Reactive oxygen species (ROS) are versatile molecules mediating a variety of cellular responses in plant cells, including programmed cell death (PCD), development, gravitropism, and hormone signaling. A picture showing how ROS function in signal transduction networks has started to emerge as the result of recent studies providing genetic, cell biological, and physiological evidence describing roles for ROS in signaling (Apel and Hirt, 2004; Laloi et al., 2004; Mittler et al., 2004; Mori and Schroeder, 2004). However, further efforts are necessary to characterize the targets and molecular functions of ROS, as well as the complex interplay of ROS-generating and ROS-scavenging mechanisms. Moreover, the interactions of nitric oxide with other ROS species in hormone signaling is a subject of interest (Desikan et al., 2004; Wendehenne et al., 2004; Guo and Crawford, 2005; Bright et al., 2006). Due to limited space, in this Update article we focus on recent progress made in understanding the roles of ROS in hormone signaling.
AUXIN, ETHYLENE, AND ROS
ROS have been implicated as second messengers in several plant hormone responses. Joo et al. (2001) showed that ROS are asymmetrically generated in roots by gravistimulation to regions of reduced growth. A function for ROS in root curvature was reported by inhibiting cell growth, thus contributing to tropisms. Auxin also induced ROS production in roots and the auxin transport inhibitor N-1-naphthylphthlamic acid did not inhibit hydrogen peroxide (H2O2)-induced root curvature, leading to the suggestion that ROS play a role downstream of transport in auxin signaling and gravitropism (Joo et al., 2001).
A pharmacological study suggested that ethylene and ROS are required for root nodule initiation and function as positive transducers downstream of the Nod factor response in a semiaquatic legume (D'Haeze et al., 2003). Additional studies have suggested roles of ethylene in either stomatal opening or closing, depending on the plant species (Giulivo, 1986). Recently, it was reported that H2O2-mediated stomatal closure is completely disrupted in the loss-of-function mutant of the ethylene receptor etr1-7, suggesting a role for ETR1 in guard cell ROS signaling and stomatal closure (Desikan et al., 2005). Interestingly, in another recent study, ethylene was proposed to counteract stomatal closure (Tanaka et al., 2005). Abscisic acid (ABA)-induced stomatal closure was inhibited by ethylene or the hormone precursor 1-aminocyclopropane-1-carboxylic acid and by the ethylene-overproducing mutation eto1-1 (Tanaka et al., 2005). Moreover, this ethylene-induced inhibition of stomatal closure was suppressed in two ethylene-insensitive mutants, the dominant etr1-1 allele, and ein3-1 (Tanaka et al., 2005). Reverse transcription-PCR analysis with guard cell-enriched epidermal strips showed that ETR1 is expressed in guard cells (Desikan et al., 2005). ATH1 and AG oligonucleotide-based (Affymetrix) microarray analyses of guard cell-expressed genes suggest that at least the ETR1, ERS1, and EIN4 ethylene receptor genes are expressed in guard cells, with ERS1 apparently showing the highest expression level in these experiments (Leonhardt et al., 2004; Heggie and Gray, 2005; J. Kwak, N. Leonhardt, Y. Yang, and J. Schroeder, unpublished data). As these receptors share overlapping redundant negative regulator functions (Hua and Meyerowitz, 1998), it would be interesting to analyze stomatal responses in etr1ein4, etr1ers1, and ers1ein4 double mutants in addition to the recessive loss-of-function etr1-7 line (Desikan et al., 2005). The dual functions of ethylene receptors proposed in these two studies—transduction of stomatal closure and inhibition of ABA-induced stomatal closure in Arabidopsis (Arabidopsis thaliana)—will require further analyses (Desikan et al., 2005; Tanaka et al., 2005).
