Abstract
Francisella tularensis is the intracellular gram-negative coccobacillus that causes tularemia, and its virulence and infectiousness make it a potential agent of bioterrorism. Previous studies using mononuclear leukocytes have shown that the lipopolysaccharide (LPS) of F. tularensis is neither a typical proinflammatory endotoxin nor an endotoxin antagonist. This inertness suggests that F. tularensis LPS does not bind host LPS-sensing molecules such as LPS-binding protein (LBP). Using priming of the polymorphonuclear leukocyte (PMN) oxidase as a measure of endotoxicity, we found that F. tularensis live vaccine strain LPS did not behave like either a classic endotoxin or an endotoxin antagonist in human PMNs, even when the concentration of LBP was limiting. Furthermore, F. tularensis LPS did not compete with a radiolabeled lipooligosaccharide from Neisseria meningitidis for binding to LBP or to the closely related PMN granule protein, bactericidal/permeability-increasing protein. Our results suggest that the inertness of F. tularensis LPS and the resistance of F. tularensis to oxygen-independent PMN killing may result from the inability of F. tularensis LPS to be recognized by these important LPS-sensing molecules of the innate immune system.
Francisella tularensis is the gram-negative coccobacillus that causes tularemia, a life-threatening zoonotic infection of humans (17). The natural reservoir of the bacterium is not known for certain, but rodents and lagomorphs can become infected and transmit the infection to humans directly via exposure to carcasses or indirectly via arthropod vectors (17, 35). Infection is acquired by inoculation on mucosa or in broken skin, and as few as 10 organisms acquired by the aerosol route can cause overwhelming sepsis and a high rate of mortality. The organism's virulence and infectiousness spurred its development as a biowarfare agent beginning in the 1930s, and both the United States and the Soviet Union actively weaponized F. tularensis during the Cold War. For these reasons, F. tularensis is considered a category A bioterrorism agent and a high priority for research into rapid diagnosis, pathogenesis, treatment, and prevention (13, 35).
Two subspecies of F. tularensis, F. tularensis subsp. holarctica and F. tularensis subsp. tularensis, are virulent in humans and can replicate in macrophages as they disseminate throughout the reticuloendothelial system (17). The fact that a few organisms at cutaneous or mucosal sites can cause overwhelming disease suggests that the organism is able to elude innate immune clearance efficiently. Because of the practical and regulatory complications of working with fully virulent clinical isolates, most of the available studies of the early host response to F. tularensis have used the live vaccine strain (LVS), which is attenuated in humans but causes a fatal infection in mice. In this model system, cytokines such as gamma interferon and tumor necrosis factor alpha are important to host defense, especially early in the response to primary infection (16, 27, 42), perhaps through their ability to activate macrophages for more efficient killing of intracellular bacteria (19). Polymorphonuclear leukocytes (PMN) also play an important role in initial host responses, as neutropenic mice are extremely susceptible to primary infection with a small intradermal inoculum of F. tularensis LVS (41); control mice are able to attenuate replication of the organism in the spleen, liver, and lungs, whereas replication in neutropenic mice proceeds inexorably until death. Thus, although adaptive immunity is crucial to the eventual resolution of the infection with F. tularensis LVS (44), the available data on pathogenesis suggest that soluble and cellular innate immune effectors slow dissemination until a specific immune response is formed.
How the organism eludes the potent antimicrobial effectors of the innate immune system is unknown. Typically, the host immune system relies upon recognition of unique pathogen-associated molecular patterns in order to initiate protective inflammatory responses, and among the most important of these recognized pathogen-associated molecular patterns for the defense against gram-negative organisms is lipopolysaccharide (LPS) (24). Host proteins such as lipopolysaccharide-binding protein (LBP), CD14, and MD-2 bind to LPS and, in concert with Toll-like receptor 4 (TLR4), initiate intracellular signaling cascades that result in the protective elaboration of cytokines and the mobilization of antimicrobial effectors (5). However, the LPS of F. tularensis is unusual in structure and biological activity. Whereas the LPSs of many gram-negative bacteria function as potent proinflammatory endotoxins, the LPS of F. tularensis is apparently inert. It is unable to stimulate mononuclear cells to release cytokines or nitric oxide or to upregulate surface immunoglobulins on B cells (3, 39). Conversely, it does not act as an endotoxin antagonist for mononuclear cells (3). These observations suggest that F. tularensis LPS may not interact with host LPS recognition proteins, thus depriving the host of potentially protective inflammatory responses. Structural studies of the lipid A portions of LPSs from both the LVS strain and a virulent F. tularensis subsp. holarctica strain indicate that the acyl chains are unusual in length and in configuration (36, 46), and these and other unusual aspects of F. tularensis LPS structure may be responsible for its unusual biological activity.
Studies of knockout mice deficient in the phagocyte oxidase and in vitro studies of the interactions between PMN and F. tularensis LVS suggest that reactive oxidant species (ROS) are indispensable for PMN killing of the bacterium (28, 31). Because typical proinflammatory LPS potently primes the neutrophil oxidase for subsequent release of ROS in response to stimuli, it is possible that the inert LPS of F. tularensis deprives the host of maximal stimulation of important host neutrophil oxidative responses. Given that virulent strains of F. tularensis are less efficiently killed by PMN than is F. tularensis LVS and are more resistant ROS in vitro (30, 31), the failure to stimulate host PMN may be particularly important during interactions between the host and more virulent strains. The inability of oxygen-independent bactericidal mechanisms of PMN to kill ingested F. tularensis LVS suggests that the azurophilic granule protein bactericidal/permeability-increasing protein (BPI) is unable to bind F. tularensis LPS and cause lethal injury (48). BPI is closely related to LBP, an LPS recognition protein that is required for induction of proinflammatory responses to low concentrations of LPS. Thus, the failure of F. tularensis LPS to act as a proinflammatory endotoxin and the resistance of F. tularensis to oxygen-independent PMN killing could reflect the failure of F. tularensis LPS to bind these proteins. In this study, we demonstrate the inert properties of F. tularensis LPS in human PMN and show that these two closely related molecules involved in the host response to endotoxin, LBP and BPI, are unable to bind F. tularensis LPS.
