Abstract
Needle biopsy samples were taken from vastus lateralis muscle (VL) of five male body builders (BB, age 27.4 ± 0.93 years; mean ±s.e.m.), who had being performing hypertrophic heavy resistance exercise (HHRE) for at least 2 years, and from five male active, but untrained control subjects (CTRL, age 29.9 ± 2.01 years). The following determinations were performed: anatomical cross-sectional area and volume of the quadriceps and VL muscles in vivo by magnetic resonance imaging (MRI); myosin heavy chain isoform (MHC) distribution of the whole biopsy samples by SDS-PAGE; cross-sectional area (CSA), force (Po), specific force (Po/CSA) and maximum shortening velocity (Vo) of a large population (n= 524) of single skinned muscle fibres classified on the basis of MHC isoform composition by SDS-PAGE; actin sliding velocity (Vf) on pure myosin isoforms by in vitro motility assays. In BB a preferential hypertrophy of fast and especially type 2X fibres was observed. The very large hypertrophy of VL in vivo could not be fully accounted for by single muscle fibre hypertrophy. CSA of VL in vivo was, in fact, 54% larger in BB than in CTRL, whereas mean fibre area was only 14% larger in BB than in CTRL. MHC isoform distribution was shifted towards 2X fibres in BB. Po/CSA was significantly lower in type 1 fibres from BB than in type 1 fibres from CTRL whereas both type 2A and type 2X fibres were significantly stronger in BB than in CTRL. Vo of type 1 fibres and Vf of myosin 1 were significantly lower in BB than in CTRL, whereas no difference was observed among fast fibres and myosin 2A. The findings indicate that skeletal muscle of BB was markedly adapted to HHRE through extreme hypertrophy, a shift towards the stronger and more powerful fibre types and an increase in specific force of muscle fibres. Such adaptations could not be fully accounted for by well known mechanisms of muscle plasticity, i.e. by the hypertrophy of single muscle fibre (quantitative mechanism) and by a regulation of contractile properties of muscle fibres based on MHC isoform content (qualitative mechanism). Two BB subjects took anabolic steroids and three BB subjects did not. The former BB differed from the latter BB mostly for the size of their muscles and muscle fibres.
It is well established that skeletal muscle can adapt to the variable functional requirements through a quantitative mechanism based on changes in muscle mass and fibre size, and a qualitative mechanism based on a change in fibre type distribution. Human muscles are in fact mixed muscles expressing three main fibre types, type 1, 2A and 2X in variable proportions (Harridge et al. 1996). Type 1, 2A and 2X fibres, in turn, differ in contractile and energetic properties that are known to depend on their myosin heavy chain (MHC) isoform content (Bottinelli & Reggiani, 2000). Type 1 fibres contain the myosin heavy chain 1 (MHC-1) isoform and have lower maximum shortening velocity (Vo), maximum power (Wmax) and ATPase activity (ATPase), and slower kinetics of stretch activation (Hilber et al. 1999) than type 2X fibres, which contain MHC-2X (Bottinelli et al. 1996; Stienen et al. 1996). Type 2A fibres contain MHC-2A and are intermediate. Moroever, specific force (Po/CSA) is also lower in type 1 than in type 2A and 2X fibres, whereas no difference is seen between 2A and 2X fibres.
Exercise training is a major factor shaping muscle phenotype. Resistance training has been studied extensively and it is now well known that it determines both muscle hypertrophy (quantitative mechanism) and a shift of fibre type distribution (qualitative mechanism) (Schiaffino & Reggiani, 1996; Bottinelli & Reggiani, 2000; Fluck & Hoppeler, 2003). However, several open issues remain.
According to a long-lasting (Morpurgo, 1879) and well supported belief (Gollnick et al. 1981; Gollnick et al. 1983), it is generally assumed that the increase in muscle mass can be fully accounted for by single muscle fibre hypertrophy. It should be noted, however, that several findings suggest that hyperplasia can also occur, at least in some animals (rat, cat, chicken) and in some conditions (compensatory hypertrophy due to synergist ablation and tenotomy; chronic stretch; resistance training) (Antonio & Gonyea, 1993). Moreover, the few studies performed on body builders have generally reported a limited and inconsistent hypertrophy of muscle fibres, failing to account for the obvious and extreme hypertrophy of the muscles, although a precise quantitative analysis was not performed (MacDougall et al. 1982; Tesch & Larsson, 1982).
