Abstract
High grade malignant gliomas are genetically unstable, heterogeneous and highly infiltrative; all characteristics that lend glioma cells superior advantages in resisting conventional therapies. Unfortunately, the median survival time for patients with glioblastoma multiforme remains discouraging at 12–15 months from diagnosis. Neuroimmunologists/oncologists have focused their research efforts to harness the power of the immune system to improve brain tumor patient survival. In the past 30 years, small numbers of patients have been enrolled in a plethora of experimental immunotherapy Phase I and II trials. Some remarkable anecdotal responses to immune therapy are evident. Yet, the reasons for the mixed responses remain an enigma. The inability of the devised immunotherapies to consistently increase survival may be due, in part, to intrinsically-resistant glioma cells. It is also probable that the tumor compartment of the tumor-bearing host has mechanisms or produces factors that promote tumor tolerance and immune suppression. Finally, with adoptive immunotherapy of ex vivo activated effector cell preparations, the existence of suppressor T cells within them theoretically may contribute to immunotherapeutic failure. In this review, we will summarize our own studies with immunotherapy resistant glioma cell models, as well as cover other examined immunosuppressive factors in the tumor microenvironment and immune effector cell suppressor populations that may contribute to the overall immune suppression. An in-depth understanding of the obstacles will be necessary to appropriately develop strategies to overcome the resistance and improve survival in this select population of cancer patients.
Keywords: T regulatory cells, cellular immunotherapy, immunotherapy, brain tumors, CTL resistance, effector T lymphocytes, FasL, immunosuppressive mechanisms, apoptosis, adhesion molecules, extracellular matrix proteins, cytokines
Abbreviations: alloreactive cytotoxic T lymphocytes, (aCTL); antigen presenting cells (APC); blood brain barrier (BBB); central nervous system (CNS); cyclooxygenase (COX); dendritic cells (DCs); extracellular matrix (ECM); Fas-associated death domain-like IL-1b-converting enzyme inhibitory protein c (cFLIP); Glioblastoma multiforme (GBM); Glioma associated antigens (GAA); Human leukocyte antigens (HLA); Immunotherapy resistant (ITR); Indoleamine 2,3-dioxygenase, (IDO); Inhibitors of apoptosis proteins (IAP); Interferon (IFN); Interleukin (IL); Intercellular adhesion molecule-1 (ICAM-1); Killer immunoglobulin-like receptors (KIRs); Leukocyte function antigen-1 (LFA-1); Major histocompatibility complex (MHC); MHC class I-related (MIC); Mixed lymphocyte reaction (MLR); Natural killer (NK); Peripheral blood mononuclear cells (PBMNC); Prostaglandin E2, (PGE2); Signal transducer and activator of transcription-3 (STAT-3); T cell receptor (TCR); T helper, (Th); T regulatory cell (Treg); Transforming growth factor (TGF); Tumor associated antigens (TAA); Tumor infiltrating lymphocytes (TIL); Tumor necrosis factor (TNF)
I. Introduction
The majority of primary tumors of the central nervous system (CNS) are of astrocytic lineage (CBTRUS, 2005). Of the astrocytomas, the most malignant form, glioblastoma multiforme (GBM), is diagnosed at a much higher frequency than lower grade astrocytomas. The median survival time for GBM patients is poor, approximating 12–15 months even with aggressive upfront treatment (Stupp et al, 2005). Several obstacles prevent the complete eradication of GBM by conventional therapies. GBMs locally but diffusely infiltrate neighboring brain tissue through white matter tracts, perivascular, and periventricular spaces, and often invading cells are found centimeters away from the primary tumor mass (Hochberg and Pruitt, 1980). As a consequence, GBM patients are rarely cured of their tumors by surgical intervention. The significant degree of genetic instability (Louis and Gusella, 1995) and cellular heterogeneity within GBM ensures that not all cellular variants will respond to radiation or chemotherapy. For example, glioblastoma cells downregulate p53 (Shu et al, 1998) or upregulate DNA repair enzymes such as O6-methylguanine-DNA methyltransferase (Bandres et al, 2005) as a means to avoid radiation and chemotherapy induced-cell death, respectively. In addition, the physical isolation of brain tumors by the blood–brain barrier (BBB) and drug efflux pumps integrated into the membranes of endothelial cells at the BBB interface prevents efficient delivery of systemically administered chemotherapeutic agents (Doolittle et al, 2005).
To circumvent these limitations, researchers have tried to make the tumor cells more visible to the immune system (Paul and Kruse, 2001). To date, knowledge of the complex coordination of anti-tumor immune responses within the brain remains limited. Often translation of brain tumor immunotherapies is partially based upon knowledge of anti-tumor immune responses from tissues outside the brain or in xenograft models with defective endogenous immune compartments. Despite these restrictions, promising results have been observed in brain tumor patients treated with a variety of immunotherapeutic approaches (Kruse et al, 1997; Quattrocchi et al, 1999; Kruse and Rubinstein, 2001; Yu et al, 2004; Liau et al, 2005). Unfortunately, as is often the case with radiation and chemotherapy, patients might initially respond to a biologic therapy but then fail to respond to subsequent administrations of the immune therapeutic agent (Restifo et al, 1996; Rosenberg et al, 2003). Indeed, tumors have employed multiple mechanisms of immune evasion both in vitro and in vivo (Walker et al, 1997; Medema et al, 1999, 2001; Teitz et al, 2000; Wiendl et al, 2002).
Here, we review the unique immunologic aspects of the brain, intrinsic tumor tolerance mechanisms, and glioma-associated immune suppression and evasion. The generation and characterization of immunotherapy resistant (ITR) glioma models may allow for the development of strategies to overcome the resistance. Last, extensive characterization of immune infiltrates or immune effector cell populations for the presence of significant numbers of suppressor T cells may indicate that selective depletion of the suppressor T cell compartment is warranted before adoptive transfer into brain tumor patients, or before ex vivo activated cytotoxic effectors are generated.
II. Immunologic aspects of the brain
The brain was defined long ago as an immune privileged organ (Medewar, 1948). This term was coined to describe the observed higher degree of tolerance to allografts exhibited by the brain relative to those placed in other anatomical sites. The concept appeared to be logically based since the brain would require protection from uncontrolled and/or severe inflammatory events that would raise intracranial pressure and lead to death of neurons within vital CNS structures. The physical isolation of brain parenchyma from systemic circulation by the BBB (Doolittle et al, 2005), low or absent expression of human leukocyte antigens (HLA) on brain cells (Lampson and Hickey, 1986; Read et al, 2003), absence of lymphatic drainage (Walker et al, 2002) and resident dendritic cells (DCs) (Hickey, 2001), all suggest that the requirements for initiation of immune responses within the brain are significantly more stringent. Collectively, these observations led many to accept the idea that immune surveillance does not occur within the brain.
Other studies have demonstrated that the brain is not as completely immunologically silent as was once thought. The induction of CNS autoimmune diseases (De Simone et al, 1995) and anti-viral immune reactions to neurotropic viruses (Klein et al, 2005) were reported. Although non-activated T lymphocytes are incapable of penetrating the BBB, activated T lymphocytes are capable of traversing the BBB. The presence of tumor infiltrating lymphocytes (TIL) suggests that anti-tumor responses are engendered in response to malignant lesions within the brain (Quattrocchi et al, 1999).
Controversy exists with respect to the ability of microglia, CNS macrophage, to initiate immune responses. Recent studies have shown that brain resident microglia process antigen (Aloisi et al, 1998), and express class II major histocompatibility complex (MHC) and co-stimulatory molecules (De Simone et al, 1995; Aloisi et al, 1998). In addition, our laboratory showed that microglia ingest T cell damaged glioma cells in vitro and in vivo (Kulprathipanja and Kruse, 2004). They can stimulate T cell proliferation, maturation (Carson et al, 1999) and cytokine secretion (Aloisi et al, 1998). Microglia can also produce anti-inflammatory molecules such as IL-10 (Seo et al, 2004) and prostaglandin E2 (Watters et al, 2005), both of which may inhibit antigen presentation by microglial cells. Schartner and colleagues have shown that tumor-associated microglia display an impaired capacity to upregulate class II MHC antigens relative to normal brain microglia, even upon stimulation with potent microglial activators (Schartner et al, 2005).
It has been postulated that cervical lymph nodes act as the drainage site of the brain interstitial fluid, thus constituting the afferent arm of immune responses within the CNS (Karman et al, 2004). In agreement with this notion, Tsugawa and colleagues demonstrated that DCs injected into intracerebral tumors localized to cervical lymph nodes in animal models (Tsugawa et al, 2004). Migration of DCs to the cervical lymph nodes may facilitate the activation of peripheral anti-tumor T cell responses. It has become clear that the brain is more immunologically active than originally thought.
