Abstract
Leaf growth in monocotyledons results from the flux of newly born cells out of the division zone and into the adjacent elongation-only zone, where cells reach their final length. We used a kinematic method to analyze the effect of phosphorus nutrition status on cell division and elongation parameters in the epidermis of Lolium perenne. Phosphorus deficiency reduced the leaf elongation rate by 39% due to decreases in the cell production rate (−19%) and final cell length (−20%). The former was solely due to a lower average cell division rate (0.028 versus 0.046 cell cell−1 h−1) and, thus, a lengthened average cell cycle duration (25 versus 15 h). The number of division cycles of the initial cell progeny (five to six) and, as a result, the number of meristematic cells (32–64) and division zone length were independent of phosphorus status. Accordingly, low-phosphorus cells maintained meristematic activity longer. Lack of effect of phosphorus deficiency on meristematic cell length implies that a lower division rate was matched to a lower elongation rate. Phosphorus deficiency did not affect the elongation-only zone length, thus leading to longer cell elongation duration (99 versus 75 h). However, the substantially reduced postmitotic average relative elongation rate (0.045 versus 0.064 mm mm−1 h−1) resulted in shorter mature cells. In summary, phosphorus deficiency did not affect the general controls of cell morphogenesis, but, by slowing down the rates of cell division and expansion, it slowed down its pace.
Although essential for plant growth and development, inorganic phosphorus is one of the least available nutrients in soils of many terrestrial ecosystems (Vance et al., 2003). Plants are profoundly affected by phosphorus deficiency because phosphorus is an indispensable constituent of nucleic acids and membrane phospholipids. Moreover, phosphorus plays a pivotal role in energy transfer, as a regulator of enzyme activity, and in signal transduction. Thus, not surprisingly, low phosphorus availability activates a series of morphological and physiological responses that maximize phosphorus acquisition (Raghothama, 1999) and are directed to maintain internal phosphorus homeostasis (Ticconi and Abel, 2004). Leaf growth depression under phosphorus deficiency is well documented (Radin and Eidenbock, 1984; Chiera et al., 2002; Assuero et al., 2004; Kavanová et al., 2006). Ultimately, this growth reduction must be due to an alteration of cell division or cell elongation parameters.
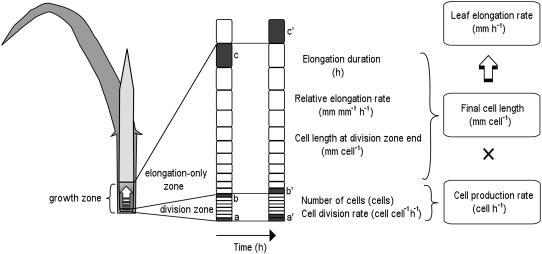
We have chosen a grass leaf system to investigate the cellular bases of growth reduction under phosphorus deficiency. In grasses, growth is confined to a short tissue segment located at the base of the developing leaf enclosed by older sheaths (Kemp, 1980). Here, meristematic cells proliferate, undergoing a number of cell cycles before entering a phase of elongation-only growth. This creates a clearly defined spatial pattern of cell development along the longitudinal axis, giving place to a basal division zone, where meristematic cells elongate and divide, and an elongation-only zone, where cells undergo postmitotic elongation. Together, the two zones form the leaf growth zone (Fig. 1). Kinematic analysis provides the appropriate analytical tools to translate back the spatial patterns into the time history of an individual cell, making it possible to derive, from the spatial profiles of cell length and displacement velocity, rates and durations of cell division and elongation (Green, 1976; Silk and Erickson, 1979; Silk, 1992). The leaf elongation rate (mm h−1), the flux of leaf tissue out of the growth zone, can then be analyzed in terms of the cell production rate (cell h−1) and final cell length (mm cell−1; Volenec and Nelson, 1981). In turn, the cell production rate is determined by the number of cells in the division zone and their division rate (cell cell−1 h−1), whereas the final cell length is determined by the length of cells leaving the meristem (mm cell−1), and their relative elongation rate (mm mm−1 h−1) and elongation duration (h; Fig. 1).
Figure 1.
Growth zone of a grass leaf. Growth is limited to the basal part of the growing leaf, the growth zone, which is enclosed by the sheaths of expanded leaves. Meristematic cells in the division zone elongate and divide simultaneously (a→a′). The progeny of the initial cell at the base of the meristem goes through a certain number of division cycles, thus determining the number of cells per meristematic file. Upon entering the elongation-only zone (b→b′), cells elongate without further divisions until they reach their final length at the distal end of the growth zone (c→c′).
The contribution of the different cellular parameters to leaf growth reduction under nutrient stress is not well understood. Few studies addressed the effects of phosphorus deficiency and gave different results. In cotton (Gossypium hirsutum), Radin and Eidenbock (1984) concluded that reduced cell expansion underlay reduced leaf size, whereas, in soybean (Glycine max), Chiera et al. (2002) concluded that reduced cell division was the major cause. Although this divergence may be related to a different species response, it may also arise from the fact that neither of the studies directly measured these parameters. Instead, the role of cell expansion was inferred from smaller leaf cells and the role of cell division was inferred from reduced cell number. In maize (Zea mays), the first monocot studied, Assuero et al. (2004) attributed the reduction in leaf growth to a decreased cell production rate. In the only other kinematic study of phosphorus effects on leaf growth in the grass Lolium perenne, low relative elongation rates along the elongation-only zone caused a severe reduction of leaf growth (Kavanová et al., 2006).
This study provides a comprehensive analysis of the cellular responses underlying reduction of the leaf elongation rate in L. perenne leaves growing under phosphorus deficiency. Using a kinematic approach, we evaluated which parameters determining the number of produced cells and their final length responded to changes in phosphorus status and which did not. This included (1) number of meristematic cells as controlled by a (constant) number of division cycles of the initial cell progeny; (2) duration of cell elongation as determined by a spatially controlled elongation-only zone length; (3) rate of cell division as determined by the growth rate of meristematic cells and a (constant) mitotic cell length; and (4) rate of mitotic and postmitotic elongation.
