Abstract
The function of a Gα protein in the elicitation of phytoalexin (benzophenanthridine) biosynthesis was characterized in cultured cells of California poppy (Eschscholzia californica). Both the decrease of Gα content via antisense transformation and the expression of recombinant anti-Gα single-chain antibodies strongly impaired the induction of alkaloid biosynthesis by low elicitor concentrations. All transgenic cell types were deficient in two elicitor-triggered early signal events: activation of phospholipase A2 (PLA2) and efflux of vacuolar protons. The lacking H+ efflux could be restored (1) by adding lysophosphatidylcholine (LPC), a product of PLA2 activity, to vacuoles in situ and (2) by exposing intact cells to isotonic, near-neutral HEPES buffers. The latter treatment induced alkaloid biosynthesis in the absence of elicitor and in Gα-deficient cells. We conclude that Gα mediates the stimulation of PLA2 by low elicitor concentrations and that the resulting peak of LPC initiates a transient efflux of vacuolar protons. In this way, an acidic peak of the cytoplasmic pH is generated that causes the expression of enzymes of phytoalexin production independent of the hypersensitive response.
INTRODUCTION
Several signal pathways of plant cells are known or supposed to involve the activity of heterotrimeric G proteins (for reviews, see Ma, 2001; Millner, 2001; Assmann, 2002, 2005; Jones, 2002; Jones and Assmann, 2004; Perfus-Barbeoch et al., 2004). The broadest evidence exists for essential roles of G proteins in growth and developmental processes, for example, cell cycle control (Ullah et al., 2001; Colucci et al., 2002; Apone et al., 2003), and the signal cascades initiated by auxin (Nato et al., 2000; Ullah et al., 2003), gibberellic acid (Ashikari et al., 1999; Ueguchi-Tanaka et al., 2000; Ullah et al., 2002; Iwasaki et al., 2003), and abscisic acid (Wang et al., 2001; Pandey and Assmann, 2004).
G proteins are also involved in the control of pathogen defense, but the accumulated evidence points to selective rather than analogous or ubiquitous signal functions. Early pharmacological data suggested a less-defined role of heterotrimeric G proteins in the generation of reactive oxygen by plasma membrane redox processes (in Beta vulgaris [Vera-Estrella et al., 1994] and in Glycine max [Rajasekhar et al., 1999]) and the overproduction of phytoalexins (in Eschscholzia californica [Roos et al., 1999] and in Daucus carota [Kurosaki et al., 2001]). By contrast, Gα-deficient rice (Oryza sativa) mutants were not impaired in the generation of reactive oxygen, medium alkalinization, and expression of early genes of phytoalexin production (Tsukada et al., 2002). Actual data indicate that distinct elements of the hypersensitive response are selectively controlled by Gα or Gβ proteins. In Arabidopsis thaliana, null mutants of the respective genes were used to show that the first peak of the oxidative burst, arising mainly from chloroplasts, requires signaling through the heterotrimer or the Gβγ complex, whereas the late peak reflecting the activation of membrane-bound NADPH oxidases requires solely Gα. This subunit is therefore involved in intercellular signaling and cell death (Joo et al., 2005).
The variety of signaling pathways that require G protein activities contrasts with the presence of only one canonical Gα and Gβ subunit, respectively, in the Arabidopsis and rice proteomes, each encoded by a single-copy gene (e.g., Ma, 2001; Assmann, 2002; Jones, 2002; Kato et al., 2004). Even if two γ subunits are present in rice and Arabidopsis, there is no doubt that plants establish the selectivity of G protein–dependent signal transfers in a way different from animal cells, which contain the expected multitude of α, β, and γ subunits (23, 6, and 12, respectively, in the human genome; Jones, 2002). Up to now, only a few plant targets are known to be directly controlled by heterotrimeric G proteins, including ion influx channels for potassium (Wang et al., 2001) and calcium (Aharon et al., 1998) and phospholipase D (PLD) (Ritchie and Gilroy, 2000; Lein and Saalbach, 2001; Zhao and Wang, 2004). New studies suggest a role for Gα or the Gβγ complex in modulating the sensitivity of hormone-triggered signal cascades without being directly involved in the signal transduction sequence. This might explain their conditioning effect at greater areas of growth and development (Ullah et al., 2001, 2003; Assmann, 2004). To gain a broader understanding of the multitude of plant G protein functions, not only more of their molecular targets need to be uncovered but also their functional specificity deserves attention (i.e., the dynamics, signatures, and downstream effects of second messengers that arise from a distinct G protein–target interaction). Here, we provide an example of how the G protein control of phospholipase A2 (PLA2) indirectly gives rise to transient fluxes of intracellular protons and in this way initiates a signal path toward phytoalexin biosynthesis.
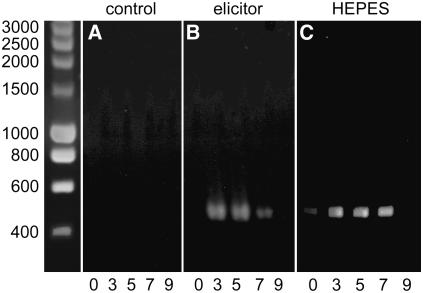
Cultured cells of the California poppy (E. californica) react to a yeast glycoprotein elicitor with the overproduction of benzophenanthridine alkaloids (Schumacher et al., 1987; Blechert et al., 1995; Roos et al., 1998, 1999). The elicitor-triggered alkaloid formation is known to reflect the induction of biosynthetic enzymes and thus is a convenient indicator of gene activation (Haider et al., 2000). This was demonstrated, for example, with the berberine bridge enzyme (BBE), which catalyzes the completion of the berberine ring system, a key reaction of benzophenanthridine biosynthesis. Transcriptional activation of the BBE gene by high elicitor concentrations (250 μg/mL of yeast glycoprotein preparation) has been shown as a time-dependent increase of the gene-specific poly(A)+ RNA prior to alkaloid production (Dittrich and Kutchan, 1991). Here, we demonstrate by RT-PCR the increase of the BBE transcript following contact with low elicitor concentrations (1 μg/mL) or treatment with external buffers that impose distinct shifts of the intracellular pH (see below). Under such conditions, the cellular content of BBE-mRNA peaks between 3 and 5 h (Figure 1). The increase of alkaloid content starts off between 8 and 10 h after elicitor contact (Färber et al., 2003).
Figure 1.
Expression Analysis of the BBE mRNA by RT-PCR.
DNA agarose electrophoresis showing a 456-bp fragment of the BBE gene as amplified from RNA of wild-type cells by RT-PCR (see Methods). The RNA was extracted at 0, 3, 5, 7, or 9 h after a 30-min treatment with 1-μg/mL elicitor (B) or 60 mM HEPES buffer, pH 7.4 (C). Nonstimulated cells are shown in (A). Size calibration of DNA fragments is at left (SmartLadder; Eurogentec). Data from one typical experiment are displayed. The result was confirmed by two repetitions. Similar experiments were performed with RNA from the transformed cell lines TG11, TG14 (antisense Gα), TGA6b, or TGA6e (anti-Gα-scFv). In these cultures, no elicitor-triggered increase of BBE mRNA could be detected. Because most transformants, like nonstimulated wild-type cultures, produce small amounts of alkaloids, the levels of BBE mRNA in these cells are supposed to stay below the detection limit.
Prior to gene activation, elicitor contact triggers two activities with potential signal character: (1) the activation of PLA2 as detected both in intact cells and isolated plasma membranes by the hydrolysis of an artificial fluorogenic phospholipid or by the transient accumulation of lysophosphatidylcholine (LPC), a hydrolysis product of genuine phospholipids (Roos et al., 1999; Viehweger et al., 2002); and (2) a transient efflux of vacuolar protons that gives rise to cytoplasmic acidification. These pH shifts have been visualized in intact cells by confocal pH topography (Roos et al., 1998; Färber et al., 2003). Similar proton fluxes can be triggered by adding ∼1 μM LPC to vacuoles in situ (i.e., cells with permeabilized plasma membrane). Most probably the effect of LPC reflects the activation of vacuolar Na+/H+ antiporters, as indicated by its Na+ dependence and inhibition by amiloride (Viehweger et al., 2002).
The question arose as to whether these reactions to the glycoprotein elicitor are causally linked and represent intermediary signals required for the induction of benzophenanthridine alkaloid biosynthesis. A clue for our experimental strategy was provided by earlier findings suggesting the involvement of a G protein in the control of phospholipase activity and pH shifts: Gα and Gβ subunits were detected immunologically in plasma membrane preparations that displayed an elicitor-dependent binding of GTPγS; in intact cells, both phospholipase A and cytoplasmic acidification were stimulated by <5 μM mastoparan (Roos et al., 1999). In order to substantiate and characterize the role of a heterotrimeric G protein in the early signal transduction, we attempted to reduce both the amount and the functionality of the Gα subunit. Cell lines were established that expressed either antisense Gα mRNA or anti-Gα short-chain antibodies. This study reports the consequences of these transformations that confirmed a tight connection between the stimulation of PLA2 and a pH-dependent signal path.
