Abstract
Interstitial lung macrophages from tuberculosis-susceptible I/St and tuberculosis-resistant A/Sn mice demonstrated significant constitutive differences in gene expression levels, whereas in vitro infection of these cells with Mycobacterium tuberculosis had only a modulatory impact on gene expression. We conclude that intrinsic gene expression profiles are an important determinant of tuberculosis pathogenesis in mice.
The primary host cells for Mycobacterium tuberculosis, which is the causative agent of tuberculosis (TB), are mature tissue macrophages. The specific host response pathways allowing M. tuberculosis to take up residence in macrophages and the host cell factors that underlie the M. tuberculosis-macrophage interplay are largely unknown. We have previously demonstrated that in A/Sn and I/St mice, which are genetically resistant and susceptible to tuberculosis, respectively (7, 16), only freshly isolated interstitial lung macrophages, and not peritoneal or spleen- or bone marrow-derived macrophages, strictly followed the genetic pattern of tuberculosis susceptibility/resistance (11). In addition, the resistance phenotype can be readily transferred with bone marrow cells from resistant F1 donors into irradiated susceptible I/St recipients (12). To further identify host response genes involved in early M. tuberculosis-macrophage interactions, we conducted a series of microarray gene expression experiments employing lung macrophages from A/Sn and I/St mice.
Interstitial lung macrophages, isolated as described earlier (11), were either infected with M. tuberculosis H37Rv at a multiplicity of infection of 5:1 for 24 h or cultured under the same conditions without infection (control). The efficiency of infection was 50% to 60%, as demonstrated by auramine staining of fixed macrophages, with no observed interstrain difference in mycobacterial uptake (data not shown). RNA extracted from infected and control macrophages of I/St and A/Sn mice (RNeasy minikit; QIAGEN, California) was hybridized to murine genome U74Av2 microarrays (www.affymetrix.com). The data were analyzed with the Significance Analysis of Microarrays software (SAM; http://www-stat.stanford.edu/∼tibs/SAM//index.html). For the analysis, the gene expression levels in macrophages of I/St and A/Sn mice were compared either before or after infection. We considered genes that had d scores (absolute values) of ≥2.0 to be significant. The d score is similar to a t statistic, but a small constant is added to the standard error to reduce the variability in its estimate. A better measure of statistical significance can be obtained by examining the false detection rate (FDR) associated with the magnitude of the differences between strains, with adjustment for the number of genes tested (24). Accurate empirical estimates of the FDR were obtained from the permutation analysis built into the SAM software, employing a d score (absolute value) of 2.0 corresponding to an estimated FDR of 1% (24). Microarray data analysis led to the identification of 152 genes with significant differentials in expression either before or after infection of lung macrophages of the two strains (Table 1; see also supplemental material S1).
TABLE 1.
Partial list of genes differentially expressed in I/St and A/Sn macrophagesa
| Gene | Score (d) forb:
|
Protein or genee | Accession no. | Gene name | |
|---|---|---|---|---|---|
| Control cellsc | Infected cellsd | ||||
| Chemokine and cytokine genes | |||||
| Scyb14f | 10.4 | 5.3 | Cxcl14, macrophage inflammatory protein 2 gamma | AW120786 | 96953_at |
| Il-11f | 9.9 | 3.4 | Interleukin 11 | U03421 | 92266_at |
