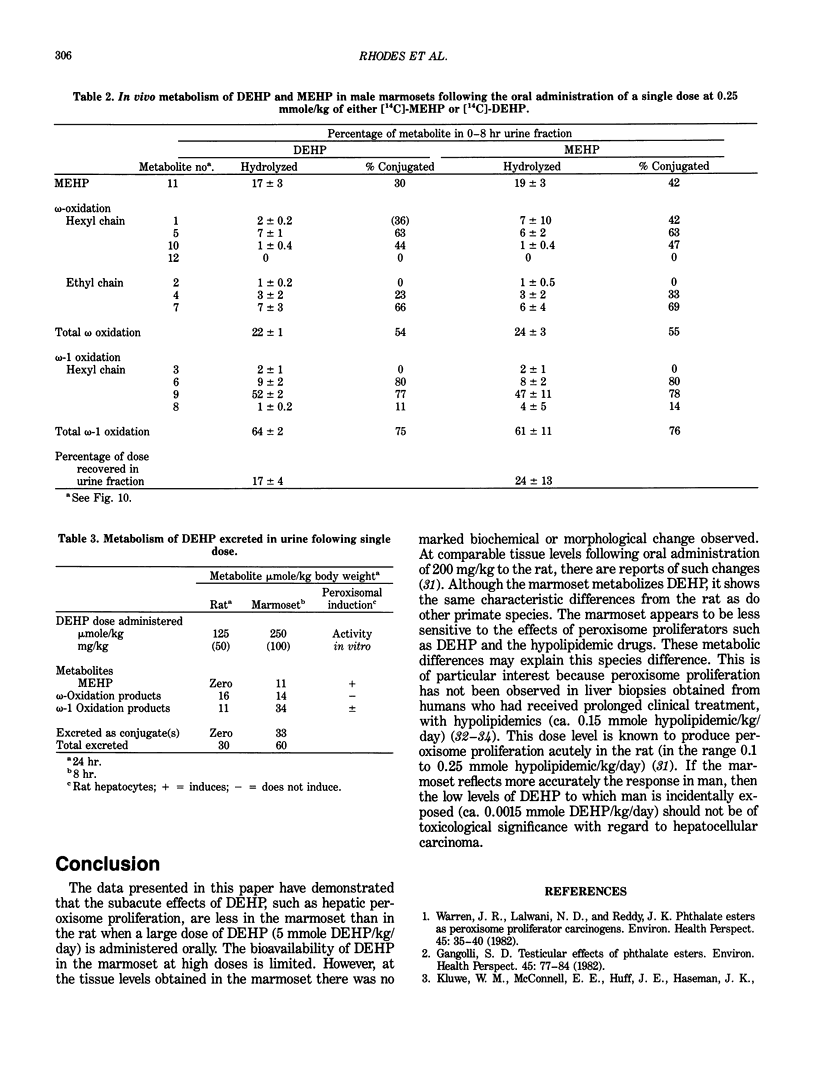
Abstract
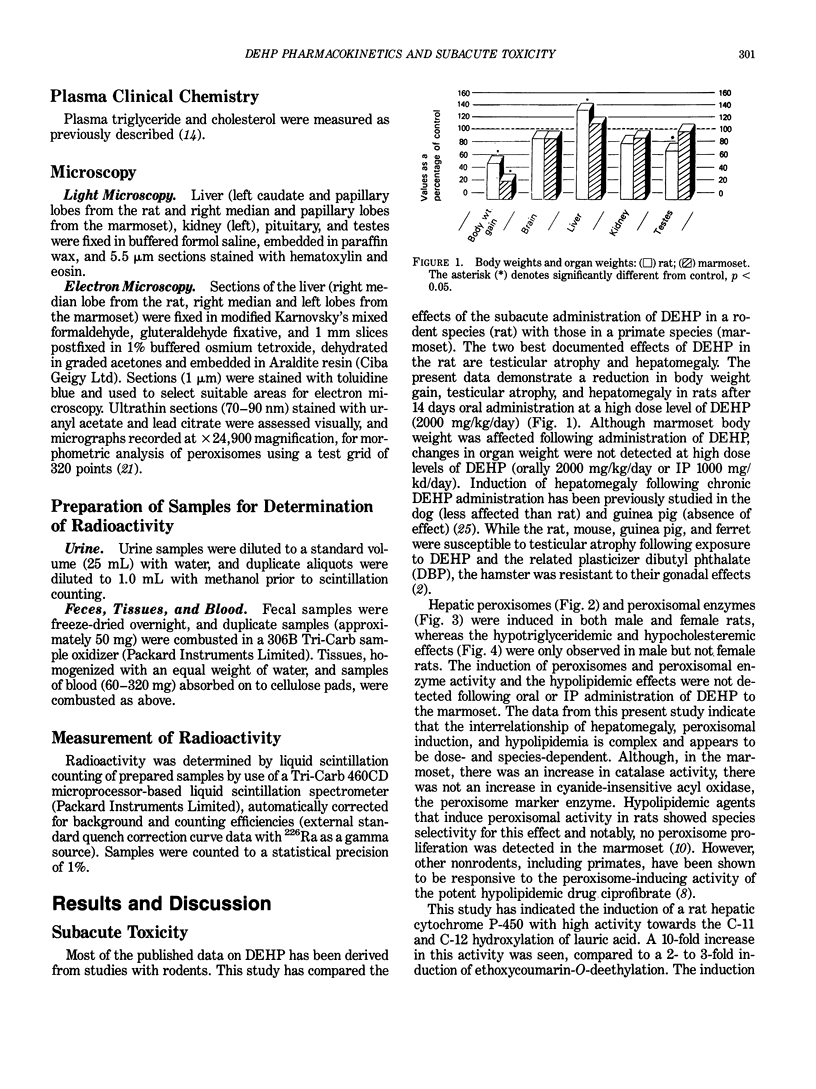
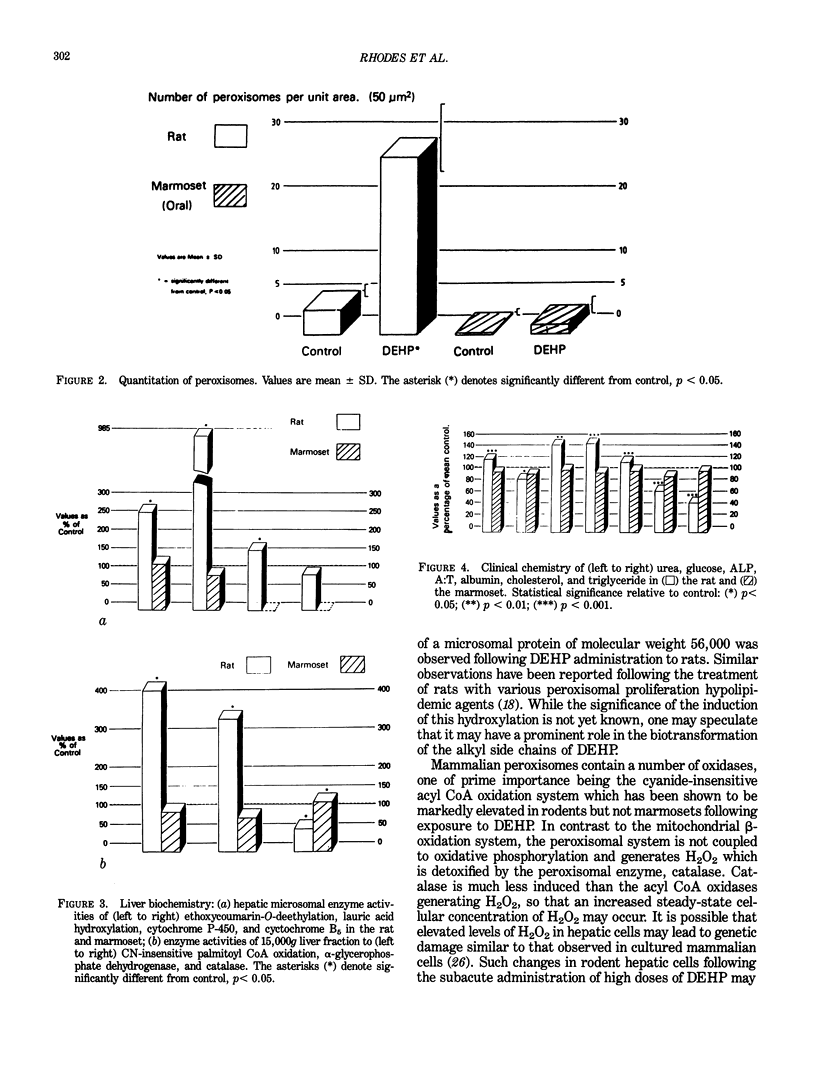
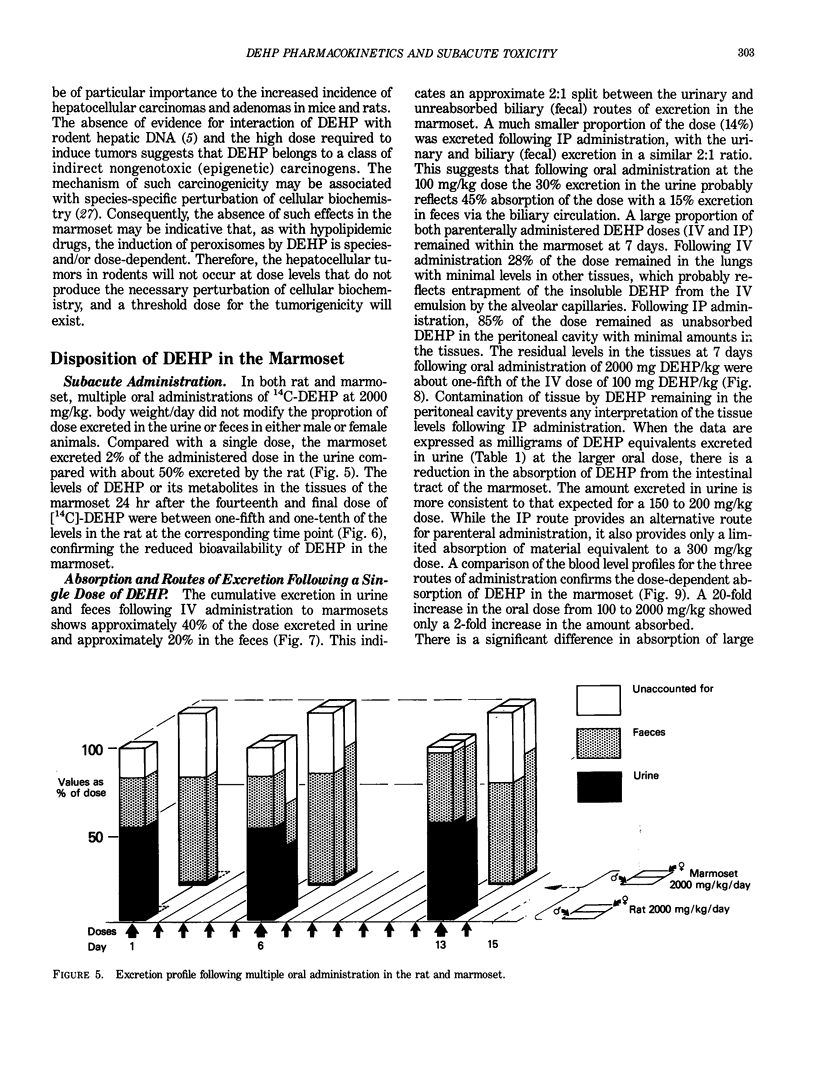
Certain phthalate esters and hypolipidemic agents are known to induce morphological and biochemical changes in the liver of rodents, which have been associated with an increased incidence of hepatocellular tumors in these species. There is evidence that hypolipidemic agents do not induce these effects in either subhuman primates or man. The oral and intraperitoneal administration of di(2-ethylhexyl) phthalate (DEHP) to the marmoset monkey at doses up to 5 mmole DEHP/kg body weight/day for 14 days did not induce morphological or biochemical changes in the liver or testis comparable with those obtained in rats given the same amount of DEHP. In the marmoset, the excretion profile of [14C]-DEHP following oral, IP, and IV administration and the lower tissue levels of radioactivity demonstrated a considerably reduced absorption in this species compared to the rat. The urinary metabolite pattern in the marmoset was in many respects qualitatively similar to but quantitatively different from that in the rat; the marmoset excreted principally conjugated metabolites derived from omega- 1 oxidation. The pharmacokinetic differences between these two species indicate that the tissues of the marmoset