Abstract
Chloride (Cl−) channels expressed in vascular smooth muscle cells (VSMC) are important to control membrane potential equilibrium, intracellular pH, cell volume maintenance, contraction, relaxation and proliferation. The present study was designed to compare the expression, regulation and function of CFTR Cl− channels in aortic VSMC from Cftr+/+ and Cftr−/− mice. Using an iodide efflux assay we demonstrated stimulation of CFTR by VIP, isoproterenol, cAMP agonists and other pharmacological activators in cultured VSMC from Cftr+/+. On the contrary, in cultured VSMC from Cftr−/− mice these agonists have no effect, showing that CFTR is the dominant Cl− channel involved in the response to cAMP mediators. Angiotensin II and the calcium ionophore A23187 stimulated Ca2+-dependent Cl− channels in VSMCs from both genotypes. CFTR was activated in myocytes maintained in medium containing either high potassium or 5-hydroxytryptamine (5-HT) and was inhibited by CFTRinh-172, glibenclamide and diphenylamine-2,2′-dicarboxylic acid (DPC). We also examined the mechanical properties of aortas. Arteries with or without endothelium from Cftr−/− mice became significantly more constricted (∼2-fold) than that of Cftr+/+ mice in response to vasoactive agents. Moreover, in precontracted arteries of Cftr+/+ mice, VIP and CFTR activators induced vasorelaxation that was altered in Cftr−/− mice. Our findings suggest a novel mechanism for regulation of the vascular tone by cAMP-dependent CFTR chloride channels in VSMC. To our knowledge this study is the first to report the phenotypic consequences of the loss of a Cl− channel on vascular reactivity.
The vascular tone is regulated by vasoactive agonists that modulate, directly or indirectly, the activity of membrane ion channels located in vascular smooth muscle cells (VSMC) (Gurney, 2000). Among the ion channels expressed by VSMC, Cl− channels are important regulators controlling membrane potential equilibrium, intracellular pH, cell volume maintenance, contraction, relaxation and proliferation (Large & Wang, 1996; Nelson et al. 1997; Chipperfield & Harper, 2000; Jackson, 2000; Kitamura & Yamazaki, 2001; Wang et al. 2002). The calcium-activated Cl− current contributes significantly to agonist-induced VSMC contraction (Large & Wang, 1996; Lamb & Barna, 1998). A member of the CLCA gene family, mCLCA4, is thought to be responsible for the calcium-activated Cl− current in mice aortic VSMC (Elble et al. 2002). In addition to the CLCA genes, bestrophin genes are considered to encode calcium-activated Cl− channels (reviewed in Hartzell et al. 2005). Also, ClC-3 a member of the voltage-dependent Cl− channel family ClC (Jentsch et al. 2002) has been involved in VSMC proliferation (Wang et al. 2002) as well as in the regulation of cellular volume (Yamazaki et al. 1998; Lamb et al. 1999; Yamamoto-Mizuma et al. 2004). The cystic fibrosis transmembrane conductance regulator (CFTR) is a low-conductance, cAMP-regulated and ATP-gated Cl− channel located in the apical membrane of epithelial cells (Riordan et al. 1989; Gadsby & Nairn, 1999; Sheppard & Welsh, 1999). In addition, CFTR expression has been observed in a variety of other cell types, including lymphocytes, ventricular heart cells, endothelial cells and rat aortic VSMC (Tousson et al. 1998; Gadsby & Nairn, 1999; Robert et al. 2004).
The physiological functions of vascular smooth muscles are affected through phosphorylation of specific substrate proteins by cAMP- and cGMP-dependent protein kinases (Werstiuk & Lee, 2000; Henning & Sawmiller, 2001; Woodrum & Brophy, 2001). The increase of cAMP and cGMP levels has been implicated in the relaxation of vascular smooth muscle cells in response to vasodilators such as β-adrenergic agonists or the neuropeptide vasoactive intestinal peptide (VIP) (Henning & Sawmiller, 2001). VIP increases the cellular cAMP in smooth muscle cells but the precise contribution of cAMP to the vasodilatation effect of VIP is not fully understood (Henning & Sawmiller, 2001). However, the cAMP accumulation associated with vascular relaxation through β-adrenoceptor stimulation activates the cAMP-dependent protein kinase A (PKA) with subsequent phosphorylations of unknown target proteins (Werstiuk & Lee, 2000).
The present experiments were designed to investigate the role of CFTR in the cAMP-dependent Cl− transport as well as in the control of smooth muscle cell functions in response to β-adrenoceptor stimulation, VIP and other agonists of the cAMP pathway. We used Cftr+/+ and Cftr−/− mice to study mechanical properties of aortic rings and determined with primary cultures of VSMC the cAMP- and Ca2+-dependent Cl− transports using robotic equipment measuring iodide efflux.
Methods
Vessel preparation
All experiments were performed on wild-type (Cftr+/+) or CFTR knock-out (Cftr−/−) mice B6; 129-CFTRtm1Unc (Snouwaert et al. 1992) obtained from CNRS-CDTA (Orleans, France). Animals were killed by cervical dislocation, a procedure approved by the local animal ethics committee of the University of Poitiers. The homozygous mutant mice were originally obtained by a targeted mutation of the CFTR gene with insertion of a neomycin-resistance cassette into exon 10 via homologous recombination (Snouwaert et al. 1992). The thoracic aorta of mice was dissected out and placed into Krebs solution containing (mm): 120 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 15 NaHCO3, 1.2 KH2PO4, 11 d-glucose and 10 Hepes, pH 7.4, as described (Robert et al. 2004).
Dissociation of vascular smooth muscle cells (VSMC)
Aortas from Cftr+/+ or Cftr−/− mice were cut into strips ∼1 mm wide. VSMC were isolated from each preparation using a low-Ca2+ dissociation medium (DM) of the following composition (mm): 110 NaCl, 5 KCl, 15 NaHCO3, 0.16 CaCl2, 2 MgCl2, 0.5 NaH2PO4, 0.5 KH2PO4, 10 glucose, 15 Hepes, 0.04 Phenol Red, 0.49 EDTA, 10 taurine; equilibrated with air and adjusted to pH 7.0 with NaOH. Vessel pieces were washed in DM and then placed in 5 ml DM containing 8 mg collagenase (type 1A, Sigma), 1 mg protease (type XXIV, Sigma), 10 mg bovine serum albumin and 10 mg trypsin inhibitor (Sigma) for 15 min at 37°C. After transferring the tissue to enzyme-free DM, single cells were released by gentle trituration.