RESULTS
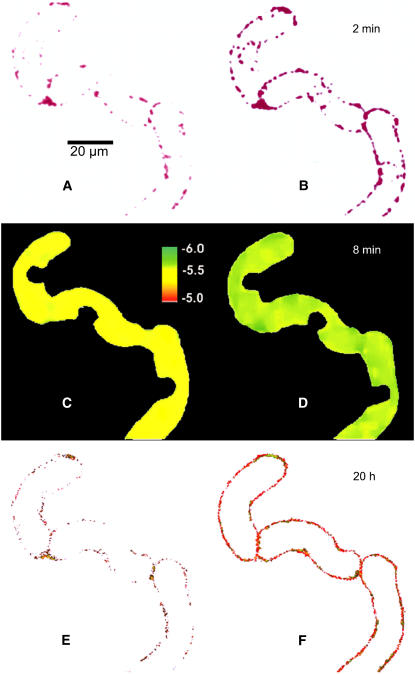
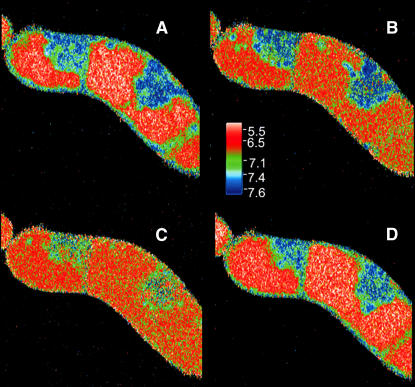
Figure 2 provides a synopsis of elicitor-triggered activities that can be visualized in cultured cells of E. californica: (1) activation of PLA2, (2) efflux of vacuolar protons, and (3) overproduction of benzophenanthridine alkaloids. In a 6-d culture, elicitor-triggered alkaloid formation was seen in approximately two-thirds of the cells. The majority of them displayed the activation of PLA2, loss of vacuolar protons, and excretion of benzophenanthridines as consecutive events in the same cell after contact with 1 μg/mL yeast elicitor. The increase of PLA2 activity prior to the pH shift is compatible with the generation by PLA2 of LPC and its subsequent functioning as a trigger of vacuolar proton efflux as suggested from previous in situ experiments (Viehweger et al., 2002).
Figure 2.
Increase of PLA2 Activity, Vacuolar pH Shifts, and Alkaloid Production in the Same Cell after Elicitor Contact.
Confocal images scanned immediately after addition of 1 μg/mL of yeast elicitor (t = 0, left) and after the times indicated (right).
(A) and (B) Stimulation of PLA2 visualized as the elicitor-triggered increase of fluorescence during incubation with the artificial substrate bis-BODIPY-FL-C11-phosphatidylcholine (BPC). For better visibility, the original yellow-green fluorescence was converted into magenta.
(C) and (D) pH maps of the vacuolar areas (ratio images of DM-NERF fluorescence).
(E) and (F) Fluorescence of benzophenanthridines at emission of 580 to 630 nm. For better visibility, the fluorescence of DM-NERF (emission 535 to 555 nm) that resided in the vacuoles was subtracted. The series is a typical example out of 89 images of alkaloid-producing cells that were examined from different culture batches. For details, see Methods.
Expression of Gα Is Suppressed by Antisense Transformation
To suppress the production of Gα, callus cultures of E. californica were transformed biolistically by a plasmid DNA that contained an 893-bp sequence of the E. californica Gα gene in antisense orientation together with the neomycin phosphotransferase gene and the 35S promoter. Selection for paromomycin-resistant growth resulted in several stable cell lines that all showed reduced growth and alkaloid production (cf. below).
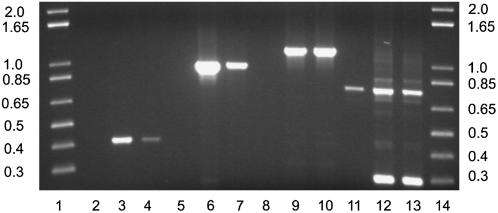
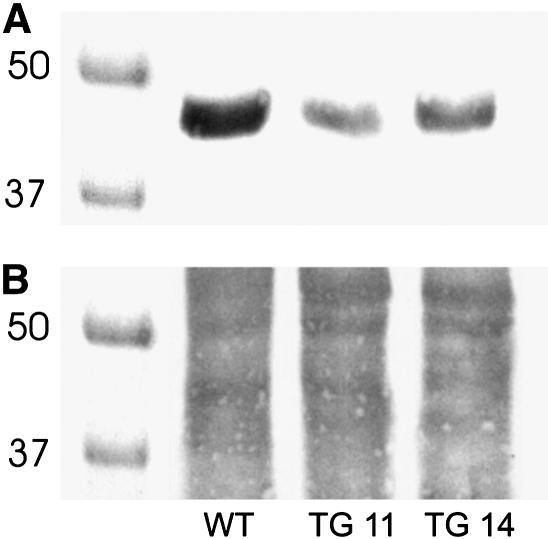
The integration into the Eschscholzia genome of the antisense Gα construct was demonstrated by stringent PCR with gene-specific oligonucleotide primers that were constructed to align either with sequences of the Gα gene (i.e., in both antisense and sense directions) as well as with Gα sequences, plus flanking regions of the promoter or terminator, respectively (Figure 3, Table 1). In the latter cases, PCR products of the predicted lengths were obtained that were not detectable in the wild type. At the protein level, protein gel blotting revealed a significant reduction in the amount of Gα in the TG11 and TG14 strains (Figure 4). Thus, the cultured cells were effectively transformed with the antisense Gα gene and displayed a severe yet not complete suppression of Gα formation.
Figure 3.
PCR Reactions to Confirm Antisense Gα Transformation of E. californica Cells.
DNA agarose electrophoresis of PCR products. Lanes 1 and 14 contain marker DNA (1-kb plus DNA ladder; Gibco BRL). The lengths of expected and found PCR products are specified in Table 1. This experiment was done with DNA extracted from the cell line TG11. DNA from the cell line TG14 yielded the same results.
Table 1.
Expected and Observed PCR Products from Figure 3
| Oligonucleotide Combination | Wild-Type DNA | Antisense Gα DNA | ||||
|---|---|---|---|---|---|---|
| Expected (Position) | Observed | Lane No. | Expected (Position) | Observed | Lane Nos. | |
| 35S/EcGA4 | – | – | 2 | 418 (429–847) | 418 | 3 and 4 |
| 35S/EcGA3 | – | – | 5 | 972 (429–1401) | 972 | 6 and 7 |
| 35S/OCS | – | – | 8 | 1167 (429–1596) | 1167 | 9 and 10 |
| EcGA1/EcGA3 | Genomic DNA (intron) | 800 | 11 | 301 (1100–1401) | 301 and 800 | 12 and 13 |
Figure 4.
Immunological Detection of Gα in the Plasma Membrane.
Proteins were extracted from isolated plasma membranes with SDS, separated by SDS-PAGE, blotted onto nitrocellulose membranes, and probed with anti-Gα antibodies as described previously (Roos et al., 1999). Gα bands obtained from wild-type and antisense Gα lines TG11 and TG14 are shown in (A). Each lane received similar amounts of solubilized protein as demonstrated by fast green protein staining of the same blot (B). The relative content of Gα in relation to the wild type was estimated at 15% in TG11 and at 24% in TG14 cells, based on a densitometric evaluation of the digitized spots.
Anti-Gα-scFv Antibodies Can Be Stably Expressed in E. californica Cells
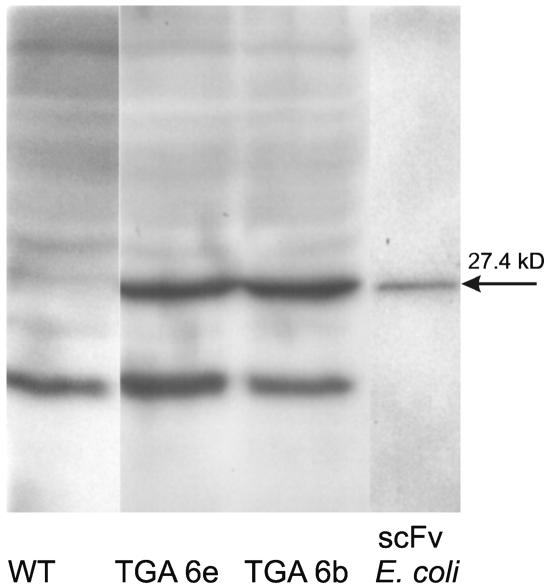
In an alternative approach, the deactivation of Gα by an endogenously produced single-chain antibody (scFv fragment) was attempted. Expression of antibody fragments has been successfully used to modulate the function of low molecular weight regulators and a few regulatory proteins in plants (Artsaenko et al., 1999; Conrad and Manteuffel, 2001) but was not yet applied to G proteins. In our experiments, eight different scFv antibodies with affinity to a conserved 300-bp sequence of the E. californica Gα protein were selected from a phage library of human IgG sequences and cloned in bacteria. The scFv with the highest Gα binding affinity was then expressed in E. californica cells (see Methods). Five cell lines were obtained that showed a stable expression of the antibody (Figure 5) and a near-normal growth rate. The Gα binding affinity of the purified plant-made antibody, assayed by ELISA (see Methods), was ∼16 nM compared with 6 nM of the scFv protein produced in the bacteria used for cloning.
Figure 5.
Immunological Detection of Anti-Gα-scFv Antibodies.
The proteins of the 90,000g supernatant obtained during cell fractionation (Roos et al., 1999) were separated by SDS-PAGE, blotted onto nitrocellulose membranes, and probed with anti-cMyc antibodies (Santa Cruz Biotechnology). TGA6a and TGA6b, cell lines transformed by anti-Gα-scFv-cMyc DNA; rightmost lane, protein extract of the transformed Escherichia coli strain HB 2151 used in the production of anti-Gα-scFv-cMyc antibody (see Methods). The scFv-cMyc band is of the predicted size (27.4 kD); the lower band represents an unknown cross-reacting plant protein. Prior to analysis, all samples were assayed by the Bradford method and diluted to equal protein contents.