RESULTS
Leaf Elongation Rate
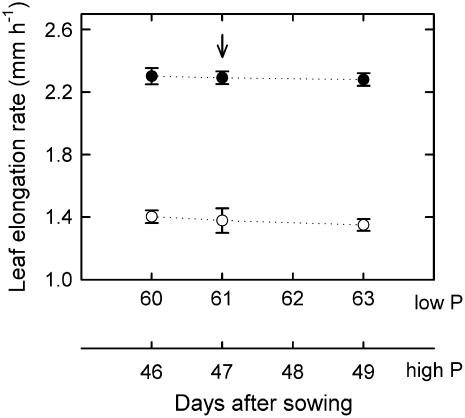
L. perenne plants grew at low (0.02 mm) or high (1 mm) phosphorus supply. Growth at low phosphorus supply caused a 42% reduction in the phosphorus concentration in the leaf growth zone (P < 0.001; Table I) and a 39% reduction in the leaf elongation rate (P < 0.001; Fig. 2). In both treatments, leaves selected for measurement elongated at a steady rate over time (Fig. 2).
Table I.
Phosphorus status, tiller size, and developmental stage of leaves selected for analysis of leaf growth and underlying cellular dynamics
L. perenne plants were grown for 47 d at high (1 mm) and 61 d at low (0.02 mm) phosphorus supply. Data are averages of six plants (±se), along with the significance of the difference between phosphorus treatments based on a t test. ***, P ≤ 0.001; NS, not significant, P > 0.05.
| Parameter | High Phosphorus | Low Phosphorus | Significance |
|---|---|---|---|
| Phosphorus in the growth zone (mg g−1 fresh weight) | 1.07 ± 0.01 | 0.61 ± 0.01 | *** |
| Number of green leaves per tiller | 4.8 ± 0.19 | 4.7 ± 0.24 | NS |
| Sheath length of the youngest expanded leaf (mm) | 90 ± 5 | 101 ± 5 | NS |
| Blade length of the youngest expanded leaf (mm) | 305 ± 14 | 342 ± 20 | NS |
| Blade length of the growing leaf (mm) | 206 ± 13 | 190 ± 24 | NS |
| Blade length expanded: blade length growing leaf | 0.68 ± 0.05 | 0.57 ± 0.08 | NS |
Figure 2.
Effect of phosphorus supply on the leaf elongation rate. L. perenne plants were grown at high (1 mm, •) and low (0.02 mm, ○) phosphorus supply. The arrow indicates the time when the kinematic analysis was performed. Data are means of five to six plants on each date (±se).
The treatment effect on the leaf elongation rate was entirely due to the different phosphorus nutrition status because selected tillers of low- and high-phosphorus plants did not differ in size or developmental variables: Leaf blades and sheaths had similar lengths, and the tillers held a similar number of leaves (Table I). Further, growing leaves were in the same developmental stage, indicated by the ratio of the growing blade length to the blade length of the youngest expanded leaf (Table I). This ensured that effects of phosphorus status on growth were not confused with effects of size and development (Kavanová et al., 2006).
Cell Proliferation
The reduction of leaf growth under low phosphorus originated partly from decreased cell proliferation in the division zone. Phosphorus deficiency reduced the cell production rate (i.e. the cell flux out of the division zone estimated from the leaf elongation rate and final cell length [Eq. 2]) by 19% (P < 0.01; Table II).
Table II.
Effect of phosphorus deficiency on kinematic parameters
L. perenne plants were grown for 47 d at high (1 mm) and 61 d at low (0.02 mm) phosphorus supply. Data are averages for epidermal cells of six plants (±se), along with the significance of the difference between phosphorus treatments based on a t test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; NS, not significant, P > 0.05.
| Parameter | High Phosphorus | Low Phosphorus | Significance |
|---|---|---|---|
| Leaf elongation rate (mm h−1) | 2.30 ± 0.05 | 1.41 ± 0.05 | *** |
| Cell production rate (cell h−1) | 1.91 ± 0.10 | 1.52 ± 0.10 | ** |
| Average cell division rate (cell cell−1 h−1) | 0.046 ± 0.008 | 0.028 ± 0.005 | ** |
| Average cell cycle duration (h) | 15.1 ± 3.1 | 24.9 ± 2.0 | *** |
| Number of cells per meristematic cell file | 41 ± 7 | 55 ± 9 | NS |
| Number of division cycles | 5.4 ± 2.7 | 5.8 ± 2.3 | NS |
| Final cell length (mm) | 1.208 ± 0.057 | 0.929 ± 0.052 | ** |
| Cell length in the meristem (μm) | 19.3 ± 3.2 | 18.8 ± 2.4 | NS |
| Postmitotic relative elongation rate (mm mm−1 h−1) | 0.064 ± 0.005 | 0.045 ± 0.005 | * |
| Elongation duration (h) | 75 ± 6 | 99 ± 9 | * |
This difference arose entirely from a different average cell division rate, that is, the number of cells produced per cell present in the division zone per unit time. Meristematic cells divided at a 39% lower rate in low-phosphorus plants (P < 0.01; Table II). As a result, the average cell cycle duration (Eq. 8) was 10 h longer in low-phosphorus plants (Table II).
Phosphorus deficiency did not affect the average number of cells in a meristematic cell file (P > 0.1; Table II). Cell division was confined to the basal 0.9 ± 0.1 mm in low-phosphorus plants and to 0.6 ± 0.1 mm in high-phosphorus plants, but this difference was not statistically significant (P = 0.06). It is important to note that we did not derive the length of the division zone and the number of meristematic cells from cell deposition rates. Instead, we counted all cells present in individual meristematic cell files from the leaf base to the position of the last recently formed perpendicular cell wall. A closer examination of these data revealed that the number of cells per meristematic cell file was a weighted average of two major groups of files: files with approximately 32 cells and files with approximately 64 cells. Low- and high-phosphorus plants had a similar frequency distribution of these two groups (data not shown).
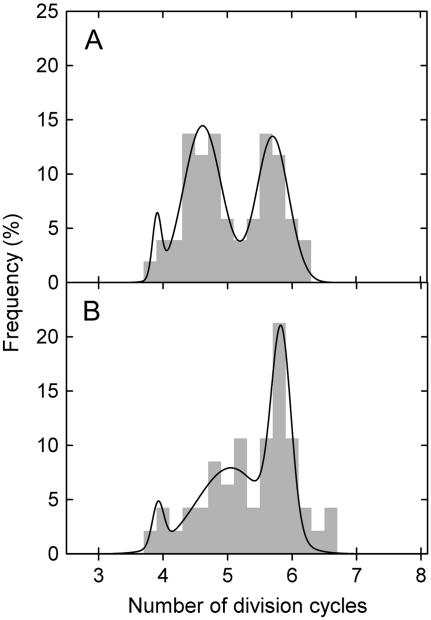
The number of division cycles necessary to displace a transversal cell wall from the basal to the distal boundary of the division zone (i.e. the average number of division cycles of the progeny of a cell formed by the division of the initial cell at the base of the meristem) can be derived from the number of cells in the division zone (Eq. 10). In both phosphorus treatments, the number of division cycles was, on average, five to six (P > 0.1; Table II). Frequency distribution of the number of division cycles in different meristematic cell files revealed distinct peaks around four, five, and six, indicating that variability exists between cell files within one division zone (Fig. 3). Whereas in high-phosphorus plants cell files were equally distributed around five and six division cycles, low-phosphorus plants tended to have a frequency distribution shifted toward six division cycles.