Primary culture of vascular smooth muscle cells
For each culture, thoracic aortas from eight mice (Cftr+/+ or Cftr−/−) were excised and placed in modified Krebs solution containing (mm): 120.8 NaCl, 5.9 KCl, 0.2 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 2 NaHCO3, 11 d-glucose and 10 Hepes, pH 7.4. Cleaned aortas were placed for 20 min at 37°C in the above solution containing 10 U ml−1 elastase (Serva, Germany), 170 U ml−1 collagenase (CLS2, Worthington, USA), 0.6 mg ml−1 trypsin inhibitor (Sigma), 1 mg ml−1 bovine serum albumin (Sigma). Tunica adventicia and endothelium were removed and the remaining medial layers were minced and digested for 20 min in Minimum Essential Medium (α-MEM; Gibco) containing 0.024 m Hepes, 300 U ml−1 collagenase, 65 U ml−1 elastase. The suspension was filtered, collected in α-MEM containing 0.024 m Hepes + 10% fetal calf serum (FCS) and centrifuged at +4°C. Cells were resuspended in α-MEM + 10% FCS, plated in 24-well plates and incubated at 37°C in 5% CO2. Characteristics of cultured VSMC were verified by positive immunostaining with the monoclonal anti-α-smooth muscle actin antibody (clone 1A4, Sigma) and by their inability to react with von Willebrand Factor VIII antibody (Sigma), a marker of endothelial cells. All solutions contained 100 U ml−1 penicillin and 0.1 mg ml−1 streptomycin.
Immunofluorescence study
An immunolocalization study of CFTR was performed using VSMC dissociated from either Cftr+/+ or Cftr−/− mice. Cells were rinsed in Tris-buffered saline (TBS) and fixed with 3% paraformaldehyde in TBS for 20 min at room temperature. Samples were then rinsed in TBS, permeabilized with 0.1% Triton X-100 in TBS for 10 min, and again rinsed with TBS. After fixation, non-specific binding sites on VSMC were blocked with TBS containing 0.5% BSA and 0.05% saponin for 1 h at room temperature. Cells were then stained with the anti-CFTR C-terminal monoclonal primary antibody (1 : 100, clone 24–1, R&D Systems, Minneapolis, MN, USA) for 90 min at room temperature and rinsed in TBS containing 0.5% BSA and 0.05% saponin. Cells were incubated with the alexa 488-conjugated secondary antibody (1 : 400, Molecular Probes, Inc., Eugene, OR, USA) to detect CFTR. Nucleic acids were stained with TO-PRO-3 iodide (Molecular Probes) for 15 min at room temperature (1 : 200 in TBS). Preparations were mounted in an anti-fade solution, Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, USA). Fluorescence was detected using confocal laser scanning microscopy on an Olympus FV1000. In all experiments, CFTR appears in green and nucleus in red.
Contraction measurement on isolated aortic rings
After separation of connective tissues, the thoracic segment of aorta was cut into rings of 3 mm length. In some preparations, the endothelium was removed by rubbing its luminal surface. Aortas were mounted between a fixed clamp at the base of a water-jacketed 5 ml organ bath containing an oxygenated (95% O2 and 5% CO2) Krebs solution and an IT1-25 isometric force transducer (Emka Technologies, Paris, France) (Robert et al. 2004). All experiments were performed at 37°C. A basal tension of 1 g was applied in all experiments. Endothelium integrity or functional removal was verified by the presence or absence, respectively, of the relaxant response to 10−5m acetylcholine. During 1 h, tissues were rinsed three times in Krebs solution and the basal tone was always monitored and adjusted to 1 g. High K+ solution (Krebs solution with 80 mm of KCl where Na+ was replaced by an equimolar concentration of K+ to maintain a constant ion strength; hereafter denoted 80 mm K+) was used to evoke the sustained contractile response. Once the sustained tension was established, the tissues were allowed to equilibrate further for 30 min before cumulative or single addition of agonist to the bath. Cumulative concentration–response relationships for the relaxant effect of CFTR agonists were determined with aortic rings following stable contraction and were expressed as the percentage contraction of the agonist-constricted arterial rings. The concentration IC50 was calculated as the drug concentration inducing a half-maximal vasorelaxation (or inhibition of contraction).
Functional study of CFTR chloride channel activity
Chloride channel activity was assayed at 37°C by measuring the rate of iodide (125I) efflux using primary cultures of VSMC using a high capacity robotic system (MultiProbe II EXT, PerkinElmer Life Sciences, Courtaboeuf, France) as previously described (Marivingt-Mounir et al. 2004; Robert et al. 2004). Cells were incubated in Krebs solution containing 1 μm KI and 1 μCi Na125I ml−1 (NEN, Boston, MA, USA) during 1 h at 37°C to permit the 125I to reach equilibrium. Cells were then washed with the Krebs solution to remove extracellular 125I. The loss of intracellular 125I was determined by removing the medium with Krebs solution containing either 80 mm K+ or 10 μm 5-hydroxytryptamine (5-HT) every 1 min for up to 9 min. The first three aliquots were used to establish a stable baseline in efflux buffer alone. A medium containing the appropriate drug was used for the remaining aliquots. Residual radioactivity was extracted with a mixture of 0.1 n NaOH and 0.1% SDS, and determined using a gamma counter (Cobra II, PerkinElmer Life Sciences). The fraction of initial intracellular 125I lost during each time point was determined and time-dependent rates (k= peak rate, min−1) of 125I efflux were calculated from the following equation:
where 125It is the intracellular 125I at time t, and t1 and t2 are successive time points (Marivingt-Mounir et al. 2004; Robert et al. 2004). Curves were constructed by plotting kversus time. Relative rates, kpeak−kbasal (min−1), were calculated for each set of experiments. In experiments using the transport inhibitors CFTRinh-172, glibenclamide, diphenylamine-2-carboxylic acid (DPC) and TS-TM calix[4]arene, the inhibitor was present (during 30 min) in the loading solution and in the efflux buffer. CFTRinh-172 (Ma et al. 2002) is highly soluble in dimethylsulphoxide (DMSO). It was dissolved as 100 mm stock solution under agitation and prepared freshly at the final concentration of 100 μm in a large volume of efflux buffer. Although the compound is active at much lower concentration (Ma et al. 2002) and up to 20 μm, we used a high concentration of CFTRinh-172 for our study.
Chemicals
The CFTR chloride channel activating benzo[c]quinolizinium compounds MPB-07 (10-chloro-6-hydroxybenzo[c]quinolizinium chloride) and MPB-91 (5-Butyl-10-chloro-6-hydroxybenzo[c]quinolizinium chloride) were prepared as previously described (Marivingt-Mounir et al. 2004; Robert et al. 2004). TS-TM calix[4]arene (5,11,17,23-tetrasulphonato-25,26,27,28-tetramethoxy-calix[4]arene), an inhibitor of outwardly rectifying Cl− channels (Singh et al. 1995), and 3-[(3-trifluoromethyl)phenyl]-5-[(4-carboxyphenyl)methylene]-2-thioxo-4-thiazolidinone (CFTRinh-172), a specific CFTR inhibitor (Ma et al. 2002), were generously provided by Drs Singh and Bridges, University of Pittsburgh, Pittsburgh, USA. All other chemicals were purchased from Sigma. All compounds were dissolved in DMSO (final DMSO concentration < 0.1%) except isoproterenol, VIP and MPB-07, which were dissolved in water.