III. Intrinsic mechanisms of tumor tolerance
Given the ability of T lymphocytes and natural killer (NK) cells to injure tumor cells, it is surprising that cancers are prevalent in the human population. So what prevents an immunocompetent animal from rejecting brain tumors? Immunological tolerance to cancer, in part, is mediated by the expression and presentation of self-antigens by neoplastic cells. The tolerance mechanisms designed to inhibit autoimmunity also protect tumors from their rejection.
A. Central tolerance
Central tolerance is mediated by macrophages, DCs, and epithelial cells of the thymus, all of which participate in the processing and display of self-antigens to immature T cells within the thymus (Gallegos and Bevan, 2004). Self-peptide is expressed and displayed in the thymus, an activity that plays an essential role in shaping the T cell repertoire of the host. Immature T cells expressing T cell receptors (TCRs) with extremely low avidity to MHC: self antigen complexes survive the process of positive selection while those with high avidity are signaled to undergo apoptosis during the process of negative selection (Gett et al, 2003; Gallegos and Bevan, 2004; Gronski et al, 2004; Mathis and Benoist, 2004). In this way, the thymus purges the host of autoreactive T cells. Under experimental conditions, the frequency of CD8+ T cells recognizing gp100, tyrosinase and MAGE, all self derived peptides that are also expressed by malignant gliomas, was less than 1 in 10,000 (Zippelius et al, 2002). With the exception of high thymic output of MART-1 melanoma antigen-specific T cells (Gallegos and Bevan, 2004), the result of this selective process is a population of T cells with only low to intermediate reactivity to self-tumor antigens. Thus, autoreactive T cells of sufficiently low avidity to survive negative selection are incapable of responding to tumor antigens with high avidity.
B. T cell anergy
Cells surviving thymic selection are subjected to peripheral tolerance mechanisms. Tumor cells, including glioma cells, express MHC:self-peptide ligands, but do not express the necessary co-stimulatory molecules to effectively activate naive T cells (Wintterle et al, 2003). T cells can become anergic if they bind to MHC:self antigen ligands in the absence of costimulatory signals. Anergic T cells do not proliferate or differentiate into armed effector cells upon re-encounter of self antigen even if they receive costimulatory signals. These interactions lead to tumor specific T cell ignorance. Two additional mechanisms used to maintain tumor tolerance are T cell anergy induced by tumor-associated immature DCs (Kusmartsev et al, 2005) and activation-induced T cell death due to repeated tumor antigen stimulation (Saff et al, 2004). Recent data suggest that DCs loaded with self-antigen migrate into the thymus to induce tolerance to self antigens expressed in peripheral organs (Gallegos and Bevan, 2004).
C. T regulatory (Treg) cells
1. CD4+/CD25+ T cells
Sakaguchi and colleagues were the first to identify a subset of CD4+ T cells expressing the CD25 activation marker, IL-2 receptor α-chain, that when depleted in vivo, resulted in severe autoimmune diseases (Sakaguchi et al, 1995). Reconstitution with CD4+CD25+ T cells reversed the autoimmunity. Treg cells also inhibit DC maturation and their antigen presentation function (Misra et al, 2004), and T cell activation and proliferation (Sakaguchi et al, 1995; Misra et al, 2004). The mechanism of T cell suppression is contact-dependent and often mediated by IL-10 and TGF-β (Sakaguchi et al, 1995).
2. CD8+/CD25+ & CCR7+/CD45RO+/CD8+ T cells
Recently identified human CD8+CD25+ lymphocytes were capable of suppressing allogeneic and autologous T cell proliferation in a cell contact-dependent manner. TCRs on CD8+CD25+ cells are thought to bind to HLA-E/self peptide complexes displayed on the cell surface of self-antigen activated T cells (Maggi et al, 2005; Jiang and Chess, 2006). The TCR/HLA-E/peptide interactions bring the Treg and autoreactive T cells in direct physical contact and subsequently autoreactive T cell activity is downregulated. Breakdown of this peripheral tolerance mechanism is thought to contribute to the pathogenesis of various autoimmune diseases. Another type of suppressor CD8+ Treg cell was identified in the tumor environment in patients with ovarian carcinoma. Plasmacytoid DCs in tumor ascites induced IL-10+/CCR7+/CD45RO+/CD8+ cells that were found to significantly inhibit mature myeloid DC-mediated tumor associated antigen (TAA)-specific CTL effector function through IL-10 production (Wei et al, 2005).
Recent studies have shown that in vivo depletion of T reg cells mediates regression of tolerogenic tumors (Liyanage et al, 2002; Ghiringhelli et al, 2004). In humans, Treg cells are elevated in the peripheral blood and tumor microenvironment of cancer patients, suggesting that Treg cells may prevent the initiation of anti-tumor responses directed towards shared self-antigens (Woo et al, 2001). Preliminary results of a phase I/II immunotherapy trial employing Ontak (IL-2 fused to diphtheria toxin) to deplete Treg cells in ovarian cancer patients are promising (Curiel et al, 2006). Ontak therapy significantly depleted Treg cells in peripheral blood and increased the percentage of IFN-γ expressing CTL without inducing autoimmune reactions.
IV. Mechanisms of glioma immune suppression and resistance
It is clear that intrinsic tumor tolerance mechanisms represent a significant obstacle to the induction of potent and persistent anti-tumor immune responses in immunocompetent hosts. There is evidence however that high avidity tumor-antigen-specific T cells do exist (Zippelius et al, 2002) and furthermore that immunogenic tumors are efficiently rejected in immunocompetent hosts (Rubinstein et al, 2004). Failure of the intrinsic tumor tolerance mechanisms allows for potent immunoselective pressure to nascent transformed cells. The genetic instability of tumors and their repeated exposure to immune selective pressures increase the potential for selection of tumor cell variants with an enhanced capacity to evade immune attack. Many studies have demonstrated that tumor cells utilize multiple immune evasion strategies and the strategies are described below.
A. Secreted immunosuppressive factors
1. PGE2
The non-steroidal anti-inflammatory drugs target the cyclooxygenase enzymes, COX-1 and COX-2, which convert arachidonic acid to prostaglandins and thromboxane (Wang and Dubois, 2006). COX-2 derived prostaglandin E2 (PGE2) promotes tumor cell invasion, motility and angiogensis upon binding to its receptor, EPI-4. In addition, PGE2 induces immunosuppression by downregulating the production of T helper (Th) 1 cytokines (IL-2, IFN-γ and TNF-α) and upregulating Th2 cytokines (IL-4, IL-10 and IL-6) (Wang and Dubois, 2006). As well, PGE2 inhibits T cell activation and suppresses the anti-tumor activity of NK cells (Baxevanis et al, 1993; Chemnitz et al, 2006). A recent report indicates that tumor derived supernatants containing abundant levels of PGE2 enhanced the suppressive activity of Treg cells, induced expression of the Treg specific transcription factor, Foxp3, in non-Treg cells, and COX-2 inhibition reduced Treg activity and tumor burden in vivo (Sharma et al, 2005). Coculture of human primary glioma cells overexpressing COX-2 with mature DCs, induced mature DCs to overexpress IL-10. IL-10 overexpressing DCs then induced a CD4+ Treg type anti-tumor response that was abrogated with COX-2 inhibition (Akasaki et al, 2004).
2. TGF-β
There are three closely related mammalian TGF-β isoforms (TGF-β1, 2, and 3), all of which signal through transmembrane serine/threonine kinase receptors (Govinden and Bhoola, 2003). Upon receptor binding, Smad 2 is phosphorylated and associates with Smad 4. The resulting Smad2/4 complexes then enter the nucleus and mediate the transcription of target genes. TGF-β is involved in regulating inflammation, angiogenesis and proliferation (Govinden and Bhoola, 2003). In addition, TGF-β is expressed by a variety of cancers including astrocytomas (Bodmer et al, 1989). TGF-β2 appears to be the major isoform expressed by glioblastomas, although more recent studies indicate that TGF-β1 expression is predominately restricted to glioblastomas (Constam et al, 1992; Kjellman et al, 2000). Rarely do gliomas express TGF-β3 (Constam et al, 1992). Unlike gliomas, normal glial cells secrete TGF-β1 and -β2 in a latent form that must be proteolytically cleaved to have biological activity (Constam et al, 1992; Kjellman et al, 2000). TGF-β inhibits T cell activation, proliferation (Ranges et al, 1987; Gorelik and Flavell, 2000), and the maturation and function of professional antigen presenting cells (APCs) (Letterio and Roberts, 1998; Smyth et al, 1991; Thomas and Massague, 2005). As well, TGF-β inhibits the synthesis of cytotoxic molecules including perforin, granzymes A and B, IFN-γ, and FasL in activated CTL (Smyth et al, 1991; Thomas and Massague, 2005). Recently it has been shown that TGF-β1 deficient mice develop lethal autoimmunity due to a lack of sustained Treg function (Marie et al, 2005). It is likely that TGF-β plays a role in tumor tolerance by facilitating the conversion of naive T cells to a Treg phenotype.. Alternatively, TGF-β may recruit Tregs towards the primary tumor site as a means of immune evasion.