MATERIALS AND METHODS
Materials.
Endotoxin-free phosphate-buffered saline and Hanks' balanced salt solution (HBSS) with or without divalent cations were purchased from Mediatech, Inc. (Herndon, VA). Endotoxin-free distilled water, normal saline, and human serum albumin were purchased from Baxter International Inc. (Deerfield, IL). Bovine serum albumin (BSA), lucigenin, and proteinase K were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Recombinant human LBP and BPI were obtained from Xoma (Berkeley, CA). Clinical-grade dextran was purchased from MP Biomedicals, Inc. (Irvine, CA).
PMN isolation.
Venous blood was obtained from healthy volunteers in accordance with a protocol approved by the Institutional Review Board for Human Subjects at the University of Iowa. PMN were isolated from heparinized samples as described previously, with a slight modification using dextran sedimentation followed by Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ) density gradient separation and hypotonic lysis with distilled water (6). Purified cell preparations were >96% PMN and were kept in normal saline without exposure to calcium until use in priming experiments.
LOS preparations.
Neisseria meningitidis lipooligosaccharide (LOS) was isolated from a type B strain as previously described (32); [3H]LOS biosynthetically radiolabeled to high specific activity (6,000 cpm/ng) was obtained from an acetate auxotroph of the N. meningitidis type B strain as described previously (20). Penta-acyl LOS was obtained from a late acyltransferase mutant (msbB) of Neisseria gonorrhoeae as previously described (37). LPS from F. tularensis was isolated as follows. The live vaccine strain of Francisella tularensis (ATCC 29684) was grown on densely streaked cystine heart agar (Becton Dickinson, Franklin Lakes, NJ) supplemented with 9% sheep blood (Colorado Serum Co., Denver, Colo.) for 48 h at 37°C in 5% CO2. Bacteria were harvested into phosphate-buffered saline with Ca2+, Mg2+, and 0.5% phenol to kill the bacteria. Cells were washed and resuspended in buffer A (10 mM EDTA, 0.06 M Tris base, 2% sodium dodecyl sulfate [SDS]), pH 6.8) and boiled for 5 min. This sample was incubated with 65 μg/ml of proteinase K for 1 hour at 65°C and then overnight at 37°C. It was then subjected to cold ethanol precipitation four times before being resuspended in distilled water. This suspension was centrifuged at 48,000 × g for 20 minutes at 20°C, and the resulting supernatant was brought up to 16 ml in distilled water and centrifuged at 147,000 × g for 75 min at 20°C. The resulting pellet was resuspended in distilled water and again centrifuged at 147,000 × g for 75 min. The sample was lyophilized for accurate weighing prior to suspension in high-performance liquid chromatography-grade distilled water (Fisher Scientific International Inc., Hampton, NH).
F. tularensis LPS was analyzed by SDS-polyacrylamide gel electrophoresis and silver staining as previously described (45). Although F. tularensis LPS stains poorly in silver-treated SDS-polyacrylamide gels (10), we were able to visualize F. tularensis LPS in silver-stained gels by applying large quantities per lane (i.e., 2.5 μg) (data not shown). Immunoblotting was performed after transfer of F. tularensis LPS to a 0.2-μm polyvinylidene difluoride membrane (Millipore, Billerica, MA) in a NuPage XCell II blot module per the manufacturer's instructions (Invitrogen, Carlsbad, CA). Membranes were blocked in Tris-buffed saline (pH 7.5) with 0.1% Tween 20 and 3% BSA. LPS was probed with a monoclonal antibody specific for the O antigen of F. tularensis LPS (clone FB11; QED Bioscience, San Diego, CA). Bound monoclonal antibody was detected with anti-murine immunoglobulin G (IgG) antibody conjugated to horseradish peroxidase (Bio-Rad, Hercules, CA), and membranes were developed for visualization using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). Using the described methods, the typical ladder pattern of a smooth LPS was apparent (data not shown).
Priming and assay of the oxidative burst.
PMN (9 × 105) were suspended in 180 μl of reaction buffer (HBSS with divalent cations, 0.1% dextrose, 1% human serum albumin, and 100 ng/ml LBP). LPS or LOS was added to this mix to the desired concentration and incubated in a 96-well polystyrene plate (Perkin-Elmer, Wellesley, MA) at 37°C in a BMG Lumistar Galaxy luminometer (BMG Labtechnologies, Offenberg, Germany). Formyl-methionyl-leucyl-phenylalanine (f-Met-Leu-Phe) (Sigma-Aldrich; final concentration, 1 μM) and lucigenin (bis-N-methylacridinium nitrate) (Sigma-Aldrich, St. Louis, MO; final concentration, 100 μM) were injected after 30 min of incubation, and measurement of luminescence commenced in cycles of 16 or 36 seconds. In preliminary experiments, the peak of luminescence consistently occurred between 30 and 45 seconds. Under these conditions, no appreciable priming occurred in the absence of LBP (data not shown). The limiting concentration of LBP was defined as the concentration that allowed easily discernible priming yet was not maximal.
Assay of LPS binding to adsorbed LBP.
One hundred microliters of LBP suspended at 10 μg/ml in an alkaline buffer (0.1 M NaCO3, 20 mM EDTA, pH 9.6) was adsorbed to Nunc MaxiSorp 96-well plates (Nalge Nunc International, Rochester, NY) by overnight incubation at 4°C. This LBP concentration was the lowest that allowed maximal binding of N. meningitidis [3H]LOS. Wells were washed three times with wash buffer (0.1% BSA in 10 mM HEPES in HBSS) and then blocked with blocking buffer (3% BSA in 10 mM HEPES in HBSS) for 1 hour at room temperature; control wells were coated with BSA during the blocking step. Wells were washed three times. N. meningitidis [3H]LOS was suspended in 100 μl of wash buffer at 5 ng/ml with or without competing LPS. This solution was incubated in the coated wells at 37°C for 3 h. Wells were washed twice with wash buffer before being treated with 2% SDS at 90°C for 10 min to remove bound N. meningitidis [3H]LOS. The resulting solutions were analyzed by liquid scintillation spectroscopy in a Beckman LS liquid scintillation counter (Beckman Coulter, Fullerton, CA). Counts per minute in BSA-coated control wells were indistinguishable from background, and recovered counts per minute in positive control wells were 8 to 9% of the total applied.