Figure 3.
Frequency distribution of cell files with different numbers of division cycles. L. perenne plants were grown for 47 d at high (1 mm; A) and 61 d at low (0.02 mm; B) phosphorus supply. For the analysis, data for eight to 10 cell files of each of the six plants per treatment were combined. In every file, the number of division cycles of the progeny of the cell formed by the division of the initial cell at the base of the meristem was calculated as log2 (number of cells in the meristem; Eq. 10). Triple Gaussian normal distribution curves best fitted the frequency distributions (r2 = 0.95 for high-phosphorus plants; r2 = 0.91 for low-phosphorus plants), with peaks located at 3.9 ± 3.46, 4.6 ± 0.03, and 5.7 ± 0.03 divisions per cell (high phosphorus), and 3.9 ± 0.38, 5.1 ± 0.15, and 5.8 ± 0.02 divisions per cell (low phosphorus).
As a consequence of a similar number of division cycles but longer average cell cycle duration, the average residence time of a cell in the division zone tended to be longer under phosphorus deficiency (144 ± 55 h versus 81 ± 37 h in high-phosphorus plants). Thus, cells in low-phosphorus plants tended to maintain meristematic activity for a longer period of time.
Relative Elongation Rate of Meristematic Cells
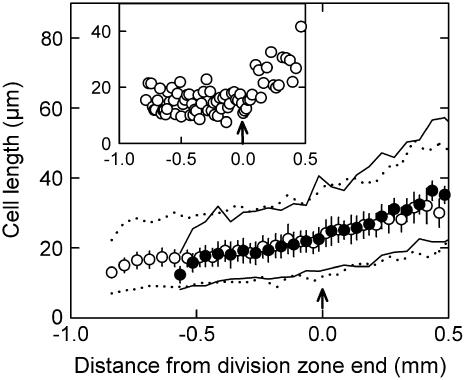
Cell length was typically constant within each individual meristematic cell file up to the position where division stopped (Fig. 4, inset). When averaged over each treatment, average cell lengths were stable along the first half of the division zone and phosphorus deficiency did not affect this pattern (Fig. 4), but average cell length increased in the second half of the division zone by 23% in low- and 17% in high-phosphorus plants. This increase was due to the fact that in some meristematic cell files the number of division cycles of the initial cell progeny was five (meristem length approximately 32 cells), whereas in others it was six (meristem length approximately 64 cells). Hence, in the second half of the meristem, dividing cells (maintaining their average length unaltered) coexisted with nondividing cells (that were increasing in length). This is also appreciated by comparing the stability of minimal cell lengths against the increase in maximal cell lengths.
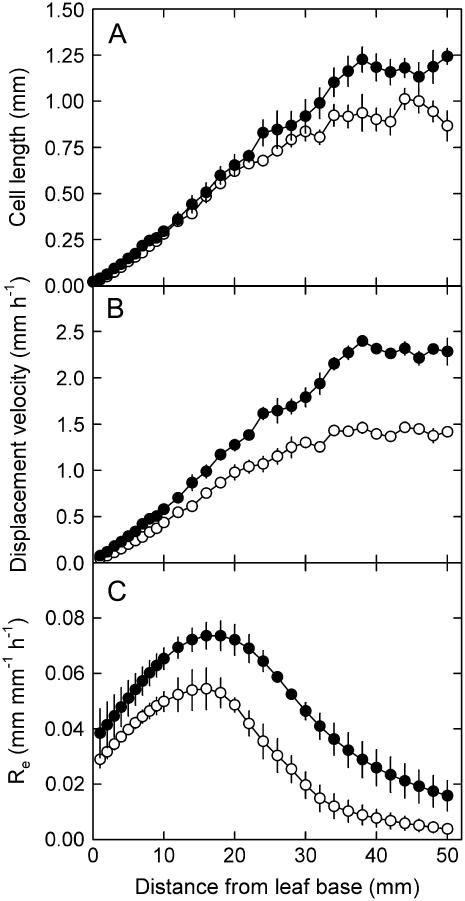
Figure 4.
Effect of phosphorus supply on epidermal cell length along the basal part of the leaf growth zone. L. perenne plants were grown for 47 d at high (1 mm, •) and 61 d at low (0.02 mm, ○) phosphorus supply. The length of the shortest and the longest cell over 50-μm intervals is indicated by continuous lines (1 mm phosphorus) and dashed lines (0.02 mm phosphorus). Data are means of six plants (±se). Arrows indicate the distal end of the division zone. Inset shows the raw cell length data for an individual cell file of a low-phosphorus plant.
The stability of cell length along the division zone provides important information on the balance between relative rates of cell division and elongation (for discussion, see Green, 1976). It implies that the relative rates of meristematic cell elongation were very close to the average cell division rates (0.028 ± 0.005 h−1 in low- versus 0.046 ± 0.008 h−1 in high-phosphorus plants). Phosphorus deficiency did not affect the size at which cells divided or, consequently, their length at birth: The 39% higher average cell division rate of high-phosphorus meristematic cells means they had a 39% higher relative elongation rate.
Final Cell Length
Whereas one-half of the leaf growth reduction under phosphorus stress was due to a reduced cell production rate, the other half originated from a decrease in final cell length. Mature epidermal cells were 20% shorter in low-phosphorus plants (P < 0.01; Table II; Fig. 5A). The final length of a cell depends on three factors: the length of the cell leaving the meristem (i.e. when it enters the elongation-only zone) and the relative rate and duration of the elongation-only phase. Phosphorus deficiency affected the latter two, but cell size at the position where elongation started was not different: 24.2 ± 3.8 μm in low- versus 22.5 ± 3.4 μm in high-phosphorus plants (P > 0.1; Fig. 4).
Figure 5.
Effect of phosphorus supply on spatial profiles of epidermal cell length (A), displacement velocity (B), and relative elongation rate (Re; C) along the base of the growing leaf. L. perenne plants were grown for 47 d at high (1 mm, •) and 61 d at low (0.02 mm, ○) phosphorus supply. Data are means of six plants (±se).