| Scyb13f | 7.4 | 6.1 | Cxcl13, B-cell homing chemokine | AF030636 | 102025_at |
| Ccr5 | NS | 2.4 | Chemokine (C-C motif) receptor 5 | AF022990 | 161968_f_at |
| Scya11 | 3.6 | NS | Small chemokine (C-C motif) ligand 11 (eotaxin-1) | U77462 | 92742_at |
| Il-6f | 3.5 | NS | Interleukin 6 | X54542 | 102218_at |
| Scyb2 MIP-2a | 3.2 | NS | Cxcl-2, macrophage inflammatory protein 2 alpha | X53798 | 101160_at |
| Scyb1 MIP-2 | 2.8 | NS | Cxcl-1, macrophage inflammatory protein 2 | L12030 | 95349_g_at |
| Il-17 | −5.6 | NS | Interleukin 17 | U35108 | 99349_at |
| Scyb10 | −3.0 | NS | Cxcl-10, macrophage interferon-activated protein 10 (IP-10) | M33266 | 93858_at |
| Scyb9 | −2.9 | NS | Cxcl-9, monokine induced by gamma interferon (Mig) | M34815 | 101436_at |
| Immune response genes | |||||
| Ifi205 | 7.7 | 3.0 | Interferon-activated gene 205 | M74123 | 94224_s_at |
| Ifi202a | 5.3 | 2.0 | Interferon-activated gene 202A | AV229143 | 94774_at |
| Saa3f | 5.0 | 3.5 | Serum amyloid A 3 | X03505 | 102712_at |
| Gbp2 | −4.8 | −2.0 | Guanylate nucleotide binding protein 2 | AJ007970 | 104597_at |
| Chi3l3 | NS | 3.9 | Chitinase 3-like 3 | M94584 | 92694_at |
| Gbp1 | −5.1 | NS | Guanylate nucleotide binding protein 1 | M55544 | 95974_at |
| Psmb9 | −4.8 | NS | Proteosome (prosome macropain) subunit beta type 9 | D44456 | 93085_at |
| Ifit2 | −4.4 | NS | Interferon-induced protein with tetratricopeptide repeats 2 | U43085 | 103639_at |
| Mx2 | −4.2 | NS | Myxovirus (influenza virus) resistance 2 | J03368 | 102699_at |
| Gbp3 | −3.4 | NS | Guanylate nucleotide binding protein 3 | AW047476 | 103202_at |
| Mx1 | −3.4 | NS | Myxovirus (influenza virus) resistance 1 | M21038 | 98417_at |
| Ifi47 | −3.2 | NS | Gamma interferon-inducible protein | M63630 | 104750_at |
| Cytoskeletal/extracellular matrix genes | |||||
| Mglap | 3.9 | 2.4 | Matrix gamma-carboxyglutamate (gla) protein | D00613 | 93866_s_at |
| Csrp1 | NS | 2.9 | Cysteine-rich protein 1 | D88793 | 92608_at |
| Adam8 | NS | 2.7 | A disintegrin and metalloprotease domain 8 | X13335 | 103024_at |
| Coro1a | NS | 2.1 | Coronin actin binding protein 1A | AW122039 | 96648_at |
| Mmp8f | NS | −2.4 | Matrix metalloproteinase 8 | U96696 | 94769_at |
| Cldn4 | 3.2 | NS | Claudin 4 | AB000713 | 101410_at |
| Receptor/cell surface genes | |||||
| Fpr1 | 5.2 | 2.3 | Formyl peptide receptor 1 | L22181 | 99387_at |
| Tm7sf1 | −6.7 | −7.0 | Transmembrane 7 superfamily member 1 | AI060729 | 103017_at |
| Marco | −5.1 | −2.1 | Macrophage receptor with collagenous structure | U18424 | 102974_at |
| Cd22 | −5.0 | −3.0 | CD22 antigen | L02844 | 102939_s_at |
| Il-7r | −3.6 | −5.5 | Interleukin 7 receptor | M29697 | 99030_at |
| Il1rl1 | NS | −2.5 | Interleukin 1 receptor-like 1 | Y07519 | 98501_at |
| Taa1 | 7.8 | NS | Tumor-associated antigen 1 | U35836 | 94643_at |
| Tnfrsf9 | 4.6 | NS | Tumor necrosis factor receptor superfamily member 9 | AA798611 | 103509_at |
| Bdkrb | 3.6 | NS | Bradykinin receptor beta | L26047 | 101748_at |
| Raet1c | 3.3 | NS | Retinoic acid early transcript gamma | D64162 | 102649_s_at |
| Pira1 | 3.1 | NS | Paired-immunoglobulin-like receptor A1 | U96682 | 95784_at |
| Ly6a | −4.3 | NS | Lymphocyte antigen 6 complex locus A | X04653 | 93078_at |
| Itpr5 | −3.2 | NS | Inositol 145-triphosphate receptor 5 | AF031127 | 101441_i_at |
| Ifngr | −3.2 | NS | Gamma interferon receptor | M28233 | 99334_at |
| Csf2rb1 | −3.0 | NS | Colony-stimulating factor 2 receptor beta 1 | M34397 | 94748_g_at |
Expression levels of genes in I/St macrophages are given relative to A/Sn macrophages, with negative numbers indicating that the gene is expressed at a higher level in A/Sn macrophages.