Immune capture of LPS-LBP or LPS-BPI complexes.
Rabbit antiserum to recombinant human LBP (Synpep, Dublin, CA) was purified for immunoglobulins with a HiTrap rProtein A affinity column as per the manufacturer's instructions (Amersham Biosciences). Immune and nonimmune antibodies were adsorbed to 96-well Nunc MaxiSorp plates in 100 μl of adsorption buffer overnight at 4°C at the lowest concentration that allowed maximal binding of LPS-LBP complexes (5 μg/ml). Wells were washed, blocked, and finally washed again as described above. LPS-LBP complexes were formed as follows. In a silicated tube, 100 μl of N. meningitidis [3H]LOS (3 ng/ml), with or without competing LPS, was coincubated with 1 ng/ml LBP in wash buffer for 30 min at 37°C with gentle shaking. The solution of LPS-LBP complexes was then incubated in the coated 96-well plate for 2 h at 37°C. After the wells were washed, samples were recovered for scintillation counting in 2% SDS as described above. Recovered counts per minute were 9 to 10% of the total applied. The assay for BPI-LPS complexes was performed as described above using anti-recombinant human BPI antibodies isolated from goat serum as described above (47). The BPI concentration used was 8 ng/ml, but otherwise reagent concentrations were identical. Wells coated with antibody to LBP or BPI did not bind N. meningitidis [3H]LOS that had not been previously incubated with LBP or BPI, respectively. Statistical analysis and representation of the data from the adsorbed LBP and immune capture assays were performed with Prism 4 software (GraphPad Software, San Diego, CA). Average counts per minute ± the standard errors of the means are reported.
Statistical analysis.
Statistical comparisons in priming experiments focused on the peak luminescence. Statistical comparisons between two groups were performed using a two-sided Student t test. Three or more groups were compared using one-way analysis of variance followed by Bonferroni analysis as calculated with Prism 4 software. P values of less than 0.05 were considered statistically significant.
RESULTS
LPS from the live vaccine strain of F. tularensis did not prime the PMN oxidative burst.
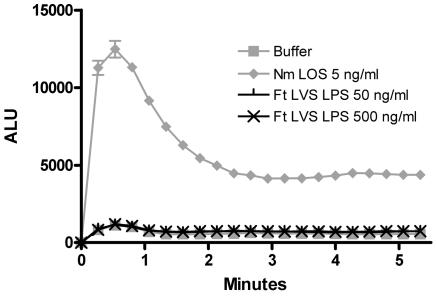
PMN freshly isolated from the bloodstream produce relatively low levels of ROS when exposed to the bacterial peptide analog f-Met-Leu-Phe. However, after priming, exposure to f-Met-Leu-Phe results in a brisk oxidative response (22). Among the most potent of such priming agents are the endotoxins of a variety of gram-negative organisms, including N. meningitidis, Escherichia coli, Klebsiella pneumoniae, and Salmonella enterica. Given that previous studies have observed a lack of endotoxicity of F. tularensis LPS in mononuclear cells (3, 39), we hypothesized that F. tularensis LPS would also lack endotoxicity in human PMN and would fail to prime for the f-Met-Leu-Phe-triggered oxidative burst. Using lucigenin-enhanced chemiluminescence to measure the respiratory burst (2), we compared priming by F. tularensis LVS LPS to that induced by a potent endotoxin, N. meningitidis lipooligosaccharide. Whereas unprimed PMN produced minimal chemiluminescence when exposed to 1 μM f-Met-Leu-Phe, PMN incubated with 5 ng/ml of N. meningitidis LOS for 30 minutes at 37°C prior to f-Met-Leu-Phe exposure produced a more than 12-fold-higher peak luminescence (Fig. 1). Even 1 ng/ml of N. meningitidis LOS resulted in an increase of six- to eightfold over unprimed PMN (data not shown). By contrast, as much as 500 ng/ml of F. tularensis LPS resulted in chemiluminescence that was indistinguishable from that produced by PMN incubated in buffer alone (Fig. 1). Thus, as measured by the ability to prime the PMN oxidative burst, F. tularensis LVS LPS lacked endotoxicity in human PMN.
FIG. 1.
F. tularensis LVS LPS did not prime the PMN oxidative burst in response to f-Met-Leu-Phe. PMN (9 × 105) were incubated in buffer with LPS at the indicated concentrations and 100 ng/ml of LBP in 96-well plates for 30 min at 37°C. f-Met-Leu-Phe and lucigenin were injected at time zero. Light emission is expressed as arbitrary light units (ALU). Results are the averages for triplicate samples ± standard errors of the means; P < 0.001 for comparisons between N. meningitidis (Nm) LOS at 5 ng/ml and each concentration of F. tularensis (Ft) LVS LPS, and P > 0.05 for comparisons between each concentration of F. tularensis LVS LPS and buffer alone. These results are representative of those from three experiments.
LPS from the live vaccine strain of F. tularensis did not antagonize priming of the PMN oxidative burst by a potent endotoxin.