3. Interleukin-10 (IL-10)
IL-10, originally named cytokine synthesis inhibitory factor, is similar to TGF-β in many respects (Grutz, 2005). IL-10 inhibits IL-2 induced T cell proliferation (Grutz, 2005), the DC and macrophage activation of T cells (Hishii et al, 1995), downmodulates class II MHC on APCs (Hishii et al, 1995), and is expressed by Treg cells (Sakaguchi, 2005) and human gliomas (Hishii et al, 1995). Other studies suggest that IL-10 is not always immunosuppressive but acts by promoting brain tumor growth inhibition in vivo (Book et al, 1998).
B. Impairment of adhesive effector: tumor cell interactions and protective tumor cloaks
1. Extracellular Matrix (ECM) Proteins
Adhesive interactions and membrane triggered signals induced upon cell-cell contact play an important role in immune cell function. One interaction required for efficient effector cell lysis of tumor cells is effector binding to tumor cell surfaces. Tumor cells have developed strategies to prevent their adhesion by immune effector cells. ECM proteins have recently been recognized to participate in T cell activation (Hemesath et al, 1994). In particular, tenascin-C extracts from the U251 glioma cell line were shown to inhibit T lymphocyte proliferation and cytokine production (Hemesath et al, 1994; Puente Navazo et al, 2001). In addition, TIL proliferation and IFN-γ production was inhibited by tenascin C (Parekh et al, 2005). Other studies convincingly established that glioma cells producing thick glycosaminoglycan coats, composed predominately of hylauronic acid, are protected from allogeneic CTL responses (Gately et al, 1982; Dick et al, 1983; Oberc-Greenwood et al, 1986).
2. Intercellular adhesion molecule-1 (CD54)
Adhesion molecules are known to mediate cell-cell interactions, particularly those between T cells and antigen-presenting or target cells. Intercellular adhesion molecule-1 (ICAM-1) functions as a cell surface receptor for leukocyte function antigen-1 (LFA-1) present on CTL and NK cells. LFA-1/ICAM-1 interactions facilitate T cell recognition of TAA presented class I MHC (Kikuchi et al, 2004; Fiore et al, 2002). Multiple studies have shown that ICAM-1 expression is required for tumor rejection in vivo (Kikuchi et al, 2004) and in vitro tumor cell lysis by multiple effector cell types (Kikuchi et al, 2004; Fiore et al, 2002; Schiltz et al, 2002). Disruption of LFA-1/ICAM-1 interactions inhibits target cell lysis and consequently constitutes one mechanism of evasion from tumor specific T and NK cell lysis (Schiltz et al, 2002; Fiore et al, 2002).
3. HLA class I defects
MHC class I molecules, also known as human leukocyte antigens (HLA), are required for presentation of endogenous or foreign antigenic peptides to cytotoxic T lymphocytes (Slingluff et al, 2000; Read et al, 2003) and for the engagement of receptors that regulate NK cell activity (O’Connor et al, 2006). In humans, MHC class I molecules comprise the classical HLA-A,B,C (class Ia) and several non-classical HLA molecules (class Ib) that include HLA-E, -F, -G and -H, MHC class I-related (MIC)-A and –B and CD1 (Bjorkman and Parham, 1990; Braud et al, 1999).
Classical HLA class I genes code for a 45 kDa α chain that folds into three domains (α1, α2 and α3). The α chain binds to proteolytically processed peptides (8–10 amino acids) and establishes noncovalent bonds with the 12 kDa β2-microglobulin (β2-m) protein to form a trimolecular complex displayed at the plasma membrane (Restifo et al, 1996). Cells presenting immunogenic peptides in the context of classical HLA class I molecules are susceptible to CTL-mediated lysis. All classical HLA-A,-B and -C and a significant proportion of non-classical HLA class I genes are located on chromosome 6 while β2-m is encoded by a gene mapped to chromosome 15 (Braud et al, 1999). Detailed studies of human tissues revealed that the majority of nucleated cells express classical HLA class I molecules (Daar et al, 1984). Some tissues such as cornea, testis, thyroid and parathyroid glands and the brain, display low or absent levels of class I HLA (Daar et al, 1984; Lampson and Hickey, 1986; Read et al, 2003).
In contrast to classical HLA genes, non-classical HLA genes are less widely expressed and polymorphic (Braud et al, 1999). Much like the classical HLA class I molecules, HLA-E, -F, -G, and –H molecules all noncovalently associate with β2-m and antigenic peptides. With the exception of HLA-F, the functions of the class Ib molecules were recently clarified, as detailed in the comprehensive review by Braud and colleagues (Braud et al, 1999).
Tumor cells displaying aberrant HLA class I expression may evade T cell detection and subsequently induced cytotoxicity. There are several molecular or genotypic phenotypes to describe abnormal HLA class I presentation in transformed cells (Figure 1). A complete HLA class I loss may be caused by mutations of both β2-m alleles. This would inhibit translation of β2-m mRNA (Restifo et al, 1996; Rosenberg et al, 2003). In the absence of β2-m expression, HLA class I heavy chain/β2-m/peptide complexes will not form nor be transported to the cell surface (Figure 1B). β2-m mutations leading to total loss of HLA class I expression have been identified in melanomas obtained from recurrent patients who initially experienced clinical responses to T cell-based immunotherapy (Rosenberg et al, 2003; Restifo et al, 1996; Jager et al, 1997; Chang et al, 2005). In HLA class I allelic loss, such as the loss of one HLA-A allele (Figure 1C), the phenotype produced may result from loss of a gene(s) encoding for the heavy chain of the lost HLA class I allele(s) or by mutations that inhibit their transcription or translation (Marincola et al, 1994; Wang et al, 1999; Facoetti et al, 2005; Demanet et al, 2004). Immunotherapy refractory tumors often demonstrate selective HLA class I allelic loss (Chang et al, 2003; Jager et al, 2002), indicating that the HLA defects contributed to the lack of response in the patients. In contrast to these findings, Hiraki and colleagues recently demonstrated that chondrosarcoma cells with HLA-A11 loss were lysed by autologous CTL thus indicating that the tumor was able to present antigenic peptides with the remaining HLA-A24 allele (Hiraki et al, 2001). Loss of a HLA class I haplotype (Figure 1D) may be caused by loss of portions of the short arm of chromosome 6 (Maeurer et al, 1996; Chang et al, 2003). In the phenotypes presented (Figure 1), it is more reasonable to assume that with the phenotype shown in Figure 1B the CTL susceptibility may be lost, with little possibility that it could be restored with exogenous IFN-γ treatment (Read et al, 2003); whereas the CTL susceptiblity would be reduced with the phenotypes presented in Figures 1C and 1D.
Figure 1.
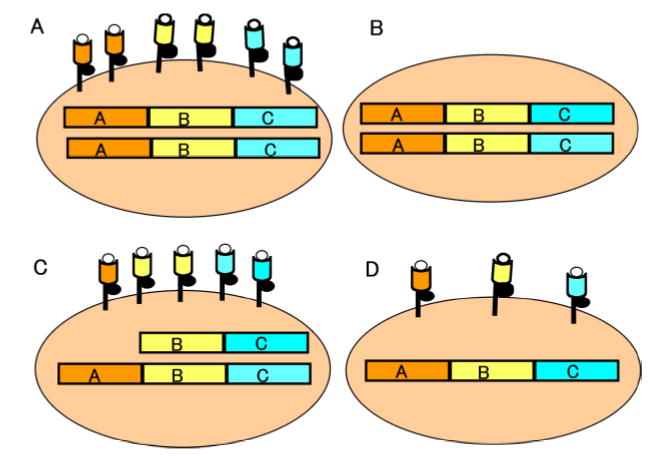
Depiction of normal class I HLA presentation and other aberrant HLA phenotypic defects in tumors. (A) Normal HLA class I allele presentation is shown on a tumor cell. The heavy HLA class I α chains associate with the invariant β2-m chain (black circle) and antigenic peptide (open circle) at the plasma membrane surface. (B) Loss of HLA antigen expression at the tumor cell surface is shown. Inactivating mutations of the β2-m genes may prevent β2-m expression, leading to a total loss of HLA class I. (C) Specific HLA allelic dropout is demonstrated. In this instance we show HLA class I A allelic downregulation that decreases the number of HLA/peptide complexes at the cell surface. (D) HLA haplotype loss is shown. As a result of loss of portions of chromosome 6, a complete loss of maternal or paternal HLA would be displayed as a HLA class I downregulation that would more drastically decrease the number of HLA/peptide complexes at the cell surface than what is depicted in C.
