Abstract
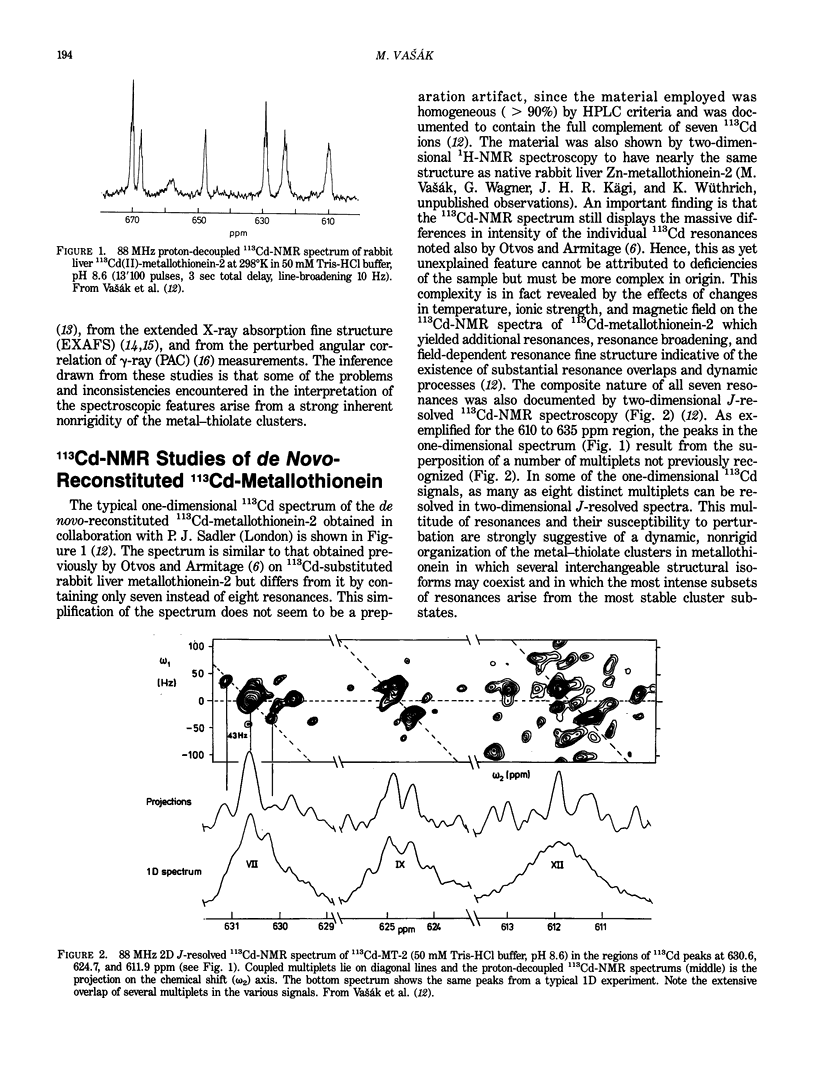
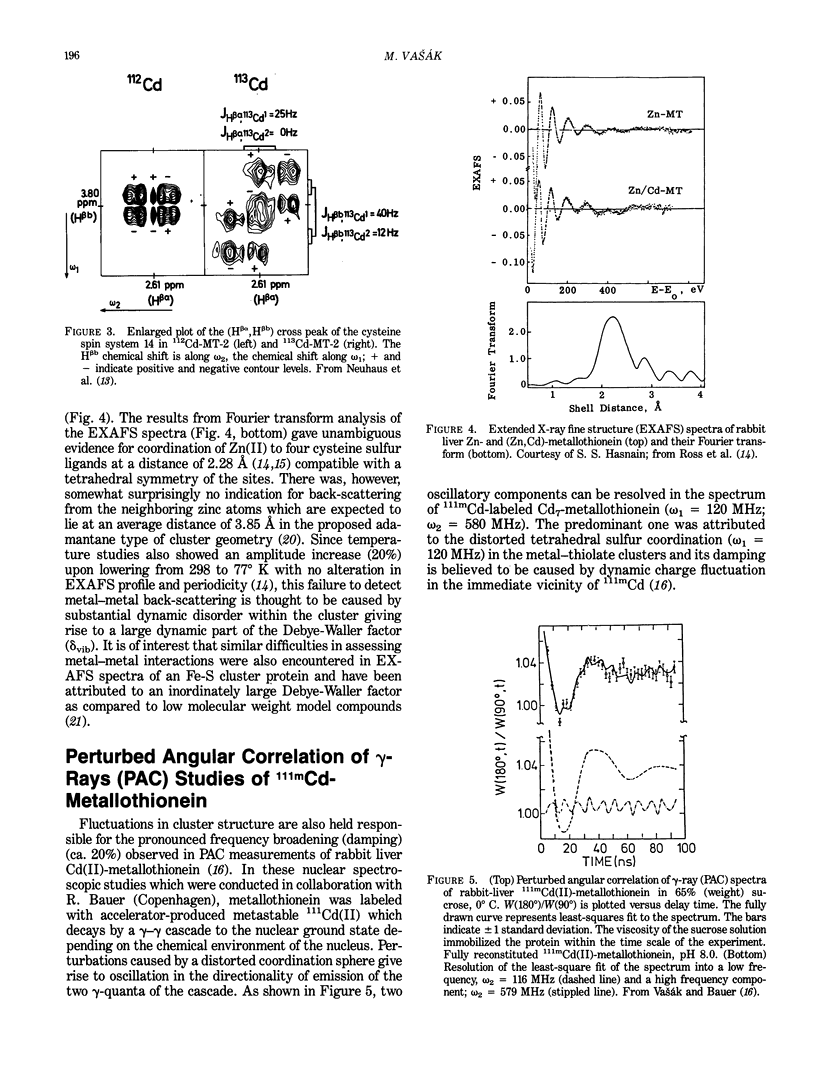
113Cd-NMR studies have established that vertebrate and invertebrate metallothioneins contain two unique metal-thiolate clusters. However, it proved to be difficult to account theoretically for all features of the 113Cd-NMR spectra. In a reinvestigation of these features using chromatographically homogeneous 113Cd7-metallothionein we have identified a total of seven 113Cd resonances and have confirmed the massive intensity difference among these signals. From the effects of variations in temperature, ionic strength, and magnetic field on one-dimensional 113Cd-NMR spectra and from two-dimensional J-resolved 113Cd-NMR spectrum it was concluded that the seven 113Cd signals are composed of several overlapping multiplets and indicative of a dynamic organization of the metal-thiolate clusters. The same nonrigid structure is also indicated individually by recent two-dimensional correlated (COSY) 1H-NMR studies of 113Cd7-metallothionein. While showing 113Cd-1H coupling for 19 of the 20 cysteine residues, these studies have evidence for only two "bridging" cysteine ligands of the clusters instead of the expected eight suggesting interferences by metal-thiolate exchange process at the 1H-NMR time scale. Analogous indirect evidence for structural mobility of the clusters comes from EXAFS measurements of Zn7-metallothionein and