ROLES OF ROS IN GA3 SIGNALING IN THE ALEURONE LAYER AND CELL DEATH
ROS have been shown to play a central role in PCD of barley (Hordeum vulgare) aleurone cells, which offer a well-developed system for studying cell biological and physiological functions of GA3 responses. GA3 initiates cell death of aleurone cells, whereas ABA inhibits cell death (Wang et al., 1996; Appleford and Lenton, 1997). H2O2 was shown to represent a major reactive oxygen leading to cell death in aleurone cells (Bethke and Jones, 2001). Furthermore, transcript levels and activities of ROS-scavenging enzymes, including catalase, ascorbate peroxidase, and superoxide dismutase, were significantly down-regulated in GA3-treated aleurone cells, thus rendering these cells sensitive to oxidative damage and cell death, whereas ABA caused increases in catalase activity and CAT2 mRNA (Fath et al., 2001). Aleurone cells are devoid of functional chloroplasts (Jones, 1969), one of the ROS sources in plant cells. In aleurone cells, mitochondria are abundant and a cyanide-insensitive electron transport pathway switches to a cyanide-sensitive one in response to GA3 that may contribute to ROS production (Fath et al., 2002). In addition, a reduction in the number of lipid-storing oleosomes correlates with increases in the enzyme activities of the glyoxylate cycle in aleurone cells, suggesting that mitochondria and glyoxysomes are major sources of ROS in aleurone cells (Fath et al., 2002).
ROS also play an important signaling role as regulators of PCD in response to pathogens (Levine et al., 1994). Two Arabidopsis NADPH oxidase genes, AtrbohD and AtrbohF, were reported to be a major source of pathogen-induced ROS generation (Torres et al., 2002). Interestingly, atrbohD/atrbohF double mutants showed reduced cell death in response to a bacterial pathogen, but enhanced cell death in response to a fungal pathogen. These opposite responses may derive from interaction with salicylic acid. Torres et al. (2005) reported that ROS produced by NADPH oxidases antagonize salicylic acid and suppress cell death in cells that are more distantly located from the cells at the site of infection. The cells at the site of infection undergo hypersensitive cell death. These results indicate that ROS play dual functions in both driving and suppressing PCD in different contexts in the pathogen response.
ROS AND ABA SIGNALING
Research on ABA signal transduction has characterized cell biological and genetic mechanisms upstream and downstream of ROS production, and we therefore describe these in further detail in this article. ROS were shown to induce increases in cytosolic Ca2+ and stomatal closure (McAinsh et al., 1996; Lee et al., 1999). Oligogalacturonic acid and chitosan treatments caused H2O2 production in guard cells, resulting in stomatal closure in tomato (Lycopersicon esculentum) and Commelina. These responses were suppressed by EGTA, catalase, and ascorbic acid (Lee et al., 1999). ABA activation of plasma membrane Ca2+-permeable channels contributes to ABA-induced cytosolic Ca2+ increases in guard cells of Vicia and Arabidopsis (Hamilton et al., 2000; Pei et al., 2000), together with organellar Ca2+ release and Ca2+-independent mechanisms (Schroeder et al., 2001; Hetherington and Woodward, 2003; Fan et al., 2004). Application of H2O2 to guard cells activates hyperpolarization-regulated Ca2+-permeable (ICa) channels and produces simultaneous cytosolic calcium elevations, and this activation is impaired in the ABA-insensitive gca2 mutant (Pei et al., 2000). ABA regulation of ICa channels requires cytoplasmic NADPH in whole-cell recordings of Arabidopsis guard cells (Murata et al., 2001). Furthermore, ABA enhances cellular ROS levels in Arabidopsis guard cells, in Vicia faba guard cells, and in maize (Zea mays) embryos (Guan et al., 2000; Pei et al., 2000; Zhang et al., 2001c). These studies define new ABA signal transduction events.
It was reported that H2O2 induces cytosolic alkalization in Vicia guard cells (Zhang et al., 2001a), whereas in another study, cytoplasmic alkalization preceded ROS production during stomatal responses to ABA and methyl jasmonate in Arabidopsis (Suhita et al., 2004). In V. faba guard cells, initial ABA-induced ROS increases were observed within 30 s of ABA application (Zhang et al., 2001c). The findings of cytosolic pH changes both before and after ROS may reflect the parallel and branched nature of the ABA-signaling network (Leung and Giraudat, 1998; Schroeder et al., 2001; Hetherington and Woodward, 2003; Fan et al., 2004). Nevertheless, further research into cellular ROS homeostasis and the timing of ROS production would be of interest and may require the development of time-resolved ratiometric reporters that show a high sensitivity to ROS, as have been developed for other second messengers (Allen et al., 1999; Zacharias et al., 2000; Zhang et al., 2002).