Spatial Analysis of Postmitotic Elongation
Cell elongation was confined to the basal 31 to 36 mm of the growing leaf in low- and high-phosphorus plants, respectively (P > 0.1; Fig. 5A). The number of cells in the elongation-only zone was also not affected by phosphorus deficiency (136 ± 9 in low- and 117 ± 9 in high-phosphorus plants; P > 0.1). This confirms our previous observation (Kavanová et al., 2006) that phosphorus deficiency has no (direct) effect on the length of the elongation-only zone.
Relative elongation rates along the elongation-only zone obtained by differentiating displacement velocity profiles (Fig. 5B) were uniformly lower at all positions in low-phosphorus plants (Fig. 5C). Thus, phosphorus deficiency did not modify the spatial distribution of relative elongation rates, which were, on average, 30% lower in low-phosphorus plants (P < 0.05; Table II).
Temporal Analysis of Postmitotic Elongation
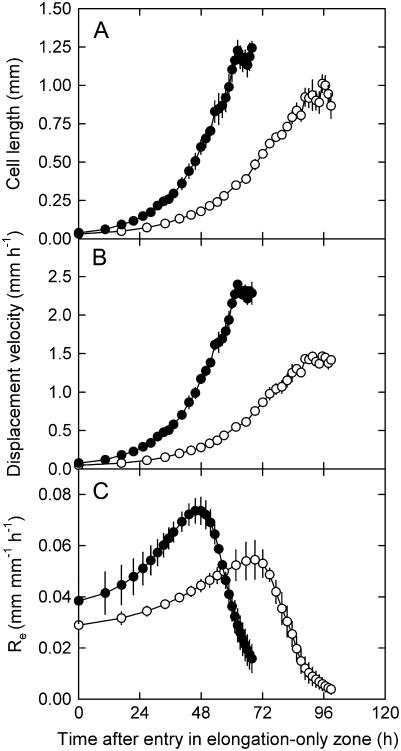
Furthermore, we carried out a temporal analysis of the elongation of an individual cell from the moment it enters the elongation-only zone. The spatial profiles of cell length, displacement velocity, and relative elongation rate were transformed into time courses using the growth trajectory function, which relates spatial position of a cell to time coordinates (Eq. 5). This analysis revealed that cells expanded for a substantially shorter period in high-phosphorus plants (P < 0.05; Table II) because they moved more rapidly through the elongation-only zone. Thus, the higher relative elongation rate of high-phosphorus plants was partially offset by a shorter elongation duration (Fig. 6).
Figure 6.
Effect of phosphorus supply on temporal profiles of cell length (A), displacement velocity (B), and relative elongation rate (Re; C) of an individual epidermal cell from the time it enters the elongation-only zone. L. perenne plants were grown for 47 d at high (1 mm, •) and 61 d at low (0.02 mm, ○) phosphorus supply. Data are means of six plants (±se).
DISCUSSION
Growth regulation constitutes a major field of interest in plant physiology. However, the cellular bases of growth reduction under stress conditions are not fully understood. This kinematic study showed that, under phosphorus deficiency, the reduction of leaf growth in the grass L. perenne arose from inhibition of cell division and elongation rates, leading to reductions in both the cell production rate and the final cell length. The lengthened average cell cycle duration in low-phosphorus plants was linked to a slower elongation rate so that meristematic cell length was not modified. Notably, other variables were unrelated to phosphorus status. In the division zone, phosphorus deficiency did not affect the number of division cycles (of the progeny of a cell formed by the division of the initial cell at the base of the meristem). In the elongation-only zone, phosphorus deficiency did not modify the position where postmitotic elongation stopped. Hence, phosphorus deficiency did not affect the putative controls of the cell morphogenetic program, but, by slowing down the rates of cell division and elongation (and thus increasing the residence time in both zones), it slowed down the pace at which it was carried out.
Cell Proliferation Is Modulated in Response to Phosphorus Status
Coupling between cell elongation and cell division has been observed under undisturbed conditions in plant meristems, where cells double in size from birth until the next division (Cánovas et al., 1990; Korn, 2001; Ivanov et al., 2002). However, only scarce knowledge exists on the links between cell growth and cell division (Li et al., 2005) and how cell size, nutrient status, or other signals impinge upon cell cycle progression in multicellular plants (De Veylder et al., 2003).
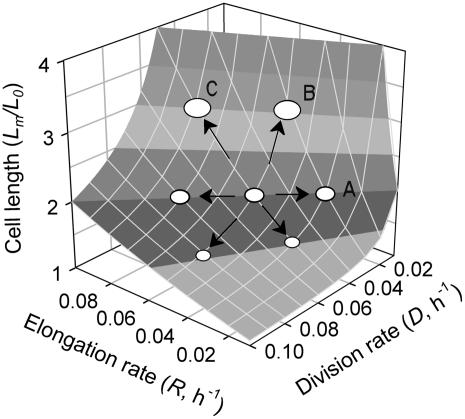
Our study shows that phosphorus deficiency decreased the average division rate of meristematic cells. But phosphorus deficiency did not affect meristematic cell length (Fig. 4), implying that a decrease in the division rate was accompanied by an equivalent reduction in the elongation rate. Therefore, phosphorus deficiency did not affect the close coordination between cell growth and cell division in the leaf meristem (see Fig. 7, trajectory A).
Figure 7.
Coordination between rates of cell division, elongation, and cell length. A cell growing exponentially at a relative rate R (h−1) and dividing at a relative rate D (h−1) increases its size during one cell cycle from the initial length (L0, mm) to its mitotic length (Lm, mm): Lm = L0 × exp (Rm × ln 2/D). Solving this equation for the relative increase in length gives: Lm/L0 = 2^(Rm/D). The ratio of Lm/L0 = 2 indicates that the cell progeny maintains constant size; the ratio of Lm/L0 > 2 indicates that R is higher than D, resulting in longer cells; and the ratio of Lm/L0 < 2 indicates that D is higher than R, resulting in shorter cells. Three cases are shown. A, Equal decrease in D and R does not affect meristematic cell length. B, Lower D with no change in R leads to longer meristematic cells. C, Faster R with no change in D results in longer meristematic cells.
What would be the result of uncoupling cell division from elongation in the meristem? Figure 7 illustrates the possible outcomes. A factor that decreases cell division rate but does not affect elongation will increase meristematic cell length (Fig. 7, trajectory B). This would also increase the initial length at which cells start expanding, and result in longer mature cells, even though their elongation rate is not affected. The same will occur when a factor increases the elongation rate but does not affect the division rate (Fig. 7, trajectory C). This analysis also illustrates the risks of inferring changes in cell division or elongation rates based only on meristematic or mature cell length.