Antisense Gα- and Anti-Gα-scFv Cells Are Deficient in the Elicitor Activation of PLA2
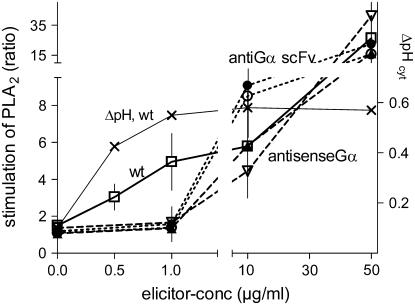
Antisense Gα- and anti-Gα-scFv transformants displayed a common deficiency: the activity of PLA2 was little or not stimulated by low elicitor concentrations, whereas the response to high elicitor concentrations remained similar to the wild type (Figure 6). The basal, nonelicitable activity of PLA2 was not significantly diminished. This indicated that (1) low but not high elicitor concentrations require Gα to activate PLA2, and (2) the total activity of PLA2 that is responsive to elicitor stimulation was not significantly impaired by the transformation procedure.
Figure 6.
PLA2 Activity of Wild-Type and Transformed Cell Suspensions.
Suspensions of wild-type and transformed cell lines were incubated with the fluorogenic substrate BPC and yeast elicitor at the indicated concentrations in microplate compartments (see Methods). The fluorescence development resulting from PLA2 activity is averaged from three experiments with different culture batches, each comprising 48 minisuspensions. Data are fold increase, expressed as the ratio of the PLA2 activity of elicitor–to–elicitor-free cell suspensions. Open triangles, antisense Gα line TG11; closed triangles, antisense Gα line TG14; open circles, anti-Gα-scFv line TGA6b; closed circles, anti-Gα-scFv line TGA6e; exes, elicitor-triggered pH shifts. Data are taken in part from Roos et al. (1998).
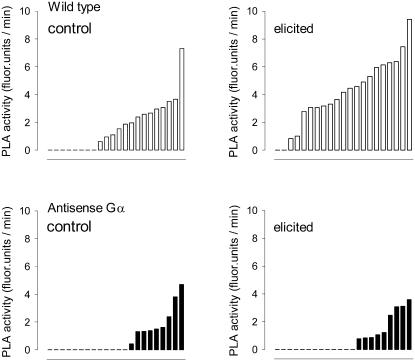
Since two different modes of attacking Gα, the reduction of the available Gα protein (Figure 4) and the presence of an additional Gα binding protein (Figure 5), resulted in a similar malfunction, it is likely that PLA2 is a target of control by the Gα protein. In order to corroborate the above data obtained with minisuspensions in a microplate (see Methods), the elicitor-triggered activation of PLA2 was also assayed by confocal microscopy of single cells. In the analysis shown in Figure 7, the antisense Gα cell line TG11 was compared with the wild type. The data confirm the lack of elicitor-triggered activation of PLA2 in antisense Gα cells: neither the number of responding cells nor the enzyme activity per cell is enhanced by elicitor contact, while both parameters increase in the wild type.
Figure 7.
Activity of PLA2 in Single Cells of Elicited and Nonelicited Cultures.
PLA2 activities of individual cells that were randomly selected from a wild type or an antisense Gα cell suspension (strain TG11) were measured by confocal microscopy assay (see Methods). Data are sorted by magnitude; each bar refers to one cell. Positions with y = 0 represent cells with nondetectable enzyme activity. The experiment was repeated twice (once in the same and once in another culture batch) and yielded a similar relationship between elicited and nonstimulated cells.
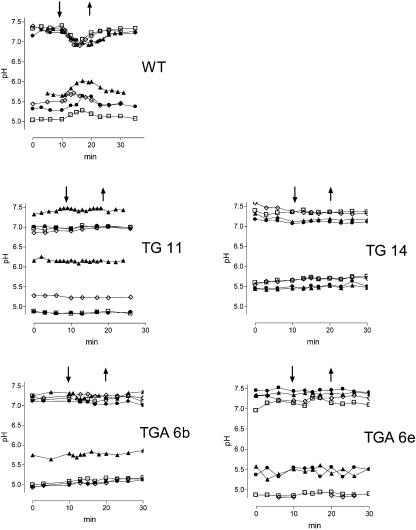
Antisense Gα- and Anti-Gα-scFv Cells Lack Elicitor-Triggered Fluxes of Vacuolar Protons
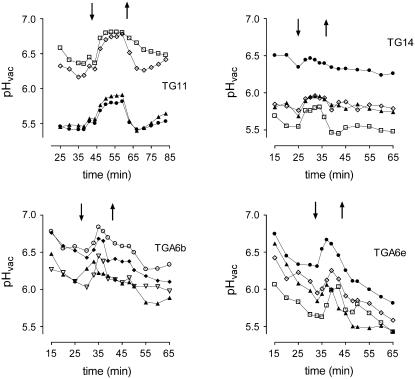
As outlined in the Introduction and shown in Figure 2, elicitor contact not only activates PLA2 but also triggers an efflux of vacuolar protons. It is already known that this response saturates at low elicitor concentrations (Roos et al., 1998) and thus contrasts with the activation of PLA2 shown in Figure 6. We have found earlier that elicitor-triggered fluxes of vacuolar H+ can be mimicked by adding LPC, a product of PLA2 activity, to vacuoles in situ (Viehweger et al., 2002). If this mechanism is relevant for the control of vacuolar proton fluxes in intact cells, the lacking elicitor activation of PLA2 in either antisense Gα or anti-Gα-scFv cells should be accompanied by a lack of elicitor-triggered proton fluxes. To prove this expectation, each two of the antisense Gα- and the anti-Gα-scFv strains were tested in detail by confocal pH mapping (Figure 8). Neither antisense Gα nor anti-Gα-scFv transformants showed an elicitor-triggered increase of vacuolar pH or an acidification of the cyctoplasm that is typical of the wild type. This result, along with the lack of PLA2 stimulation, suggests a tight connection between both activities, although it does not yet indicate a causal link.
Figure 8.
Time Course of Vacuolar and Cytoplasmic pH in Wild-Type, Antisense Gα, and Anti-Gα-scFv Cells.
pH traces are shown of cells in a flow chamber during perfusion with 75% phosphate-free culture liquid. The pH traces were obtained by confocal pH mapping (ratio imaging with the probe carboxy-seminaphthorhodafluor-4F [SNARF-4F]). In the time period indicated by the arrows, 1 μg/mL of yeast elicitor was present in the perfusion medium. Each trace represents the pH of a cytoplasmic/nuclear area (top curves) or the corresponding central vacuole (bottom curves, same symbols). TG11 and TG14, antisense Gα cell lines; TGA6b and TGA6e, cell lines expressing anti-Gα-scFv antibodies. Each experiment was repeated twice with the indicated cell strain and yielded similar pH traces.
LPC Compensates for the Lack of Elicitor Activation of Vacuolar Proton Fluxes
In order to ascertain whether the lack of elicitor activation of PLA2 caused the lack of pH shifts, a gain-of-function experiment was performed. Proton fluxes at the vacuole of E. californica can be studied in situ (i.e., after the selective permeabilization of the plasma membrane). Using this technique, we monitored vacuolar proton fluxes in response to LPC. This compound is liberated from phosphatidylcholine by an active PLA2 and peaks in intact cells after contact with low elicitor concentrations (Viehweger et al., 2002).
As shown in Figure 9, the vacuoles of the transgenic cell lines, despite some kinetic variations, are able to react to LPC by a loss of protons, similar to the wild type (cf. Figure 7A in Viehweger et al., 2002). Thus, neither the antisense suppression of Gα nor the expression of anti-Gα-scFv antibodies severely impaired the activating effect of LPC on the vacuolar proton transport. This indicates that the lack of LPC, due, in turn, to the lack of PLA2 activation, was the cause of the missing pH shifts in the antisense Gα strain.
Figure 9.
Efflux of Vacuolar Protons Triggered by LPC in Situ.
Each trace represents the pH of an individual vacuole (pHvac), measured in situ by classic fluorescence microscopy (ratio imaging with 5-carboxyfluorescein according to Viehweger et al., 2002). The perfusion medium contained 1 mM Mg ATP; during the period indicated by arrows, 1 μM LPC (16:0) was also present. Cells with vacuoles of differing initial pH were included in this figure to demonstrate that the efflux triggered by LPC does not depend on the vacuolar pH at the time LPC is added, but rather on the pH gradient across the tonoplast. The vacuolar pH of intact cells ranges from 4.3 to 6.5. TG11 and TG14, antisense Gα cell lines; TGA6b and TGA6e, cell lines expressing anti-Gα-scFv antibodies.
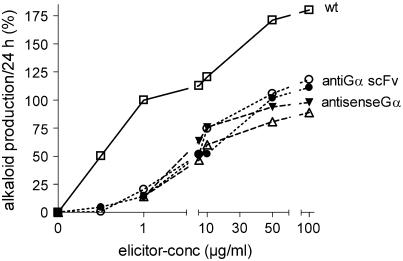
Antisense Gα and Anti-Gα-scFv Cells Show a Common Deficiency in the Alkaloid Response
The elicitor-triggered alkaloid responses of both antisense Gα and anti-Gα-scFv strains did not reach the level of the wild type. When the reactivity of the cells was titrated with a broad range of elicitor concentrations, it became obvious that the difference was mostly due to lack of response of all transformants to low elicitor concentrations (Figure 10). At 1 μg/mL, the elicitor triggered 10 to 20% of the wild type response; at 50 μg/mL, this percentage was 47 to 60% and saturated at this level (up to 400 μg/mL elicitor was tested). Thus, the transgenic cell lines are commonly impaired not only in the early responses, as PLA2 activation (see Figure 6) and elicitation of vacuolar proton efflux (see Figure 9), but also lack the alkaloid response to low elicitor concentrations. The latter deficiency is accompanied by the loss of elicitor-activated transcription of the BBE gene, as has been shown in RT-PCR analyses like that of Figure 1.