NS, not significant.
Relative interstrain difference in gene expression in uninfected macrophages.
Relative interstrain difference in gene expression in infected macrophages.
Protein name abbreviation is given in case it differs from the gene name.
Results for these genes were confirmed by quantitative real-time assay.
Generally, lung macrophages from susceptible I/St mice demonstrated significantly higher expression levels of cytokine/chemokine genes, including the genes for interleukin 11 (Il-11), Il-6, Cxcl-13, and Cxcl-14 (Table 1), than did their A/Sn counterparts. In contrast, only three cytokine/chemokine genes (Cxcl-10, Cxcl-9, and Il-17) were expressed at significantly higher levels in macrophages from resistant A/Sn mice. In the group of immune response genes, I/St macrophages expressed only three genes (Ifi205, Ifi202, and Saa3) at a higher level than did A/Sn macrophages. Conversely, a large number of genes belonging to this class were expressed at significantly higher levels in A/Sn macrophages (Table 1), suggesting their critical role in the development of the immune response at an early stage of infection. The majority of genes encoding receptor/cell surface molecules that are potentially important for the on-time activation of protective mechanisms after infection were highly expressed in lung macrophages of A/Sn mice. Likewise, genes encoding signal transduction molecules were generally expressed at higher levels in A/Sn macrophages (see supplemental material S1). Interestingly, matrix metalloproteinase 8, one of the extracellular matrix proteins involved in the processing of extracellular matrices and wound healing (20), was shown to be expressed at significantly higher levels in A/Sn macrophages. Differences in constitutive expression levels for selected genes (Il-11, Il-6, Mmp8, Cxcl-14, Cxcl-13, and Saa3) were confirmed by real-time reverse transcription-PCR (RT-PCR) (Table 2; see also supplemental material S2) using mRNAs obtained in three additional independent experiments.
TABLE 2.
Real-time reverse transcription-PCR confirmation of array data
| Gene | In vitro infection with M. tuberculosis | Expression level for I/St vs A/Sn macrophages
|
|
|---|---|---|---|
| PCRa | Microarrayb | ||
| Il-11 | − | 8.9 | 5.0 |
| + | 7.6 | 3.7 | |
| Il-6 | − | 3.6 | 2.1 |
| + | 4.5 | 2.2 | |
| Mmp8 | − | −3.4c | NSd |
| + | −5.4 | −2.1 | |
| Cxcl14 | − | 11.1 | 4.2 |
| + | 9.6 | 3.5 | |
| Cxcl13 | − | 28.1 | 5.1 |
| + | 30.7 | 7.2 | |
| Saa3 | − | 9.3 | 2.5 |
| + | 12.3 | 2.3 | |
Mean fold change in expression level for three experiments.
Fold change in gene expression revealed by SAM analysis of three microarray hybridization sets.
Negative numbers indicate that the gene is expressed at a higher level in A/Sn macrophages.
NS, not significant.
Constitutive higher expression of Il-6 by macrophages of susceptible I/St mice is consistent with the data of Keller and colleagues, who demonstrated an approximately 10-fold increase in Il-6 expression in infected macrophages from TB-susceptible but not from TB-resistant mice (10). IL-6 is a pleiotropic cytokine which is produced by a variety of cells, including macrophages (14, 26), with numerous types of cell targets. M. tuberculosis-infected macrophages produce IL-6, which inhibits gamma interferon-responsive genes in macrophages and inhibits eradication of infection (14).
Remarkably, the high expression level of Il-6 by macrophages of I/St mice is accompanied by elevated levels of Cxcl-13 (Scyb13) expression (Tables 1 and 2). Cxcl-13, the B-cell-homing chemokine, is produced by macrophages (2, 9) and dendritic cells (3). Goya and colleagues (8) have shown that prolonged production of IL-6 in the lungs leads to formation of pulmonary lesions that have lymphoid tissue-like structure, where the chemokine gene Cxcl-13 is highly expressed. Significantly higher expression levels of Il-6 and Cxcl-13 by lung macrophages of susceptible I/St mice (Tables 1 and 2), in conjunction with extremely high levels of specific immunoglobulin G2a antibody responses in these mice (18), strongly suggest that severe TB inflammation in the lungs of these mice involves a nonprotective B-cell component. This suggestion is further supported by a recent finding of Ulrichs et al. (25), who demonstrated the formation of well-organized B-cell foci in the vicinity of tuberculous lesions in lung tissue surgically removed from TB patients with a rapidly progressing severe form of the disease.