The coupling of cell growth and division in proliferating cells may be achieved by alternative means: The cell division rate may affect the cell growth rate, the cell growth rate may influence the cell division rate, or both processes may respond to a common signal. In the first scenario, phosphorus deficiency would have inhibited cell cycle progression, and the reduced cell division rate would have decreased the cell elongation rate. Some authors have indeed suggested that cell division might affect cell growth (Doerner et al., 1996; Cockcroft et al., 2000). However, several studies showed that cell cycle modulators, either accelerating or slowing cell division rates, decoupled cell division from cell growth. Overexpression of the cyclin-dependent kinase inhibitors KRP1 and KRP2 (De Veylder et al., 2001) and expression of a dominant negative allele of the Arabidopsis (Arabidopsis thaliana) CDKA gene in tobacco (Nicotiana tabacum; Hemerly et al., 1995) resulted in fewer, but bigger, meristematic cells. Similarly, meristematic and mature cells of plants overexpressing cyclin D3 were increased in number, but were of smaller size due to accelerated progression through the G1 phase (Dewitte et al., 2003). Thus, it seems improbable that a lower cell division rate drove reductions in the elongation rate of meristematic cells under phosphorus stress.
The second scenario puts forward that a lower elongation rate of meristematic cells lengthened the average cell cycle duration. This view is supported by the fact that the probability of G1-to-S transition, a major cell size checkpoint, increased with increasing cell size (Cánovas et al., 1990). Further, Pien et al. (2001) showed that local induction of expansin expression led to the formation of normal leaf primordia, suggesting that increased cell expansion was driving cell division. In our study, a decrease in the elongation rate of meristematic cells under phosphorus deficiency would have prolonged the time needed to reach the critical length (see Fig. 7, trajectory A) and thus would have lengthened the average cell cycle duration. Consequently, phosphorus deficiency may primarily affect cell elongation and the effect on cell cycle duration may be a consequence of the reduction in growth rate. Nonetheless, a further study is needed to determine whether phosphorus deficiency extends the G1 phase specifically or all cell cycle phases.
It is not clear which signal could regulate both cell growth and division rate in the third scenario. Cytokinins are a putative candidate because phosphorus deficiency decreases their shoot levels (Horgan and Wareing, 1980), and they affect both cell cycle progression at the G1-to-S and G2-to-M transitions (del Pozo et al., 2005) and have an effect on the expression of expansins and thereby on cell wall expansibility as well (Downes et al., 2001).
Relative Elongation Rate Is Related to Phosphorus Status
A decrease in the relative elongation rate along the elongation-only zone led to shorter mature epidermal cells in phosphorus-deficient plants and thus contributed to a decrease in the leaf elongation rate (Fig. 5). Other parameters influencing final cell length were little affected by phosphorus status (the length of cells leaving the division zone; Fig. 4) or even increased under phosphorus stress (elongation duration; Table II).
Proliferating cells grow primarily by an increase in the cytoplasmic volume, whereas cells in the postmitotic phase expand primarily through an increase in the vacuolar volume (Fagerberg, 1984). Thus, it is telling that a reduction in the relative elongation rate was of similar magnitude in both the division and elongation-only zones (30%–40%), raising the question of whether this was due to the same mechanism. The relative elongation rate depends on cell wall extensibility, tissue hydraulic conductance, and turgor pressure in excess of the yield threshold of the cell wall (Fricke, 2002). No information exists concerning phosphorus effects on these processes. Changes in turgor have been found to play only a minor role in leaf growth responses to nitrogen and carbon stress and salinity (Fricke, 2002, and refs. therein). Thus, it is more likely that phosphorus status induced either changes in cell wall properties (mediated by expansins, for example) or changes in tissue hydraulic conductivity (possibly mediated by aquaporins, which are highly expressed in dividing and elongating cells; Chaumont et al., 1998). We believe that understanding the effects of phosphorus deficiency on leaf growth will progress little until the mechanism of reduction in the relative elongation rate is understood.
Phosphorus Deficiency Does Not Affect Cell Number But Increases Residence Time in the Growth Zone
Whereas division and elongation rates varied in response to phosphorus status, the size of the division and elongation-only zones remained unaffected. Two main models of growth zone regulation have been proposed for roots and may also be valid for grass leaves. The first one proposes that a spatial gradient of growth regulators determines the developmental state of cells at any position along the growth zone (Barlow, 1984). An alternative model claims that spatial patterns result from a certain developmental program followed by each cell (González-Fernández et al., 1968).
We evaluated whether the length of the cell division zone could be determined by a temporally limited proliferation of meristematic cells. Under low phosphorus, cells were proliferative for a longer time than under high phosphorus, suggesting either that the termination of cell proliferation was not time regulated or that the temporal control changed. The spatial dimensions of the division zone might be related to the constant number of division cycles of the progeny of a cell formed by the division of the initial cell at the base of the meristem. Regardless of phosphorus status, the number of division cycles before cells entered into the elongation-only zone was four to six. There was more variation between cell files within a division zone than between plants of different phosphorus status, showing the importance of evaluating meristem parameters for individual cell files.
A review of the literature provided further support for the observed constancy. The length of the division zone in leaves of different C3 grass species has been reported to vary between 1 and 8 mm (Beemster et al., 1996; Fiorani et al., 2000; Masle, 2000; Bultynck et al., 2003). The range of number of division cycles of the progeny of the initial cell is, however, narrower. We calculated from the published data that the number of division cycles was six to eight, which suggests that this parameter may be relatively conservative.
Interestingly, this study indicated that the length of the elongation-only zone was not affected by phosphorus deficiency. As discussed previously, phosphorus deficiency decreased the flux of cells through this zone but did not affect the elongation-only zone length, thus increasing the duration of an individual cell's elongation. This contradicts the view that the termination of cell elongation is time regulated. Support for the temporal regulation has been obtained by finding the opposite; namely, that the size of the elongation-only zone is proportional to the number of cells flowing through it (i.e. cell production rate) because each cell has a temporal program of elongation to execute (Beemster and Baskin, 1998). Following this reasoning, a change in cell flux should lead to a change in elongation-only zone length. This did not happen in our study, suggesting either that termination of cell elongation was not time regulated or that this control changed. Previously, we have shown that the elongation-only zone length correlates with tiller size (Kavanová et al., 2006) and suggested that morphogenic effects of light quality could provide a mechanism for the spatial control of its length (Barlow, 1984).