are exposed to a level of DEHP metabolites equivalent to the complete absorption of a dose of Ca. 0.1 to 0.25 mmole DEHP/kg body weight/day without significant toxicological effects. These exposure levels are at least 100-fold greater than the worst estimates of incidental human exposure (ca. 0.0015 mmole/kg/day). They are comparable with the human therapeutic dose of many hypolipidemic drugs (ca. 0.15 mmole/kg/day), a dose at which it is claimed that there is an absence of morphological or biochemical changes to human or subhuman primate liver.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF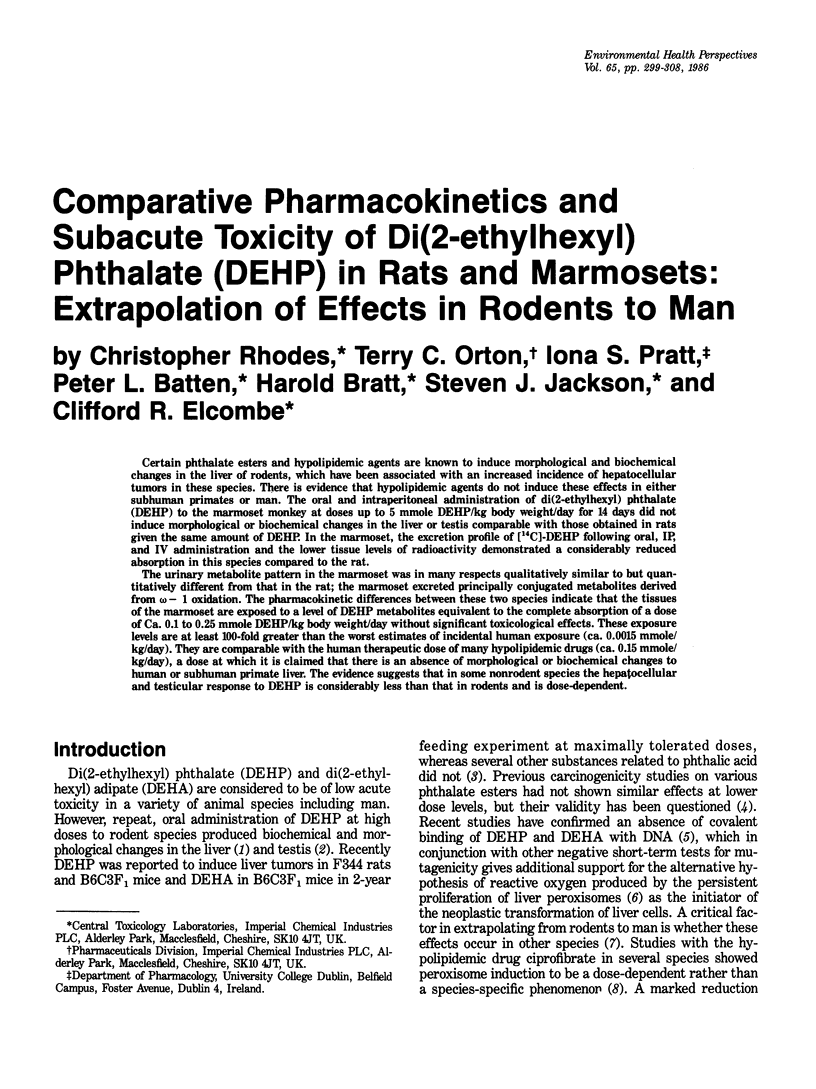


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albro P. W., Corbett J. T., Schroeder J. L., Jordan S., Matthews H. B. Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect. 1982 Nov;45:19–25. doi: 10.1289/ehp.824519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro P. W., Hass J. R., Peck C. C., Odam D. G., Corbett J. T., Bailey F. J., Blatt H. E., Barrett B. B. Identification of the metabolites of di-(2-ethylhexyl) phthalate in urine from the African green monkey. Drug Metab Dispos. 1981 May-Jun;9(3):223–225. [PubMed] [Google Scholar]
- Albro P. W., Jordan S. T., Schroeder J. L., Corbett J. T. Chromatographic separation and quantitative determination of the metabolites of di-(2-ethylhexyl) phthalate from urine of laboratory animals. J Chromatogr. 1982 Jul 23;244(1):65–79. doi: 10.1016/s0021-9673(00)80123-5. [DOI] [PubMed] [Google Scholar]