Ca2+-permeable channels are activated by hyperpolarization in guard cells, root hair cells, Fucus rhizoids, root epidermal cells, and mesophyll cells (Gelli and Blumwald, 1997; Hamilton et al., 2000; Kiegle et al., 2000; Pei et al., 2000; Véry and Davies, 2000; Coelho et al., 2002; Demidchik et al., 2003; Foreman et al., 2003; Stoelzle et al., 2003). ROS regulation of these channels has been found in several systems (Pei et al., 2000; Coelho et al., 2002; Demidchik et al., 2003; Foreman et al., 2003; for review, see Mori and Schroeder, 2004). An open question is whether ROS directly regulate Ca2+-permeable channels and/or upstream modulators (Mori and Schroeder, 2004). In vitro biochemical studies revealed that H2O2 directly inactivates the ABI1 and ABI2 type 2C protein phosphatase (PP2C) enzymes that function in ABA signaling (Meinhard and Grill, 2001; Meinhard et al., 2002). ABI1 and ABI2 function as negative regulators of ABA signaling (Merlot et al., 2001; Yoshida et al., 2006a). Knockout mutants for two other PP2Cs, PP2C-HAB and PP2CA, show strong hypersensitivity to ABA, showing that PP2C-HAB and PP2CA also negatively regulate ABA signaling (Leonhardt et al., 2004; Saez et al., 2004; Kuhn et al., 2006; Yoshida et al., 2006b). Thus, all four of these PP2Cs may be prime candidates as targets for ROS in mediating ABA responses.
ABA activation of Ca2+-permeable channels is disrupted in the two dominant PP2C mutants, abi1-1 and abi2-1 (Murata et al., 2001). ABA-induced ROS production and ABA activation of ICa channels is impaired in the abi1-1 mutant. The dominant abi2-1 mutation causes disruption of both H2O2 activation of Ca2+-permeable channels and H2O2-induced stomatal closing (Murata et al., 2001; Fig. 1). The ABA-activated protein kinase OST1 was identified to function upstream of ROS production in ABA signal transduction because the ost1 mutation disrupted ABA-induced ROS generation in guard cells (Mustilli et al., 2002; Fig. 1). OST1 is the Arabidopsis homolog of the Vicia ABA-activated protein kinase, AAPK (Li et al., 2000; Mustilli et al., 2002). Consistent with the disruption of ABA-induced ROS, increases in abi1-1 and ost1, but not abi2-1 (Murata et al., 2001; Mustilli et al., 2002), a recent study revealed that the ABI1 protein interacts with OST1 and the abi1-1 mutation, but not abi2-1 mutation, disrupts ABA activation of the OST1 protein kinase activity (Yoshida et al., 2006a). These data and the relatively strong ABA insensitivity of abi1-1 and ost1 mutants indicate that abi1-1 and OST1 may function upstream in the ABA-signaling network before the proposed branch points (Fig. 1).
Figure 1.
Simplified working model showing genetic interactions and putative functions and putative locations within the ROS-mediated ABA-signaling network in guard cells. Positive and negative regulators are shaded in green and red, respectively. PI3K, Phosphatidylinositol-3 kinase; PI4K, phosphatidylinositol-4 kinase.
An electrophysiological study provides evidence for a protein kinase-dependent and ROS-independent activation of ICa channels in V. faba guard cells (Fig. 1), showing that the PP1/PP2A protein phosphatase inhibitors, okadaic acid and calyculin A, enhanced ICa channel activity (Köhler and Blatt, 2002). In addition, phospholipids have been implicated in ABA-triggered cytosolic Ca2+ increases and stomatal closure (Staxen et al., 1999; Lemtiri-Chlieh et al., 2000). Treatment of epidermal strips with the phosphatidylinositol (PI)-3-kinase and PI-4-kinase inhibitors wortmannin and LY294002 inhibited PI kinase enzyme activities, enhanced stomatal opening, and impaired ABA-induced cytosolic Ca2+ increases, indicating that PI-3-P and PI-4-P modulate ABA signaling in guard cells (Jung et al., 2002). Furthermore, wortmannin and LY294002 inhibited ABA-triggered ROS production and stomatal closure, suggesting that PI-3-P (or products thereof) may function in the ABA-ROS-signaling branch in guard cells (Park et al., 2003).