In contrast to the only other kinematic study of phosphorus effects on leaf growth (Assuero et al., 2004), we found no difference in the division and elongation-only zone lengths in plants differing in their phosphorus status. This might be due to a species difference. However, the length of both zones varies during leaf development, and with changing tiller size (Durand et al., 1999; Kavanová et al., 2006). Thus, the discrepancy might arise from size differences between phosphorus treatments in the study of Assuero et al. (2004). In the primary root of Arabidopsis, phosphorus deficiency did not affect meristem length but decreased the length of the rapid elongation zone (Ma et al., 2003). Similar to leaves, regulation of the growth zone length in roots is not well understood. The comparison between phosphorus deficiency effects on leaf and root elongation suggests that root and leaf growth zones may differ in the cellular mechanisms underlying the growth response.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Surface-sterilized seeds of Lolium perenne L. cv Condesa were sown in pots (diameter 5 cm, height 35 cm) on a mixture of quartz sand with 63 mg phosphorus per pot in the form of finely ground Hyperphos (Deutsche HyperPhos-Gesellschaft), providing a source of phosphorus with low availability. Each pot contained one plant. Plants grew in a growth chamber (E15; Conviron), with 20°C (day)/15°C (night), 70% relative air humidity, and 525 μmol m−2 s−1 photosynthetic photon flux density at plant height for 16 h/d. Plants were irrigated for 21 d after sowing four times a day with 25 mL of modified one-half-strength Hoagland solution [0.02 mm KH2PO4, 2.5 mm KNO3, 2.5 mm Ca(NO3)2, 1 mm MgSO4, 0.5 mm KCl, 0.5 mm NaCl, 0.125 mm Fe-EDTA, 23 μm H3BO3, 4.5 μm MnSO4, 0.38 μm ZnSO4, 0.16 μm CuSO4, and 0.05 μm Na2MoO4]. Thereafter, two levels of soluble phosphorus were applied: 0.02 mm KH2PO4 (low phosphorus) and 1 mm KH2PO4 (high phosphorus).
Leaf Elongation Rate
To avoid confounding phosphorus status with tiller size effects (see Kavanová et al., 2006), the leaf elongation rate (mm h−1) and its components were analyzed in tillers with similar sheath length of the youngest fully expanded leaf (Table I). To this end, the leaf elongation rate was measured 46 to 49 d after sowing in high- and 60 to 63 d after sowing in low-phosphorus plants.
In five to six plants per treatment, representative tillers with at least three fully expanded leaves were selected at each date. The leaf elongation rate was determined on the youngest, most rapidly growing blade during the phase of maximal growth, when the leaf elongation rate was near constant. During this developmental stage, leaf growth is due exclusively to the activity of the blade growth zone, and cell division in the blade meristem and blade expansion are approximately steady (developmental stage A→B; Schnyder et al., 1990). The leaf elongation rate was calculated as the rate of change of the distance between the tip of the elongating blade and the ligule of the youngest fully expanded leaf, which was measured with a ruler every 24 h.
Sampling and Phosphorus Analysis
Twelve plants per treatment were sampled at the end of the light period 49 d after sowing in high- and 63 d after sowing in low-phosphorus plants. Leaf growth zones were dissected from leaves similar to those used for leaf elongation rate measurements. Fresh weight was recorded, samples were frozen in liquid N2, freeze-dried, weighed, ground, and stored at −25°C. Phosphorus concentration was determined on 10- to 20-mg pooled samples as described by Kavanová et al. (2006).
Cell Length Measurement
The growing blade was carefully freed from surrounding older leaves in six plants per treatment 47 d after sowing in high- and 61 d after sowing in low-phosphorus plants. A transparent replica of the abaxial epidermis along the basal 50 mm of the growing leaf was taken as described by Schnyder et al. (1990). Briefly, a thin layer of 4% (w/w) polyvinylformaldehyde (Formvar 1595 E; Merck) in chloroform was spread along the basal part of the growing leaf. Then the film was transferred with transparent adhesive tape to a microscope slide.
Images were captured using a digital camera (Camedia C-5050Z; Olympus) fitted to an optical microscope (Olympus BX50). Leaves were excluded if the ligule was situated more than 1 mm from the leaf insertion to ensure that only the blade growth zone was contributing to the leaf elongation (Schnyder et al., 1990). Starting from the base of the growing blade (i.e. the ligule), images were taken every 1 mm (0–10 mm from the base) or 2 mm (>10 mm from the base). Images were captured at magnifications of 400× to 40× (according to increasing cell lengths), and subsequently analyzed in Sigma Scan Pro 5.0 (SPSS). The mean epidermal cell length at each distance from the base was determined by measuring the length of 20 to 80 cells in cell files located midway between files containing stomata.
In addition, a sequence of overlapping images was taken along the basal 2 mm (starting from the ligule), and composite images were created. The length and distance from the leaf base of every cell in eight to 12 cell files located midway between files containing stomata were recorded in each leaf. Mean epidermal cell length over 50-μm intervals was determined for each plant and then averaged over plants of the same treatment. Similarly, the length of the longest and shortest epidermal cell over 50-μm intervals was determined for each plant and then averaged over plants of the same treatment. In each cell file, we recorded the most distal position of a newly formed (visually thinner) perpendicular cell wall, which was used as a marker for the distal end of the cell division zone.
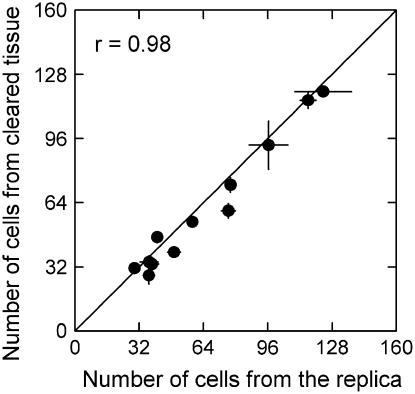
This is a novel method based on the same rationale as that introduced by Beemster et al. (1996) and used by Masle (2000). The latter method assessed newly formed perpendicular cell walls on cleared fixed tissue instead of replicas of the leaf surface. We validated the new method by comparison with that of Beemster et al. (1996). To this end, the base of 12 growing leaves in different stages of development of L. perenne cv Agenta was halved along the midrib. One-half of the leaf was treated as in Beemster et al. (1996); from the other half, a Formvar replica was taken. The position of the last newly formed perpendicular wall in epidermal cell files midway between files with stomata was measured in both sets of samples. The two methods yielded virtually identical results (Fig. 8).
Figure 8.
