Abstract
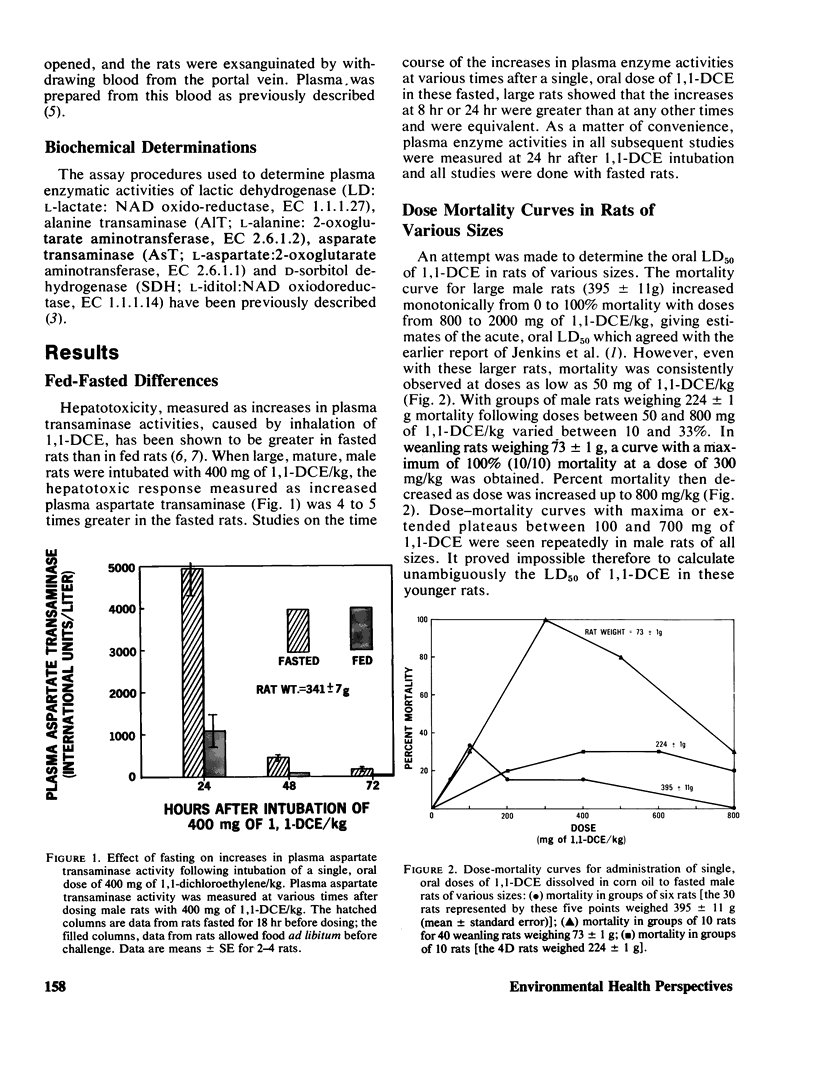
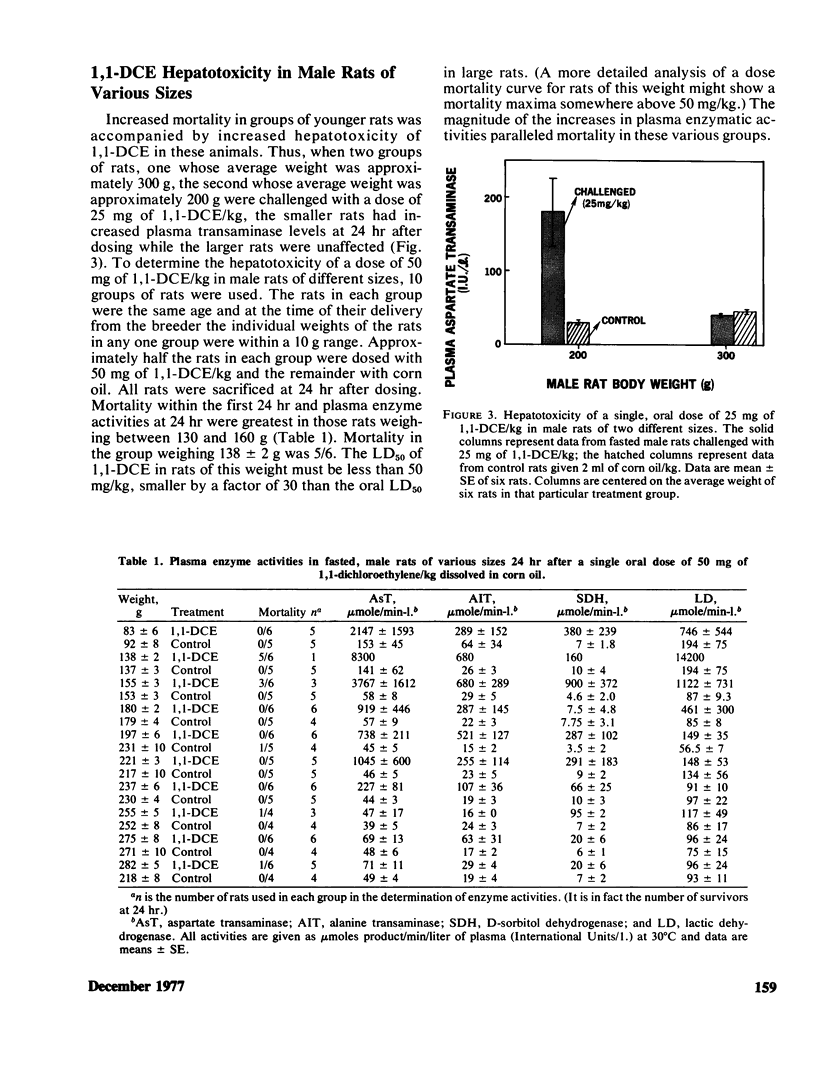
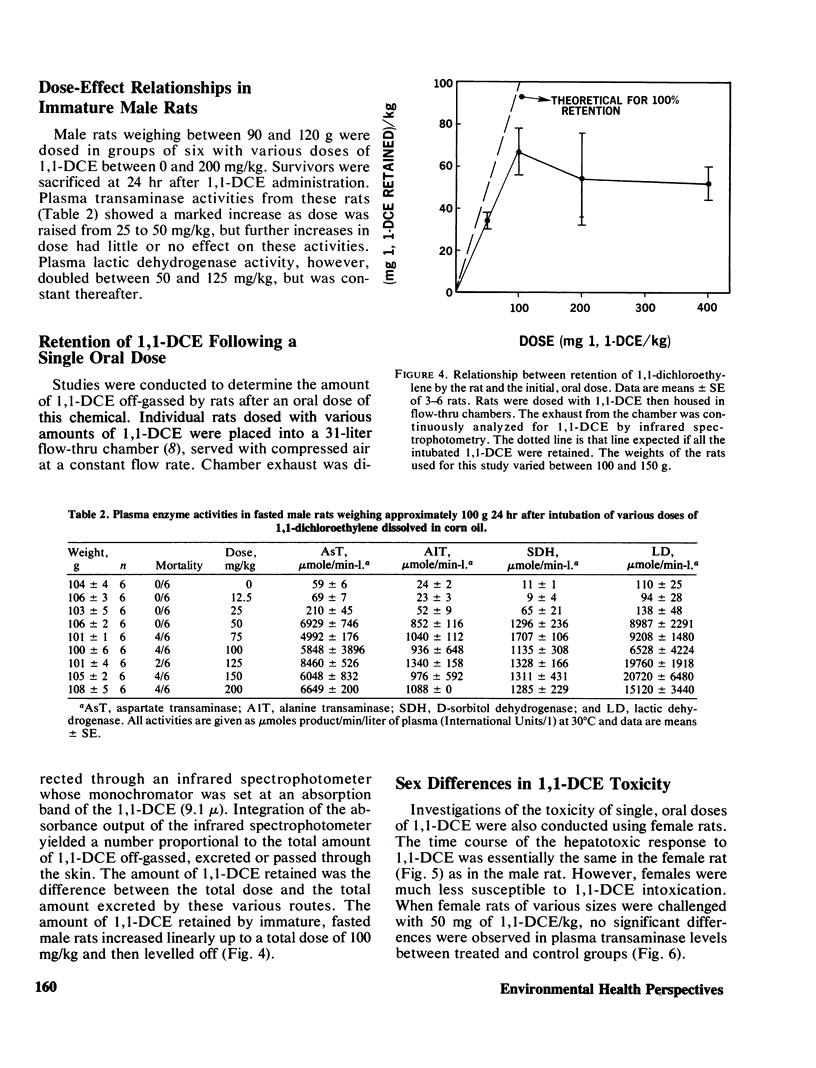
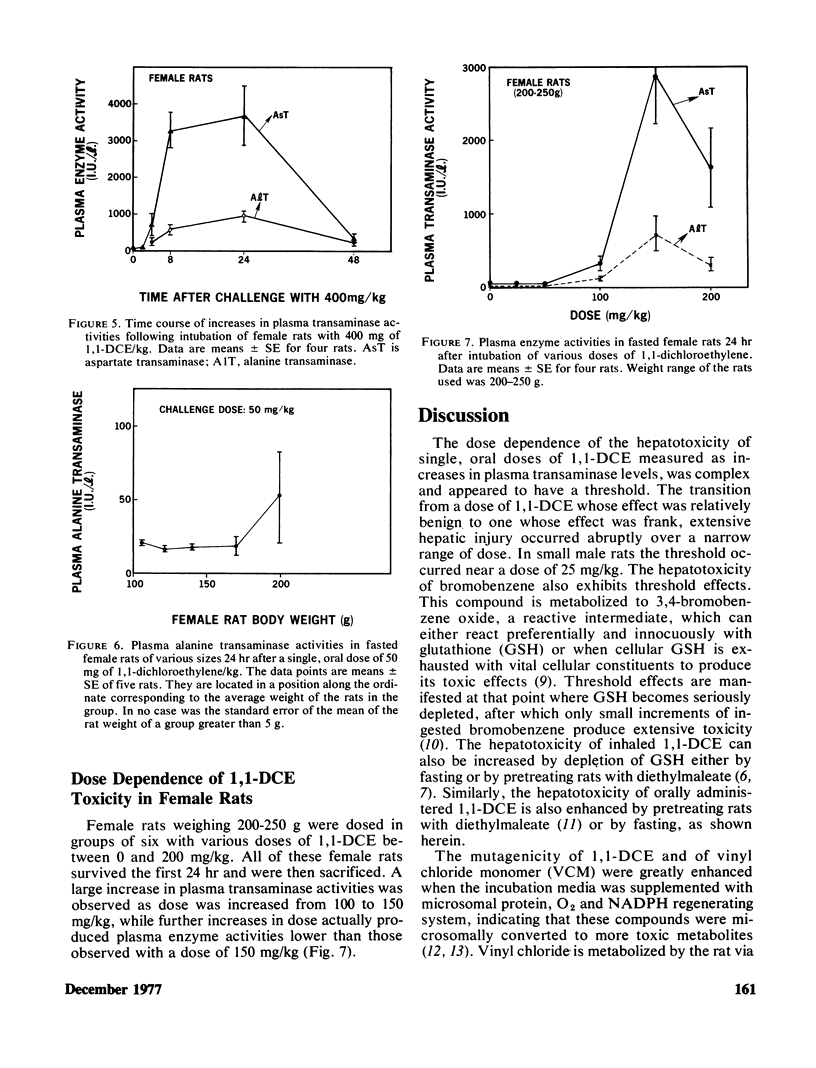
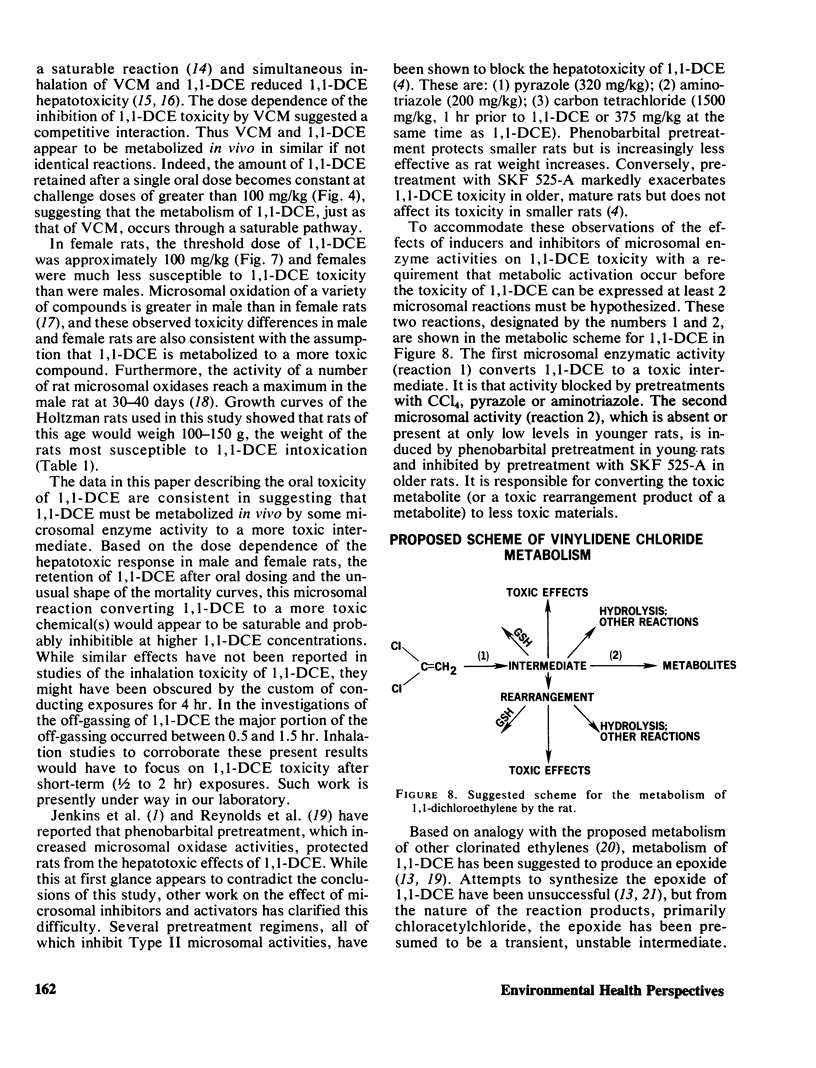
Mortality curves for groups of fasted male rats treated with single, oral doses of 1,1-dichloroethylene (1,1-DCE, vinylidene chloride) were not monotonically increasing sigmoids, but were complex with maxima or extended plateaus in the region of dose between 100 and 700 mg of 1,1-DCE/kg. The exact shape was a function of the size (age) of the rat used. When groups of rats of various sizes were dosed with 50 mg/kg, mortality and hepatotoxicity were greatest for those groups whose average weight was between 100 and 150 g. Smaller and larger male rats were less susceptible to 1,1-DCE intoxication. The toxicity of 1,1-DCE was less severe in female rats and there was no significant effect of rat size on 1,1-DCE toxicity in females. In rats of both sexes the dose dependence of the hepatotoxic response was complex, possessing a threshold level, a region of precipitous increase, and a plateau, where larger doses