Figure 10.
Alkaloid Responses Triggered by Different Elicitor Concentrations.
The increase in alkaloid content of a cell suspension during 24 h after a 30-min contact with elicitor is compiled for the wild type (7-d culture), the antisense Gα cell lines TG11 (closed triangles) and TG14 (open triangles) (10-d cultures), and the anti-Gα-scFv cell lines TGA6b (open circles) and TGA6e (closed circles) (5-d cultures). Experiments were done at the culture age that usually allowed the highest alkaloid response of either cell line. A typical experiment is shown that was repeated three times with different culture batches (four times with TG11) and yielded essentially similar ratios of response to low and high elicitor concentrations. To address the variability of alkaloid response between different culture batches, data are normalized to the average alkaloid response of wild-type cells evoked by 1 μg/mL of elicitor that was estimated from >20 experiments and set to 100%.
Shifts of Cytoplasmic pH Can Partially Restore the Alkaloid Response of Antisense Gα Cells
The previous data have shown that the deficiencies of both antisense Gα and anti-Gα-scFv cell lines coincide in the lack of elicitor-triggered proton fluxes, most probably as a result of the lack of PLA2 activation (Figures 8 and 9). This result encouraged experiments aimed to generate controlled shifts of the cytoplasmic pH that are able to mimic the inducing effect of elicitor treatment and to compensate the impaired elicitation of alkaloid biosynthesis in Gα-deficient cells.
The generation of pH shifts that resemble the elicitor-triggered acidification of the cytoplasm was attempted by several procedures. Administration of LPC (1 to 20 μM) to intact cells caused some detectable efflux of vacuolar protons and alkaloid production. Both effects were, however, not easily reproducible and often were much less efficient than low elicitor treatment. This was not totally unexpected since intact cells can rapidly metabolize externally added LPC (Viehweger et al., 2002).
Incubation with permeant acids (butyric and pivalic) caused a rapid decrease of cytosolic pH to 6.0 to 5.2. However, this shift was not completely reversible even after thorough optimization of external concentration and pH and extended perfusion with culture medium or neutral buffers. Alkaloid production after such treatments was clearly elevated but again did not reach the level of low-elicitor-treated cultures (Roos et al., 1998).
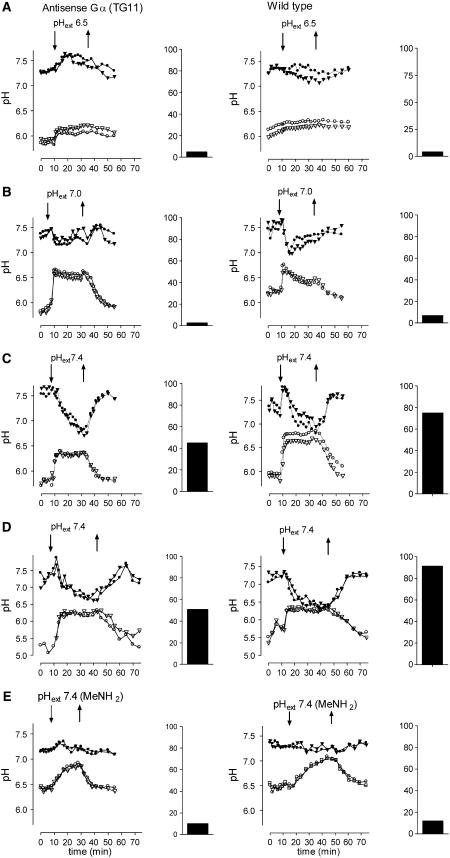
Controlled intracellular proton movements could be finally achieved by a transient exposure of cells to isotonic culture media containing HEPES, BTP, or MES buffers of ∼60 mM at a near-neutral pH (Figure 11). This method exploits the ability of many plant cells to counteract an increase of the apoplastic pH beyond its (usually acidic) optimum by the export of protons (Brauer et al., 1997; Armero and Tena, 2001). Our pH traces indicate that this export requires the supply of H+ from the vacuolar pool and includes the transient acidification of the cytoplasm. Under the above conditions, setting the external pH to 7.4 caused a strong acidification of the cytoplasm that at maximum resulted in a pH of ∼6.3. This process was paralleled by an efflux of vacuolar protons. Upon replacement of the external buffer by fresh culture medium (pH 5.6), both the vacuolar and cytoplasmic pH returned close to their original levels (Figure 11). The imposed changes of cytoplasmic and vacuolar pH strongly depended on the pH of the external buffer and the time of exposure. This allowed the establishment of distinct signatures of intracellular pH. Figures 12A to 12D show typical traces of pHcyt and pHvac generated by transiently exposing cells to external HEPES buffers of pH 6.5, 7.0, and 7.4.
Figure 11.
Proton Fluxes in Antisense Gα Cells Provoked by Extracellular HEPES Buffer.
Confocal pH maps are presented as scanned by confocal ratio imaging with SNARF-4F. Cells were fixed in a microscopic flow chamber that was perfused with the following media: 75% phosphate-free culture liquid ([A]; this pH distribution remained fairly constant up to 1 h); 2 and 30 min, respectively, after replacement with 60 mM K-HEPES, pH 7.4, in 50% phosphate-free culture liquid ([B] and [C]); 10 min after a further replacement with 75% phosphate-free culture liquid (D).
Figure 12.
Intracellular Proton Fluxes and Subsequent Alkaloid Production Caused by External Buffers.
The effect of buffer treatment on vacuolar and cytoplasmic pH was assayed by confocal pH mapping as exemplified in Figure 11. The resulting pH traces are shown alongside percentage of alkaloid production elicited by the same buffer treatment in batch cultures. For pH traces, cell suspensions in a 140-μL cell chamber were perfused with 75% phosphate-free culture liquid, pH 6.5. For the time periods indicated by arrows, it was replaced by 50% phosphate-free nutrient solution containing 60 mM HEPES buffer ([A] to [D]) or 60 mM methylamine (E) of the specified pH. Cells in (C) and (D) were treated wit the same buffer, pH 7.4, over different time periods. Confocal pH maps were obtained at 2-min intervals. pH traces representing two cytosolic areas (top curves) and the corresponding vacuoles (bottom curves) are shown for each experiment. Data from typical experiments were selected; repeated pH mapping with cells of different batches yielded similar pH traces. The bar graphs show alkaloid production per 24 h of 10-mL cell suspensions (4 mg dry weight/mL) that had received the same buffer treatment as specified with the pH traces (exchange of media occurred by no-suction filtration on a nylon mesh). Alkaloid content was assayed 24 h after buffer treatment. Data were normalized to the average alkaloid response of wild-type cells evoked by 1 μg/mL of elicitor that was set to 100% as explained in Figure 10. Typical experiments are compiled. Repetitions with cells of the same and different batches (three times each) yielded alkaloid responses (at an external pH [pHext] of 7.4) between 40 and 60% (TG11) and 65 and 110% (wild type).
Cells of the antisense Gα strain TG11 and of the wild type reacted with similarly shaped pH traces in the vacuolar and the cytoplasmic compartment. Thus, the lack of Gα did not significantly impair the underlying mechanisms of pH regulation. As the pH shifts could be evoked in the great majority of the examined cells (close to 100%) irrespective of culture age, the measured intracellular pH signature must have been observed in all elicitor-responding cells (∼66%). Consequently, we compared buffer treatments that caused different profiles of cytoplasmic acidification for their ability to mimic the alkaloid-inducing effect of the elicitor in both wild-type and antisense Gα cell suspensions. pH traces and related alkaloid responses of a typical set of experiments are compiled in Figure 12. It turns out that pHcyt is required to stay below 7.1 for at least 10 min in order to evoke a clear alkaloid response (Figures 12C and 12D). The activation of the BBE gene after such treatment was corroborated via RT-PCR by showing a transient accumulation of the specific transcript (3 to 7 h after buffer treatment; see Figure 1). This exemplifies again that alkaloid overproduction indicates enhanced transcription of biosynthetic genes.
The maximum alkaloid production that can be triggered by artificial pH shifts is ∼90% in the wild type and 50% in the antisense Gα strain TG11, each related to the alkaloid response of wild-type cells to low elicitor treatment. Thus, at least a significant part of the alkaloid response that is lacking in Gα-deficient cells could be compensated for by artificially triggered pH shifts. As discussed below, the incomplete reconstitution of the wild type reactivity likely reflects the involvement of Gα-dependent growth and developmental processes in the expression of alkaloid biosynthesis.
The percentage of wild-type cells that did not respond by alkaloid production to the imposed pH regime was roughly similar to the portion of cells that were nonreactive to the elicitor. Although it cannot easily be proven that the same cells are reactive to both elicitor and pH treatment, this is likely the case, as the alkaloid response of the culture to external buffers, pH 7.4, or to 1 μg/mL of elicitor showed similar periods of competence (6 to 7 d of culture) and similar magnitudes.