Comparison of the number of cells in the division zone determined from the transparent Formvar replica versus the number of cells determined from fixed and cleared leaf tissue. Each leaf was cut into two equal pieces along the central midrib. One-half was fixed and cleared following the procedure of Beemster et al. (1996). A Formvar replica was taken from the other half. The number of cells in the meristem was counted from the basal end (the ligule) to the position of the last recently formed (visually thinner) cell wall. Each data point represents the average of five to 10 cell files per leaf along with its se. The line indicates the y = x relationship.
Analysis of Cell Elongation
Final cell length (Lf, mm) and leaf growth zone length (LLGZ, mm) were determined by fitting a Richards function (Morris and Silk, 1992) to plant cell length profiles (TableCurve 2D; SYSTAT):
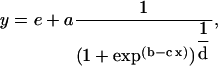 |
(1) |
where y is the cell length, x is the distance from the leaf base, e + a is the asymptotic final cell length, e is the average meristematic cell length, and b, c, and d are constants. Because a reaches the maximal value only at an infinite distance, Lf was estimated as 95% of the value of a and LLGZ as the position where this was reached.
Cell flux (F, cells h−1), the rate at which cells are displaced past a particular position, was estimated at the distal end of the elongation-only zone from the leaf elongation rate (LER) and final cell length (Lf):
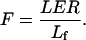 |
(2) |
Under steady-state growth, when the leaf elongation rate and the cell length profiles do not change with time, cell flux is uniform beyond the division zone, and equal to the cell production rate (Silk, 1992).
In the elongation-only zone, the displacement velocity of a cell at a certain position is the result of the elongation of all cells located more basally in the growth zone. Therefore, displacement velocity increases with distance from the leaf base and finally becomes constant and equal to the leaf elongation rate. Under steady-state growth, there is strict correspondence between local cell length [L(x), mm] and local displacement velocity [v(x), mm h−1] in the elongation-only zone (Morris and Silk, 1992; Silk, 1992):
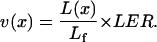 |
(3) |
The relative elongation rate in the elongation-only zone (Re, mm mm−1 h−1; synonymous terms that have been used before are strain rate, relative elemental growth rate, and segmental elongation rate) was estimated by differentiating numerically the displacement velocity with respect to position. This parameter provides a measure to compare the magnitude of the elongation rate independently from the absolute cell length at a given position (Silk, 1992).
The average relative elongation rate in the elongation-only zone ( , mm mm−1 h−1) was calculated as:
, mm mm−1 h−1) was calculated as:
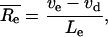 |
(4) |
where ve and vd are displacement velocity (mm h−1) at the end of the elongation-only zone and division zone, respectively, and Le is the elongation-only zone length (mm).
The spatial profiles of cell length, displacement velocity, and relative elongation rate were transformed in temporal profiles by calculating the trajectory function that describes the time it takes for a cell located at position x to be displaced to the end of the elongation-only zone (Silk et al., 1989):
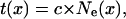 |
(5) |
where c, the cellochron (h cell−1), is the time required to displace a cell forward by one position in a cell file within the elongation-only zone and is equal to the inverse of cell flux, and Ne(x) is the number of cells present between position x and the distal limit of the elongation-only zone.
The average elongation duration (i.e. the residence time of a cell in the elongation-only zone; Te, h) was then calculated as:
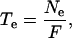 |
(6) |
where Ne is the total number of cells present in the elongation-only zone and F is the cell flux.
Analysis of Cell Division
The average division rate of cells in the meristem can be determined by relating the cell production rate to the number of cells per meristematic cell file in the division zone (Ivanov and Dubrovsky, 1997). This estimation assumes all cells in the meristem are proliferative, which is supported by studies showing that the proliferative fraction is close to 1 (Ivanov and Dubrovsky, 1997; Ivanov et al., 2002, and refs. therein). Also, the constancy of the division rate along the meristem has been shown (Beemster et al., 1996) and discussed (Baskin, 2000).
The average cell division rate (D, cell cell−1 h−1) was calculated as:
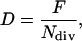 |
(7) |
where F is the cell production rate and Ndiv is the number of cells in a meristematic cell file in the division zone (Green, 1976; Ivanov and Dubrovsky, 1997).
The number of cells in a meristematic cell file (Ndiv) was directly counted from the basal end of the division zone (i.e. the ligule) to the position of the last recently formed perpendicular cell wall. Meristematic cell length was determined as the average cell length between the basal and the distal end of the cell division zone.
The average cell cycle duration (Tc, h), the time from a cell's formation to the next cytokinesis that yields two daughter cells, was calculated as follows (Green, 1976; Ivanov and Dubrovsky, 1997):
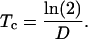 |
(8) |
The real residence time for an individual cell in the division zone is equal to Tc. However, it is possible to estimate the time needed for a perpendicular cell wall situated at the basal end of the division zone to reach the distal end of it. The residence time in the cell division zone (Tdiv, h) is then related to the number of division cycles necessary to form all cells in the division zone (Korn, 1993; Beemster and Baskin, 1998):
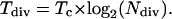 |
(9) |
The average number of division cycles of the progeny of a cell formed by the division of the initial cell at the base of the meristem (i.e. the number of division cycles necessary to displace a transversal cell wall from the basal to the distal boundary of the division zone) was determined as follows (González-Fernández et al., 1968):
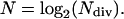 |
(10) |
Statistical Analysis
Differences between treatments were tested by Student's t test (Statistica 6.0; Statsoft). The error associated with parameters calculated from averages (e.g. cell production and average cell division rate) was estimated by Gaussian error propagation. Results are shown as means ± se.
Acknowledgments
The technical staff at Lehrstuhl für Grünlandlehre provided invaluable assistance, particularly Wolfgang Feneis, Anja Schmidt, and Angela Ernst-Schwärzli. We especially thank Milan Baláž (Department of Plant Physiology and Anatomy, Masaryk University, Brno, Czech Republic) and Stefan Raidl (Department Biology I, Systematic Botany and Mycology, Ludwig-Maximilians-Universität, Munich, Germany) for their hospitality and access to microscopes, and Tobias Baskin and an anonymous reviewer for valuable comments on a previous version of this manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 607).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hans Schnyder (schnyder@wzw.tum.de).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.079699.