- Albro P. W., Thomas R., Fishbein L. Metabolism of diethylhexyl phthalate by rats. Isolation and characterization of the urinary metabolites. J Chromatogr. 1973 Feb 28;76(2):321–330. doi: 10.1016/s0021-9673(01)96915-8. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bronfman M., Inestrosa N. C., Leighton F. Fatty acid oxidation by human liver peroxisomes. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1030–1036. doi: 10.1016/0006-291x(79)91512-2. [DOI] [PubMed] [Google Scholar]
- CARPENTER C. P., WEIL C. S., SMYTH H. F., Jr Chronic oral toxicity of di-(2-ethylhexyl) phthalate of rats, guinea pigs, and dogs. AMA Arch Ind Hyg Occup Med. 1953 Sep;8(3):219–226. [PubMed] [Google Scholar]
- Cohen A. J., Grasso P. Review of the hepatic response to hypolipidaemic drugs in rodents and assessment of its toxicological significance to man. Food Cosmet Toxicol. 1981 Oct;19(5):585–605. doi: 10.1016/0015-6264(81)90509-5. [DOI] [PubMed] [Google Scholar]
- Daniel J. W., Bratt H. The absorption, metabolism and tissue distribution of di(2-ethylhexyl)phthalate in rats. Toxicology. 1974 Mar;2(1):51–65. doi: 10.1016/0300-483x(74)90042-0. [DOI] [PubMed] [Google Scholar]