Statistics
All results are expressed as means ±s.e.m. of n observations. Sets of data were compared with either an analysis of variance (ANOVA) or Student's t test. Differences were considered statistically significant when P < 0.05. ns: non significant difference, *P < 0.05, **P < 0.01, ***P < 0.001. All statistical tests were performed using GraphPad Prism version 3.0 for Windows (GraphPad Software Inc., San Diego, CA, USA).
Results
Expression of CFTR protein in dissociated vascular smooth muscle cells of Cftr+/+ mice
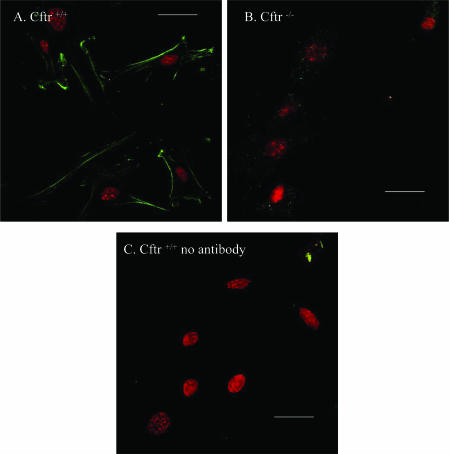
To study the expression of CFTR in mice aortic VSMC, CFTR localization was performed by indirect immunofluorescence confocal microscopy. VSMC dissociated from either Cftr+/+ or Cftr−/− mice were stained for CFTR using anti-CFTR C-terminal monoclonal antibody and appropriate secondary antibody. CFTR protein was detected in the plasma membrane and within cytoplasmic compartments of isolated VSMC from Cftr+/+ mice (Fig. 1A). No staining was detected with Cftr−/− mice (Fig. 1B) or when the primary antibody was omitted with VSMC of Cftr+/+ mice (Fig. 1C). The comparison of immunofluorescence images between Cftr+/+ and Cftr−/− mouse VSMC provides evidence that CFTR is expressed in the plasma membrane of aortic smooth muscle cells.
Figure 1. Expression of CFTR in mice vascular smooth muscle cells.
Immunofluorescence study of CFTR proteins in VSMC from Cftr+/+ (A) and Cftr−/− mice (B) and from Cftr+/+ mice in absence of primary antibody (C). Scale bars are 20 μm. CFTR is stained green and nucleus (TOPRO-3 staining) red.
Analysis of chloride transports in primary cultures of mouse VSMC
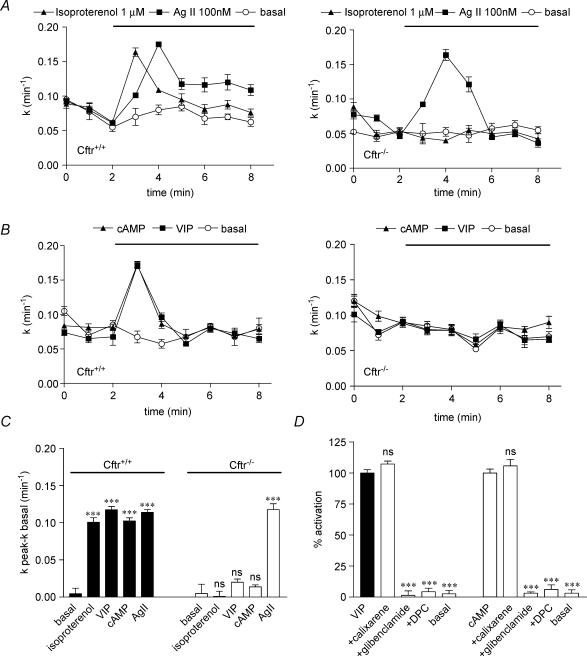
In epithelial cells, CFTR functions as a cAMP-regulated Cl− channel (Riordan et al. 1989; Gadsby & Nairn, 1999; Sheppard & Welsh, 1999). Cl− transport stimulated by cAMP and Ca2+ agonists in VSMC cultured from Cftr+/+ and Cftr−/− mice was measured using the iodide efflux method in high K+ solution (Robert et al. 2004). A significant stimulation (P < 0.001) of iodide efflux by isoproterenol (1 μm, n = 8, Fig. 2A left), VIP (300 nm, n = 8, Fig. 2B left) and cAMP agents (10 μm forskolin, 500 μm IBMX and 500 μm cpt-cAMP, n = 8, Fig. 2B left) was obtained in Cftr+/+ mouse VSMC as compared to resting cells (noted basal, n = 8). The stimulation of iodide efflux results in a rapid increase of the rate of efflux (k) within the first minute after addition of the agonist. The peak efflux occurred at the same time with isoproterenol (Fig. 2A left), VIP and cAMP agonists (Fig. 2B left). In contrast, no stimulation of Cl− channels in VSMC preparations from Cftr−/− mice could be recorded with isoproterenol (n = 8, Fig. 2A right), VIP (n = 8, Fig. 2B right) or cAMP agonists (n = 8, Fig. 2B right).
Figure 2. Functional analysis of CFTR and Ca2+-activated Cl− channels in Cftr+/+ and Cftr−/− mice smooth muscle cells.
The stimulation of iodide efflux as a function of time was evoked in solution containing 80 mm K+ by 100 nm angiotensin II (denoted AgII) and 1 μm isoproterenol (A), and cAMP agonists (10 μm forskolin, 500 μm IBMX and 500 μm cpt-cAMP) and 300 nm VIP (B) in Cftr+/+ (left) and Cftr−/− (right) mice as compared to basal. C, summary of the relative rates presented as means ±s.e.m.D, effect of 100 μm glibenclamide, 500 μm DPC and 100 nm calixarene on the efflux stimulated by 300 nm VIP or cAMP agonists in Cftr+/+ mouse smooth muscle cells as indicated. Basal was vehicle alone. Data are presented as means ±s.e.m. All experimental conditions have been repeated: n = 8. ***P < 0.001. ns: non-significant difference.
Angiotensin II stimulates Ca2+-dependent Cl− channels in Cftr+/+ and Cftr−/− mice
In vascular smooth muscle cells, the vasoconstrictor agent angiotensin II (Ag II) stimulates Ca2+-dependent Cl− channels activity via increase in [Ca2+]i (White et al. 1995; Guibert et al. 1997). Figure 2A indicates that Ag II (100 nm, n = 8) stimulated a significant (P < 0.001) iodide efflux in VSMC from both Cftr+/+ and Cftr−/− mice as compared to control without Ag II. The response was maximum (peak) after 2 min following the addition of Ag II. A similar response was obtained with the calcium ionophore A23187 used at 1 μm (n = 4, not shown). In the presence of isoproterenol, VIP and cAMP agonists, the response was faster and maximum after only 1 min after drug addition (compare Fig. 2A and 2B), probably reflecting different molecular mechanisms. Figure 2C is a bar graph summarizing the data collected from experiments with Cftr+/+ and Cftr−/− mice indicating that only the cAMP- but not the Ca2+-dependent Cl− transport is absent in mice lacking CFTR. It is interesting to note that the amplitude of the response observed with VSMC from Cftr+/+ and Cftr−/− mice stimulated by Ag II is similar (Fig. 2B) demonstrating that the absence of CFTR in the knock-out mice apparently does not alter the Ca2+-dependent Cl− transport.