Interestingly, outward K+ channels are inhibited by H2O2, which could cause inhibition of ABA and/or ROS-induced stomatal closure (Torsethaugen et al., 1999; Zhang et al., 2001b; Köhler et al., 2003). Similarly, ozone causes secondary ROS production in guard cells and inhibits outward and inward K+ channels (Torsethaugen et al., 1999; Joo et al., 2005). Ozone inhibits stomatal opening, but does not inhibit stomatal closing (Torsethaugen et al., 1999). The residual outward K+ currents after ROS application may be sufficient to function in K+ efflux during stomatal closing, as previous analyses have shown that roughly 25% of K+ channel activities are sufficient for physiological K+ fluxes during stomatal movements (Schroeder et al., 1984; Kelly et al., 1995; Kwak et al., 2001). In addition to inhibiting outward K+ channels, H2O2 and ozone inhibited inward K+ channels in guard cells, which correlates with ABA maintenance of stomatal closure (Torsethaugen et al., 1999; Zhang et al., 2001b; Köhler et al., 2003). Nevertheless, the dual down-regulation of both outward and inward K+ channels by ROS either implicates localized oxidative bursts in guard cells or reversible regulation of ROS-dependent protein modifications during signaling.
NADPH OXIDASES AND CELL CALCIUM SIGNALING
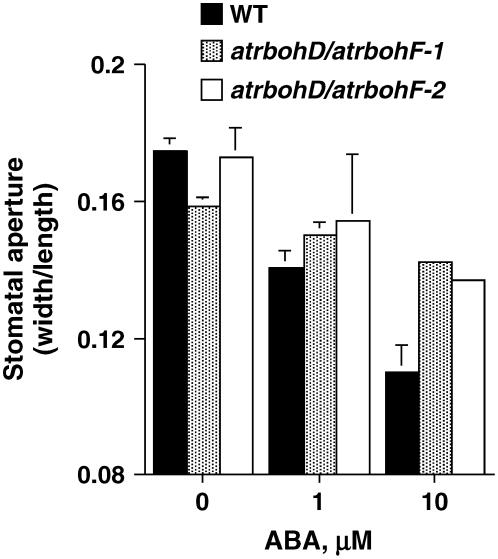
Plant cells have a diverse array of enzymatic mechanisms responsible for ROS production (Apel and Hirt, 2004; Mittler et al., 2004). Among those possible cellular mechanisms, NADPH oxidases have been suggested to function in ABA signaling (Pei et al., 2000; Murata et al., 2001; Jiang and Zhang, 2002, 2003). Genetic evidence for an important source of ABA-triggered ROS production in guard cells came from the analysis of two guard cell-expressed transmembrane NADPH oxidase catalytic subunit genes, AtrbohD and AtrbohF (Kwak et al., 2003). Analyses of stomatal movement responses show that ABA-induced stomatal closure is partially impaired in two independent alleles of the atrbohD/atrbohF double mutant (Fig. 2). Cellular events were impaired in atrbohD/atrbohF double-mutant guard cells, including ABA-induced ROS increases, ABA activation of ICa channels, and ABA-induced cytosolic Ca2+ increases (Kwak et al., 2003; Fig. 1). Exogenous application of ROS recovered ICa channel activation and stomatal closing in atrbohD/atrbohF double-mutant guard cells. The partial impairment in ABA-induced stomatal closing of atrbohD/atrbohF double mutants is consistent with models in which parallel pathways function in the early ABA-signaling network (Leung and Giraudat, 1998; Schroeder et al., 2001; Hetherington and Woodward, 2003; Fan et al., 2004; Figs. 1 and 2). These findings on atrbohD/atrbohF double mutants provide molecular genetic evidence for a function of ROS as a second messenger in ABA signaling in guard cells.
Figure 2.
ABA-induced stomatal closure in two double-mutant alleles of the NADPH oxidases atrbohD and atrbohF. ABA-induced stomatal closure is partially impaired in the two independent alleles of atrbohD/atrbohF double mutants. atrbohD/atrbohF-1 carries a dSpm transposon insertion in the fifth exon of AtrbohD (insertion D3) and a dSpm insertion in the first exon of AtrbohF (insertion F3; Torres et al., 2002). atrbohD/atrbohF-2 carries a dSpm insertion in the second intron of AtrbohF (insertion F5; Torres et al., 2002) and a T-DNA insertion in the fourth intron of AtrbohD (Salk JP65_4B03L). Double-blind experiments were performed in which the ABA concentration and the genotype were unknown. Error bars represent se of n = 3 experiments, 60 stomata per bar. Error bars are not visible when they are too small.