- Gangolli S. D. Testicular effects of phthalate esters. Environ Health Perspect. 1982 Nov;45:77–84. doi: 10.1289/ehp.824577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanefeld M., Kemmer C., Leonhardt W., Kunze K. D., Jaross W., Haller H. Effects of p-chlorophenoxyisobutyric acid (CPIB) on the human liver. Atherosclerosis. 1980 Jun;36(2):159–172. doi: 10.1016/0021-9150(80)90225-7. [DOI] [PubMed] [Google Scholar]
- Jäckh R., Rhodes C., Grasso P., Carter J. T. Genotoxicity studies on di-(2-ethylhexyl) phthalate and adipate and toxicity studies on di-(2-ethylhexyl) phthalate in the rat and marmoset. Food Chem Toxicol. 1984 Feb;22(2):151–155. doi: 10.1016/0278-6915(84)90096-6. [DOI] [PubMed] [Google Scholar]
- Kluwe W. M., McConnell E. E., Huff J. E., Haseman J. K., Douglas J. F., Hartwell W. V. Carcinogenicity testing of phthalate esters and related compounds by the National Toxicology Program and the National Cancer Institute. Environ Health Perspect. 1982 Nov;45:129–133. doi: 10.1289/ehp.8245129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- Lake B. G., Gray T. J., Foster J. R., Stubberfield C. R., Gangolli S. D. Comparative studies on di-(2-ethylhexyl) phthalate-induced hepatic peroxisome proliferation in the rat and hamster. Toxicol Appl Pharmacol. 1984 Jan;72(1):46–60. doi: 10.1016/0041-008x(84)90248-5. [DOI] [PubMed] [Google Scholar]