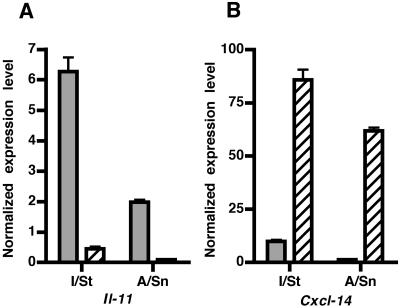
An exciting new finding obtained in this study is the high level of Il-11 expression by lung macrophages. IL-11 is a pleiotropic cytokine with anti-inflammatory activity when expressed at moderate levels (23, 27), but its overexpression may have a significant proinflammatory effect (22, 28). The production of IL-11 had previously been described for lung fibroblasts, airway epithelial cells (5, 6), and antigen-presenting cells after infection with respiratory syncytial virus (1). To demonstrate that Il-11 is indeed expressed by lung macrophages and not by contaminating lung fibroblasts, we developed fibroblast cultures from lung stroma of I/St and A/Sn mice and compared the levels of expression of Il-11 and Cxcl-14 in these cells and in interstitial lung macrophages. Cxcl-14 is the mouse ortholog of the human breast- and kidney-expressed chemokine gene (BRAK) and is constitutively expressed by fibroblasts in a number of mouse organs, including lungs. The results of this comparison are presented in Fig. 1. I/St and A/Sn lung macrophages expressed, respectively, 60- and 30-fold-higher levels of Il-11 than their corresponding lung fibroblasts. Conversely, I/St and A/Sn lung fibroblasts expressed 8- and 50-fold-higher levels of Cxcl-14 than their corresponding lung macrophages. These results show that lung macrophages are major producers of Il-11 and that the high expression levels of Il-11 in macrophages of tuberculosis-susceptible I/St mice compared to expression levels of Il-11 in tuberculosis-resistant A/Sn mice offer a possible explanation for the development of severe pathology in the lungs of M. tuberculosis-infected I/St mice (7, 16, 18).
FIG. 1.
Expression of Il-11 and Cxcl-14 by lung macrophages and fibroblasts isolated from TB-susceptible I/St mice and TB-resistant A/Sn mice. Normalized Il-11 and Cxcl-14 gene expression levels are shown as severalfold differences relative to Hprt gene expression. (A) Lung macrophages (gray bars) express higher levels of Il-11 than lung fibroblasts (hatched bars) from both I/St and A/Sn mice. (B) Lung fibroblasts (hatched bars) express higher levels of Cxcl-14 than macrophages (gray bars) from mice of both strains. In each experiment, syngeneic lung macrophages or lung fibroblasts extracted from several mice were pooled. Expression levels of Il-11 and Cxcl-14 were measured by quantitative RT-PCR. Results are expressed as means (±standard errors) of triplicate assays from one of three experiments with similar results.
While several studies have analyzed the response of host macrophages to mycobacterial infection (4, 10, 13, 15, 17, 19, 21,), none of these studies employed ex vivo-isolated lung macrophages, the predominant cell type naturally infected with M. tuberculosis, and only one study used a combination of resistant and susceptible strains of mice (10). However, all of these studies reported significant M. tuberculosis-triggered host gene expression changes. Surprisingly, we did not observe major changes in gene expression by lung macrophages of either I/St or A/Sn mice following 24-h infection with M. tuberculosis H37Rv. Hence, we tested whether overly conservative criteria for significant gene expression changes underlie this finding. It appeared that even a reduction in stringency of the analysis with an FDR up to 75% did not allow the reproduction of previously reported results (4, 10, 13, 17, 21). In a final set of experiments, we selected eight genes (Il-6, Saa3, Slpi, Ccl5, Cxcl-5, Cxcl-10, Mrc1, and Mmp9) that had been reported to undergo significant expression changes following M. tuberculosis infection of murine bone marrow-derived macrophages (4, 21, 10, 17). We found that infection of interstitial lung macrophages with M. tuberculosis does not lead to changes in the expression level of these genes (change of ≤1.5-fold [absolute value]) (data not shown). These results support the hypothesis that different types of macrophages respond differently to M. tuberculosis infection and argue against the suggestion that too-stringent criteria had been used in the microarray analysis.
