Abstract
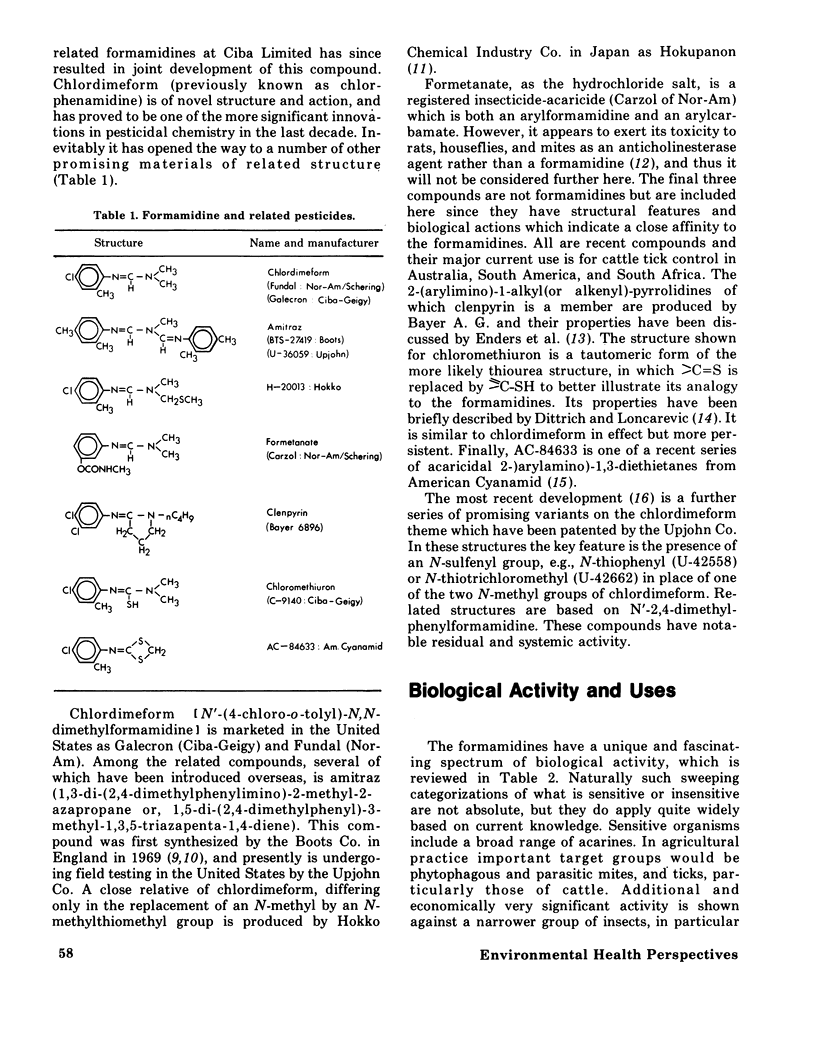
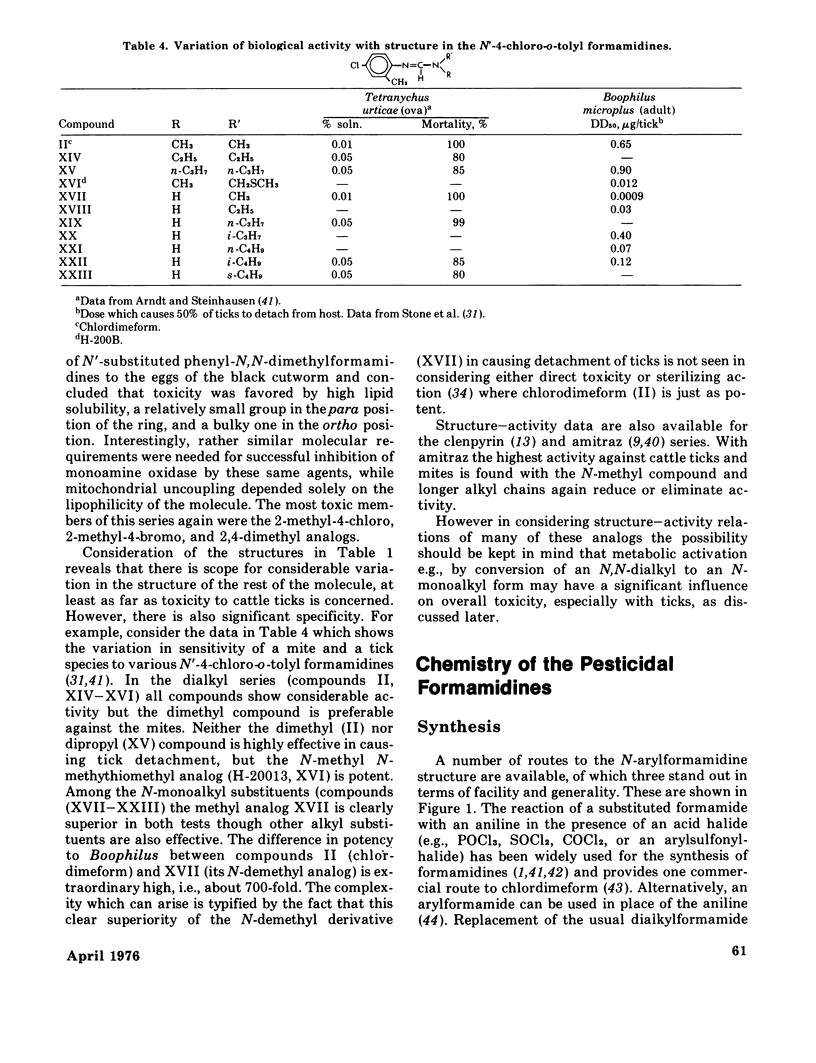
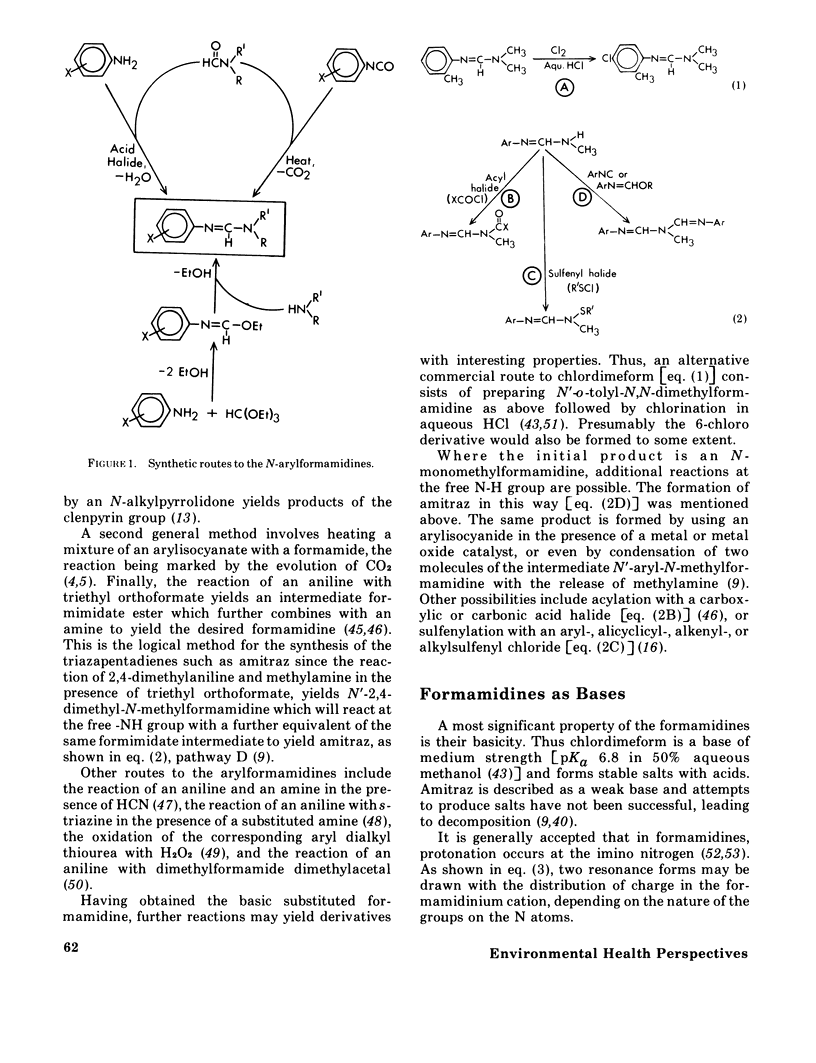
The formamidines, a relatively new group of acaricide-insecticides, are novel both in their range of biological activities and in their mode of action, which is presently unknown. This paper is a review of the historical development, properties, structures, uses, and chemistry of this group of pesticides, with particular emphasis on chlordimeform (Galecron or Fundal), N'-4-chloro-o-tolyl-N,N-dimethylformamidine, and amitraz, 1,3=di-(2,4-dimethylphenylimino)-2-methyl-2-azapropane. Their biological activity and uses are defined by their toxicity to spider mites, ticks, and certain insects, and they are particularly effective against juvenile and resistant forms of these organisms. A significant, but poorly understood feature of their field effectiveness is their breadth of toxic action which includes direct lethality, excitant-repellant behavioral effects, and chemosterilization. They are generally of low hazard for nontarget species with the significant exception of predaceous mites. Several aspects of the chemistry of these compounds are considered, including structure--activity relations, synthetic pathways, isomerism and configuration, and their chemical and environmental stability. A significant feature of the metabolism and toxicity of these agents is the possible activation of chlordimeform by N-demethylation in vivo. Strong evidence for this has been presented with the cattle tick, but recent results discussed here suggest that in other species, i.e., mice, German cockroaches or black cutworm eggs, N-demethylation is neither a strong activation nor a detoxication reaction.