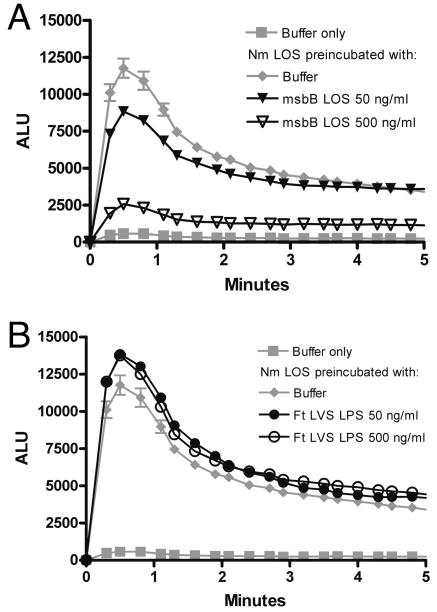
Many species of LPS that fail to induce typical cellular responses to endotoxin demonstrate antagonism of potent species of LPS. For example, the tetra-acyl LPS analogue lipid 406 or the penta-acyl LPS of late acyl transferase mutants reduces or eliminates the endotoxicity of potent LPS when present in excess (1). The penta-acyl LOS of a late acyl transferase (msbB) mutant of N. gonorrhoeae (msbB LOS) antagonized priming of the PMN oxidase by N. meningitidis LOS; a 10-fold excess of msbB LOS coincubated with N. meningitidis LOS reduced peak chemiluminescence by approximately 35% (95% confidence interval [CI]), 3% to 46%), and a 100-fold excess reduced chemiluminescence by approximately 85% (95% CI, 56% to 100%) (Fig. 2A). To determine if F. tularensis LPS, which is tetra-acylated (36, 46), shared this capacity in PMN, we assessed its ability to antagonize priming of the oxidase by N. meningitidis LOS. Ten- and 100-fold excesses of F. tularensis LVS LPS did not antagonize priming by N. meningitidis LOS (Fig. 2B).
FIG. 2.
F. tularensis LVS LPS did not antagonize PMN priming by a potent endotoxin. Conditions are as described for Fig. 1 except that F. tularensis (Ft) LVS LPS, msbB LOS, or buffer was added to PMN 5 minutes before N. meningitidis (Nm) LOS was added. Cells were then incubated at 37°C for 30 min, and lucigenin and f-Met-Leu-Phe were injected at time zero. The results from one experiment are presented in two panels for clarity. A, Penta-acylated LOS from an msbB mutant antagonized priming by 5 ng/ml of N. meningitidis LOS at 10- and 100-fold excesses (P < 0.05 for each concentration of msbB LOS in comparison with N. meningitidis LOS at 5 ng/ml). B, F. tularensis LVS LPS had no significant effect on priming by N. meningitidis LOS at the same excess concentrations (P > 0.05 for the comparison between each concentration of F. tularensis LVS LPS and N. meningitidis LOS). Results are the averages for duplicate samples ± standard errors of the means and are representative of those from three experiments. ALU, arbitrary light units.
The host inflammatory response to low concentrations of LPS depends upon a series of interactions between LPS and host proteins, including LBP, CD14, and MD-2, and we next set out to determine whether the inertness of F. tularensis LPS could be explained by its failure to bind to one or more of them. LBP binds to LPS aggregates or to gram-negative bacterial membranes and transfers individual LPS molecules to CD14 (49); CD14 in turn transfers one molecule of LPS to MD-2, resulting in TLR4 activation and signal transduction (1, 21). Endotoxin species that act as antagonists can do so by occupying either LBP, CD14, and/or MD-2 in nonstimulatory interactions, thereby reducing the ability of endotoxin agonists to activate TLR4 (25). The inability of F. tularensis LPS to prime the PMN oxidative burst or antagonize priming by a potent endotoxin could therefore be explained by an inability of LBP to interact with F. tularensis LPS. To confirm that the absence of F. tularensis LPS-mediated antagonism was not due to the presence of excess LBP in the assay, we repeated priming experiments with a concentration of LBP that resulted in detectable but submaximal priming. Even when LBP was limiting (10 ng/ml), we detected no antagonism by a 100-fold excess of F. tularensis LVS LPS, whereas a 100-fold excess of msbB LOS abrogated priming by N. meningitidis LOS (Fig. 3). When competing LPS was preincubated with buffer and LBP at 37°C for as long as 30 min (rather than 5 minutes at room temperature), we still observed no antagonism by F. tularensis LVS LPS (data not shown). These results suggest that F. tularensis LPS did not bind to LBP and that its inertness in our system reflected a failure to interact with the first of the series of host proteins required for the normal host response to LPS.
FIG. 3.
F. tularensis LVS LPS did not antagonize PMN priming by a potent endotoxin when the LBP concentration was reduced to 10 ng/ml, which was limiting. Conditions were otherwise as described for Fig. 2. The results from one experiment are presented in two panels for clarity. A, Penta-acylated LOS from an msbB mutant efficiently antagonized priming by 5 ng/ml of N. meningitidis (Nm) LOS (P < 0.05 for each concentration of msbB LOS in comparison with N. meningitidis LOS at 5 ng/ml). B, F. tularensis (Ft) LVS LPS had no significant effect on priming by 5 ng/ml of N. meningitidis LOS (P > 0.05 for the comparison between each concentration of F. tularensis LVS LPS and N. meningitidis LOS). Results are the averages for sextuplicate samples ± standard errors of the means and are representative of those from three experiments. ALU, arbitrary light units.
LPS from the live vaccine strain of F. tularensis did not bind to LBP.
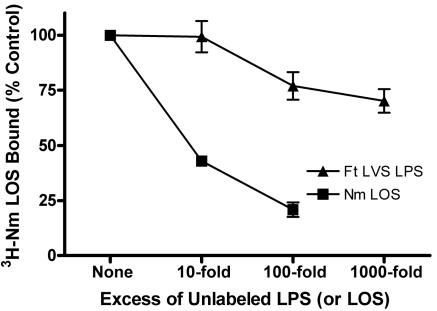
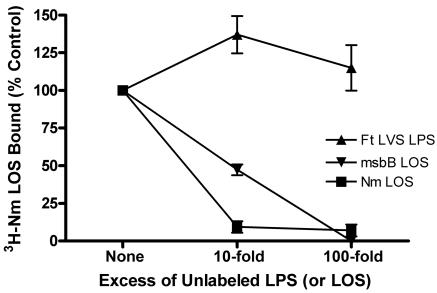
In order to test more directly the ability of F. tularensis LPS to bind LBP, we designed a competition binding assay in which we measured the ability of unlabeled N. meningitidis LOS or F. tularensis LPS to inhibit binding of N. meningitidis [3H]LOS to LBP-coated wells of a polystyrene plate. Nonspecific interactions were minimized by blocking wells with BSA, since N. meningitidis [3H]LOS did not bind to wells coated with BSA alone. When N. meningitidis [3H]LOS was coincubated with unlabeled N. meningitidis LOS in LBP-coated wells, 10- and 100-fold excesses of the unlabeled LOS reduced binding of N. meningitidis [3H]LOS to LBP by 57% (95% CI, 41% to 74%) and 79% (95% CI, 60% to 98%), respectively (Fig. 4). By contrast, even a 1,000-fold excess of F. tularensis LVS LPS reduced N. meningitidis [3H]LOS binding by only 30%. The failure of F. tularensis LVS LPS to impair N. meningitidis LOS binding demonstrated that it bound adsorbed LBP poorly compared to a typical endotoxin.