In summary, by employing global analysis of gene expression, we observed a statistically well-defined signature of gene expression differences among interstitial macrophages from A/Sn and I/St mice. These interstrain gene expression differences provide a rational basis for a mechanistic framework of the genetically controlled tuberculosis resistance and susceptibility displayed by A/Sn and I/St mice. By contrast, we were unable to reveal significant M. tuberculosis-triggered gene expression changes in interstitial lung macrophages. It is possible that the in vitro infection experiments are not a correlate of the response of the whole animal. This possibility appears unlikely since lung macrophages faithfully repeat the pattern of resistance and susceptibility observed at the whole-animal level. It is more likely that intrinsic gene expression levels are an important determinant of TB pathogenesis in the mouse and that constitutive genetically controlled gene expression in lung macrophages is an area that requires more careful consideration in the study of TB pathogenesis.
Supplementary Material
Acknowledgments
This work was supported by NIH grant HL 68532 and the Canadian Genetic Disease Network.
We thank Scotty Adams (Trudeau Institute, Saranac Lake, NY) for help with the quantitative RT-PCR primers and Serge Mostowy (McGill University) for helpful comments on the experimental design of the study.
Editor: A. D. O'Brien
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bartz, H., F. Buning-Pfaue, O. Turkel, and U. Schauer. 2002. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin. Exp. Immunol. 129:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlsen, H. S., E. S. Baekkevold, H. C. Morton, G. Haraldsen, and P. Brandtzaeg. 2004. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood 104:3021-3027. [DOI] [PubMed] [Google Scholar]
- 3.Cupedo, T., F. E. Lund, V. N. Ngo, T. D. Randall, W. Jansen, M. J. Greuter, R. de Waal-Malefyt, G. Kraal, J. G. Cyster, and R. E. Mebius. 2004. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J. Immunol. 173:4889-4896. [DOI] [PubMed] [Google Scholar]
- 4.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias, J. A., T. Zheng, N. L. Whiting, T. K. Trow, W. W. Merrill, R. Zitnik, P. Ray, and E. M. Alterman. 1994. IL-1 and transforming growth factor-beta regulation of fibroblast-derived IL-11. J. Immunol. 152:2421-2429. [PubMed] [Google Scholar]
- 6.Elias, J. A., T. Zheng, O. Einarsson, M. Landry, T. Trow, N. Rebert, and J. Panuska. 1994. Epithelial interleukin-11. Regulation by cytokines, respiratory syncytial virus, and retinoic acid. J. Biol. Chem. 269:22261-22268. [PubMed] [Google Scholar]
- 7.Eruslanov, E. B., K. B. Majorov, M. O. Orlova, V. V. Mischenko, T. K. Kondratieva, A. S. Apt, and I. V. Lyadova. 2004. Lung cell responses to M. tuberculosis in genetically susceptible and resistant mice following intratracheal challenge. Clin. Exp. Med. 135:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goya, S., H. Matsuoka, M. Mori, H. Morishita, H. Kida, Y. Kobashi, T. Kato, Y. Taguchi, T. Osaki, I. Tachibana, N. Nishimoto, K. Yoshizaki, I. Kawase, and S. Hayashi. 2003. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J. Pathol. 200:82-87. [DOI] [PubMed] [Google Scholar]
- 9.Ito, T., S. Ishikawa, T. Sato, K. Akadegawa, H. Yurino, M. Kitabatake, S. Hontsu, T. Ezaki, H. Kimura, and K. Matsushima. 2004. Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J. Immunol. 172:3628-3634. [DOI] [PubMed] [Google Scholar]
- 10.Keller, C., J. Lauber, A. Blumenthal, J. Buer, and S. Ehlers. 2004. Resistance and susceptibility to tuberculosis analyzed at the transcriptome level: lessons from mouse macrophages. Tuberculosis 84:144-158. [DOI] [PubMed] [Google Scholar]