Finally, other defects have been implicated as providing mechanisms of T cell evasion in animal models (Mukherjee et al, 2003): 1) total HLA class I downregulation may be the result of epigenetic gene silencing, e.g. hypermethylation (Coral et al, 1999), 2) altered chromatin structure of the HLA class I promoter may occur (Nie et al, 2001), or 3) loss of antigen processing machinery components such as transporter associated with antigen processing-1 may cause aberrancy (Facoetti et al, 2005). In some cases, alteration or absence of one of the components of the trimolecular structure, such as the loss of TAA peptides, rather than HLA class I loss, might also occur (Slingluff et al, 2000).
C. How do HLA class I deficient tumor cells evade NK cell killing?
NK cells are capable of killing cancer and virally-infected cells without prior sensitization (O’Connor et al, 2006). NK cells are responsible for the direct killing of HLA class I deficient tumor cells (O’Connor et al, 2006). The binding of inhibitory killer immunoglobulin-like receptors (KIRs) expressed by NK cells to class I HLA molecules on normal cells maintains NK cell tolerance. Under pathological conditions including viral infections or neoplasms, HLA class I expression is often altered, thus breaking NK cell tolerance. As such, the rapid growth of HLA class I defective tumors in the face of NK cell immune selective pressure seems contradictory to the established anti-tumor function of NK cells.
Ectopic HLA-G expression is a recently described mechanism of tumor evasion of T and NK cell lysis (Wiendl et al, 2002). HLA-G was initially believed to display antigenic peptides to T cells at the materno-fetal interface since placental trophoblasts do not express classical HLA class I antigens (Rouas-Freiss et al, 1999). It is now thought that HLA-G protects the fetus from allorejection by the maternal NK and T cells. HLA-G binds to the NK and T cell inhibitory receptor, ILT2 to mediate its immunotolerant function (Hofmeister and Weiss, 2003). Not surprisingly, HLA-G is expressed by several tumors including primary glioblastomas, and as well, by established glioma cell lines (Wiendl et al, 2002). HLA-G expression rendered glioma cells resistant to alloreactive CTL lysis (Wiendl et al, 2002). HLA-G-mediated inhibitory signals are strong enough to counteract NK activating signals. It is conceivable that ectopic tumor HLA-G expression may provide concurrent protection to T and NK cell lysis.
D. Mechanisms of protection to Fas induced apoptosis
The Fas receptor and its ligand, FasL, are members of the tumor necrosis factor (TNF) family of receptors and ligands (Ozoren and El-Deiry, 2003). Binding of FasL to Fas initiates a signaling cascade resulting in apoptosis of cells expressing Fas. The Fas apoptosis pathway constitutes one mechanism by which NK and activated T cells regulate tumor growth. Tumor cells may disrupt the Fas pathway at many levels within this signaling cascade (Figure 2).
Figure 2.
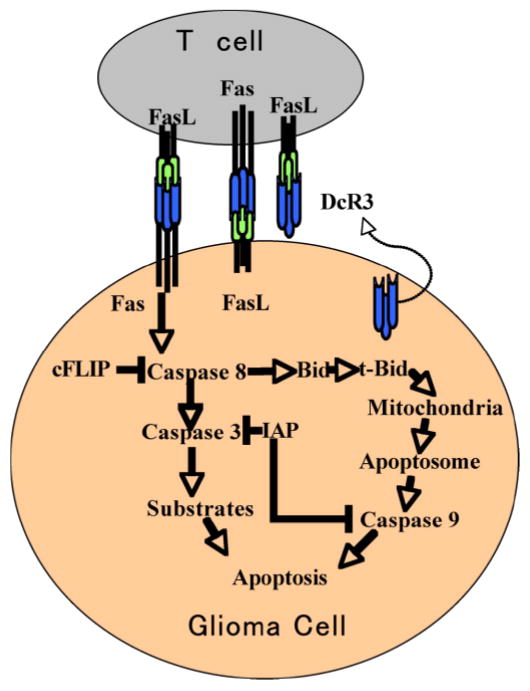
Disruption of Fas-induced apoptosis or upregulation of FasL may provide tumor cell protection to T lymphocyte induced cell injury. Decreased Fas expression by the glioma cells or their secretion of the FasL decoy receptor, DcR3, can inhibit death receptor induced apoptosis. The transduction of apoptotic signals by way of the Fas receptor is inhibited when tumor cells express cFLIP or IAPs. The cFLIP protein inhibits caspase 8 activity. The IAP family members suppress caspase 3 and caspase 9 activity. Tumor cells may counterattack T cells by expressing FasL, which can engage Fas on the T cell plasma membrane to initiate T cell apoptosis.
At the receptor level, downregulation of Fas surface expression (Leithauser et al, 1993) or secretion of the soluble decoy for FasL, decoy receptor 3 (DcR3), lacking a transmembrane region may inhibit Fas-induced apoptosis (Pitti et al, 1998; Roth et al, 2001). DcR3 is expressed by lung, colon and brain tumors (Pitti et al, 1998, Roth et al, 2001). In 9L experimental gliomas, DcR3-expressing tumors displayed reduced numbers of tumor infiltrating CD4 and CD8 T cells (Roth et al, 2001).
During signal transduction, expression of Fas-associated death domain-like IL-1b-converting enzyme inhibitory protein (cFLIP) inhibits the activation of caspase-8, rendering tumor cells resistant to apoptotic signals transduced by Fas and other death receptors (Medema et al, 1999; Kamarajan et al, 2003). Inhibition of Fas-induced glioma cell apoptosis can also be mediated by the family of apoptosis inhibitory proteins (IAPs). IAPs can inhibit caspase activity (French and Tschopp, 2002).
E. Tumors expressing FasL may counterattack activated effector T lymphocytes
The observation of tolerance to FasL+ testis grafts bolstered the hypothesis that FasL could grant tissues an immune privileged status (Bellgrau et al, 1995). Researchers in the transplantation field sought to capitalize on this finding by introducing the FasL gene into cells or tissues prior to their transplantation (Duke et al, 1999; Nelson et al, 2000). Tumor immunologists focused their attention on the role of FasL as a mechanism of immunoresistance. In several studies FasL was detected on a variety of tumor cell types both in vitro and in vivo (Saas et al, 1997; Walker et al, 1997; Husain et al, 1998; Gastman et al, 1999). Primary tumor explants and tumor cell lines expressing FasL induced apoptosis of Fas+ target cells including T lymphocytes (Saas et al, 1997; Walker et al, 1997; Husain et al, 1998; Gastman et al, 1999). A similar counterattack mechanism involving CD70, also a TNF family member, on gliomas and its receptor, CD27 on activated T cells, was recently described (Chahlavi et al, 2005).
Controversy exists as to the role of FasL in suppressing immune reactions. Allison and colleagues documented a rapid rejection of transplanted islet β cells that was accompanied by granulocytic infiltrates (Allison et al, 1997). Other researchers also found that FasL gene-modified tumors were rapidly rejected by what appeared to be a granulocyte-dependent mechanism. Some malignant glioma cells were observed to coexpress Fas and FasL, suggesting the possibility that Fas/FasL interactions may induce tumor cell apoptosis as well (Arai et al, 1997; Husain et al, 1998).
F. Novel tumor immunosuppressive mechanisms
1. B7-H1
The recently identified B7 homologue 1, B7-H1, possesses costimulatory and immunomodulatory activity (Wintterle et al, 2003). B7-H1 binds PD-1 to exert its immune modulation. Glioma cell lines and primary glioma specimens but not normal brain tissue exhibit B7-H1 expression (Wintterle et al, 2003; Wilmotte et al, 2005). In gliomas, it appears that B7-H1 inhibits allogeneic T cell activation and cytokine secretion (Wintterle et al, 2003; Wilmotte et al, 2005). Interestingly, under inflammatory conditions such as experimental allergic encephalomyelitis, microglial cells upregulate B7-H1 expression, suggesting that microglial associated B7-H1 plays a role in limiting autoimmune-induced tissue damage (Wintterle et al, 2003; Magnus et al, 2005).
2. Signal transducer and activator of transcription-3 (STAT-3)
Multiple tumor cell types display a persistent activation of STAT-3 (Kortylewski et al, 2005; Rahaman et al, 2005; Wang et al, 2004). Constitutive STAT-3 activation in tumor cells suppresses proinflammatory cytokine and chemokine release (Wang et al, 2004). As a result, the induction of tumor-specific T cell responses is inhibited. Blockade of STAT-3 signaling leads to the release of cytokines such as TNF-α, IL-6, IFN-β, chemokine related proteins such as RANTES and interferon-gamma-inducible 10 kD protein or IP10, and the activation of innate immunity and DCs.