Pharmacological inhibition of CFTR in Cftr+/+ mouse VSMC
Different classes of Cl− channel inhibitors were used to characterize the channel activity of CFTR: glibenclamide and diphenylamine-2-carboxylic acid (DPC), two non-specific inhibitors of CFTR channels (Sheppard & Welsh, 1992; Schultz et al. 1999; Robert et al. 2004) and TS-TM calix[4]arene, an inhibitor of outwardly rectifying Cl− channels but not of CFTR (Singh et al. 1995; Schultz et al. 1999; Robert et al. 2004). We found that the stimulation of iodide efflux from Cftr+/+ VSMC, with VIP or cAMP agonists was fully inhibited by 100 μm glibenclamide and 500 μm DPC but not by 100 nm calixarene (Figs 2D, n = 8 for each). This pharmacological profile of inhibition is in perfect agreement with that determined for epithelial CFTR (Sheppard & Welsh, 1992; Schultz et al. 1999) and rat aortic VSMC (Robert et al. 2004).
Pharmacological activation of CFTR channels in mouse VSMC
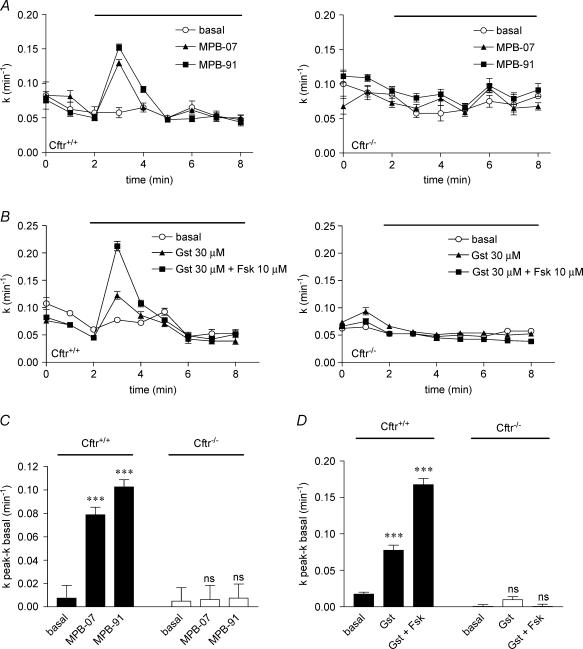
If CFTR is the major Cl− channel activated in VSMC, then we should observed its stimulation using known pharmacological activators of the epithelial CFTR, such as the benzo[c]quinolizinium derivatives MPB-07 and MPB-91, and the isoflavone genistein (Illek et al. 1995; Derand et al. 2002; Marivingt-Mounir et al. 2004; Robert et al. 2004). MPB-07 and MPB-91 (100 μm, n = 8, Fig. 3A left and Fig. 3C) and genistein (30 μm, n = 8, Fig. 3B left and Fig. 3D) stimulated iodide efflux from Cftr+/+ VSMC preparations. In contrast, no stimulation with any of these agents could be detected with Cftr−/− mice (n = 8, Fig. 3A and B right). Moreover, in the presence of 10 μm forskolin, a significant potentiation (P < 0.001) with genistein was observed with Cftr+/+ VSMC (n = 8, Fig. 3B left and Fig. 3D) but not with Cftr−/− VSMC (n = 8, Fig. 3B right). Again, this synergistic effect is in perfect agreement with previous observations made on the epithelial CFTR (Derand et al. 2002). All effluxes stimulated by MPB derivatives and genistein were inhibited by 100 μm glibenclamide (not shown) as observed with VIP and β-adrenergic agonists. Figure 3C and D summarizes results of these experiments suggesting that the benzo[c]quinolizinium derivatives MPB-07 and MPB-91 and the isoflavone genistein stimulated specifically CFTR.
Figure 3. Pharmacological activation of CFTR Cl− channels activity in mice cultured smooth muscle cells.
Iodide efflux responses as a function of time evoked in solution containing 80 mm K+ by MPB-07 (100 μm) and MPB-91 (100 μm) (A), 30 μm genistein (denoted Gst) or 30 μm genistein plus 10 μm forskolin (denoted Gst + Fsk) (B) in Cftr+/+ (left) and Cftr−/− (right) mouse VSMC. All agents: n = 8. Basal was vehicle alone in high K+. C and D, summary of the data for each experimental condition with 80 mm K+ solution after stimulation by MPB-07 or MPB-91 (C) and by genistein or genistein + forskolin (D) in Cftr+/+ and Cftr−/− mice as indicated. Basal was vehicle alone. Data are presented as means ±s.e.m. and compared to the basal. ***P < 0.001. ns: non-significant difference.
Effect of the thiazolidinone CFTR blocker CFTRinh-172
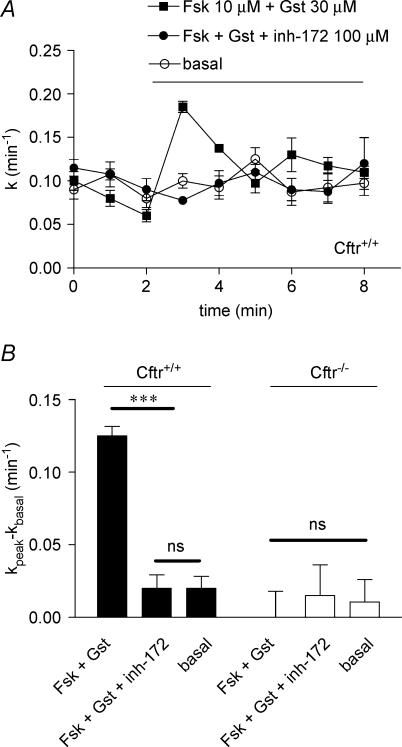
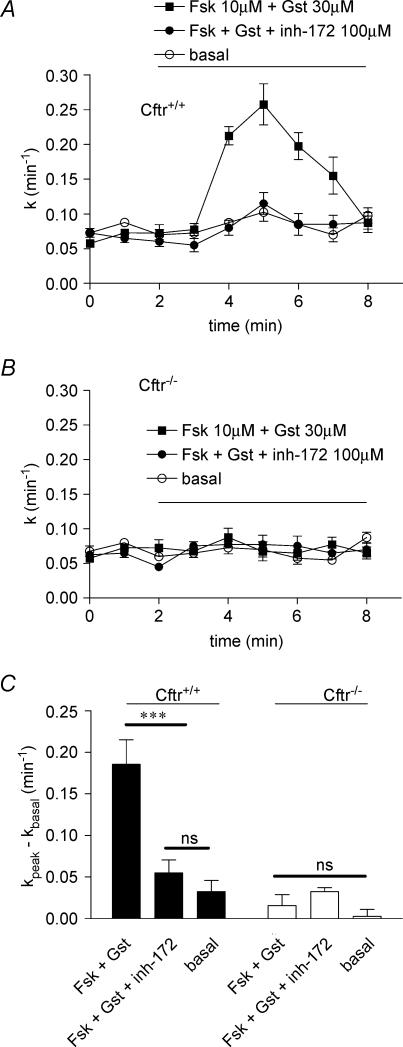
The thiazolidinone compound CFTRinh-172 has been recently developed as a specific CFTR blocker with no significant inhibitory action on other chloride channels, and specifically on the volume- and calcium-activated chloride channels (Ma et al. 2002). It was thus important to test this agent on myocytes maintained in high potassium-rich Krebs medium. With VSMC of Cftr+/+, the iodide efflux was stimulated by the cocktail forskolin–genistein in the presence or absence of CFTRinh-172 used at 100 μm. It is clear from the results presented in Fig. 4 that the compound fully inhibited the efflux. No effect was noted with VSMC of Cftr−/− mice (Fig. 4B). These results, together with those obtained with glibenclamide and DPC, complete the pharmacological profile for inhibition of CFTR and confirm that both epithelial and vascular CFTR have similar properties.