FIG. 4.
F. tularensis LVS LPS competed poorly with N. meningitidis LOS for binding to adsorbed LBP. Recombinant human LBP was adsorbed to 96-well plates, and N. meningitidis (Nm) [3H]LOS (5 ng/ml) was incubated in the wells for 3 hours at 37°C in the presence of 0.1% BSA. Unlabeled N. meningitidis LOS or F. tularensis (Ft) LVS LPS was coincubated at the indicated excess concentrations. Binding of N. meningitidis [3H]LOS was measured as counts per minute in duplicates and was normalized within each experiment. Counts per minute in the absence of unlabeled LPS (or LOS) were defined as 100% (i.e., the control value). Background counts per minute were subtracted from the results for each sample. P < 0.001 for comparisons between F. tularensis LVS LPS and N. meningitidis LOS at 10- and 100-fold excess concentrations. Error bars are the standard errors of the means; n = 3.
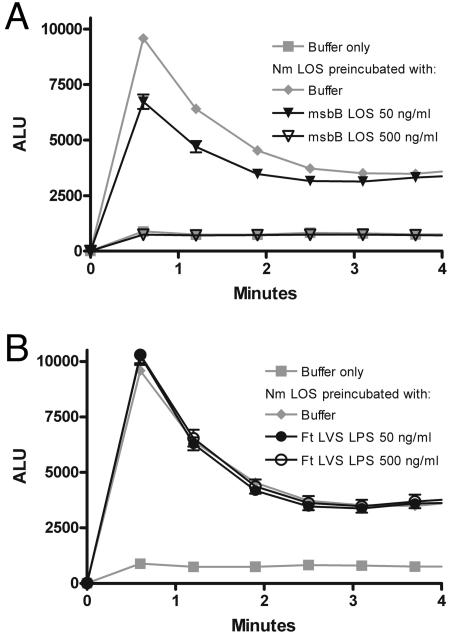
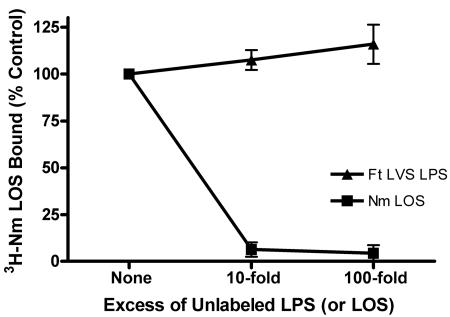
Reasoning that LBP bound to a polystyrene plate might not accurately reproduce the behavior of LBP in solution, as it is normally presented physiologically, we designed an immune capture assay in which N. meningitidis [3H]LOS was allowed to complex with LBP in solution in the presence or absence of competing LPS. These LBP-LPS complexes were then recovered using polystyrene plates coated with polyclonal rabbit anti-human LBP antibody. In order to minimize competition between free LBP and LBP-LPS complexes, the LBP concentration was kept at the lowest concentration that allowed a good signal-to-background ratio (1 ng/ml). When wells were coated with preimmune IgG, no LBP-dependent capture of N. meningitidis [3H]LOS was observed. A 10-fold excess of unlabeled N. meningitidis LOS almost completely inhibited capture of N. meningitidis [3H]LOS. Penta-acylated LOS (msbB LOS) competed less avidly for LBP, with 47% inhibition (95% CI, 31% to 75%) at a 10-fold excess and complete inhibition at a 100-fold excess (Fig. 5). In contrast, F. tularensis LVS LPS did not compete for binding to LBP at a 100-fold excess, indicating that F. tularensis LVS LPS bound soluble LBP poorly, if at all. These experiments support the notion that F. tularensis LPS did not act as an endotoxin agonist or antagonist in PMN at least in part because it did not interact with the first of a series of host proteins required for the host response to LPS.
FIG. 5.
F. tularensis LVS LPS did not compete for binding to LBP in solution. N. meningitidis (Nm) [3H]LOS (3 ng/ml) was coincubated with unlabeled N. meningitidis LOS, F. tularensis (Ft) LVS LPS, msbB LOS, or buffer for 30 min at 37°C with a limiting concentration of LBP (1 ng/ml) and 0.1% BSA. The resulting N. meningitidis [3H]LOS-LBP complexes were then incubated in duplicate in wells coated with polyclonal rabbit anti-human LBP IgG or preimmune IgG. Binding of N. meningitidis [3H]LOS was measured as counts per minute in duplicates and was normalized within each experiment after subtraction of counts from preimmune antibody-coated wells. Counts per minute in the absence of unlabeled LPS (or LOS) were defined as 100% (i.e., the control value) P < 0.001 for comparisons among each LPS at 10- and 100-fold excess concentrations, except P > 0.05 for the comparison between msbB LOS and N. meningitidis LOS at a 100-fold excess. Error bars are the standard errors of the means; n = 3.
LPS from the live vaccine strain of F. tularensis did not bind to BPI.