The AtrbohC NADPH oxidase was demonstrated to function in root hair growth and plays an important role in mediating the tip-focused Ca2+ gradient in Arabidopsis root hair cells (Foreman et al., 2003). Reactive oxygen also activated ICa-like channels in root hairs and mediated Ca2+ influx in the Fucus rhizoid, suggesting that ROS activation of Ca2+-permeable channels is a more general signaling mechanism in plants (Coelho et al., 2002; Foreman et al., 2003). In animal cells, NADPH oxidases are regulated by cytosolic components, including Rho small G proteins (for review, see Bokoch and Knaus, 2003). Recent studies identified Rho small G proteins as regulators of ROS-generating enzymes in plants too. Carol et al. (2005) demonstrated that a RhoGTPase GDP dissociation inhibitor functions upstream of ROS produced by AtRbohC NADPH oxidase during root hair growth. Dominant mutations in the small G-protein ROP2 were shown to regulate ROS generation and phosphatidic acid-induced cell death (Park et al., 2004). Moreover, Joo et al. (2005) showed that heterotrimeric G-protein α- and β-subunits are differentially involved in ozone-triggered oxidative stress responses.
Two plant enzymes, xanthine dehydrogenase and aldehyde oxidase, could provide sources for ROS production during water stress and/or ABA signaling (Yesbergenova et al., 2005). Interestingly, in contrast to their animal counterparts, xanthine dehydrogenase is capable of producing only O2−, whereas aldehyde oxidase produced only H2O2 (Yesbergenova et al., 2005). A T-DNA insertional mutation in Arabidopsis xanthine dehydrogenase 1 showed loss of O2−-producing activity. Furthermore, transcript levels of these enzymes were up-regulated upon ABA treatment and water stress (Yesbergenova et al., 2005). Additional ROS scavengers and/or ROS-producing enzymes are likely to function in ROS-signaling networks to ensure that a transient ROS burst does not trigger events that lead to cell damage and death.
ROS SIGNALING REQUIRES REGULATION OF ROS-SCAVENGING MECHANISMS
The findings that oxidative bursts function in various cellular signaling responses in plants, highlighted in this issue of Plant Physiology, suggest that ROS-scavenging mechanisms will be important for mediating and controlling these responses (Mittler et al., 2004), as illustrated in barley aleurone cells (Fath et al., 2001). For example, a strong oxidative burst will cause cellular damage and death (Mittler, 2002; Apel and Hirt, 2004). Furthermore, constitutive ROS elevations, even if not very high, could cause malfunction or desensitization of ROS-dependent signaling responses. Several studies suggest that ROS scavenger proteins play central roles in ABA signaling. Several ROS scavenger mRNAs are regulated in response to GA3, ABA, and oxidative stress (Desikan et al., 2001; Fath et al., 2001; Vranova et al., 2002; Vandenabeele et al., 2003).
The cellular redox state influences diverse cellular functions and enzyme activities. An antisense suppression of catalase activity in transgenic tobacco (Nicotiana tabacum) plants resulted in increased ascorbate peroxidase and glutathione peroxidase levels and a 4-fold decrease in ascorbate (Willekens et al., 1997), a major H2O2-scavenging antioxidant in plant cells. Interestingly, transgenic tobacco plants in which both ascorbate peroxidase and catalase are suppressed were shown to be less sensitive to oxidative stress compared to single antisense plants suppressing either catalase or ascorbate peroxidase alone, suggesting that lack of H2O2-scavenging mechanisms might have turned on an alternative mechanism by which cells are protected (Rizhsky et al., 2002). A knockout mutation in ascorbate peroxidase 1 (APX1) caused the accumulation of H2O2 in Arabidopsis and decreases in the transcript levels of catalase and chloroplast superoxide dismutase and no significant changes in glutathione peroxidase transcript levels (Pnueli et al., 2003). Furthermore, stomates of the apx1 knockout plants neither closed in response to darkness nor opened in response to light (Pnueli et al., 2003). APX1 was also shown to play a role in protecting the chloroplast from oxidative damage (Davletova et al., 2005). Arabidopsis plants with increased dehydroascorbate reductase, which generates ascorbate from dehydroascorbate, showed an increased ascorbate redox state and a reduction in H2O2 levels in guard cells. Consistent with guard cell-signaling analyses, this resulted in increased stomatal conductance and reduced sensitivity to H2O2 and ABA (Chen and Gallie, 2004). This result suggests that ABA/ROS signaling in guard cells is controlled by the ascorbic acid redox state.