were ineffective in increasing hepatotoxicity. The threshold in male rats of 100–150 g occurred near 50 mg/kg, and for females it was closer to 100 mg/kg. Considered in their entirety these data suggest that 1,1-DCE is metabolized to a toxic intermediate via some saturable pathway. Based on the effects of pretreatment with microsomal enzyme inhibitors and activators on 1,1-DCE toxicity in rats of various sizes, it appears that there are at least two microsomal reactions involved in 1,1-DCE metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. E., Mehl R. G. A comparison of the toxicology of triethylene glycol dinitrate and propylene glycol dinitrate. Am Ind Hyg Assoc J. 1973 Dec;34(12):526–532. doi: 10.1080/0002889738506893. [DOI] [PubMed] [Google Scholar]
- BOYLAND E., WILLIAMS K. AN ENZYME CATALYSING THE CONJUGATION OF EPOXIDES WITH GLUTATHIONE. Biochem J. 1965 Jan;94:190–197. doi: 10.1042/bj0940190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch H., Malaveille C., Montesano R., Tomatis L. Tissue-mediated mutagenicity of vinylidene chloride and 2-chlorobutadiene in Salmonella typhimurium. Nature. 1975 Jun 19;255(5510):641–643. doi: 10.1038/255641a0. [DOI] [PubMed] [Google Scholar]