The method used here also allowed a change in vacuolar pH, independent of the cytosolic pH. The nonmetabolizable, permeant base methylamine rapidly accumulates in plant vacuoles and thus equilibrates external and vacuolar pH without severely changing the cytoplasmic H+ concentration (Brauer et al., 1997; Roos et al., 1998). As expected, replacement of the HEPES buffers by isotonic methylamine solutions of the same pH caused the vacuolar pH to increase, whereas the cytoplasmic pH remained fairly constant. Under such conditions, no alkaloid production was induced (Figure 12E). This underscores the importance of cytoplasmic acidification, rather than the mere loss of vacuolar protons, for the induction of alkaloid biosynthesis.
DISCUSSION
The suppression of Gα at the levels of either protein amount (antisense approach) or protein function (antibody approach) at the same time impaired two potential signal steps and a distinct cellular response to the yeast glycoprotein elicitor, respectively: stimulation of PLA2 activity, transient fluxes of vacuolar protons into the cytoplasm, and induction of a significant part of the alkaloid biosynthetic capacity. The release of vacuolar protons, a typical reaction in elicitor-treated wild-type cells, disappeared concomitantly with the activation of PLA2. It was restored by allowing vacuolar contact with LPC, a product of this enzyme activity, irrespective of the amount or activity of Gα (Figure 9). This tight coherence, together with the gene-activating potential of distinct cytoplasmic pH shifts (Figures 1 and 12), suggests a causal sequence: elicitor activation of Gα, followed by stimulation of PLA2 and release of LPC, followed by LPC-triggered efflux of vacuolar protons. This sequence results in a transient decrease in cytoplasmic pH yielding a signature that is sufficient for the induction of alkaloid biosynthesis. We discuss several lines of evidence supporting this causal sequence.
PLA2: A Target of Control by Gα
There is little doubt that PLA2 requires Gα for its control by low elicitor concentrations. The lack of stimulation of this enzyme in Gα-deficient cells is not only seen by the activity assay with an artificial substrate (Figures 6 and 7). It also emerges indirectly from the lack of vacuolar pH shifts in the transformed cells (Figure 8), although they have functional, LPC-sensitive tonoplast H+ transporters (Figure 9), thus indicating the absence of the elicitor-triggered LPC peak known from the wild type (Viehweger et al., 2002).
The precise role that Gα plays in the activation of PLA2 remains a goal of further work. The actual data would be consistent either with a direct interaction of both proteins at the plasma membrane or a modulating influence of Gα at the elicitor-initiated (and probably GPCR-initiated) activation of PLA2. Both alternatives appear to be realized in plants. First, a well-evidenced physical interaction exists between Gα and PLD. Lein and Saalbach (2001) found an inhibition of recombinant PLD activity by adding Gα to the enzyme assay. Zhao and Wang (2004) demonstrated the site-specific binding of Gα (glutathione S-transferase fusion protein) to the recombinant PLD protein (isoform 1) by pull-down experiments. This binding resulted in an inhibition of PLDα1 and could be relieved by GTP. PLD and its product phosphatidic acid, via their interaction with distinct protein kinases and phosphatases, play integrating roles in the response to abscisic acid and oxidative stress (Zhang et al., 2005). Another target that physically interacts with Gα was just identified as a cytosolic prephenate dehydratase protein, functioning in the blue light--activated synthesis of Phe (Warpeha et al., 2006). A direct interaction of Gα with PLA2 in our E. californica cells would be consistent with actual immunoprecipitation data. An antibody raised against the whole recombinant protein coprecipitated Gα together with a PLA2, whereas an antibody raised against the effector coupling site of Gα precipitated this protein without PLA2 activity (J. Steighardt and W. Roos, unpublished data). However, the nature of the coprecipitated isoform of PLA2 and the specificity of binding to Gα are yet to be characterized.
The second alternative (i.e., Gα acting as a modulator rather than an immediate signal-transducing component) would be analogous to the role that G proteins play in the response of Arabidopsis to several hormones. Mutant studies suggest that Gα potentiates the effects of gibberellic acid on seed germination, most probably in concert with brassinosteroids (Ullah et al., 2002). The Gβγ complex appears to attenuate the auxin-triggered cell division and in this way influences the development of root primordia. This effect is counteracted by Gα, most probably via the sequestration of Gβγ (Ullah et al., 2003).
Although not central to this study, there are indications that the proliferation of the cultured E. californica cells requires functional Gα as well. All transgenic cell lines showed some growth retardation. The time (days) required to double the cell dry mass was as follows: wild type, 6 d; antisense Gα strains TG11, 11 d, and TG14, 10 d; anti-Gα-scFv–expressing lines TGA6b and TGA6e, each 7 d. The expression of alkaloid biosynthesis and elicitor-triggered signaling appears to be embedded into the developmental program of the cultured cells. This is indicated by the existence of a period of competence within the cultivation cycle that allows a maximum alkaloid response to the elicitor (1 μg/mL) that is seen at 6 to 7 d of culture in the wild type. We assume that the requirement for Gα in growth and developmental processes of the cultured cells is the main reason why artificial pH shifts do not completely reconstitute the alkaloid response of antisense G strains (Figures 12C and 12D, left).
The Cytoplasmic pH Signature: An Intermediate Signal toward Gene Expression
The Gα-mediated activation of PLA2 is conveyed to a transient shift of cytoplasmic pH that in turn functions as an inducing signal for alkaloid biosynthesis. This is indicated by the parallel lack of PLA2 activation and pH shifts in different Gα-deficient cell lines (see Figures 6 to 8), together with the pH shift–generating effect of LPC at vacuoles in situ (Figure 9; Viehweger et al., 2002).
pH traces of shape and magnitude that resemble those seen in elicitor-treated wild-type cells (cf. Figures 12C and 12D with Figure 5 of Roos et al., 1998) or LPC-treated in situ vacuoles (Figure 9) are generated by exposing cells to near-neutral external buffers (i.e., under conditions that mimic the stimulating effect of low elicitor treatment on gene expression [Figure 1] and alkaloid biosynthesis). Moreover, the imposed, transient acidification of the cytoplasm compensates not only for the absence of elicitor but also (to a significant degree) for the deficiency of Gα (Figure 12).
Signal sequences that connect or combine G proteins with regulatory pH shifts are not yet known from plant cells, although both elements are involved, for example, in the complex signaling networks initiated by some plant hormones, such as auxin (Hooley, 1998; Chen, 2001), abscisic acid (Grill and Himmelbach, 1998; Wang et al., 2001), and gibberellins (Gehring et al., 1994; Hooley, 1998; Ashikari et al., 1999). As an example, alkaline shifts of the cytoplasmic pH and G protein (GTP binding) activity are both initiated by abscisic acid. However, silencing the gene encoding Gα in Arabidopsis guard cells did not affect the cytosolic alkalinization, although it relieved inwardly rectifying K+ channels from the inhibition by this hormone (Wang et al., 2001).
On the other hand, an inducing effect of acidic shifts of the cytoplasmic pH was reported earlier for some pathogenesis-related enzymes, including well-known examples from the early biosynthesis of phytoalexins as Phe ammonia lyase and hydroxymethylglutaryl-CoA reductase (i.e., the initial enzymes of phenylpropane or terpenoid metabolism, respectively) (He et al., 1998; Lapous et al., 1998). In these cases, the extra protons enter the cell from outside as indicated by the simultaneous alkalinization of the medium. In E. californica, on the contrary, the cytoplasmic acidification leading to the induction of phytoalexin biosynthesis originates from the efflux of vacuolar protons. The reversible dissipation of the pH gradient across the tonoplast prevents the elicitor induction of alkaloid production (Roos et al., 1998).
To our knowledge, the shifts of cytoplasmic and vacuolar pH generated in this study by treatment with near-neutral external buffers have not yet been documented in other plants. It appears, however, that the inductive effects of similar treatments on phytoalexin biosynthesis have already been observed. Etiolated chickpea (Cicer arietinum) seedlings responded to an incubation with 80 mM MES or PIPES buffers at pHext > 6.5 with an enhanced rate of phytoalexin (medicarpin, maackianin, and formononetin) production and excretion. While Armero and Tena (2001), in the absence of pH maps, argue for proton efflux as a key step in the signaling pathway, our data would suggest that a transient acidification of the cytoplasm, which supposedly occurred in the cited experiments, constitutes the inducing effect.
Gα-Dependent and -Independent Signal Paths Lead to the Phytoalexin Response
The responses of the Gα-deficient transformants clearly indicate that low and high elicitor concentrations use different signal mechanisms to induce alkaloid biosynthesis. Lack of PLA2 activation and the strongest constraints on the alkaloid response are seen at concentrations up to 1 μg/mL elicitor, conditions that saturate the stimulation of proton efflux from the vacuole (Roos et al., 1998). Thus, signaling via Gα-dependent activation of PLA2 and related pH shifts is selectively initiated by low elicitor treatment.
In addition, high elicitor concentrations likely invoke another signal mechanism that does not require Gα, but might well involve PLA2 activity, as suggested from the strong stimulation of this enzyme by high elicitor concentrations in Gα-deficient cells (see Figure 6). We have already shown that such concentrations trigger a peak of jasmonate (Färber et al., 2003), a known inducer of the biosynthesis of benzophenanthridines (Blechert et al., 1995; Haider et al., 2000), other alkaloids (van der Fits and Memelink, 2001), and the hypersensitive response (Roos et al., 1998). An involvement of PLA2 in the oxidative burst has been suggested by inhibitor studies in ergosterol-treated tobacco cell cultures (Kasparovsky et al., 2004). In contrast with elicitor treatment, the induction of alkaloid biosynthetic enzymes by addition of jasmonate is not accompanied by an efflux of vacuolar protons or cytoplasmic acidification (Färber et al., 2003).