Figure 4. Effect of CFTRinh-172 on stimulated iodide efflux in Cftr+/+ and Cftr−/− mouse smooth muscle cells.
A, the stimulation of iodide efflux as a function of time was evoked in solution containing 80 mm K+ by 10 μm forskolin plus 30 μm genistein without or with 100 μm CFTRinh-172 as indicated. B, summary of the relative rates presented as means ±s.e.m. with statistical analysis. Basal was vehicle (DMSO). All experimental conditions have been repeated: n = 8. ***P < 0.001. ns: non-significant difference.
CFTR is functional in myocytes stimulated by 5-hydroxytryptamine
In rat (Robert et al. 2004) and mouse (this study), cAMP-dependent CFTR Cl− channel activity in VSMC appears to be dependent on the presence of high K+ solution, suggesting that CFTR could be only observed in depolarized myocytes. To determine whether the activation of CFTR channels occurs when VSMC are challenged with other constrictors, we studied the effect of 5-hydroxytryptamine (5-HT), a potent vasoconstrictor (Vanhoutte, 1988). With myocytes maintained for 30 min in Krebs medium supplemented with 10 μm 5-HT, iodide efflux was successfully stimulated by the cocktail forskolin–genistein (Fig. 5A). The implication of CFTR in this response was established for two reasons. First, no stimulation occurred with VSMC of Cftr−/− mice (Fig. 5B). Second, the response was fully inhibited by 100 μm CFTRinh-172 in Cftr+/+ (Fig. 5A and B). However, the increase of the rate of efflux (see Fig. 5A squares) occurs only during the second minute after addition of the agonists. Also, the peak efflux is reached after 3 min, i.e. slower than with Krebs medium with high potassium content (compare Fig. 4A to Fig. 5A). This difference in the rate of activation of CFTR-dependent iodide efflux may be related to the nature of the vasoconstrictor (high potassium solution versus 5-HT) and to the underlying mechanism leading to contraction.
Figure 5. Chloride transport activity of CFTR in VSMC with 5-hydroxytryptamine (5-HT).
The stimulation of iodide efflux as a function of time was evoked in solution containing 10 μm 5-HT by 10 μm forskolin plus 30 μm genistein without or with 100 μm CFTRinh-172 as indicated for Cftr+/+ mice (A) and Cftr−/− mice (B). C, summary of the relative rates presented as means ±s.e.m. with statistical analysis. Basal was vehicle (DMSO). All experimental conditions have been repeated: n = 8. ***P < 0.001. ns: non significant difference.
The vascular tone is higher in Cftr−/− mice compared to Cftr+/+
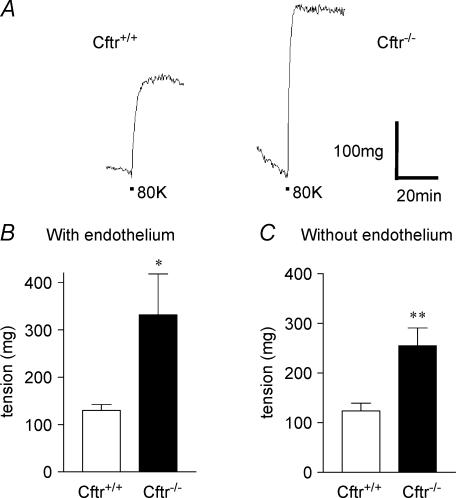
To begin to understand the physiological consequences of an absence of CFTR for the reactivity of VSMC, we compared the mechanical properties of aortas from both genotypes. Mouse aortic rings were dissected out and constricted by the high K+ solution as described (Robert et al. 2004). We found two major differences between Cftr+/+ and Cftr−/− mice. First, when aortic rings are constricted by high K+ solution, the vascular tone was significantly higher in the absence of CFTR in Cftr−/− mice compared to Cftr+/+ individuals with or without endothelium. A typical experiment is illustrated Fig. 6A showing a ∼2-fold higher constriction magnitude of an aortic ring constricted in high K+ saline from a knock-out animal (right trace) as compared (P < 0.01) to a control Cftr+/+ animal (left trace) (see also Fig. 7A for another illustration in different animals). Mean tensions have been determined as 130 ± 12 mg (n = 6 Cftr+/+ mice) and 331 ± 86 mg (n = 6 Cftr−/− mice) for preparations with endo-thelium (Fig. 6B) and 123 ± 16 mg (n = 7 Cftr+/+ mice) and 255 ± 36 mg (n = 5 Cftr−/− mice) without endo-thelium (Fig. 6C). No significant difference was obtained between preparations with and without endothelium indicating that the control of the vascular tone by CFTR is endothelium independent.
Figure 6. Measurement of the wall tension of mice aorta.
A, continuous traces from experiments performed with denuded aortic rings preconstricted with 80 mm K+ solution (denoted 80K beneath traces). B and C, bar graphs showing mean tensions in response to 80 mm K+ solution for Cftr+/+ and Cftr−/− mice with (B) or without (C) endothelium.