In addition to regulating the expression of immunomodulatory factors, STAT-3 positively regulates the expression of anti-apoptotic proteins such as Bcl-2, Bcl-XL, Mcl-1, survivin and cFLIP in glioma cells (Rahaman et al, 2005; Konnikova et al, 2003; Akasaki et al, 2006). Silencing of STAT-3 expression in glioma cells induced their apoptosis in the absence of an apoptotic stimulus, but did not in normal human astrocytes (Konnikova et al, 2003). As expected, STAT-3 knockdown led to the downregulation of the pro-apoptotic proteins.
It is plausible that the upregulation of pro-apoptotic proteins as a result of constitutive STAT-3 activation may contribute to glioma cell resistance to radiation, chemotherapy, and CTL-based immunotherapies. The use of STAT-3 inhibitors would clarify the role of STAT-3 in providing gliomas with protection to the cytotoxic therapies. Interpretations of such experiments require careful consideration of the fact that STAT-3 inhibitors may also have inhibitory effects on other pathways, such as that dealing with epidermal growth factor receptor signaling (Gazit et al, 1991). In addition, the tyrosine kinase inhibitor, AG-490, once thought to be a STAT-3 pathway specific inhibitor, was recently shown to suppress T cell proliferation (Wang et al, 1999).
3. Indoleamine 2,3-dioxygenase (IDO)
IDO is required for degradation of the essential amino acid, tryptophan. In conditions of tryptophan shortage, T cells undergo cell cycle arrest. Interestingly, expression of IDO in the placenta plays an essential role in preventing rejection of semi-allogeneic fetuses (Munn and Mellor, 1999, 2004). Based upon these observations, Uytennhove and colleagues postulated that IDO expression may create a local tryptophan shortage that could starve T cells and thus induce tumor tolerance (Uyttenhove et al, 2003). IDO expression was detected in multiple primary human tumors including glioblastomas. In immunocompetent syngeneic animals, local tryptophan degradation by IDO+ tumors provided a mechanism of immune resistance. In turn, the pharmacologic inhibition of IDO expression resulted in the rejection of IDO+ tumors. As predicted, IDO+ tumor-bearing mice display a reduced number of TAA specific CTL (Uyttenhove et al, 2003). Treatment of tumor cells with exogenous IFN-γ results in increased IDO activity. Conceivably, T cell secretion of IFN-γ may inadvertently stimulate glioma cells to upregulate their IDO activity thus creating a local tryptophan shortage (Shirey et al, 2006).
4. Galectin-1 (Gal-1)
Gal-1 is a secreted β-galactoside binding protein with wide tissue distribution and immunomodulatory functions (Liu, 2000; Yamaoka et al, 2000). A role for Gal-1 in establishing tumor tolerance was recently reported by Rubinstein and colleagues in a B16 melanoma tumor model (Rubinstein et al, 2004). Gal-1 expressing melanomas evaded immune-mediated rejection by inducing T cell apoptosis. Established human and rat glioma cell lines express Gal-1 and Gal-1 mRNA levels correlated with the tumor grade (Rubinstein et al, 2004; Camby et al, 2002). The role of Gal-1 in suppressing glioma specific T cell responses has yet to be determined. Thus far, it appears that Gal-1 promotes glioma growth, migration, and invasion (Camby et al, 2002).
V. Know your enemy: immunotherapy resistant glioma variants and Treg cells
The same mechanisms in place that protect gliomas from rejection by the host immune system may also impede the ability of brain tumor immunotherapies to eradicate the tumors or to suppress their growth. In agreement with this notion, the emergence of immunotherapy refractory melanoma cells has been observed among patients treated with activated autologous T lymphocytes (Restifo et al, 1996; Rosenberg et al, 2003). As well, brain tumor patients receiving immunotherapy often fail to respond to subsequent administrations of the immunotherapeutic agent or biologic (Kruse et al, 1997; Quattrocchi et al, 1999; Kruse and Rubinstein, 2001; Yu et al, 2004). It is important to develop strategies to circumvent the resistance if an improvement in survival is to occur. Our understanding of the obstacles can be enhanced with the generation and use of glioma cell models of immunotherapy resistance (ITR). The ITR models would allow for the determination of the reasons why not all brain tumor cells succumb to the therapy. With such knowledge the therapy may be improved upon. In cases involving adoptive T cell immunotherapy, the presence of immunosuppressive T cells such as Tregs within the starting effector cell populations may limit the efficacy of the therapy. In theory, selective depletion of the suppressor T cell compartment in ex vivo activated effector cell preparations might improve the T cell therapy.
A. Glioma cell models resistant to alloreactive CTL
The observation that glioma cells, unlike normal neurons and glia, express relatively abundant levels of class I HLA indicated that gliomas might be amenable to local adoptive immunotherapy with HLA-restricted alloreactive cytotoxic T lymphocytes (aCTL) (Read et al, 2003; Lampson and Hickey, 1986). In a pilot clinical trial, six recurrent malignant glioma patients were treated over a ten-month period with multiple intracranial infusions of recombinant human interleukin-2 and aCTL, sensitized to patient HLA antigens (Kruse et al, 1997; Kruse and Rubinstein, 2001). One patient survived 40 months, and the remaining two are >11 years from the start of immune therapy and entrance into protocol. Although the results of this study were promising, we wanted to explore the reason(s) why not all patients responded well to the therapy. One possibility was the existence of intrinsically ITR cells within the heterogeneous primary glioblastoma cell mass.
We generated aCTL resistant glioma cell models for study using in vitro immunoselection. Immunoselective pressure was applied with multiple aCTL populations to 13-06-MG glioblastoma cells obtained from a patient at initial diagnosis (Gomez et al, 2006). Two glioma cell clones, 13-06-IR29 and 13-06-IR30, were isolated from continuously immunoselected 13-06-MG cell populations. Compared to the immunosensitive 13-06-MG parental cells, the ITR clones resisted aCTL lysis for some time in the absence of selective pressure (Gomez et al, 2006). Relative chromosomal imbalances and structural abnormalities were identified that were associated with the ITR phenotype (Gomez et al, 2006).
Additional studies showed no impairment of aCTL adhesion to ITR derived ECM proteins and ITR cells (Gomez and Kruse, 2006b). Downregulation of HLA class I or ICAM-1 molecules that would inhibit aCTL recognition also was not detected in the ITR clones. Changes in HLA-G and FasL expression also were not observed. Statistically significant upregulation of the immunosuppressive cytokine TGF-β was associated with the ITR phenotype and corresponded to ITR clone gains (based upon triploid as the reference ploidy) of chromosome 1 and arm 19q where encoding of TGF-β2 and β1 lies, respectively. This finding is intriguing since TGF-β inhibits the synthesis of the cytotoxic molecules perforin, granzymes A and B, IFN-γ and FasL in activated CTL (Smyth et al, 1991; Thomas and Massague, 2005).
The ITR variants exhibited cross resistance to two other allogeneic, non-HLA-restricted effector cells (Gomez and Kruse, 2006b). After noting an inhibition in ITR clone apoptosis induction, stimulated by their coincubation with aCTL, we performed pathway specific cDNA array analysis to detect gene expression changes of apoptosis-related genes between the aCTL sensitive parental cells and the two ITR clones (Gomez and Kruse, 2006a). Downregulations of key proapoptotic genes involved in apoptosis such as Apaf-1, ATM, Asc, Caspases 3 and 8 were observed. The downregulation of the majority of the genes correlated with the chromosome losses observed in the ITR clones. The downregulation of Apaf-1 at the protein level was verified in clone 13-06-IR29 (Gomez and Kruse, 2006a), which makes it valuable for ascertaining the role that Apaf-1 might play in the ITR phenotype by knock-in experiments.
The isolation of these and more ITR glioma cell models will act as tools for further examining the factors and mechanisms by which glioma cells may resist immunotherapy with aCTL. Thus far the data indicate that disruption of the TGF-β signaling pathway, or perhaps upregulation of proapoptotic proteins using gene therapy may circumvent the resistance. The generation of more ITR glioma cell models from recurrent gliomas previously subjected to in situ radiation and chemotherapy selective pressures, and those derived from patients at initial diagnosis, would help us more comprehensively understand the factors involved in determining the sensitivity of glioma cells to adoptive T cell immunotherapy. Moreover, such models would also be useful in improving immunotherapies aimed at activating glioma specific T cell responses.
B. Selective depletion of Treg cells to improve cellular immunotherapy of brain tumors
Given the ability of Treg cells in maintaining tolerance to self-antigens displayed by normal and neoplastic cells, it is conceivable that selective Treg cell depletion of the tumor host prior to treatment with ex-vivo-sensitized autologous T lymphocytes to glioma associated antigens (GAA) (Powell et al, 2005), or to vaccination with GAA-pulsed autologous DCs could extend survival of glioma patients (Yajima et al, 2005; Zhang et al, 2006). Both approaches have mediated tumor regressions in animal models. Preliminary results of a phase I/II immunotherapy trial employing Ontak (IL-2 fused to diphtheria toxin) significantly depleted the number of Treg cells in the peripheral blood of ovarian carcinoma patients and increased the percentage of IFN-γ expressing CTL without inducing autoimmune reactions (Curiel et al, 2006). Alternatively, selective depletion of the suppressor T cell compartment may be achieved with cyclophosphamide (CPA). CPA depletion of Treg cells allowed adoptive T cell immunotherapy to be curative of established tumors in mice (Kruse et al, 1993; Ghiringhelli et al, 2004).