In summary, the alkaloid production of E. californica cells in response to saturating elicitor concentrations most probably combines the outcome of at least two different signal pathways that converge prior to the induction of benzophenanthridine biosynthesis. The Gα/pH path saturates at ∼1 μg/mL of elicitor and mobilizes 50 to 60% of the stimulatable biosynthetic capacity, while the jasmonate path saturates at ∼50 μg/mL of elicitor and activates 40 to 50% of this capacity. In Figure 10, the extent of alkaloid biosynthesis induced by either signal pathway is displayed roughly by the range between 0 to 100% and 100 to 180%, respectively. Whereas the high-sensitivity Gα/pH pathway requires initiation via Gα and includes vacuolar proton fluxes, the low-sensitivity jasmonate pathway does not depend on either of these events. This signal path is active in Gα-deficient cell strains and accounts for their alkaloid response to high elicitor treatment.
This situation is reminiscent of the response of rice cells to gibberellic acid, which most probably includes two converging signal paths triggered by different gibberellic acid concentrations, of which only the high-sensitivity pathway is blocked in antisense Gα lines (Ueguchi-Tanaka et al., 2000). Furthermore, the suggested existence of a second, Gα-independent branch that induces phytoalexin biosynthesis in concert with the hypersensitive response is concordant with data from other mutant rice cell cultures. Challenging with oligochitin elicitors showed that phytoalexin (momilolactone) production, external alkalinization, browning, and generation of reactive oxygen species (i.e., elements of the hypersensitive response) were not deficient in Gα mutants (Tsukada et al., 2002).
METHODS
Plant Material
Suspension cultures of California poppy (Eschscholzia californica) were grown and maintained in a modified Linsmayer-Skoog medium on gyrotary shakers as described previously (Viehweger et al., 2002). Antisense Gα and scFv cell lines were treated similarly but in growth cycles of 14 or 10 d, respectively, instead of 9 d in the wild type.
Generation of Antisense Gα and Anti-Gα-scFv–Transformed Cell Lines
Cloning of a cDNA Fragment Coding for EcGα
Nucleic acid isolations and manipulations were performed using standard methods. The Enhanced Avian RT-PCR kit (Sigma-Aldrich) was used for cDNA synthesis and PCR reaction. The TA cloning kit (with the pCR 2.1 vector) from Invitrogen was used for subcloning. DNA sequencing was performed using an A.L.F. DNA sequencer (Amersham Pharmacia).
Using total RNA isolated from cell cultures of E. californica with the degenerated oligonucleotides 5′-GGIAAA(G)A(T)G(C)IACIATA(C/T)TTC(T)AAA(G)CAA(G)AT-3′ and 5′-CA(G)AAC(T)TTC(T)TTC(T)TTIACA(G)AAC(T)TCA(G)TAIGC-3′ (I = inosin), an internal cDNA fragment (847 bp) of EcGα was generated by RT-PCR and ligated into the pCR 2.1 vector, resulting in the plasmid pCR 2.1/EcGα.
The plasmid pCR 2.1/EcGα was digested with BamHI and XhoI. The resulting cDNA fragment (927 bp) coding for EcGα and part of the polylinker of pCR2.1 was inserted into a plant transformation vector using the BamHI and SalI sites of BinAr (Höfgen and Willmitzer, 1990) such that the EcGα fragment was inserted in antisense orientation and under the control of the cauliflower mosaic virus 35S promoter. The resulting plasmid was named BinAr/EcGα-As.
Cloning of a cDNA Fragment Coding for Anti-Gα-svFv Antibodies
The aforementioned cDNA fragment of EcGα in BinAr was digested with EcoRI and SalI, and the resulting 300-bp fragment (594 to 894) that showed >90% homology to Gα fragments of Pisum sativum, Arabidopsis thaliana and Solanum lycopersicum was cloned into the vector pET 23a containing coding sequences of T7 and His tags. This plasmid was expressed in Escherichia coli HB 2151, and the resulting Gα fragment was isolated on a nickel-nitrilotriacetic acid agarose column. The protein was used as an antigen to screen a scFv phage library (phagemid library; Tomlinson et al., 1996). The antibodies produced by preselected bacterial clones were characterized by ELISA for specific binding to the Gα fragment and for lacking binding to T7 and His tags. The resulting anti-Gα-scFv clone was retransformed from the original pIT-Vektor into pRTRA 7/3 by NcoI-NotI cleavage and ligation in order to allow for the presence of the KDEL sequence and a cMyc tag coding region (Artsaenko et al., 1995). In pRTRA 7/3, the signal peptide coding sequence is removed to facilitate accumulation of the scFv in the cytosol. After HindIII cleavage, the resulting sequence containing the expression cassette (1592 bp) was introduced into the plant expression vector pcB 301, which is known for its high transformation efficiency (Xiang et al., 1999).
Plant Transformation
The plasmids described above were transferred into callus cultures of E. californica by following a recently published protocol of biolistic gene transfer (Popelka et al., 2003). Calli were pregrown on culture medium with 2% agar. Two days after particle bombardment, they were carefully disintegrated and transferred to liquid culture media containing 200 μM paromomycin, which completely prevented growth of wild-type cells. Samples that developed growing suspension cultures were propagated on the same media for at least two passages prior to their use in these experiments.
Assay of Binding Affinity of scFv Proteins
The scFv protein present in the 90,000g supernatant (obtained during cell fractionation according to Roos et al., 1999) was bound to an affinity column made of rProtein L agarose (ACTIgen) and eluted by following the manufacturer's instructions. rProtein L binds antibodies and fragments that contain κ light chains.
For an ELISA analysis, a 96-well microtiter plate (Immulon) was coated with the E. coli–made recombinant Gα fragment (10 mg/mL in PBS) that previously served as antigen in the phage library screening for the scFv antibody (see above). The plate was successively incubated with the following solutions in PBS: (1) increasing concentrations of isolated scFv (90 min), (2) mouse anti-cMyc antibody (Santa Cruz Biotechnology) diluted 1:75, 90 min, (3) washed five times with 0.1% Tween 20, (4) rabbit anti-mouse-IgG, alkaline phosphatase–conjugated dilution (1:2000; Sigma-Aldrich), 60 min, and (5) p-nitrophenyl phosphate substrate system for ELISA (same supplier), 20 min at 37°C. Finally, the absorption at 405 nm was read with an MRX microreader (Dynatech). Plotting the absorption (which reflects the bound scFv protein) against the log of scFv concentration yielded the concentration required for half-maximum binding. A similar procedure was done with the recombinant scFv produced in E. coli.
Assay of Alkaloid Production
Cell suspensions were filtered without suction through a nylon mesh of 50-μm2 pore size (this procedure was used for all washes), washed with 100 mM sorbitol, and resuspended in phosphate-free culture liquid diluted to 75% (50 mg fresh weight/mL). Yeast elicitor was added, and after 30 min, the cells were washed with 100 mM sorbitol and resuspended in the same elicitor-free culture liquid.
The alkaloid content was measured in 500-μL samples that were taken immediately after elicitor treatment and after another 24 h. Benzophenanthridines were extracted by adding 1 mL methanol containing 1% concentration HCl and quantified in the filtrate by their characteristic fluorescence (excitation 485 nm; emission 580 nm) as detailed elsewhere (Färber et al., 2003).
Yeast elicitor was prepared from bakers' yeast by autoclaving and ethanol precipitation according to Schumacher et al. (1987). The obtained glycoprotein preparation was further purified by ultrafiltration (30 kD), fast protein liquid chromatography (anion exchange and size exclusion), and SDS-PAGE. The active fraction is a mixture of glycoproteins of 30 to 42 kD with a mannose content of ∼40% (J. Steighardt and W. Roos, unpublished data).
RT-PCR Detection of Increased Levels of BBE mRNA
After the indicated treatments, cells were harvested by mild suction and frozen in liquid nitrogen, and total RNA was extracted with the RNeasy plant mini kit (Invitrogen). Reverse transcription was performed with 2 μg RNA using M-MuLV reverse transcriptase (Fermentas). A cDNA fragment of the BBE gene of 456 bp was then amplified by PCR (36 cycles) with specific primers (BBE sense, 5′-AGCTGATAACGTTGTCGA-3′; BBE antisense, 5′-AGAAACTGTTTCTAATCCTGC-3′) using AccuPrime Taq DNA polymerase (Invitrogen). All other chemicals were from Ambion. PCR products were separated by DNA agarose electrophoresis.
Confocal Fluorescence Microscopy
The following assays were performed on a Leica TCS-SP confocal microscope equipped with an argon/krypton laser.
pH Mapping of Intact Cells
After the indicated days of growth, 500 μL of cell suspension received 2 μM SNARF-4F acetoxymethyl ester (Molecular Probes) and 100 μM eserin (to inhibit hydrolysis of the AM ester by extracellular esterases; cf. Kuchitsu et al., 2002). After 1 h, 10 μL of cell suspension was mixed with 50 liters of 75% culture liquid containing 3% low melting agarose at 40°C. Spreading the mixture on a cover glass yielded an agarose film with embedded cells that was placed in a flow-through cell of 140-μL volume, mounted on the confocal microscope, and perfused with phosphate-free 75% culture liquid at a flow rate of 300 μL/min.