- Orton T. C., Adam H. K., Bentley M., Holloway B., Tucker M. J. Clobuzarit: species differences in the morphological and biochemical response of the liver following chronic administration. Toxicol Appl Pharmacol. 1984 Mar 30;73(1):138–151. doi: 10.1016/0041-008x(84)90062-0. [DOI] [PubMed] [Google Scholar]
- Orton T. C., Parker G. L. The effect of hypolipidemic agents on the hepatic microsomal drug-metabolizing enzyme system of the rat. Induction of cytochrome(s) P-450 with specificity toward terminal hydroxylation of lauric acid. Drug Metab Dispos. 1982 Mar-Apr;10(2):110–115. [PubMed] [Google Scholar]
- Reddy J. K., Lalwai N. D. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12(1):1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Lalwani N. D., Qureshi S. A., Reddy M. K., Moehle C. M. Induction of hepatic peroxisome proliferation in nonrodent species, including primates. Am J Pathol. 1984 Jan;114(1):171–183. [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Lalwani N. D., Reddy M. K., Qureshi S. A. Excessive accumulation of autofluorescent lipofuscin in the liver during hepatocarcinogenesis by methyl clofenapate and other hypolipidemic peroxisome proliferators. Cancer Res. 1982 Jan;42(1):259–266. [PubMed] [Google Scholar]
- Rhodes C., Soames T., Stonard M. D., Simpson M. G., Vernall A. J., Elcombe C. R. The absence of testicular atrophy and in vivo and in vitro effects on hepatocyte morphology and peroxisomal enzyme activities in male rats following the administration of several alkanols. Toxicol Lett. 1984 Apr;21(1):103–109. doi: 10.1016/0378-4274(84)90230-3. [DOI] [PubMed] [Google Scholar]