LBP is 45% identical by amino acid sequence to the bactericidal/permeability-increasing protein, an antibacterial protein found in the azurophilic granules of PMN that binds to LPS with even greater affinity than does LBP. However, BPI antagonizes cell signaling by LPS rather than facilitating it and, unlike LBP, can induce lethal changes in the membranes of gram-negative bacteria to which it binds (49). Previous studies have documented the importance of ROS to the successful killing of F. tularensis by PMN, indicating that the nonoxidative systems alone are relatively ineffective against F. tularensis (29, 31, 43). We hypothesized that the failure of nonoxidative killing by PMN might result in part from the inability of LPS recognition proteins such as BPI to bind F. tularensis LVS LPS. To test this hypothesis, we adapted the immune capture assay described above using recombinant BPI and goat anti-BPI antibody. Ten- and 100-fold excesses of unlabeled N. meningitidis LOS abrogated capture of N. meningitidis [3H]LOS by anti-BPI antibodies (Fig. 6). Similar to the results seen for LBP, F. tularensis LVS LPS did not inhibit association between BPI and N. meningitidis [3H]LOS at a 100-fold excess. In summary, two structurally similar molecules important in proinflammatory and microbicidal host responses to endotoxin, namely, LBP and BPI, were unable to bind to F. tularensis LVS LPS.
FIG. 6.
F. tularensis LVS LPS did not compete for binding to BPI in solution. N. meningitidis (Nm) [3H]LOS (3 ng/ml) was coincubated with unlabeled N. meningitidis LOS, F. tularensis (Ft) LVS LPS, msbB LOS, or buffer for 30 min at 37°C with a limiting concentration of BPI (8 ng/ml) and 0.1% BSA. The resulting N. meningitidis [3H]LOS-BPI complexes were then incubated in duplicate in wells coated with polyclonal goat anti-human BPI IgG or normal goat IgG. Binding of N. meningitidis [3H]LOS was measured as described in the legend to Fig. 5. P < 0.001 for comparisons between F. tularensis LVS LPS and N. meningitidis LOS at 10- and 100-fold excess concentrations. Error bars are the standard errors of the means; n = 3.
DISCUSSION
We have found that the LPS of the live vaccine strain of F. tularensis failed to prime human PMN for the f-Met-Leu-Phe-triggered oxidative burst. Furthermore, F. tularensis LPS did not antagonize priming by a potent LOS from N. meningitidis in a sensitive assay, even when the concentration of LBP was limiting. These observations suggested that F. tularensis LPS did not interact with the LPS-sensing machinery of the PMN. We therefore tested binding of F. tularensis LVS LPS to LBP in competition assays and demonstrated that F. tularensis LPS did not bind to this protein or to the closely related antimicrobial PMN granule protein BPI. This unusual feature of the organism may enhance its ability to survive ingestion by host phagocytes by minimizing stimulation of the cell and by resisting the effects of antibacterial proteins and peptides. Within the context of human innate host defense against infection by F. tularensis, the inertness of F. tularensis LPS may be an important virulence factor.
The proinflammatory response to low concentrations of LPS requires a series of interactions between LPS molecules and several host proteins. Although the exact mechanism by which LPS structure variations are discriminated is not yet known, tetra-acylated lipid A antagonists can inhibit TLR4-dependent cell activation by active hexa-acylated LPS species by competitively occupying LBP, CD14, and/or MD-2, depending upon which of these host proteins are present in limiting amounts (25). Thus, it appears that, at low concentrations, LPS molecules must interact with LBP, CD14, and MD-2 to have either agonist or antagonist effects, but signal transduction relies upon a particular interaction between LPS, MD-2, and TLR4. Presumably, then, LPS that occupies binding sites on these molecules but does not result in the appropriate arrangement or conformation of the MD-2-TLR4 complex acts as an antagonist.
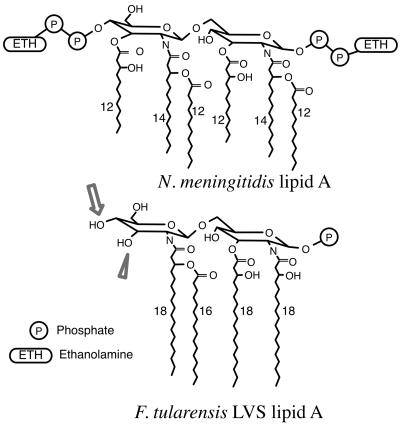
Unlike most species of LPS, F. tularensis LPS has neither agonist nor antagonist effects, as shown in this paper and in previous work (3, 39). The biological activity of LPS is associated with specific structural characteristics (1, 18), and the recently elucidated structure of the lipid A of F. tularensis LPS deviates significantly from that of typical potent endotoxins. Most typical endotoxins have two phosphate groups on the diglucosamine backbone, whereas F. tularensis LVS lipid A has none (46) or perhaps only one (36). Typical endotoxins have 12 to 14 carbon acyl chains, and the ester- and amide-linked acyl chains on each of the two glucosamines are identical, since the lipid A backbone is synthesized in a condensation reaction between two identical acylated glucosamines (38). By contrast, F. tularensis lipid A acyl chains are 16 to 18 carbons in length, and the -OH group on the third carbon of the nonreducing glucosamine is not substituted (Fig. 7) (36, 46). In these respects, F. tularensis lipid A resembles the lipid A structures of some strains of Helicobacter pylori and Porphyromonas gingivalis, which have an unsubstituted third-carbon -OH group, 16 to 18 carbon acyl chains, and only one phosphate (12, 26, 33). Interestingly, both of these LPSs have relatively low endotoxin activity, and both have been shown to bind relatively poorly to an LBP-immunoglobulin fusion protein (11). These observations suggest that a structure with four 16- to 18-carbon acyl chains asymmetrically distributed on the lipid A backbone might bind LBP poorly. Alternatively, since initial interactions between LBP (and BPI) and LPS depend upon electrostatic attraction (49, 50), the reduced charge of the lipid A region due to a lack of phosphate substitution may be most important. Further testing of such structure-function relationships would require the direct comparison of homogeneous LPS preparations, which are difficult to isolate. Whether F. tularensis LPS would also fail to bind CD14 and MD-2, if it were possible to remove the need for LBP, is not known.
FIG. 7.