Images were scanned with excitation of 488 nm (argon laser line) and dual emission at 580 to 620 nm (channel 1) and 630 to 680 nm (channel 2). Ratio images (channel 2/channel 1) were obtained and quantified in vacuolar and cytoplasmic areas with Leica confocal software. Conversion of intensity ratios into pH was done with a calibration graph established by pH mapping of cells that were incubated for 10 min with 40 mM MES buffers containing 80 mM methylamin, pH adjusted to 5.5 to 8.2. Recent test experiments as well as earlier data confirmed that SNARF shows a similar pH dependence in either vacuolar or cytoplasmic/nuclear areas (Roos et al., 1998).
pH Mapping of Vacuoles in Situ
The selective permeabilization of the plasma membrane by a combination of osmotic and cold shock and the subsequent confocal mapping of vacuolar pH with 5-carboxyfluorescein followed a previously published protocol (Viehweger et al., 2002).
Assay of PLA2 Activity, Vacuolar Proton Fluxes, and Alkaloid Formation in the Same Cell
Six-day suspensions of wild-type cells were incubated with 5 μM of the pH probe 5-(and 6)carboxy-2′,7′-dimethyl-3′-hydroxy-6′-N-ethylaminospiro[isobenzofuran-1(3H), 9′-(9H)xanthen]-3-one (DM-NERF) for 2 h. Two-milliliter samples (60 mg fresh weight) were filtered and washed with the same volume of 100 mM sorbitol, and the cells were resuspended in phosphate-free 20% culture liquid containing 4.5% sucrose. Five microliters of this suspension were spotted on a microscopic slide and covered with an Isopore membrane of 10-μm pore size (Millipore). A 0.5-mm-thick agarose slide, made from 200 μL of the above nutrient solution with 2% agarose, was placed on top of the membrane. A hole in the agarose layer allowed application of effectors or substrates. Between the microscopic observations, the preparation was covered with Biofolie (Heräus).
After 30 min of adaptation, the PLA2 assay was started by adding 5 μL of the substrate solution: 0.5 μM BPC in 75% phosphate-free culture liquid (made from a 100-mM stock solution in ethanol). Fluorescence development due to substrate hydrolysis was monitored in the cytoplasmic region of individual cells. After ∼6 min, when the fluorescence development reached a slower but constant rate, addition of elicitor (5 μL; final concentration 1 μg/mL) caused a new increase of fluorescence development that was monitored for 2 min.
pH mapping occurred in parallel with the PLA2 assay. Fluorescence excited at 488 nm was recorded simultaneously at 495 to 515 nm (PLA2; channel 1), 535 to 555 nm (pH; channel 2), and 580 to 600 nm (pH; channel 3). pH maps were obtained by determining ratios of fluorescence intensities of channel 3/channel 2. Calibration of the NERF fluorescence of vacuolar areas to pH was done similarly as described for vacuoles accumulating SNARF1 (Roos et al., 1998). Test experiments confirmed that the fluorescence originating from DM-NERF remained aligned with the vacuoles at least over 1 h. Thus, it did not interfere with the measurement of PLA2 activity that generated BODIPY-based fluorescence only at the cellular surface and later in the cytoplasm.
Sixteen hours after elicitor contact, the accumulation of benzophenanthridine alkaloids was measured in the cell wall region as an increase of fluorescence at 488 nm (excitation) and 580 to 630 nm (emission). Fluorescence spectra obtained from cell wall areas with the same microscope settings confirmed that this emission included the typical maxima of benzophenanthridines at 585 and 605 nm. The localization of this fluorescence emission in the cell wall was confirmed at the end of each experiment by plasmolyzing the cells with 3 M NaCl, under which conditions the wall region separated from the fluorescent vacuoles (K. Viehweger and W. Roos, unpublished data). At the used filter settings, the fluorescence of DM-NERF did not substantially overlap with the fluorescence emission of authentic benzophenanthridine alkaloids (sanguinarine, chelerythrine, and macarpine).
Assay of PLA2 Activity in Cell Suspensions
In a 96-well microtiter plate with a glass bottom (Greiner), each compartment received 50 μL substrate mix consisting of 75 nM BPC and 0.01% CHAPS in 100 mM sorbitol. After 2 min of equilibration, 20 μL of cell suspension (∼50 mg fresh weight/mL in 100 mM sorbitol) was added, the mixture was shaken for 2 s, and the fluorescence (excitation 485/20 nm; emission 528/20 nm) was read continuously for 3 min with an FLx800 microplate fluorescence reader (Bio-Tek Instruments). After the first assay period, the cell suspensions received 5 μL of elicitor solution to reach the desired concentration, followed by 2-s shaking and another 3 min assay period. At the end of the experiment, the amount of cells present in each well was quantified via the DNA content. For this purpose, 5 liters of Sytox Blue (Molecular Probes) and the detergent cetyl-trimethyl-ammonium bromide were added to reach final concentrations of 50 nM and 0.05%, respectively. The Sytox Blue DNA fluorescence (excitation 360 nm ± 40 nm; emission 460 nm ± 40 nm) was calibrated to the number of cell aggregates by an equation that determined the ratios of the Sytox Blue fluorescence to the cell density as assayed with a high-frequency cell counter (Casy 1; Schärfe Systems).
The increase of PLA2-derived fluorescence was normalized to the cell number and estimated by nonlinear regression. Elicitor activation of PLA2 was calculated as the ratio of fluorescence increments after and before addition of the elicitor.
The fluorescence increase reflected the extent of substrate hydrolysis. This was confirmed by thin layer chromatography of the fluorescent product BODIPY-FL-LPC according to Roos et al. (1999).
Acknowledgments
We thank U. Conrad (IPK Gatersleben, Germany) for experimental support and advice in the expression of anti-Gα-scFv antibodies, P. Millner (University of Leeds, UK) for providing the anti-Gα antiserum, J. Steighardt (University of Halle, Germany) for the protein gel blots of Gα, S. Frick (Leibniz-Institute of Plant Biochemistry, Halle, Germany) for help with the RT-PCR, and G. Kumleen (IPK Gatersleben) for help with biolistic procedures. We also thank M. Hieke and G. Danders (University of Halle) for the maintenance cell cultivation and engaged technical assistance. The work was supported by the Deutsche Forschungsgemeinschaft (SFB 363).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Werner Roos (werner.roos@pharmazie.uni-halle.de).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.035121.
References
- Aharon, G.S., Gelli, A., Snedden, W.A., and Blumwald, E. (1998). Activation of a plant plasma membrane Ca2+ channel by TG α1, a heterotrimeric G protein alpha-subunit homologue. FEBS Lett. 424 17–21. [DOI] [PubMed] [Google Scholar]
- Apone, F., Alyeshmerni, N., Wiens, K., Chalmers, D., Chrispeels, M.J., and Colucci, G. (2003). The G-protein-coupled receptor GCR1 regulates DNA synthesis through activation of phosphatidylinositol-specific phospholipase C. Plant Physiol. 133 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armero, J., and Tena, M. (2001). Possible role of plasma membrane H+-ATPase in the elicitation of phytoalexin and related isoflavone root secretion in chickpea (Cicer arietinum L.) seedlings. Plant Sci. 161 791–798. [Google Scholar]
- Artsaenko, O., Peisker, M., zur Nieden, U., Fiedler, U., Weiler, E.W., Müntz, K., and Conrad, U. (1995). Expression of a single chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant J. 8 745–750. [DOI] [PubMed] [Google Scholar]
- Artsaenko, O., Philipps, J., Fiedler, U., and Peisker, M., and Conrad, U. (1999). Intracellular immunomodulation in plants: A new tool for the investigation of phytohormones. In Recombinant Antibodies: Applications in Plant Science and Plant Pathology, K. Harper and A. Ziegler, eds (London: Taylor & Francis), pp. 145–156.