- Stich H. F., Wei L., Lam P. The need for a mammalian test system for mutagens: action of some reducing agents. Cancer Lett. 1978 Oct;5(4):199–204. doi: 10.1016/s0304-3835(78)80039-1. [DOI] [PubMed] [Google Scholar]
- Ullrich V., Weber P. The O-dealkylation of 7-ethoxycoumarin by liver microsomes. A direct fluorometric test. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1171–1177. doi: 10.1515/bchm2.1972.353.2.1171. [DOI] [PubMed] [Google Scholar]
- Warren J. R., Lalwani N. D., Reddy J. K. Phthalate esters as peroxisome proliferator carcinogens. Environ Health Perspect. 1982 Nov;45:35–40. doi: 10.1289/ehp.824535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbourn J., Montesano R. An overview of phthalate ester carcinogenicity testing results: the past. Environ Health Perspect. 1982 Nov;45:127–128. doi: 10.1289/ehp.8245127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Däniken A., Lutz W. K., Jäckh R., Schlatter C. Investigation of the potential for binding of Di(2-ethylhexyl) phthalate (DEHP) and Di(2-ethylhexyl) adipate (DEHA) to liver DNA in vivo. Toxicol Appl Pharmacol. 1984 May;73(3):373–387. doi: 10.1016/0041-008x(84)90089-9. [DOI] [PubMed] [Google Scholar]