The lipid A structures of N. meningitidis (7) and F. tularensis LVS (the 1-hydroxyl phosphate group is included as per reference 36). The arrowhead points to the unsubstituted 3′ hydroxyl group of F. tularensis lipid A. The arrow indicates the lack of a phosphate group at the 4′ position.
For decades, it has been appreciated that a vigorous inflammatory response to endotoxin can be deleterious to the host when it results in septic shock. For instance, in animal models in which purified potent LPS is injected intravenously, host mutations of TLR4 that abrogate the inflammatory response to LPS are protective. However, the same mutations that protect against injection of endotoxin render the host susceptible to minute inocula of live gram-negative bacteria, particularly at mucosal sites (14, 23, 34, 51). Thus, a vigorous inflammatory response to LPS and the subsequent recruitment of innate immune effectors are crucial to host resistance to infection with many gram-negative organisms. Since the inflammatory cytokines gamma interferon and tumor necrosis factor alpha are crucial to the host response to F. tularensis (15, 16, 19, 42), possession of an LPS which does not cause inflammation could enhance bacterial survival in the host. Consistent with this lack of endotoxicity, and in contrast to the responses to most gram-negative pathogens, TLR4-deficient mice show no deficits in their response to virulent and attenuated strains of F. tularensis unless large inocula of bacteria are used (8, 9). Thus, the failure of F. tularensis LPS to bind LBP and thereby behave like a typical endotoxin means that an important arm of the innate immune system is not engaged to defend against F. tularensis.
Although a T-cell-mediated immune response is required for resolution of F. tularensis infection, the PMN likely plays a key role in early host defense (17). It is known that PMN are prominent among the inflammatory cells recruited early to sites of infection (4, 40), and depletion of PMN in a mouse model increases bacterial dissemination and mortality after primary intradermal or intravenous challenge with F. tularensis LVS (41). These results suggest that PMN are crucial to the initial control of infection by the innate immune system until a T-cell-mediated response can develop. In vitro work on F. tularensis LVS interactions with PMN has shown that killing is strictly oxygen dependent (31), and a recent study confirmed markedly increased susceptibility to primary infection in PMN oxidase-deficient mice (29). Our observation that F. tularensis LPS failed to prime the PMN oxidase suggests that the mobilization of these important ROS during interactions between PMN and F. tularensis may be suboptimal. Furthermore, the previous work suggests that the array of antimicrobial proteins and peptides in the azurophilic granule, including BPI, defensins, and proteases, among others, is unable to kill ingested F. tularensis in the absence of ROS. This conclusion is supported by the observation that a crude PMN protein extract that possesses potent antimicrobial effects against many gram-negative organisms is unable to kill a variety of F. tularensis strains (43). Our results implicate the failure of BPI to bind F. tularensis LPS as contributing to the failure of nonoxidative killing mechanisms. Furthermore, similar structural principles may determine interactions of other PMN-derived cationic antimicrobial proteins with gram-negative bacteria and LPS. If so, our results suggest a mechanistic basis of F. tularensis resistance to the nonoxidative bactericidal systems of PMN.
We hypothesize that the failure of proteins of the innate immune system to recognize F. tularensis LPS impairs both the proinflammatory and the microbicidal host responses. Although infection with F. tularensis is frequently characterized by tremendous local and systemic immune responses, initial interactions with the host may not stimulate host phagocytes fully, thereby limiting the early response to the organism. Future work will investigate the extent to which priming of the oxidative burst enhances killing of F. tularensis and will characterize the determinants of successful PMN handling of the organism.
Acknowledgments
Jason H. Barker is supported by a Veterans Administration Associate Investigator Award, VA Medical Center, Iowa City, IA. This work was supported by U.S. Public Health Service grants P01 AI044642 (to W.M.N., M.A.A., and J.W.) and R01 AI34879 (to W.M.N.).
Editor: J. T. Barbieri
REFERENCES
- 1.Alexander, C., and E. T. Rietschel. 2001. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 7:167-202. [PubMed] [Google Scholar]
- 2.Allen, R. C. 1986. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 133:449-493. [DOI] [PubMed] [Google Scholar]
- 3.Ancuta, P., T. Pedron, R. Girard, G. Sandstrom, and R. Chaby. 1996. Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect. Immun. 64:2041-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburn, L. L., and S. E. Miller. 1945. Tularemia: a report of laboratory infection fatal on the fifth day, with early pulmonary involvement; autopsy. Arch. Pathol. 39:388-392. [Google Scholar]
- 5.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 6.Borregaard, N., J. M. Heiple, E. R. Simons, and R. A. Clark. 1983. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J. Cell Biol. 97:52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caroff, M., and D. Karibian. 2003. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 338:2431-2447. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W., R. KuoLee, H. Shen, M. Busa, and J. W. Conlan. 2004. Toll-like receptor 4 (TLR4) does not confer a resistance advantage on mice against low-dose aerosol infection with virulent type A Francisella tularensis. Microb. Pathog. 37:185-191. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., R. Kuolee, H. Shen, M. Busa, and J. W. Conlan. 2005. Toll-like receptor 4 (TLR4) plays a relatively minor role in murine defense against primary intradermal infection with Francisella tularensis LVS. Immunol. Lett. 97:151-154. [DOI] [PubMed] [Google Scholar]
- 10.Conlan, J. W., H. Shen, A. Webb, and M. B. Perry. 2002. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine 20:3465-3471. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, M. D., C. Seachord, K. Ratcliffe, B. Bainbridge, A. Aruffo, and R. P. Darveau. 1996. Helicobacter pylori and Porphyromonas gingivalis lipopolysaccharides are poorly transferred to recombinant soluble CD14. Infect. Immun. 64:3601-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darveau, R. P., T. T. Pham, K. Lemley, R. A. Reife, B. W. Bainbridge, S. R. Coats, W. N. Howald, S. S. Way, and A. M. Hajjar. 2004. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect. Immun. 72:5041-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 14.Eden, C. S., R. Shahin, and D. Briles. 1988. Host resistance to mucosal Gram-negative infection. Susceptibility of lipopolysaccharide nonresponder mice. J. Immunol. 140:3180-3185. [PubMed] [Google Scholar]