Full text
PDF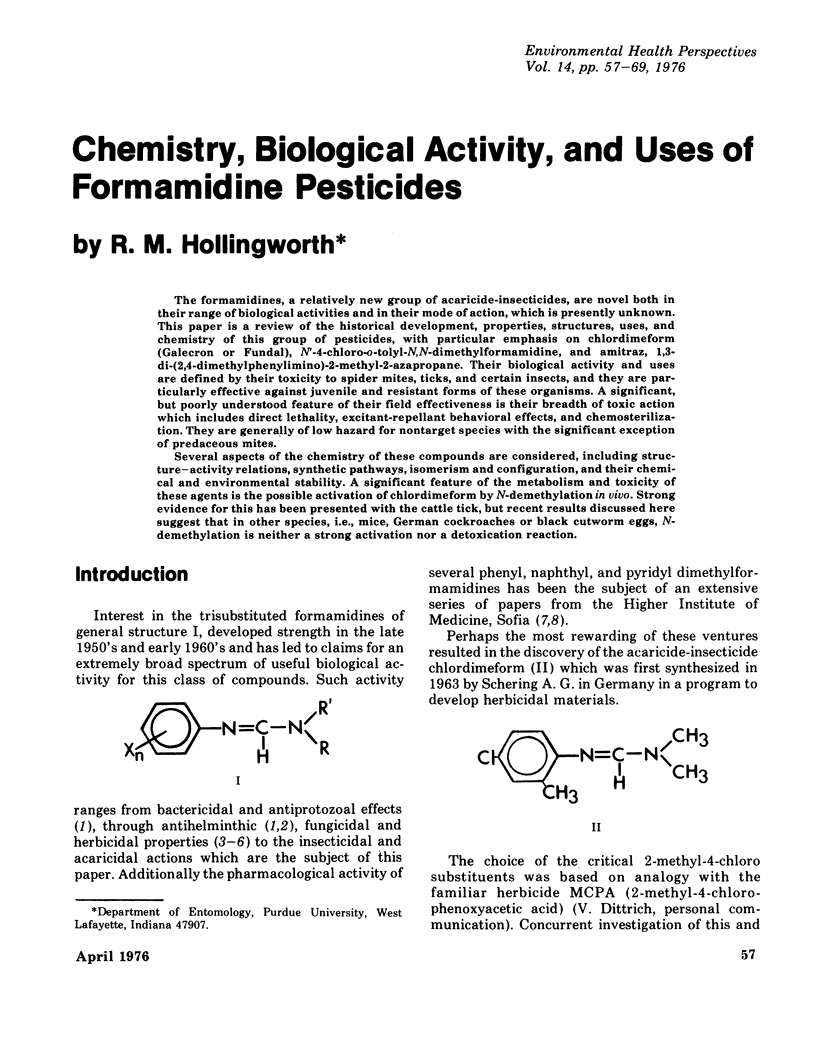






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Knowles C. O. Metabolism of N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine) and 4'-chloro-o-formotoluidide by rat hepatic microsomal and soluble enzymes. Comp Gen Pharmacol. 1971 Jun;2(6):189–197. doi: 10.1016/0010-4035(71)90010-3. [DOI] [PubMed] [Google Scholar]
- Bartha R., Linke H. A., Pramer D. Pesticide transformations: production of chloroazobenzenes from chloroanilines. Science. 1968 Aug 9;161(3841):582–583. doi: 10.1126/science.161.3841.582. [DOI] [PubMed] [Google Scholar]
- Dittrich V., Loncarević A. New insecticides for Asiatic rice borer control in paddy rice. J Econ Entomol. 1971 Oct;64(5):1225–1229. doi: 10.1093/jee/64.5.1225. [DOI] [PubMed] [Google Scholar]
- Ercegovich C. D., Witkonton S., Asquith D. Disappearance of N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine from six major fruit crops. J Agric Food Chem. 1972 May-Jun;20(3):565–568. doi: 10.1021/jf60181a005. [DOI] [PubMed] [Google Scholar]
- Gladney W. J., Ernst S. E., Drummond R. O. Chlordimeform: a detachment-stimulating chemical for three-host ticks. J Med Entomol. 1974 Nov 25;11(5):569–572. [PubMed] [Google Scholar]
- Harris C. R., Gore F. Toxicological studies on cutworms. 8. Toxicity of three insecticides to the various stages in the development of the darksided cutworm. J Econ Entomol. 1971 Oct;64(5):1049–1050. doi: 10.1093/jee/64.5.1049. [DOI] [PubMed] [Google Scholar]
- Knowles C. O. Chemistry and toxicology of quinoxaline, organotin, organofluorine, and formamidine acaricides. Environ Health Perspect. 1976 Apr;14:93–102. doi: 10.1289/ehp.761493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles C. O. Metabolism of two acaricidal chemicals, N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine) and m-([(dimethylamino)methylene]amino)phenyl methylcarbamate hydrochloride (formetanate). J Agric Food Chem. 1970 Nov-Dec;18(6):1038–1047. doi: 10.1021/jf60172a044. [DOI] [PubMed] [Google Scholar]