from measurements of the perturbed angular correlation of gamma-rays (PAC) emis-sion of 111mCd-metallothionein. Thus, while the EXAFS spectra revealed back-scattering from the thiolate ligands, lattice movements within the cluster is believed to preclude back-scattering from neighboring metals. Similarly, in the PAC time spectra the damping of the major oscillatory component was attributed to inordinately large charge fluctuation in the immediate environment of the 111mCd nucleus.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF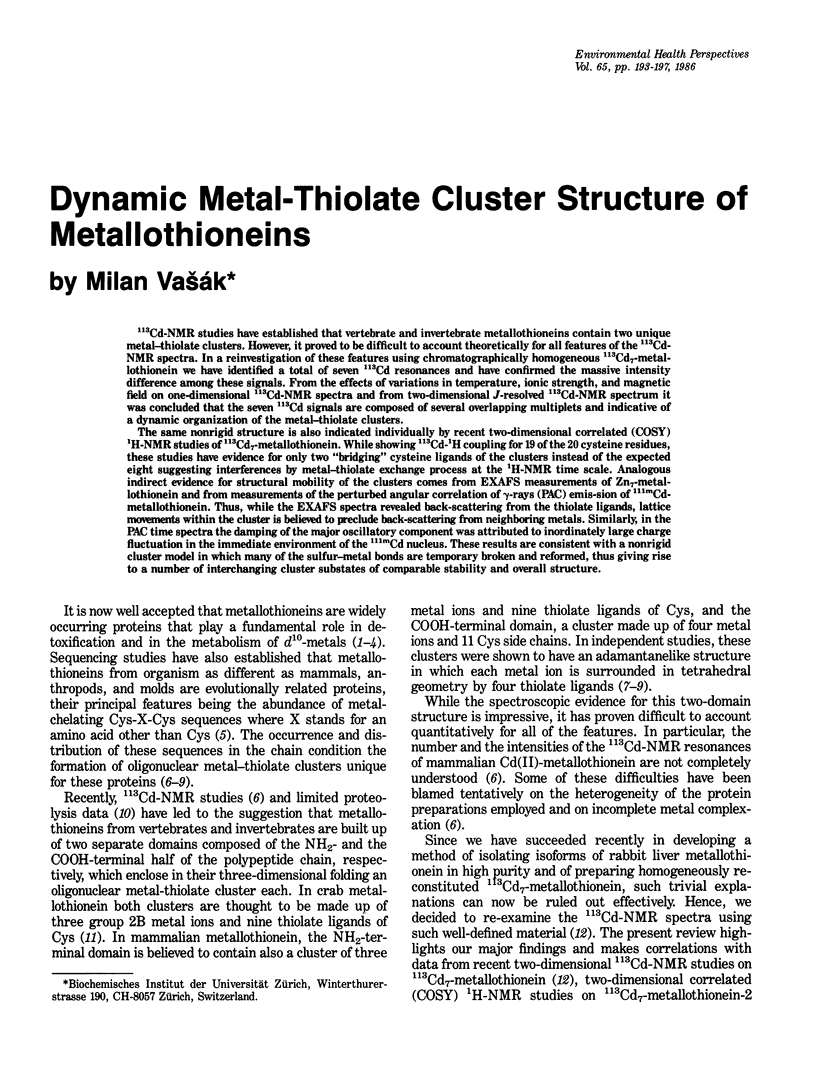


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellis P. D. Cadmium-113 magnetic resonance spectroscopy. Science. 1983 Sep 16;221(4616):1141–1146. doi: 10.1126/science.221.4616.1141. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Garner C. D., Hasain S. S., Bremner I., Bordas J. An EXAFS study of the zinc sites in sheep liver metallothionein. J Inorg Biochem. 1982 Jun;16(3):253–256. doi: 10.1016/s0162-0134(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Berger C., Vallee B. L., Kägi J. H. Amino-acid sequence of equine renal metallothionein-1B. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3413–3417. doi: 10.1073/pnas.73.10.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus D., Wagner G., Vasák M., Kägi J. H., Wüthrich K. 113CD-1H spin-spin couplings in homonuclear 1H correlated spectroscopy of metallothionein. Identification of the cysteine 1H spin systems. Eur J Biochem. 