- 15.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135-142. [DOI] [PubMed] [Google Scholar]
- 16.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erridge, C., E. Bennett-Guerrero, and I. R. Poxton. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837-851. [DOI] [PubMed] [Google Scholar]
- 19.Fortier, A. H., T. Polsinelli, S. J. Green, and C. A. Nacy. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect. Immun. 60:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giardina, P. C., T. Gioannini, B. A. Buscher, A. Zaleski, D. S. Zheng, L. Stoll, A. Teghanemt, M. A. Apicella, and J. Weiss. 2001. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J. Biol. Chem. 276:5883-5891. [DOI] [PubMed] [Google Scholar]
- 21.Gioannini, T. L., A. Teghanemt, K. A. Zarember, and J. P. Weiss. 2003. Regulation of interactions of endotoxin with host cells. J. Endotoxin Res. 9:401-408. [DOI] [PubMed] [Google Scholar]
- 22.Guthrie, L. A., L. C. McPhail, P. M. Henson, and R. B. Johnston, Jr. 1984. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J. Exp. Med. 160:1656-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagberg, L., D. E. Briles, and C. S. Eden. 1985. Evidence for separate genetic defects in C3H/HeJ and C3HeB/FeJ mice, that affect susceptibility to Gram-negative infections. J. Immunol. 134:4118-4122. [PubMed] [Google Scholar]
- 24.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 25.Kitchens, R. L., and R. S. Munford. 1995. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J. Biol. Chem. 270:9904-9910. [DOI] [PubMed] [Google Scholar]
- 26.Kumada, H., Y. Haishima, T. Umemoto, and K. Tanamoto. 1995. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J. Bacteriol. 177:2098-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindgren, H., L. Stenman, A. Tarnvik, and A. Sjostedt. 2005. The contribution of reactive nitrogen and oxygen species to the killing of Francisella tularensis LVS by murine macrophages. Microbes Infect. 7:467-475. [DOI] [PubMed] [Google Scholar]
- 29.Lindgren, H., S. Stenmark, W. Chen, A. Tarnvik, and A. Sjostedt. 2004. Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect. Immun. 72:7172-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lofgren, S., A. Tarnvik, G. D. Bloom, and W. Sjoberg. 1983. Phagocytosis and killing of Francisella tularensis by human polymorphonuclear leukocytes. Infect. Immun. 39:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lofgren, S., A. Tarnvik, M. Thore, and J. Carlsson. 1984. A wild and an attenuated strain of Francisella tularensis differ in susceptibility to hypochlorous acid: a possible explanation of their different handling by polymorphonuclear leukocytes. Infect. Immun. 43:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manthey, C. L., P. Y. Perera, B. E. Henricson, T. A. Hamilton, N. Qureshi, and S. N. Vogel. 1994. Endotoxin-induced early gene expression in C3H/HeJ (Lpsd) macrophages. J. Immunol. 153:2653-2663. [PubMed] [Google Scholar]
- 33.Moran, A. P., B. Lindner, and E. J. Walsh. 1997. Structural characterization of the lipid A component of Helicobacter pylori rough- and smooth-form lipopolysaccharides. J. Bacteriol. 179:6453-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien, A. D., D. L. Rosenstreich, I. Scher, G. H. Campbell, R. P. MacDermott, and S. B. Formal. 1980. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 124:20-24. [PubMed] [Google Scholar]
- 35.Oyston, P. C., A. Sjostedt, and R. W. Titball. 2004. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967-978. [DOI] [PubMed] [Google Scholar]
- 36.Phillips, N. J., B. Schilling, M. K. McLendon, M. A. Apicella, and B. W. Gibson. 2004. Novel modification of lipid A of Francisella tularensis. Infect. Immun. 72:5340-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Post, D. M., N. J. Phillips, J. Q. Shao, D. D. Entz, B. W. Gibson, and M. A. Apicella. 2002. Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A. Infect. Immun. 70:909-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raetz, C. R., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandstrom, G., A. Sjostedt, T. Johansson, K. Kuoppa, and J. C. Williams. 1992. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol. Immunol. 5:201-210. [DOI] [PubMed] [Google Scholar]
- 40.Schricker, R. L., H. T. Eigelsbach, J. Q. Mitten, and W. C. Hall. 1972. Pathogenesis of tularemia in monkeys aerogenically exposed to Francisella tularensis 425. Infect. Immun. 5:734-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjostedt, A., J. W. Conlan, and R. J. North. 1994. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect. Immun. 62:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjostedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369-1374. [DOI] [PubMed] [Google Scholar]
- 43.Stefanye, D., H. G. Tressalt, and L. Spero. 1961. Observations on the behavior in vitro of Pasteurella tularensis after phagocytosis. J. Bacteriol. 81:470-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11:440-451. [PubMed] [Google Scholar]
- 45.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 46.Vinogradov, E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112-6118. [DOI] [PubMed] [Google Scholar]
- 47.Weinrauch, Y., A. Foreman, C. Shu, K. Zarember, O. Levy, P. Elsbach, and J. Weiss. 1995. Extracellular accumulation of potently microbicidal bactericidal/permeability-increasing protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J. Clin. Investig. 95:1916-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss, J., M. Victor, A. S. Cross, and P. Elsbach. 1982. Sensitivity of K1-encapsulated Escherichia coli to killing by the bactericidal/permeability-increasing protein of rabbit and human neutrophils. Infect. Immun. 38:1149-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss, J. 2003. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem. Soc. Trans. 31:785-790. [DOI] [PubMed] [Google Scholar]
- 50.Weiss, J., M. Victor, and P. Elsbach. 1983. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on Gram-negative bacteria. J. Clin. Investig. 71:540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods, J. P., J. A. Frelinger, G. Warrack, and J. G. Cannon. 1988. Mouse genetic locus Lps influences susceptibility to Neisseria meningitidis infection. Infect. Immun. 56:1950-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]