References
- Assuero SG, Mollier A, Pellerin S (2004) The decrease in growth of phosphorus-deficient maize leaves is related to a lower cell production. Plant Cell Environ 27: 887–895 [Google Scholar]
- Barlow PW (1984) Positional controls in root development. In PW Barlow, DJ Carr, eds, Positional Controls in Plant Development. Cambridge University Press, Cambridge, UK, pp 281–318
- Baskin TI (2000) On the constancy of cell division rate in the root meristem. Plant Mol Biol 43: 545–554 [DOI] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI (1998) Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116: 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Masle J, Williamson RE, Farquhar GD (1996) Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L.): kinematic analysis of leaf elongation. J Exp Bot 47: 1663–1678 [Google Scholar]
- Bultynck L, Fiorani F, Van Volkenburgh E, Lambers H (2003) Epidermal cell division and cell elongation in two Aegilops species with contrasting leaf elongation rates. Funct Plant Biol 30: 425–432 [DOI] [PubMed] [Google Scholar]
- Cánovas JL, Cuadrado A, Escalera M, Navarrete MH (1990) The probability of G1 cells to enter into S increases with their size while S length decreases with cell enlargement in Allium cepa. Exp Cell Res 191: 163–170 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Herman EM, Chrispeels MJ (1998) Characterization of a maize tonoplast aquaporin expressed in zones of cell division and elongation. Plant Physiol 117: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiera J, Thomas J, Rufty T (2002) Leaf initiation and development in soybean under phosphorus stress. J Exp Bot 53: 473–481 [DOI] [PubMed] [Google Scholar]
- Cockcroft CE, den Boer BGW, Healy JMS, Murray JAH (2000) Cyclin D control of growth rate in plants. Nature 405: 575–579 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, Krols L, Terras P, Landrieu I, Van der Schueren E, Maes S, Naudts M, Inzé D (2001) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Joubès J, Inzé D (2003) Plant cell cycle transitions. Curr Opin Plant Biol 6: 536–543 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Lopez-Matas MA, Ramirez-Parra E, Gutierrez C (2005) Hormonal control of the plant cell cycle. Physiol Plant 123: 173–183 [Google Scholar]
- Dewitte W, Riou-Khamlichi C, Scofield S, Healy JMS, Jacqmard A, Kilby NJ, Murray JAH (2003) Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3. Plant Cell 15: 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner P, Jørgensen JE, You R, Steppuhn J, Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380: 520–523 [DOI] [PubMed] [Google Scholar]
- Downes BP, Steinbaker CR, Crowell DN (2001) Expression and processing of a hormonally regulated β-expansin from soybean. Plant Physiol 126: 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand JL, Schäufele R, Gastal F (1999) Grass leaf elongation rate as a function of developmental stage and temperature: morphological analysis and modelling. Ann Bot (Lond) 83: 577–588 [Google Scholar]
- Fagerberg WR (1984) Cytological changes in palisade cells of developing sunflower leaves. Protoplasma 119: 21–30 [Google Scholar]
- Fiorani F, Beemster GTS, Bultynck L, Lambers H (2000) Can meristematic activity determine variation in leaf size and elongation rate among four Poa species? A kinematic study. Plant Physiol 124: 845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W (2002) Biophysical limitation of cell elongation in cereal leaves. Ann Bot (Lond) 90: 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández A, López-Sáez JF, Moreno P, Giménez-Martin G (1968) A model for dynamics of cell division cycle in onion roots. Protoplasma 65: 263–276 [DOI] [PubMed] [Google Scholar]
- Green PB (1976) Growth and cell pattern formation on an axis: critique of concepts, terminology, and modes of study. Bot Gaz 137: 187–202 [Google Scholar]
- Hemerly A, de Almeida Engler J, Bergounioux C, Van Montagu M, Engler G, Inzé D, Ferreira P (1995) Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. EMBO J 14: 3925–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan JM, Wareing PF (1980) Cytokinins and the growth responses of seedlings of Betula pendula Roth. and Acer pseudoplatanus L. to nitrogen and phosphorus deficiency. J Exp Bot 31: 525–532 [Google Scholar]
- Ivanov VB, Dobrochaev AE, Baskin TI (2002) What the distribution of cell lengths in the root meristem does and does not reveal about cell division. J Plant Growth Regul 21: 60–67 [DOI] [PubMed] [Google Scholar]
- Ivanov VB, Dubrovsky JG (1997) Estimation of the cell-cycle duration in the root apical meristem: a model of linkage between cell-cycle duration, rate of cell production, and rate of root growth. Int J Plant Sci 158: 757–763 [Google Scholar]
- Kavanová M, Grimoldi AA, Lattanzi FA, Schnyder H (2006) Phosphorus nutrition and mycorrhiza effects on grass leaf growth. P status- and size-mediated effects on growth zone kinematics. Plant Cell Environ 29: 511–520 [DOI] [PubMed] [Google Scholar]
- Kemp DR (1980) The location and size of the extension zone of emerging wheat leaves. New Phytol 84: 729–737 [Google Scholar]
- Korn RW (1993) The geometry of elongating plant cells. Bull Math Biol 55: 345–364 [Google Scholar]
- Korn RW (2001) The geometry of proliferating dicot cells. Cell Prolif 34: 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Potuschak T, Colón-Carmona A, Gutiérrez RA, Doerner P (2005) Arabidopsis TCP20 links regulation of growth and cell division control pathways. Proc Natl Acad Sci USA 102: 12978–12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle J (2000) The effects of elevated CO2 concentrations on cell division rates, growth patterns, and blade anatomy in young wheat plants are modulated by factors related to leaf position, vernalization, and genotype. Plant Physiol 122: 1399–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AK, Silk WK (1992) Use of a flexible logistic function to describe axial growth of plants. B Math Biol 54: 1069–1081 [Google Scholar]
- Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98: 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JW, Eidenbock MP (1984) Hydraulic conductance as a factor limiting leaf expansion of phosphorus-deficient cotton plants. Plant Physiol 75: 372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Schnyder H, Seo S, Rademacher IF, Kühbauch W (1990) Spatial distribution of growth rates and of epidermal cell lengths in the elongation zone during leaf development in Lolium perenne L. Planta 181: 423–431 [DOI] [PubMed] [Google Scholar]
- Silk WK (1992) Steady form from changing cells. Int J Plant Sci 153: S49–S58 [Google Scholar]
- Silk WK, Erickson RO (1979) Kinematics of plant growth. J Theor Biol 76: 481–501 [DOI] [PubMed] [Google Scholar]
- Silk WK, Lord EM, Eckard KJ (1989) Growth patterns inferred from anatomical records—empirical tests using longisections of roots of Zea mays L. Plant Physiol 90: 708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticconi CA, Abel S (2004) Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci 9: 548–555 [DOI] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Volenec JJ, Nelson CJ (1981) Cell dynamics in leaf meristems of contrasting tall fescue genotypes. Crop Sci 21: 381–385 [Google Scholar]