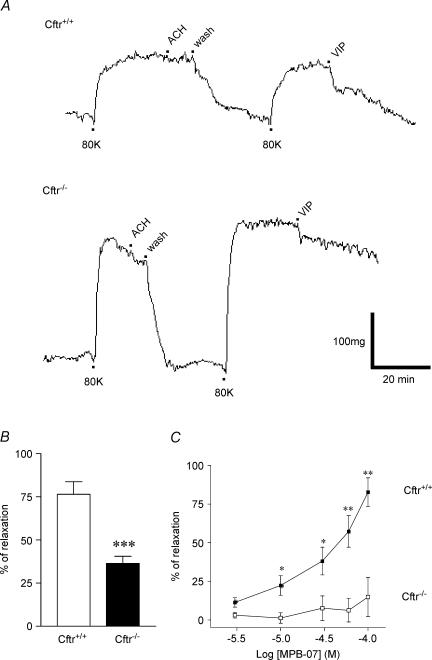
Figure 7. Effect of CFTR activators on the wall tension of mouse aorta.
A, continuous traces from experiments performed with denuded aortic rings reversibly preconstricted with 80 mm K+ solution (denoted 80K beneath traces). The absence of endothelium was verified by addition of acetylcholine (ACh, 10−5m) as indicated. Then, 300 nm VIP was added to the bath for Cftr+/+ (top trace) and Cftr−/− (bottom trace) mice. B, bar graphs of mean percentage vasorelaxation determined 30 min after adding 300 nm VIP after constriction by 80 mm K+ solution for 7 Cftr+/+ and 5 Cftr−/− mice. C, concentration-dependent curves for MPB-07-dependent vasorelaxation of aortic rings constricted by 80 mm K+ for Cftr+/+(IC50= 37 ± 1.17 μm, n = 6) and Cftr−/− mice (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 between bars for B. In C statistical differences were calculated for a given concentration between Cftr+/+ and Cftr−/− mice.
Impaired agonist-dependent vasorelaxation in Cftr−/− mice
We observed a second alteration of the vascular reactivity by comparing the vasorelaxation properties of aortas from Cftr+/+ and Cftr−/− mice. Traces presented Fig. 7A show that VIP poorly relaxed denuded aortic ring from Cftr−/− animals (bottom trace) compared to Cftr+/+ mice (top trace). On a total of seven Cftr+/+ and five Cftr−/− animals we observed significant differences (P < 0.001) as we found 77% relaxation induced by VIP with preconstricted Cftr+/+ mice aortas but only 37% relaxation with Cftr−/− mice (Fig. 7B). The partial defective response to VIP of Cftr−/− mouse aortas was confirmed with the following experiments. Figure 7C shows that MPB-07 is not able to relax denuded preconstricted aortic rings from six additional Cftr−/− animals. Nevertheless, MPB-07 significantly reduced (IC50= 37 ± 1.17 μm) KCl-induced contraction of aortic rings from Cftr+/+ mice (n = 6, Fig. 7C). Thus, aortas that lack CFTR (i) are significantly more constricted than are control arteries, (ii) are less sensitive to the relaxing action of VIP, and (iii) failed to relax in the presence of CFTR activators. These observations suggest an alteration of the vascular reactivity that is endothelium independent.
Discussion
Our results provide compelling evidence that the activation of CFTR Cl− channels is an unexpected but potentially important mechanism for contraction and relaxation of aortic smooth muscle. First, we found that disrupting the Cftr gene not only affects the response of VSMC to vasoactive agents but also prevents the cAMP-dependent vasorelaxation. Second, we observed that constricted aortic rings from Cftr−/− mice present an abnormal level of vasoconstriction, indicating that CFTR is required to limit KCl-induced vasoconstriction. Third, we showed that CFTR is responsible for the cAMP-dependent Cl− transport in VSMC and that a different Ca2+-activated Cl− channel (possibly mCLCA4 or bestrophin) (Elble et al. 2002; Hartzell et al. 2005) is preserved in myocytes from Cftr−/− mice. Fourth, because we did not observe any functional differences between aortic preparations with or without endothelium, we concluded that the role of CFTR in Cl− transport and vascular reactivity is endothelium independent. The pharmacology of CFTR in epithelial and vascular smooth muscle cells appears to be remarkably similar with respect to inhibitors (CFTRinh-172, glibenclamide, DPC), activators (cAMP agonists, benzoquinolizinium and isoflavone derivatives) and physiological agonists (isoproterenol, VIP). However, this is not proof that the molecular form of CFTR in epithelia and VSMC is the same. Indeed, it is known for example that CFTR exists in two major isoforms due to alternative splicing in cardiac and epithelial cells (Horowitz et al. 1993). Further studies will be required to identify the vascular isoform of CFTR.
In a previous studies on rat VSMC (Robert et al. 2004), we observed cAMP-dependent CFTR Cl− channel activity in the presence of high K+ solution, suggesting that CFTR could be only observed in depolarized myocytes. However, the CFTR activator MPB-07 was also shown to inhibit the contractile response to the α-adrenergic agonist noradrenaline (Robert et al. 2004). In mouse myocytes, we also found that in the presence of the vasoconstrictor 5-hydroxytryptamine (5-HT) (Vanhoutte, 1988), CFTR channels activation occurs. Thus, CFTR appears to be active upon cell contractions and not only after depolarization of myocytes. The experiments performed on mice deficient for CFTR allowed us to clearly demonstrate the implication of CFTR as the privileged target for cAMP-dependent vasoactive agent and relaxation. The elevation of intracellular cAMP following the interaction of VIP with VPAC receptors activates protein kinase A (Henning & Sawmiller, 2001), and upon phosphorylation of the R domain (Gadsby & Nairn, 1999; Sheppard & Welsh, 1999; Robert et al. 2004) opens CFTR, like in epithelia (Joo et al. 2002; Derand et al. 2004), promoting vasorelaxation as shown in this report. In addition, we compared the Cl− transport of cultured VSMC activated by cAMP and Ca2+ agonists. Angiotensin II is a locally released mediator controlling the vascular tone through stimulation of Ca2+-dependent Cl− channels via increase in intracellular [Ca2+] and depolarization leading to Ca2+ entry through voltage-dependent Ca2+ channels and contraction (White et al. 1995; Large & Wang, 1996; Guibert et al. 1997; Nelson et al. 1997). In cultured VSMC from Cftr−/− mice, we found that angiotensin II and the calcium ionophore A23187 stimulated Cl− transport consistent with a model in which cAMP- and Ca2+-dependent Cl− transports are supported by different molecules (Clarke et al. 1994). Other studies demonstrated that Ca2+-dependent Cl− currents contribute significantly to VSMC contraction (Lamb & Barna, 1998). The role of CFTR in the physiology of VSMC is still unclear on the basis of the present study on mouse or on rat (Robert et al. 2004). Importantly we found now that CFTR is readily activated once the muscle cell is contracted either through a receptor-mediated mechanism (i.e. with 5-HT) with the involvement of secondary messengers in the signal transduction pathway, or with KCl via a voltage-dependent effect. Thus CFTR is not only activated in depolarized myocytes. Interestingly, it has been shown that genistein, which we showed activates CFTR in VSMC, reduces agonist-induced contraction in an endothelium-independent manner via the cAMP-dependent signal transduction pathway in porcine coronary arterial smooth muscle (Lee et al. 2004) and rat aortic rings (Satake & Shibata, 1999). Nevertheless, the link between contraction and CFTR activation remains unknown. One possible explanation would be that while VSMC contract, cytoskeleton remodelling modifies the turnover of the protein. Chloride flow through open CFTR channels under cAMP stimulation would thus allow repolarization of the membrane and thereby relaxation.