Treg cells constitute a fairly low percentage (approximately 5 to 15%) of peripheral blood mononuclear cells (PBMNC) and variable numbers have also been detected in a wide variety of tumors (Powell et al, 2005). Upon ex-vivo expansion of TIL with exogenous IL-2, or activation of precursor allogeneic T lymphocytes in one-way mixed lymphocyte reactions (MLR), the proliferation of Treg cells may ensue. The expansion of Treg cell numbers may dampen the activation and proliferation of tumor sensitized T cells or precursor alloreactive CTL. Therefore, we plan to determine the frequency of Treg cells present in the donor PBMNC before and after stimulation of the PBMNC in one-way MLR. In addition, we are currently exploring whether selective Treg cell depletion, prior to the activation of precursor aCTL will increase the number of aCTL generated in the MLR, and whether an improvement in their cytolytic activity is achieved.
VI. Concluding remarks
Significant advances in the field of immunology have paved the way for the development of immune therapeutic strategies to combat primary intracranial neoplasms. Malignant gliomas are adept at evading the host immune system. The various immunotherapeutic regimens tested thus far have resulted in mixed responses. To overcome the immunoresistance it is necessary to determine the ways in which gliomas resist the immunotherapies. The creation and characterization of ITR glioma models should allow for the formulation of strategies to enhance the current therapies. Analysis of tumor cells and the tumor microenvironment may shed some light on two compartments that participate in tumor tolerance and the immune suppression. For cellular therapy strategies, however, investigating the immune effector cell compartment for the presence of suppressor T cells may also yield important information leading to modification of experimental procedures to eliminate suppressor cells. If an improvement in survival is to materialize for this select population of cancer patients, we need to understand the obstacles present in all three compartments to appropriately develop strategies to overcome immune resistance.

German G. Gomez and Carol A. Kruse
Acknowledgments
This work was supported by National Institutes of Health grants F31 CA94834 to GGG, NS-046463 and NS-056300 to CAK, The R. Herbert & Alma S. Manweiler Memorial Research Fund at the University of Colorado Foundation, and The La Jolla Foundation for Molecular Medicine Research. L.E. Gerschenson critically read the manuscript and provided helpful comments.
References
- Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004;173:4352–59. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- Akasaki Y, Liu G, Matundan HH, Ng H, Yuan X, Zeng Z, Black KL, Yu JS. A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and -9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J Biol Chem. 2006;281:6165–74. doi: 10.1074/jbc.M505266200. [DOI] [PubMed] [Google Scholar]
- Allison J, Georgiou HM, Strasser A, Vaux DL. Transgenic expression of CD95 ligand on islet β cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc Nat Acad Sci USA. 1997;94:3943–7. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi F, Ria F, Penna G, Adorini L. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol. 1998;160:4671–80. [PubMed] [Google Scholar]
- Arai H, Gordon D, Nabel EG, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Nat Acad Sci USA. 1997;94:13862–7. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandres E, Andion E, Escalada A, Honorato B, Catalan V, Cubedo E, Cordeu L, Garcia F, Zarate R, Zabalegui N, Garcia-Foncillas J. Gene expression profile induced by BCNU in human glioma cell lines with differential MGMT expression. J Neurooncol. 2005;73:189–98. doi: 10.1007/s11060-004-5174-5. [DOI] [PubMed] [Google Scholar]
- Baxevanis CN, Reclos GJ, Gritzapis AD, Dedousis GV, Missitzis I, Papamichail M. Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer. 1993;72:491–501. doi: 10.1002/1097-0142(19930715)72:2<491::aid-cncr2820720227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman PJ, Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–88. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Bodmer S, Strommer K, Frei K, Siepl C, de Tribolet N, Heid I, Fontana A. Immunosuppression and transforming growth factor-β in glioblastoma. Preferential production of transforming growth factor-β 2. J Immunol. 1989;143:3222–9. [PubMed] [Google Scholar]
- Book AA, Fielding KE, Kundu N, Wilson MA, Fulton AM, Laterra J. IL-10 gene transfer to intracranial 9L glioma: tumor inhibition and cooperation with IL-2. J Neuroimmunol. 1998;92:50–9. doi: 10.1016/s0165-5728(98)00172-6. [DOI] [PubMed] [Google Scholar]
- Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr Opin Immunol. 1999;11:100–8. doi: 10.1016/s0952-7915(99)80018-1. [DOI] [PubMed] [Google Scholar]
- Camby I, Belot N, Lefranc F, Sadeghi N, de Launoit Y, Kaltner H, Musette S, Darro F, Danguy A, Salmon I, Gabius HJ, Kiss R. Galectin-1 modulates human glioblastoma cell migration into the brain through modifications to the actin cytoskeleton and levels of expression of small GTPases. J Neuropathol Exp Neurol. 2002;61:585–96. doi: 10.1093/jnen/61.7.585. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55:127–34. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- CBTRUS (2005) Statistical Report: Primary Brain Tumors in the United States 1998–2002, Published by the Central Brain Tumor Registry of the United States, Hinsdale, IL.
- Chahlavi A, Rayman P, Richmond AL, Biswas K, Zhang R, Vogelbaum M, Tannenbaum C, Barnett G, Finke JH. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65:5428–38. doi: 10.1158/0008-5472.CAN-04-4395. [DOI] [PubMed] [Google Scholar]
- Chang CC, Campoli M, Ferrone S. HLA class I defects in malignant lesions: what have we learned? Keio J Med. 2003;52:220–9. doi: 10.2302/kjm.52.220. [DOI] [PubMed] [Google Scholar]
- Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot β 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005;174:1462–71. doi: 10.4049/jimmunol.174.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitz JM, Driesen J, Classen S, Riley JL, Debey S, Beyer M, Popov A, Zander T, Schultze JL. Prostaglandin E2 impairs CD4+ T cell activation by inhibition of lck: implications in Hodgkin’s lymphoma. Cancer Res. 2006;66:1114–22. doi: 10.1158/0008-5472.CAN-05-3252. [DOI] [PubMed] [Google Scholar]
- Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-β 1, -β 2, and -β 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148:1404–10. [PubMed] [Google Scholar]
- Coral S, Sigalotti L, Gasparollo A, Cattarossi I, Visintin A, Cattelan A, Altomonte M, Maio M. Prolonged upregulation of the expression of HLA class I antigens and costimulatory molecules on melanoma cells treated with 5-aza-2′-deoxycytidine (5-AZA-CdR) J Immunother. 1999;22:16–24. doi: 10.1097/00002371-199901000-00003. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Barnett B, Kryczek I, Cheng P, Zou W. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Cancer Immunity. 2006;6(Suppl 1):20. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984;38:287–92. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- De Simone R, Giampaolo A, Giometto B, Gallo P, Levi G, Peschle C, Aloisi F. The costimulatory molecule B7 is expressed on human microglia in culture and in multiple sclerosis acute lesions. J Neuropathol Exp Neurol. 1995;54:175–87. doi: 10.1097/00005072-199503000-00004. [DOI] [PubMed] [Google Scholar]
- Demanet C, Mulder A, Deneys V, Worsham MJ, Maes P, Claas FH, Ferrone S. Down-regulation of HLA-A and HLA-Bw6, but not HLA-Bw4, allospecificities in leukemic cells: an escape mechanism from CTL and NK attack? Blood. 2004;103:3122–30. doi: 10.1182/blood-2003-07-2500. [DOI] [PubMed] [Google Scholar]
- Dick SJ, Macchi B, Papazoglou S, Oldfield EH, Kornblith PL, Smith BH, Gately MK. Lymphoid cell-glioma cell interaction enhances cell coat production by human gliomas: novel suppressor mechanism. Science. 1983;220:739–42. doi: 10.1126/science.6220469. [DOI] [PubMed] [Google Scholar]
- Doolittle ND, Abrey LE, Bleyer WA, Brem S, Davis TP, Dore-Duffy P, Drewes LR, Hall WA, Hoffman JM, Korfel A, Martuza R, Muldoon LL, Peereboom D, Peterson DR, Rabkin SD, Smith Q, Stevens GH, Neuwelt EA. New frontiers in translational research in neuro-oncology and the blood-brain barrier: report of the tenth annual Blood-Brain Barrier Disruption Consortium Meeting. Clin Cancer Res. 2005;11:421–8. [PubMed] [Google Scholar]
- Duke RC, Newell E, Schleicher M, Meech S, Bellgrau D. Transplantation of cells and tissues expressing Fas ligand. Transplant Proc. 1999;31:1479–81. doi: 10.1016/s0041-1345(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M, Campoli M, Ferrone S. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11:8304–11. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–23. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- French LE, Tschopp J. Defective death receptor signaling as a cause of tumor immune escape. Semin Cancer Biol. 2002;12:51–5. doi: 10.1006/scbi.2001.0405. [DOI] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–49. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastman BR, Atarashi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, Whiteside TL. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59:5356–64. [PubMed] [Google Scholar]