- Knowles C. O., Roulston W. J. Toxicity to Boophilus microplus of formamidine acaricides and related compounds, and modification of toxicity by certain insecticide synergists. J Econ Entomol. 1973 Dec;66(6):1245–1251. doi: 10.1093/jee/66.6.1245. [DOI] [PubMed] [Google Scholar]
- Knowles C. O., Shrivastava S. P. Chlordimeform and related compounds: toxicological studies with house flies. J Econ Entomol. 1973 Feb;66(1):75–79. doi: 10.1093/jee/66.1.75. [DOI] [PubMed] [Google Scholar]
- Lingren P. D., Wolfenbarger D. A., Nosky J. B., Diaz M., Jr Response of Campoletis perdistinctus and Apanteles marginiventris to insecticides. J Econ Entomol. 1972 Oct;65(5):1295–1299. doi: 10.1093/jee/65.5.1295. [DOI] [PubMed] [Google Scholar]
- Mitsov V. Pharmacological assaying of some compounds of the formamidine group. Nauchni Tr Vissh Med Inst Sofiia. 1966;45(5):61–67. [PubMed] [Google Scholar]
- Ross J. A., Tweedy B. G. Malonic acid conjugation by soil microorganisms of a pesticide-derived aniline moiety. Bull Environ Contam Toxicol. 1973 Oct;10(4):234–236. doi: 10.1007/BF01684550. [DOI] [PubMed] [Google Scholar]
- Roulston W. J., Wharton R. H., Schnitzerling H. J., Sutherst R. W., Sullivan N. D. Mixtures of chlorphenamidine with other acaricides for the control of organ ophosphorus-resistant strains of cattle tick Boophilus microplus. Aust Vet J. 1971 Nov;47(11):521–528. doi: 10.1111/j.1751-0813.1971.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Westigard P. H., Medinger L. E., Kellogg O. E. Field evaluation of pesticides for their suitability in an integrated program for spider mites on pear. J Econ Entomol. 1972 Feb;65(1):191–192. doi: 10.1093/jee/65.1.191. [DOI] [PubMed] [Google Scholar]
- Witkonton S., Ercegovich C. D. Degradation of N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine in six different fruit. J Agric Food Chem. 1972 May-Jun;20(3):569–573. doi: 10.1021/jf60181a006. [DOI] [PubMed] [Google Scholar]