- DANIEL J. W. THE METABOLISM OF 36C1-LABELLED TRICHLOROETHYLENE AND TETRACHLOROETHYLENE IN THE RAT. Biochem Pharmacol. 1963 Aug;12:795–802. doi: 10.1016/0006-2952(63)90109-6. [DOI] [PubMed] [Google Scholar]
- Fjellstedt T. A., Allen R. H., Duncan B. K., Jakoby W. B. Enzymatic conjugation of epoxides with glutathione. J Biol Chem. 1973 May 25;248(10):3702–3707. [PubMed] [Google Scholar]
- Gillette J. R. A perspective on the role of chemically reactive metabolites of foreign compounds in toxicity. II. Alterations in the kinetics of covalent binding. Biochem Pharmacol. 1974 Nov 1;23(21):2927–2938. doi: 10.1016/0006-2952(74)90267-6. [DOI] [PubMed] [Google Scholar]
- Greim H., Bonse G., Radwan Z., Reichert D., Henschler D. Mutagenicity in vitro and potential carcinogenicity of chlorinated ethylenes as a function of metabolic oxiran formation. Biochem Pharmacol. 1975 Nov 1;24(21):2013–2017. doi: 10.1016/0006-2952(75)90396-2. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Conolly R. B., Murphy S. D. Diurnal variation of hepatic glutathione concentration and its correlation with 1,1-dichloroethylene inhalation toxicity in rats. Res Commun Chem Pathol Pharmacol. 1973 Sep;6(2):465–471. [PubMed] [Google Scholar]
- Jaeger R. J., Conolly R. B., Murphy S. D. Effect of 18 hr fast and glutathione depletion on 1,1-dichloroethylene-induced hepatotoxicity and lethality in rats. Exp Mol Pathol. 1974 Apr;20(2):187–198. doi: 10.1016/0014-4800(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Conolly R. B., Murphy S. D. Short-term inhalation toxicity of halogenated hydrocarbons: effects on fasting rats. Arch Environ Health. 1975 Jan;30(1):26–31. doi: 10.1080/00039896.1975.10666628. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Trabulus M. J., Murphy S. D. Biochemical effects of 1,1-dichloroethylene in rats: dissociation of its hepatotoxicity from a lipoperoxidative mechanism. Toxicol Appl Pharmacol. 1973 Mar;24(3):457–467. doi: 10.1016/0041-008x(73)90052-5. [DOI] [PubMed] [Google Scholar]