- Gately MK, Glaser M, McCarron RM, Dick SJ, Dick MD, Mettetal RW, Jr, Kornblith PL. Mechanisms by which human gliomas may escape cellular immune attack. Acta Neurochir (Wien) 1982;64:175–97. doi: 10.1007/BF01406052. [DOI] [PubMed] [Google Scholar]
- Gazit A, Osherov N, Posner I, Yaish P, Poradosu E, Gilon C, Levitzki A. Tyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J Med Chem. 1991;34:1896–1907. doi: 10.1021/jm00110a022. [DOI] [PubMed] [Google Scholar]
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–60. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- Gomez GG, Kruse, C.A. (2006a) Immunoresistant human glioma cell clones selected with alloreactive cytotoxic T lymphocytes: Apoptosis-pathway specific microarrays show downregulation of multiple proapoptotic factors. submitted. [PMC free article] [PubMed]
- Gomez GG, Kruse, C.A. (2006b) Cellular and functional characterization of immunoresistant human glioma cell clones selected with alloreactive cytotoxic T lymphocytes. submitted [DOI] [PMC free article] [PubMed]
- Gomez GG, Varella-Garcia M, Kruse CA. Isolation of immunoresistant human glioma cell clones after selection with alloreactive cytotoxic T lymphocytes: cytogenetic and molecular cytogenetic characterization. Cancer Genet Cytogenet. 2006;165:121–34. doi: 10.1016/j.cancergencyto.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-β. Pharmacol Ther. 2003;98:257–65. doi: 10.1016/s0163-7258(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, Gallimore A, Gascoigne NR, Ohashi PS. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–9. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leuk Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- Hemesath TJ, Marton LS, Stefansson K. Inhibition of T cell activation by the extracellular matrix protein tenascin. J Immunol. 1994;152:5199–207. [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–24. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Hiraki A, Ikeda K, Yoshino T, Kaneshige T, Kiura K, Kunisada T, Fujiwara K, Tanimoto M, Harada M. Tumor-specific cytotoxic T lymphocyte responses against chondrosarcoma with HLA haplotype loss restricted by the remaining HLA class I allele. Biochem Biophys Res Commun. 2001;286:786–91. doi: 10.1006/bbrc.2001.5411. [DOI] [PubMed] [Google Scholar]
- Hishii M, Nitta T, Ishida H, Ebato M, Kurosu A, Yagita H, Sato K, Okumura K. Human glioma-derived interleukin-10 inhibits antitumor immune responses in vitro. Neurosurg. 1995;37:1160–6. doi: 10.1227/00006123-199512000-00016. [DOI] [PubMed] [Google Scholar]
- Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurol. 1980;30:907–11. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- Hofmeister V, Weiss EH. HLA-G modulates immune responses by diverse receptor interactions. Semin Cancer Biol. 2003;13:317–23. doi: 10.1016/s1044-579x(03)00022-1. [DOI] [PubMed] [Google Scholar]
- Husain N, Chiocca EA, Rainov N, Louis DN, Zervas NT. Co-expression of Fas and Fas ligand in malignant glial tumors and cell lines. Acta Neuropathol (Berl) 1998;95:287–95. doi: 10.1007/s004010050799. [DOI] [PubMed] [Google Scholar]
- Jager E, Ringhoffer M, Altmannsberger M, Arand M, Karbach J, Jager D, Oesch F, Knuth A. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int J Cancer. 1997;71:142–7. doi: 10.1002/(sici)1097-0215(19970410)71:2<142::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jager MJ, Hurks HM, Levitskaya J, Kiessling R. HLA expression in uveal melanoma: there is no rule without some exception. Hum Immunol. 2002;63:444–51. doi: 10.1016/s0198-8859(02)00389-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354:1166–76. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- Kamarajan P, Sun NK, Chao CC. Up-regulation of FLIP in cisplatin-selected HeLa cells causes cross-resistance to CD95/Fas death signalling. Biochem J. 2003;376:253–60. doi: 10.1042/BJ20030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173:2353–61. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, Kufe DW, Ohno T. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452–9. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- Kjellman C, Olofsson SP, Hansson O, Von Schantz T, Lindvall M, Nilsson I, Salford LG, Sjogren HO, Widegren B. Expression of TGF-β isoforms, TGF-β receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89:251–8. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–66. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnikova L, Kotecki M, Kruger MM, Cochran BH. Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer. 2003;3:23. doi: 10.1186/1471-2407-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- Kruse CA and Rubinstein D (2001) In Brain Tumor Immunotherapy (Eds, Liau LM, Becker DP, Cloughesy TF, and Bigner DD), Humana Press, 149–70.
- Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol Immunother. 1997;45:77–87. doi: 10.1007/s002620050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse CA, Mitchell DH, Kleinschmidt-DeMasters BK, Bellgrau D, Eule JM, Parra JR, Kong Q, Lillehei KO. Systemic chemotherapy combined with local adoptive immunotherapy cures rats bearing 9L gliosarcoma. J NeuroOncol. 1993;15:97–112. doi: 10.1007/BF01053931. [DOI] [PubMed] [Google Scholar]
- Kulprathipanja NV, Kruse CA. Microglia phagocytose alloreactive CTL-damaged 9L gliosarcoma cells. J Neuroimmunol. 2004;153:76–82. doi: 10.1016/j.jneuroim.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–92. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson LA, Hickey WF. Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from “histologically normal” to that showing different levels of glial tumor involvement. J Immunol. 1986;136:4054–62. [PubMed] [Google Scholar]
- Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, Schmidt A, Debatin KM, Krammer PH, Moller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–29. [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Ann Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- Liu FT. Galectins: a new family of regulators of inflammation. Clin Immunol. 2000;97:79–88. doi: 10.1006/clim.2000.4912. [DOI] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Louis DN, Gusella JF. A tiger behind many doors: multiple genetic pathways to malignant glioma. Trends Genet. 1995;11:412–5. doi: 10.1016/s0168-9525(00)89125-8. [DOI] [PubMed] [Google Scholar]
- Maeurer MJ, Gollin SM, Storkus WJ, Swaney W, Karbach J, Martin D, Castelli C, Salter R, Knuth A, Lotze MT. Tumor escape from immune recognition: loss of HLA-A2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6. Clin Cancer Res. 1996;2:641–52. [PubMed] [Google Scholar]
- Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, Annunziato F. Thymic regulatory T cells. Autoimmun Rev. 2005;4:579–86. doi: 10.1016/j.autrev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, Ifergan I, Chen L, Bischof F, Bar-Or A, Wiendl H. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci. 2005;25:2537–46. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–67. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincola FM, Shamamian P, Alexander RB, Gnarra JR, Turetskaya RL, Nedospasov SA, Simonis TB, Taubenberger JK, Yannelli J, Mixon A, et al. Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J Immunol. 1994;153:1225–37. [PubMed] [Google Scholar]
- Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–16. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- Medema JP, de Jong J, Peltenburg LTC, Verdegaal EM, Gorter A, Bres SA, Franken KL, Hahne M, Albar JP, Melief CJ, Offringa R. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Nat Acad Sci USA. 2001;98:11515–20. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema JP, de Jong J, van Hall T, Melief CJ, Offringa R. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J Exp Med. 1999;190:1033–8. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medewar P. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Madsen CS, Ginardi AR, Tinder TL, Jacobs F, Parker J, Agrawal B, Longenecker BM, Gendler SJ. Mucin 1-specific immunotherapy in a mouse model of spontaneous breast cancer. J Immunother. 2003;26:47–62. doi: 10.1097/00002371-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–8. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Nelson DP, Setser E, Hall DG, Schwartz SM, Hewitt T, Klevitsky R, Osinska H, Bellgrau D, Duke RC, Robbins J. Proinflammatory consequences of transgenic fas ligand expression in the heart. J Clin Invest. 2000;105:1199–208. doi: 10.1172/JCI8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Yang G, Song Y, Zhao X, So C, Liao J, Wang LD, Yang CS. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogenesis. 2001;22:1615–23. doi: 10.1093/carcin/22.10.1615. [DOI] [PubMed] [Google Scholar]
- Oberc-Greenwood MA, Muul LM, Gately MK, Kornblith PL, Smith BH. Ultrastructural features of the lymphocyte-stimulated halos produced by human glioma-derived cells in vitro. J Neurooncol. 1986;3:387–96. doi: 10.1007/BF00165589. [DOI] [PubMed] [Google Scholar]