1984 Sep 17;143(3):659–667. doi: 10.1111/j.1432-1033.1984.tb08419.x. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos J. D., Olafson R. W., Armitage I. M. Structure of an invertebrate metallothionein from Scylla serrata. J Biol Chem. 1982 Mar 10;257(5):2427–2431. [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Influence of parenteral zinc and actinomycin D on tissue zinc uptake and the synthesis of a zinc - binding protein. Bioinorg Chem. 1975 Apr;4(3):215–224. doi: 10.1016/s0006-3061(00)80104-0. [DOI] [PubMed] [Google Scholar]
- Vasák M., Hawkes G. E., Nicholson J. K., Sadler P. J. 113Cd NMR studies of reconstituted seven-cadmium metallothionein: evidence for structural flexibility. Biochemistry. 1985 Jan 29;24(3):740–747. doi: 10.1021/bi00324a031. [DOI] [PubMed] [Google Scholar]
- Vasák M., Kägi J. H. Metal thiolate clusters in cobalt(II)-metallothionein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6709–6713. doi: 10.1073/pnas.78.11.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M., Magos L. Cadmium-thionein and the protection by cadmium against the nephrotoxicity of mercury. Chem Biol Interact. 1976 Aug;14(3-4):357–369. doi: 10.1016/0009-2797(76)90114-9. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Domain nature of metallothionein. J Biol Chem. 1982 Apr 10;257(7):3471–3476. [PubMed] [Google Scholar]