FUTURE PERSPECTIVES
ROS mediate diverse functions in a variety of cellular processes. Many exciting findings have revealed roles of ROS in hormonal responses in the past few years and many new questions arise. What are the downstream protein targets that are modified by ROS during signal transduction, enabling stimulus-specific cellular responses? Among those mechanisms responsible for ROS generation in plant cells, which combination of cellular mechanisms mediates ROS production for specific signaling cascades, and how do ROS producers and scavengers interact with each other to regulate cellular ROS levels? What are the roles for the other seven Atrboh genes whose functions are waiting to be unveiled? How is the enzyme activity of NADPH oxidases regulated in plants? Surely there is a lot more to come in this stimulating field.
Acknowledgments
We apologize to those colleagues whose contributions we did not review due to the short format of this Update.
This work was supported by grants from the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research Education and Extension Service (grant no. 2004–35100–14909 to J.M.K.), the National Institutes of Health (grant no. GM60396–P42E510337 to J.I.S.), and the National Science Foundation (grant no. MCB0417118 to J.I.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: June M. Kwak (jkwak@umd.edu).
References
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19: 735–747 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Appleford N, Lenton J (1997) Hormonal regulation of α-amylase gene expression in germinating wheat (Triticum aestivum) grains. Physiol Plant 100: 534–542 [Google Scholar]
- Bethke PC, Jones RL (2001) Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J 25: 19–29 [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Knaus UG (2003) NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci 28: 502–508 [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16: 1143–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho SM, Taylor AR, Ryan KP, Sousa-Pinto I, Brown MT, Brownlee C (2002) Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. Plant Cell 14: 2369–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies J (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Desikan R, Cheung M-K, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot 55: 205–212 [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Bright J, Harrison J, Weir I, Hooley R, Neill SJ (2005) A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol 137: 831–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, A-H-Mackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Haeze W, De Rycke R, Mathis R, Goormachtig S, Pagnotta S, Verplancke C, Capoen W, Holsters M (2003) Reactive oxygen species and ethylene play a positive role in lateral root base nodulation of a semiaquatic legume. Proc Natl Acad Sci USA 100: 11789–11794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L-M, Zhao Z, Assmann SM (2004) Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7: 537–546 [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke P, Beligni V, Jones R (2002) Active oxygen and cell death in cereal aleurone cells. J Exp Bot 53: 1273–1282 [PubMed] [Google Scholar]
- Fath A, Bethke PC, Jones RL (2001) Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol 126: 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Gelli A, Blumwald E (1997) Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J Membr Biol 155: 35–45 [DOI] [PubMed] [Google Scholar]
- Giulivo C (1986) Hormonal control of water transport in soil-plant-atmosphere continuum. Acta Hortic 179: 385–393 [Google Scholar]
- Guan LM, Zhao J, Scandalios JG (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J 22: 87–95 [DOI] [PubMed] [Google Scholar]
- Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17: 3436–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Kohler B, Blatt MR (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggie L, Gray J (2005) Experiment: identification of genes controlling stomatal number in response to rising atmospheric carbon dioxide levels. http://affymetrix.arabidopsis.info/narrays/experimentpage.pl?experimentid=29
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2002) Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215: 1022–1030 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2003) Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defense in leaves of maize seedlings. Plant Cell Environ 26: 929–939 [DOI] [PubMed] [Google Scholar]
- Jones RL (1969) The fine structure of barley aleurone cells. Planta 85: 359–374 [DOI] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126: 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein α- and β-subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JY, Kim YW, Kwak JM, Hwang JU, Young J, Schroeder JI, Hwang I, Lee Y (2002) Phosphatidylinositol 3- and 4-phosphate are required for normal stomatal movements. Plant Cell 14: 2399–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WK, Esser JE, Schroeder JI (1995) Effects of cytosolic calcium and limited, possible dual, effects of G protein modulators on guard cell inward potassium channels. Plant J 8: 479–489 [Google Scholar]
- Kiegle E, Gilliham M, Haseloff J, Tester M (2000) Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J 21: 225–229 [DOI] [PubMed] [Google Scholar]
- Köhler B, Blatt MR (2002) Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J 32: 185–194 [DOI] [PubMed] [Google Scholar]