- O’Connor GM, Hart OM, Gardiner CM. Putting the natural killer cell in its place. Immunol. 2006;117:1–10. doi: 10.1111/j.1365-2567.2005.02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozoren N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–47. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- Parekh K, Ramachandran S, Cooper J, Bigner D, Patterson A, Mohanakumar T. Tenascin-C, over expressed in lung cancer down regulates effector functions of tumor infiltrating lymphocytes. Lung Cancer. 2005;47:17–29. doi: 10.1016/j.lungcan.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Paul DB, Kruse CA. Immunologic approaches to therapy for brain tumors. Current Neurol Neurosci Reports. 2001;1:238–44. doi: 10.1007/s11910-001-0024-8. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, Godowski PJ, Wood WI, Gurney AL, Hillan KJ, Cohen RL, Goddard AD, Botstein D, Ashkenazi A. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- Powell DJ, Jr, Parker LL, Rosenberg SA. Large-scale depletion of CD25+ regulatory T cells from patient leukapheresis samples. J Immunother. 2005;28:403–11. doi: 10.1097/01.cji.0000170363.22585.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente Navazo MD, Valmori D and Ruegg C (2001) The alternatively spliced domain TnFnIII A1A2 of the extracellular matrix protein tenascin-C suppresses activation-induced T lymphocyte proliferation and cytokine production. J Immunol, 167 [DOI] [PubMed]
- Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, Gudeman S, Varia MA. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45:141–57. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- Rahaman SO, Vogelbaum MA, Haque SJ. Aberrant Stat3 signaling by interleukin-4 in malignant glioma cells: involvement of IL-13Ralpha2. Cancer Res. 2005;65:2956–63. doi: 10.1158/0008-5472.CAN-04-3592. [DOI] [PubMed] [Google Scholar]
- Ranges GE, Figari IS, Espevik T, Palladino MA., Jr Inhibition of cytotoxic T cell development by transforming growth factor β and reversal by recombinant tumor necrosis factor α. J Exp Med. 1987;166:991–8. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SB, Kulprathipanja NV, Gomez GG, Paul DB, Winston KR, Robbins JM, Kruse CA. Human alloreactive CTL interactions with gliomas and with those having upregulated HLA expression from exogenous IFN-γor IFN-γgene modification. J Int Cyt Res. 2003;23:379–93. doi: 10.1089/107999003322226032. [DOI] [PubMed] [Google Scholar]
- Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional β 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Nat Cancer Inst. 1996;88:100–8. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Robbins PF, Wunderlich JR, Hwu P, Sherry RM, Schwartzentruber DJ, Topalian SL, Restifo NP, Filie A, Chang R, Dudley ME. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. J Immunother. 2003;26:385–93. doi: 10.1097/00002371-200309000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W, Isenmann S, Nakamura M, Platten M, Wick W, Kleihues P, Bahr M, Ohgaki H, Ashkenazi A, Weller M. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–65. [PubMed] [Google Scholar]
- Rouas-Freiss N, Khalil-Daher I, Riteau B, Menier C, Paul P, Dausset J, Carosella ED. The immunotolerance role of HLA-G. Semin Cancer Biol. 1999;9:3–12. doi: 10.1006/scbi.1998.0103. [DOI] [PubMed] [Google Scholar]
- Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–51. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- Saas P, Walker PR, Hahne M, Quiquerez AL, Schnuriger V, Perrin G, French L, Van Meir EG, de Tribolet N, Tschopp J, Dietrich PY. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest. 1997;99:1173–78. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saff RR, Spanjaard ES, Hohlbaum AM, Marshak-Rothstein A. Activation-induced cell death limits effector function of CD4 tumor-specific T cells. J Immunol. 2004;172:6598–606. doi: 10.4049/jimmunol.172.11.6598. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1551–64. [PubMed] [Google Scholar]
- Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia. 2005;51:279–85. doi: 10.1002/glia.20201. [DOI] [PubMed] [Google Scholar]
- Schiltz PM, Gomez GG, Read SB, Kulprathipanja NV, Kruse CA. Effects of IFN-γ and interleukin-1β on major histocompatibility complex antigen and intercellular adhesion molecule-1 expression by 9L gliosarcoma: relevance to its cytolysis by alloreactive cytotoxic T lymphocytes. J Int Cyt Res. 2002;22:1209–16. doi: 10.1089/10799900260475731. [DOI] [PubMed] [Google Scholar]
- Seo DR, Kim KY, Lee YB. Interleukin-10 expression in lipopolysaccharide-activated microglia is mediated by extracellular ATP in an autocrine fashion. Neuroreport. 2004;15:1157–61. doi: 10.1097/00001756-200405190-00015. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- Shirey KA, Jung JY, Maeder GS, Carlin JM. Upregulation of IFN-gamma receptor expression by proinflammatory cytokines influences IDO activation in epithelial cells. J Int Cyt Res. 2006;26:53–62. doi: 10.1089/jir.2006.26.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HK, Kim MM, Chen P, Furman F, Julin CM, Israel MA. The intrinsic radioresistance of glioblastoma-derived cell lines is associated with a failure of p53 to induce p21(BAX) expression. Proc Nat Acad Sci USA. 1998;95:14453–8. doi: 10.1073/pnas.95.24.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingluff CL, Jr, Colella TA, Thompson L, Graham DD, Skipper JC, Caldwell J, Brinckerhoff L, Kittlesen DJ, Deacon DH, Oei C, Harthun NL, Huczko EL, Hunt DF, Darrow TL, Engelhard VH. Melanomas with concordant loss of multiple melanocytic differentiation proteins: immune escape that may be overcome by targeting unique or undefined antigens. Cancer Immunol Immunother. 2000;48:661–72. doi: 10.1007/s002620050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-β. Inhibition by transforming growth factor-β. J Immunol. 1991;146:3289–97. [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–35. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Massague J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tsugawa T, Kuwashima N, Sato H, Fellows-Mayle WK, Dusak JE, Okada K, Papworth GD, Watkins SC, Gambotto A, Yoshida J, Pollack IF, Okada H. Sequential delivery of interferon-αgene and DCs to intracranial gliomas promotes an effective antitumor response. Gene Ther. 2004;11:1551–8. doi: 10.1038/sj.gt.3302300. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- Walker PR, Calzascia T, Dietrich PY. All in the head: obstacles for immune rejection of brain tumours. Immunol. 2002;107:28–38. doi: 10.1046/j.1365-2567.2002.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PR, Saas P, Dietrich PY. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol. 1997;158:4521–4. [PubMed] [Google Scholar]
- Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Kirken RA, Erwin RA, Yu CR, Farrar WL. JAK3, STAT, and MAPK signaling pathways as novel molecular targets for the tyrphostin AG-490 regulation of IL-2-mediated T cell response. J Immunol. 1999;162:3897–904. [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective histocompatibility leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J Exp Med. 1999;190:205–15. doi: 10.1084/jem.190.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–55. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;168:4772–80. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- Wilmotte R, Burkhardt K, Kindler V, Belkouch MC, Dussex G, Tribolet N, Walker PR, Dietrich PY. B7-homolog 1 expression by human glioma: a new mechanism of immune evasion. Neuroreport. 2005;16:1081–5. doi: 10.1097/00001756-200507130-00010. [DOI] [PubMed] [Google Scholar]
- Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with earlystage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- Yajima N, Yamanaka R, Mine T, Tsuchiya N, Homma J, Sano M, Kuramoto T, Obata Y, Komatsu N, Arima Y, Yamada A, Shigemori M, Itoh K, Tanaka R. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res. 2005;11:5900–11. doi: 10.1158/1078-0432.CCR-05-0559. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Mishima K, Nagashima Y, Asai A, Sanai Y, Kirino T. Expression of galectin-1 mRNA correlates with the malignant potential of human gliomas and expression of antisense galectin-1 inhibits the growth of 9 glioma cells. J Neurosci Res. 2000;59:722–30. doi: 10.1002/(SICI)1097-4547(20000315)59:6<722::AID-JNR4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–9. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, Ma W, Hoa N, Minev G, Delgado C, Wespic HT, Okada H and Jadus MR (2006) Antigenic profiles of human glioma cell lines: Implications for patient CTL targeting of tumor associated antigens with allogeneic tumor cell-based vaccine or other immune-cell based therapies. submitted
- Zippelius A, Pittet MJ, Batard P, Rufer N, de Smedt M, Guillaume P, Ellefsen K, Valmori D, Lienard D, Plum J, MacDonald HR, Speiser DE, Cerottini JC, Romero P. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195:485–94. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]