- Ashikari, M., Wu, J., Yano, M., Sasaki, T., and Yoshimura, A. (1999). Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 96 10284–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann, S.M. (2002). Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell 14 (suppl.), S355–S373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann, S.M. (2004). Plant G proteins, phytohormones, and plasticity: Three questions and a speculation. Sci. STKE 264 re20. [DOI] [PubMed] [Google Scholar]
- Assmann, S.M. (2005). G proteins Go green: A plant G protein signaling FAQ sheet. Science 310 71–73. [DOI] [PubMed] [Google Scholar]
- Blechert, S., Brodschelm, W., Haider, S., Kammerer, L., Kutchan, T.M., Mueller, M.J., Xia, Z.Q., and Zenk, M.H. (1995). The octadecanoic pathway: Signal molecules for the regulation of secondary pathways. Proc. Natl. Acad. Sci. USA 92 4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer, D., Uknalis, J., Triana, R., and Tu, S.I. (1997). Effects of external pH and ammonium on vacuolar pH in maize roothair cells. Plant Physiol. Biochem. 35 31–39. [Google Scholar]
- Chen, J.G. (2001). Dual auxin signaling pathways control cell elongation and division. J. Plant Growth Regul. 20 255–264. [Google Scholar]
- Colucci, G., Apone, F., Alyeshmerni, N., Chalmers, D., and Chrispeels, M.J. (2002). GCR1, the putative Arabidopsis G protein-coupled receptor gene is cell cycle-regulated, and its overexpression abolishes seed dormancy and shortens time to flowering. Proc. Natl. Acad. Sci. USA 99 4736–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, U., and Manteuffel, G. (2001). Immunomodulation of phytohormones and functional proteins in plant cells. Trends Plant Sci. 6 399–402. [DOI] [PubMed] [Google Scholar]
- Dittrich, H., and Kutchan, T. (1991). Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack. Proc. Natl. Acad. Sci. USA 88 9969–9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber, K., Schumann, B., Miersch, O., and Roos, W. (2003). Selective desensitization of jasmonate- and pH-dependent signaling in the induction of benzophenanthridine biosynthesis in cells of Eschscholzia californica. Phytochemistry 62 491–500. [DOI] [PubMed] [Google Scholar]
- Gehring, C.A., Irving, H.R., and Parish, R.W. (1994). Gibberellic acid induces cytoplasmic acidification in maize coleoptiles. Planta 194 532–540. [Google Scholar]
- Grill, E., and Himmelbach, A. (1998). ABA signal transduction. Curr. Opin. Plant Biol. 1 412–418. [DOI] [PubMed] [Google Scholar]
- Haider, G., von Schrader, T., Füßlein, M., Blechert, S., and Kutchan, T.M. (2000). Structure-activity relationships of synthetic analogs of jasmonic acid and coronatine on induction of benzo[c]phenanthridine alkaloid accumulation in Eschscholzia californica cell cultures. Biol. Chem. 381 741–748. [DOI] [PubMed] [Google Scholar]
- He, D.Y., Yazaki, Y., Nishizawa, Y., Takai, R., Yamada, K., Sakano, K., Shibuya, N., and Minami, E. (1998). Gene activation by cytoplasmic acidification in suspension-cultured rice cells in response to the potent elicitor, N-acetylchitoheptaose. Mol. Plant Microbe Interact. 11 1167–1174. [Google Scholar]
- Hooley, R. (1998). Plant hormone perception and action: A role for G-protein signal transduction? Philos. Trans. R. Soc. Lond. B Biol. Sci. 353 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfgen, R., and Willmitzer, L. (1990). Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci. 66 221–230. [Google Scholar]
- Iwasaki, Y., Fujisawa, Y., and Kato, H. (2003). Function of heterotrimeric G protein in gibberellin signaling. J. Plant Growth Regul. 22 126–133. [Google Scholar]
- Jones, A.M. (2002). G protein-coupled signaling in Arabidopsis. Curr. Opin. Plant Biol. 5 402–407. [DOI] [PubMed] [Google Scholar]
- Jones, A.M., and Assmann, S.M. (2004). Plants: The latest model system for G protein research. EMBO Rep. 5 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo, J.H., Wang, S.Y., Chen, J.G., Jones, A.M., and Fedoroff, N.V. (2005). Different signaling and cell death roles of heterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, C., Mizutani, T., Tamaki, H., Kumagai, H., Hirobe, A., Fujisawa, Y., Kato, H., and Iwasaki, Y. (2004). Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J. 38 320–331. [DOI] [PubMed] [Google Scholar]
- Kuchitsu, K., Ward, J.M., Allen, G.J., Schelle, I., and Schroeder, J.I. (2002). Loading acetoxymethyl ester fluorescent dyes into the cytoplasm of Arabidopsis and Commelina guard cells. New Phytol. 153 527–533. [DOI] [PubMed] [Google Scholar]
- Kasparovsky, T., Blein, J.P., and Mikes, V. (2004). Ergosterol elicits oxidative burst in tobacco cells via phospholipase A(2) and protein kinase C signal pathway. Plant Physiol. Biochem. 42 429–435. [DOI] [PubMed] [Google Scholar]
- Kurosaki, F., Yamashita, A., and Arisawa, M. (2001). Involvement of GTP-binding protein in the induction of phytoalexin biosynthesis in cultured carrot cells. Plant Sci. 161 273–278. [DOI] [PubMed] [Google Scholar]
- Lapous, D., Mathieu, Y., Guern, J., and Lauriére, C. (1998). Increase of defense gene transcripts by cytoplasmic acidification in tobacco cell suspensions. Planta 205 452–458. [Google Scholar]
- Lein, W., and Saalbach, G. (2001). Cloning and direct G-protein regulation of phospholipase D from tobacco. Biochim. Biophys. Acta 1530 172–183. [DOI] [PubMed] [Google Scholar]
- Ma, H. (2001). Plant G proteins: The different faces of GPA1. Curr. Biol. 11 869–871. [DOI] [PubMed] [Google Scholar]
- Millner, P.A. (2001). Heterotrimeric G proteins in plant cell signaling. New Phytol. 151 165–174. [DOI] [PubMed] [Google Scholar]
- Nato, A., Fresneau, C., Moursalimova, N., De Buyser, J., Lavergne, D., and Henry, Y. (2000). Expression of auxin and light-regulated arrestin-like proteins, G proteins and nucleoside diphosphate kinase during induction and development of wheat somatic embryos. Plant Physiol. Biochem. 38 483–490. [Google Scholar]
- Pandey, S., and Assmann, S. (2004). The Arabidopsis putative G protein–coupled receptor GCR1 interacts with the G protein subunit GPA1 and regulates abscisic acid signaling. Plant Cell 16 1616–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus-Barbeoch, L., Jones, A.M., and Assmann, S.M. (2004). Plant heterotrimeric G protein function: Insights from Arabidopsis and rice mutants. Curr. Opin. Plant Biol. 7 719–731. [DOI] [PubMed] [Google Scholar]
- Popelka, J.C., Xu, J., and Altpeter, F. (2003). Generation of rye (Secale cereale L.) plants with low transgene copy number after biolistic gene transfer and production of instantly marker-free transgenic rye. Transgenic Res. 12 587–596. [DOI] [PubMed] [Google Scholar]
- Rajasekhar, V.K., Lamb, C., and Dixon, R.A. (1999). Early events in the signal pathway for the oxidative burst in soybean cells exposed to avirulent Pseudomonas syringae pv glycinea. Plant Physiol. 120 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S., and Gilroy, S. (2000). Abscisic acid stimulation of phospholipase D in the barley aleurone is G protein-mediated and localized to the plasma membrane. Plant Physiol. 124 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, W., Dordschbal, B., Steighardt, J., Hieke, M., Weiss, D., and Saalbach, G. (1999). A redox-dependent, G protein-coupled phospholipase A of the plasma membrane is involved in the elicitation of alkaloid biosynthesis in Eschscholzia californica. Biochim. Biophys. Acta 1448 390–402. [DOI] [PubMed] [Google Scholar]
- Roos, W., Evers, S., Hieke, M., Tschöpe, M., and Schumann, B. (1998). Shifts of intracellular pH distribution as a part of the signal mechanism leading to the elicitation of benzophenanthridine alkaloid biosynthesis. Plant Physiol. 118 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, H.M., Gundlach, H., Fiedler, F., and Zenk, M.H. (1987). Elicitation of benzophenanthridine alkaloid synthesis in Eschscholtzia cell cultures. Plant Cell Rep. 6 410–413. [DOI] [PubMed] [Google Scholar]
- Tomlinson, I.M., Walter, G., Jones, P.T., Dear, P.H., Sonnhammer, E.L.L., and Winter, G. (1996). The imprint of somatic hypermutation on the repertoire of human germline V genes. J. Mol. Biol. 256 813–817. [DOI] [PubMed] [Google Scholar]
- Tsukada, K., Ishizaka, M., Fujisawa, Y., Iwasaki, Y., Yamaguchi, T., Minami, E., and Shibuya, N. (2002). Rice receptor for chitin oligosaccharide elicitor does not couple to heterotrimeric G protein: Elicitor responses of suspension cultured rice cells from Daikoku dwarf (d1) mutants lacking a functional G protein alpha-subunit. Physiol. Plant. 116 373–382. [Google Scholar]
- Ueguchi-Tanaka, M., Fujisawa, Y., Kobayashi, M., Ashikari, M., Iwasaki, Y., Kitano, H., and Matsuoka, M. (2000). Rice dwarf mutant d1, which is defective in the alpha subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 97 11638–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, H., Chen, J.G., Temple, B., Boyes, D.C., Alonso, J.M., Davis, K.R., Ecker, J.R., and Jones, A.M. (2003). The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, H., Chen, J.G., Wang, S., and Jones, A.M. (2002). Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 129 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, H., Chen, J.G., Young, J.C., Im, K.H., Sussman, M.R., and Jones, A.M. (2001). Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292 2066–2069. [DOI] [PubMed] [Google Scholar]
- van der Fits, L., and Memelink, J. (2001). The jasmonate-inducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate-responsive promoter element. Plant J. 25 43–53. [DOI] [PubMed] [Google Scholar]
- Vera-Estrella, R., Higgins, V.J., and Blumwald, E. (1994). Plant defense response to fungal pathogens. (II. G-protein-mediated changes in host plasma membrane redox reactions). Plant Physiol. 106 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viehweger, K., Dordschbal, B., and Roos, W. (2002). Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+ pool for pH signaling via the activation of Na+-dependent proton fluxes. Plant Cell 14 1509–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.Q., Ullah, H., Jones, A.M., and Assmann, S.M. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292 2070–2072. [DOI] [PubMed] [Google Scholar]
- Warpeha, K.M., Lateef, S.S., Lapik,Y., Anderson, M., Lee, B.-S., and Kaufman, L.S. (2006). G-protein-coupled receptor 1, G-protein Gα-subunit 1, and prephenate dehydratase 1 are required for blue light-induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol. 140 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Ch., Han, P., Lutziger, I., Wang, K., and Oliver, D.J. (1999). A mini binary vector series for plant transformation. Plant Mol. Biol. 40 711–717. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Yu, L., Zhang, Y., and Wang, X. (2005). Phospholipase D in the signaling networks of plant response to abscisic acid and reactive oxygen species. Biochim. Biophys. Acta 1736 1–9. [DOI] [PubMed] [Google Scholar]
- Zhao, J., and Wang, X.M. (2004). Arabidopsis phospholipase D alpha 1 interacts with the heterotrimeric G protein alpha-subunit through a motif analogous to the DRY motif in G- protein-coupled receptors. J. Biol. Chem. 279 1794–1800. [DOI] [PubMed] [Google Scholar]