Although the knock-out of the Cftr gene in mice has revealed impairment of cAMP-dependent Cl− secretion in epithelia (Snouwaert et al. 1992; Clarke et al. 1994) no data have been reported concerning the vascular consequence of disrupting CFTR. Moreover, despite the fact that mCLCA4, CLC-3 and volume-sensitive anion channels are expressed in VSMC (Lamb & Barna, 1998; Yamazaki et al. 1998; Kitamura & Yamazaki, 2001; Elble et al. 2002; Yamamoto-Mizuma et al. 2004) only a few reports investigated the vascular role of these proteins after gene knock-out. However, studying the consequences of the loss of the voltage-dependent Cl− channel ClC-3 in rat aortas using specific antisense (Wang et al. 2002) revealed an interesting role for Cl− channels. Indeed, transfection of rat aortic VSMCs with antisense oligonucleotide specific to ClC-3 prevents rat aortic VSMC proliferation induced by endothelin-1 suggesting a link between cell proliferation and this chloride channel (Wang et al. 2002). With a different strategy using mice lacking ClC-3, Yamamoto-Mizuma et al. (2004) showed that some of the fundamental properties of the native volume-sensitive osmolyte and anion channels (VSOACs) recorded from mouse atrial cells and VSMC are affected. In particular, whereas the basic electrophysiological and pharmacological properties of VSOACs are similar in Clcn3+/+ and Clcn3−/− mice, the channel regulation by endogenous protein kinase C, sensitivities to ATP or Mg2+ and sensitivity to block by anti-ClC-3 antibodies are different (Yamamoto-Mizuma et al. 2004). Unfortunately, no data have been presented concerning the contraction/relaxation consequences for VSMC (Yamamoto-Mizuma et al. 2004). However, in another study also performed on Clcn3−/− mice, the swelling-activated chloride current was unchanged, the acidification of synaptic vesicles was impaired and a severe postnatal degeneration of the retina and of the hippocampus was described (Stobrawa et al. 2001). It is therefore difficult to reach a conclusion on the possible role of ClC-3 in the regulation of cell volume in VSMC. Other reports did not mentioned vascular abnormalities in Clcn2−/−, Clcn5−/− and Clcn7−/− mice (Piwon et al. 2000; Bosl et al. 2001; Kornak et al. 2001). In mice lacking the Na+–K+–Cl− cotransporter (Nkcc1−/−) a significantly lower systolic blood pressure was observed but no differences were noted between Nkcc1−/− and Nkcc1+/+ mice for the maximum contractility of aortic smooth muscle stimulated by phenylephrine (Meyer et al. 2002). Therefore, whereas numerous studies suggested a fundamental role for Cl− transporters in the physiology of VSMCs (Large & Wang, 1996; Nelson et al. 1997; Lamb & Barna, 1998; Chipperfield & Harper, 2000; Jackson, 2000; Kitamura & Yamazaki, 2001; Elble et al. 2002; Wang et al. 2002) only a few reports including the present study, using gene knock-out or antisense strategy, have firmly demonstrated their role in vascular reactivity (Wang et al. 2002; Yamamoto-Mizuma et al. 2004).
Finally, we observed that the absence of CFTR in VSMC is associated with a higher contraction in Cftr−/− mice, suggesting that loss or dysfunction of CFTR impairs the physiological control of the vascular tone. At this stage of our investigation it is premature to conclude about a potential link between CFTR in VSMC and vascular abnormalities. However, it is important to keep in mind that individuals affected by the genetic disease cystic fibrosis, suffering from epithelial chloride impermeability, also develop as the disease progresses disabling respiratory and non-respiratory diseases eventually leading to respiratory failure, and portal and pulmonary hypertension (Vizza et al. 1998; Debray et al. 1999; Salvi, 1999). Because CFTR is involved in vasorelaxation and since vasoconstriction of the small- and medium-sized pulmonary arteries plays an important role in the pathogenesis of pulmonary hypertension during the early stages of the disease (Vizza et al. 1998; Salvi, 1999), an altered function of CFTR may be linked to the development of pulmonary hypertension. Further investigations will be required to explore the expression of CFTR in other vascular systems (e.g. pulmonary arteries) as well as its role in pulmonary hypertension in CF. Additional investigations will be needed with the delF508-animal model to determine the consequence of the gene alteration for the vascular parameters.
In conclusion in future investigations, Cftr+/+ and Cftr−/− mice should serve as good models to study the contribution of CFTR and Ca2+-dependent Cl− channels to vascular properties. Based on our findings and those of others (White et al. 1995; Large & Wang, 1996; Guibert et al. 1997; Nelson et al. 1997; Lamb & Barna, 1998) these two Cl− channels appear to be functionally important in Ca2+-dependent vasoconstriction and cAMP-mediated vasorelaxation, respectively. Taken together, these findings demonstrate for the first time a causal relationship between the absence of CFTR and the defective vascular tone and vasorelaxation observed with Cftr−/− mice.
Acknowledgments
This work was part of the thesis project of R.R. supported by a fellowship from Vaincre la Mucoviscidose (VLM). Other grants are from CF-Pronet and CNRS. The authors would like to thank Alison Gurney and Vadim Osipenko for their precious advices on the dissociation of smooth muscle cells, Clarisse Vandebrouck for her precious help with SMC culture, Anne Cantereau for her expertise in confocal microscopy, Bruno Constantin for the alexa 488-conjugated secondary antibody and lab colleagues for stimulating discussions.