- 11.Majorov, K. B., I. V. Lyadova, T. K. Kondratieva, E. B. Eruslanov, E. I. Rubakova, M. O. Orlova, V. V. Mischenko, and A. S. Apt. 2003. Different innate ability of I/St and A/Sn mice to combat virulent Mycobacterium tuberculosis: phenotypes expressed in lung and extrapulmonary macrophages. Infect. Immun. 71:697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majorov, K. B., E. B. Eruslanov, E. I. Rubakova, T. K. Kondratieva, and A. S. Apt. 2005. Analysis of cellular phenotypes that mediate genetic resistance to tuberculosis using a radiation bone marrow chimera approach. Infect. Immun. 73:6174-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGarvey, J. A., D. Wagner, and L. E. Bermudez. 2004. Differential gene expression in mononuclear phagocytes infected with pathogenic and non-pathogenic mycobacteria. Clin. Exp. Immunol. 136:490-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagabhushanam, V., A. Solache, L. M. Ting, C. J. Escaron, J. Y. Zhang, and J. D. Ernst. 2003. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J. Immunol. 171:4750-4757. [DOI] [PubMed] [Google Scholar]
- 15.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikonenko, B. V., M. M. Averbakh, C. Lavebratt, E. Schurr, and A. S. Apt. 2000. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tuberc. Lung Dis. 80:15-25. [DOI] [PubMed] [Google Scholar]
- 17.Pai, R. K., M. E. Pennini, A. A. Tobian, D. H. Canaday, W. H. Boom, and C. V. Harding. 2004. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 72:6603-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radaeva, T. V., B. V. Nikonenko, V. V. Mischenko, M. M. Averbakh, Jr., A. S. Apt. 2005. Direct comparison of low-dose and Cornell-like models of chronic and reactivation tuberculosis in genetically susceptible I/St and resistant B6 mice. Tuberculosis 85:65-72. [DOI] [PubMed] [Google Scholar]
- 19.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro, S. D., and R. M. Senior. 1999. Matrix metalloproteinases. Matrix degradation and more. Am. J. Respir. Cell Mol. Biol. 20:1100-1102. [DOI] [PubMed] [Google Scholar]
- 21.Shi, S., C. Nathan, D. Schnappinger, J. Drenkow, M. Fuortes, E. Block, A. Ding, T. R. Gingeras, G. Schoolnik, S. Akira, K. Takeda, and S. Ehrt. 2003. MyD88 primes macrophages for full-scale activation by interferon-gamma yet mediates few responses to Mycobacterium tuberculosis. J. Exp. Med. 198:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang, W., G. P. Geba, T. Zheng, P. Ray, R. J. Homer, C. Kuhn III, R. A. Flavell, and J. A. Elias. 1996. Targeted expression of IL-11 in murine airway causes lymphocytic inflammation, bronchial remodeling and airways obstruction. J. Clin. Investig. 98:2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trepicchio, W. L., M. Bozza, G. Pedneault, and A. Dorner. 1996. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of pro-inflammatory cytokine release and nitric oxide production. J. Immunol. 157:3627-3634. [PubMed] [Google Scholar]
- 24.Tusher, V. G., R. Tibshirani, and R. Chu. 2000. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulrichs, T., G. A. Kosmiadi, V. Trusov, S. Jorg, L. Pradl, M. Titukhina, V. V. Mischenko, N. Gushina, and S. H. Kaufmann. 2004. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defense in the lung. J. Pathol. 204:217-228. [DOI] [PubMed] [Google Scholar]
- 26.Van Snick, J., A. Vink, S. Cayphas, and C. Uyttenhove. 1987. Interleukin-HP1, a T cell-derived hybridoma growth factor that supports the in vitro growth of murine plasmacytomas. J. Exp. Med. 165:914-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walmsley, M., D. M. Butler, L. Marinova-Mutafchieva, and M. Feldmann. 1998. An anti-inflammatory role for interleukin-11 in established murine collagen-induced arthritis. Immunology 95:31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, P. K. K., I. K. Campbell, L. Robb, and I. P. Wicks. 2005. Endogenous IL-11 is pro-inflammatory in acute methylated bovine serum albumin/IL-1-induced (mBSA/IL-1) arthritis. Cytokine 29:72-76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.