- Köhler B, Hills A, Blatt MR (2003) Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol 131: 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI (2006) The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol 140: 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori I, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, Bloom R, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI (2001) Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol 127: 473–485 [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7: 323–328 [DOI] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo I-S, Oh K-Y, Choi EJ, Schroeder Taylor AT, Low PS, Lee Y (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC, Brealey CA (2000) Inositol hexakisphosphate is a physiological signal regulating the K+-inward rectifying conductance in guard cells. Proc Natl Acad Sci USA 97: 8687–8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49: 199–222 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Li J, Wang X-Q, Watson MB, Assmann SM (2000) Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287: 300–303 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Clayton H, Mansfield TA, Hetherington AM (1996) Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 111: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhard M, Grill E (2001) Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett 508: 443–446 [DOI] [PubMed] [Google Scholar]
- Meinhard M, Rodriguez PL, Grill E (2002) The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214: 775–782 [DOI] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25: 295–303 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135: 702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei Z-M, Mori IC, Schroeder JI (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Gu Y, Lee Y, Yang Z (2004) Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol 134: 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KY, Jung JY, Park J, Hwang JU, Kim YW, Hwang I, Lee Y (2003) A role for phosphatidylinositol 3-phosphate in abscisic acid-induced reactive oxygen species generation in guard cells. Plant Physiol 132: 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Liang H, Rozenberg M, Mittler R (2003) Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J 34: 187–203 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inze D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32: 329–342 [DOI] [PubMed] [Google Scholar]
- Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez-Garcia MP, Nicolas C, Lorenzo O, Rodriguez PL (2004) Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J 37: 354–369 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hedrich R, Fernandez JM (1984) Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature 312: 361–362 [Google Scholar]
- Staxen I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR (1999) Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci USA 96: 1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100: 1456–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S (2005) Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 138: 2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37: 1130–1134 [DOI] [PubMed] [Google Scholar]
- Torsethaugen G, Pell EJ, Assmann SM (1999) Ozone inhibits guard cell K+ channels implicated in stomatal opening. Proc Natl Acad Sci USA 96: 13577–13582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S, Van Der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Van Montagu M, Zabeau M, et al (2003) A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc Natl Acad Sci USA 100: 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A-A, Davies JM (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranova E, Atichartpongkul S, Villarroel R, Van Montagu M, Inze D, Van Camp W (2002) Comprehensive analysis of gene expression in Nicotiana tabacum leaves acclimated to oxidative stress. Proc Natl Acad Sci USA 99: 10870–10875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Oppedijk BJ, Lu X, Van Duijn B, Schilperoort RA (1996) Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol Biol 32: 1125–1134 [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol 7: 449–455 [DOI] [PubMed] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inze D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16: 4806–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesbergenova Z, Yang G, Oron E, Soffer D, Fluhr R, Sagi M (2005) The plant Mo-hydroxylases aldehyde oxidase and xanthine dehydrogenase have distinct reactive oxygen species signatures and are induced by drought and abscisic acid. Plant J 42: 862–876 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006. a) The regulatory domain of SRK2E/OST1/SNRK2.6 interacts with ABI1 and integrates ABA and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T (2006. b) ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol 140: 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias DA, Baird GS, Tsien RY (2000) Recent advances in technology for measuring and manipulating cell signals. Curr Opin Neurobiol 10: 416–421 [DOI] [PubMed] [Google Scholar]
- Zhang J, Campbell RE, Ting AY, Tsien RY (2002) Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol 3: 906–918 [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong FC, Gao JF, Song CP (2001. a) Hydrogen peroxide-induced changes in intracellular pH of guard cells precede stomatal closure. Cell Res 11: 37–43 [DOI] [PubMed] [Google Scholar]
- Zhang X, Miao YC, An GY, Zhou Y, Shangguan ZP, Gao JF, Song CP (2001. b) K+ channels inhibited by hydrogen peroxide mediate abscisic acid signaling in Vicia guard cells. Cell Res 11: 195–202 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P (2001. c) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]