References
- Bosl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell–cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J. 2001;20:1289–1299. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2000;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr−/− mice. Proc Natl Acad Sci U S A. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray D, Lykavieris P, Gauthier F, Dousset B, Sardet A, Munck A, Laselve H, Bernard O. Outcome of cystic fibrosis-associated liver cirrhosis: management of portal hypertension. J Hepatol. 1999;31:77–83. doi: 10.1016/s0168-8278(99)80166-4. [DOI] [PubMed] [Google Scholar]
- Derand R, Bulteau-Pignoux L, Becq F. The cystic fibrosis mutation G551D alters the non-Michaelis-Menten behavior of the cystic fibrosis transmembrane conductance regulator (CFTR) channel and abolishes the inhibitory Genistein binding site. J Biol Chem. 2002;277:35999–36004. doi: 10.1074/jbc.M206121200. [DOI] [PubMed] [Google Scholar]
- Derand R, Montoni A, Bulteau-Pignoux L, Janet T, Moreau B, Muller JM, Becq F. Activation of VPAC1 receptors by VIP and PACAP-27 in human bronchial epithelial cells induces CFTR-dependent chloride secretion. Br J Pharmacol. 2004;141:698–708. doi: 10.1038/sj.bjp.0705597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble RC, Ji G, Nehrke K, DeBiasio J, Kingsley PD, Kotlikoff MI, Pauli BU. Molecular and functional characterization of a murine calcium-activated chloride channel expressed in smooth muscle. J Biol Chem. 2002;277:18586–18591. doi: 10.1074/jbc.M200829200. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev. 1999;79:S77–S107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- Guibert C, Marthan R, Savineau JP. Oscillatory Cl− current induced by angiotensin II in rat pulmonary arterial myocytes: Ca2+ dependence and physiological implication. Cell Calcium. 1997;21:421–429. doi: 10.1016/s0143-4160(97)90053-1. [DOI] [PubMed] [Google Scholar]
- Gurney AM. Electrophysiological recording methods used in vascular biology. J Pharmacol Toxicol Meth. 2000;44:409–420. doi: 10.1016/s1056-8719(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;65:519–558. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49:27–37. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- Horowitz B, Tsung SS, Hart P, Levesque PC, Hume JR. Alternative splicing of CFTR Cl− channels in heart. Am J Physiol. 1993;264:H2214–H2220. doi: 10.1152/ajpheart.1993.264.6.H2214. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268:C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem. 2002;277:50710–50715. doi: 10.1074/jbc.M208826200. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yamazaki J. Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn J Pharmacol. 2001;85:351–357. doi: 10.1254/jjp.85.351. [DOI] [PubMed] [Google Scholar]
- Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- Lamb FS, Barna TJ. Chloride ion currents contribute functionally to norepinephrine-induced vascular contraction. Am J Physiol. 1998;275:H151–H160. doi: 10.1152/ajpheart.1998.275.1.H151. [DOI] [PubMed] [Google Scholar]
- Lamb FS, Clayton GH, Liu BX, Smith RL, Barna TJ, Schutte BC. Expression of CLCN voltage-gated chloride channel genes in human blood vessels. J Mol Cell Cardiol. 1999;31:657–666. doi: 10.1006/jmcc.1998.0901. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Lee MYK, Leung SWS, Vanhoutte PM, Man RYK. Genistein reduces agonist-induced contractions of porcine coronary arterial smooth muscle in a cyclic AMP-dependent manner. Eur J Pharmacol. 2004;503:165–172. doi: 10.1016/j.ejphar.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivingt-Mounir C, Norez C, Derand R, Bulteau-Pignoux L, Nguyen-Huy D, Viossat B, Morgant G, Becq F, Vierfond JM, Mettey Y. Synthesis, SAR, crystal structure, and biological evaluation of benzoquinoliziniums as activators of wild-type and mutant cystic fibrosis transmembrane conductance regulator channels. J Med Chem. 2004;47:962–972. doi: 10.1021/jm0308848. [DOI] [PubMed] [Google Scholar]
- Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl− cotransporter. Am J Physiol Heart Circ Physiol. 2002;283:H1846–H1855. doi: 10.1152/ajpheart.00083.2002. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Conway MA, Knot HJ, Brayden JE. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. J Physiol. 1997;502:259–264. doi: 10.1111/j.1469-7793.1997.259bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ. ClC-5 Cl−-channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Robert R, Thoreau V, Norez C, Cantereau A, Kitzis A, Mettey Y, Rogier C, Becq F. Regulation of the cystic fibrosis transmembrane conductance regulator channel by beta-adrenergic agonists and vasoactive intestinal peptide in rat smooth muscle cells and its role in vasorelaxation. J Biol Chem. 2004;279:21160–21168. doi: 10.1074/jbc.M312199200. [DOI] [PubMed] [Google Scholar]
- Salvi SS. Alpha1-adrenergic hypothesis for pulmonary hypertension. Chest. 1999;115:1708–1719. doi: 10.1378/chest.115.6.1708. [DOI] [PubMed] [Google Scholar]
- Satake N, Shibata S. The potentiating effect of genistein on the relaxation induced by isoproterenol in rat aortic rings. General Pharmacol. 1999;33:221–227. doi: 10.1016/s0306-3623(99)00011-7. [DOI] [PubMed] [Google Scholar]
- Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol Rev. 1999;79:S109–S144. doi: 10.1152/physrev.1999.79.1.S109. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J General Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Singh AK, Venglarik CJ, Bridges RJ. Development of chloride channel modulators. Kidney Int. 1995;48:985–993. doi: 10.1038/ki.1995.380. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Tousson A, Van Tine BA, Naren AP, Shaw GM, Schwiebert LM. Characterization of CFTR expression and chloride channel activity in human endothelia. Am J Physiol. 1998;275:C1555–C1564. doi: 10.1152/ajpcell.1998.275.6.C1555. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Platelets, endothelium and blood vessel wall. Experientia. 1988;44:105–109. doi: 10.1007/BF01952190. [DOI] [PubMed] [Google Scholar]
- Vizza CD, Lynch JP, Ochoa LL, Richardson G, Trulock EP. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998;113:576–583. doi: 10.1378/chest.113.3.576. [DOI] [PubMed] [Google Scholar]
- Wang GL, Wang XR, Lin MJ, He H, Lan XJ, Guan YY. Deficiency in ClC-3 chloride channels prevents rat aortic smooth muscle cell proliferation. Circ Res. 2002;91:E28–E32. doi: 10.1161/01.res.0000042062.69653.e4. [DOI] [PubMed] [Google Scholar]
- Werstiuk ES, Lee RM. Vascular beta-adrenoceptor function in hypertension and in ageing. Can J Physiol Pharmacol. 2000;78:433–452. [PubMed] [Google Scholar]
- White CR, Elton TS, Shoemaker RL, Brock TA. Calcium-sensitive chloride channels in vascular smooth muscle cells. Proc Soc Exp Biol Medical. 1995;208:255–262. doi: 10.3181/00379727-208-43853. [DOI] [PubMed] [Google Scholar]
- Woodrum DA, Brophy CM. The paradox of smooth muscle physiology. Mol Cell Endocrinol. 2001;177:135–143. doi: 10.1016/s0303-7207(01)00407-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Mizuma S, Wang GX, Liu LL, Schegg K, Hatton WJ, Duan D, Horowitz TL, Lamb FS, Hume JR. Altered properties of volume-sensitive osmolyte and anion channels (VSOACs) and membrane protein expression in cardiac and smooth muscle myocytes from Clcn3-/- mice. J Physiol. 2004;557:439–456. doi: 10.1113/jphysiol.2003.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J, Duan D, Janiak R, Kuenzli K, Horowitz B, Hume JR. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. J Physiol. 1998;507:729–736. doi: 10.1111/j.1469-7793.1998.729bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]