- Jenkins L. J., Jr, Trabulus M. J., Murphy S. D. Biochemical effects of 1,1-dichloroethylene in rats: comparison with carbon tetrachloride and 1,2-dichloroethylene. Toxicol Appl Pharmacol. 1972 Nov;23(3):501–510. doi: 10.1016/0041-008x(72)90052-x. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Zampaglione N., Gillette J. R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- KATO R., VASSANELLI P., FRONTINO G., CHIESARA E. VARIATION IN THE ACTIVITY OF LIVER MICROSOMAL DRUG-METABOLIZING ENZYMES IN RATS IN RELATION TO THE AGE. Biochem Pharmacol. 1964 Jul;13:1037–1051. doi: 10.1016/0006-2952(64)90100-5. [DOI] [PubMed] [Google Scholar]
- Kato R. Sex-related differences in drug metabolism. Drug Metab Rev. 1974;3(1):1–32. doi: 10.3109/03602537408993737. [DOI] [PubMed] [Google Scholar]
- Oesch F., Kaubisch N., Jerina D. M., Daly J. W. Hepatic epoxide hydrase. Structure-activity relationships for substrates and inhibitors. Biochemistry. 1971 Dec 21;10(26):4858–4866. doi: 10.1021/bi00802a005. [DOI] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S., Moslen M. T., Szabo S., Jaeger R. J., Murphy S. D. Hepatotoxicity of vinyl chloride and 1,1-dichloroethylene. Role of mixed function oxidase system. Am J Pathol. 1975 Oct;81(1):219–236. [PMC free article] [PubMed] [Google Scholar]