As regards single muscle fibre properties, it has been recently shown that the myosin isoform-based dependence of contractile properties of muscle fibres might have relevant exceptions (Bottinelli, 2001). In ageing and disuse, in fact, specific tension (Po/CSA) and unloaded shortening velocity (Vo) of slow (type 1) and fast (type 2A and 2X) fibres have been shown to change (Larsson et al. 1997; D'Antona et al. 2003). It is unclear whether changes in Po/CSA and Vo of muscle fibres can also occur in young healthy subjects following training. In two recent studies on resistance training (Widrick et al. 2002; Shoepe et al. 2003), an increase in CSA and force (Po) of muscle fibres, but no change in Po/CSA and Vo of slow and fast fibres, was observed, suggesting that the properties of a given fibre type change mainly by a quantitative mechanism (increase in CSA and absolute force). However, an increase in Vo of type 1 and 2A fibres has been observed in highly trained swimmers following a decrease in training intensity (Trappe et al. 2000), and variations in Po/CSA have been observed in a very recent study on cross-country runners during a competitive season and changes in endurance training regime (Harber et al. 2004).
Finally, it is still unclear how large and how relevant for muscle function in vivo the adaptation of fibre type distribution to training can be (Ingalls, 2004). In longitudinal studies the training-induced shift in fibre type composition is often small and surprisingly similar for endurance (Baumann et al. 1987) (duration 8 weeks) and resistance training (Adams et al. 1993; Andersen et al. 1994; Liu et al. 2003a, b) (duration from 3 weeks to 3 months). A type 2X → 2A shift is mostly observed in spite of the different effects of the two paradigms on muscle mass and metabolism (Fluck & Hoppeler, 2003). On the contrary, comparative studies of different subject populations (cross-sectional studies) have shown a strong bias in fibre type distribution towards fast fibres in elite sprinters (∼70% type 2A and type 2X) and towards slow fibres in elite marathon runners (60–90% type 1 fibres) (Sjostrom et al. 1988; Andersen et al. 2000). The inconsistency between the former (longitudinal) and the latter (cross-sectional) studies could be due either to the longer and more intense training of elite athletes or to a genetically determined bias of fibre type distribution towards fast fibres in sprinters and slow fibres in marathon runners.
To address the above issues, we reasoned that body builders (BB) in which muscle adaptations were expected to be particularly evident due to the very long and intense resistance training could represent a valuable model. BB that performed hypertrophic heavy resistance exercise (HHRE), a type of resistance training specifically designed to increase muscle mass, for at least 2 years to compete at national and international level were enrolled in the study. Muscle phenotype was characterized by extreme muscle hypertrophy, that could not be fully accounted for by single muscle fibre hypertrophy, by a significant expression of MHC-2X, the fastest and most powerful MHC isoform which is very little present in trained young subjects (Mizuno, 1991), and by a significantly higher specific force of fast and especially 2X muscle fibres in relation to controls. Muscle phenotype was therefore clearly adapted to HHRE, but such adaptation was not fully accounted for by the known qualitative and quantitative mechanisms of muscle plasticity.
A preliminary report of the present data has been presented in abstract form (Pellegrino et al. 2003).
Methods
Subjects
Five male, body-building athletes (BB) (body weight 77.5 ± 3.4 kg; height 170.0 ± 3.3 cm; age 27.4 ± 0.93 years; means ±s.e.m.), volunteered to participate in the study. HHRE had been systematically and continuously practised for at least 2 years in order to participate in national and international competitions. The study was approved by the ethical committee of the University of Pavia and conformed to the standards set by the Declaration of Helsinki (last modified in 2000). After subjects were fully informed of the goal of the experiments and of the risks involved in the bioptic procedure, written informed consent was obtained. Five male subjects (age 29.9 ± 4.5 years; height 178 ± 3.5 cm; weight 75.3 ± 4.4 kg) performing recreational physical activity, but with no previous history of resistance training or of any other specific training, were recruited as a control group (CTRL).
Training
A careful diary of the past 2 years and specifically of the last 12 weeks of training was collected. Athletes were engaged in a classical model of progressive resistance training that targets all major upper and lower muscle groups. Over the previous year resistance training was conducted no less than three times per week. A heavy resistance hypertrophic protocol consisted of medium high loads (60–80% of one-repetition maximum strength or 1RM) with multiple repetitions (6–12) performed in each working set (4–5). There were 1–2 min rest periods between sets at moderate velocity of contraction (1–2 s concentric and 1–2 s eccentric). Five different resistance-training exercises for legs were performed in the fixed order: incline leg press; hack squat; knee extension; hamstring curl; and calf raise (Kraemer et al. 2002).
Diet
Athletes assumed a hyperproteic diet, with a protein intake of 1.5–2.5 g per kg each day.
Drug consumption and hormonal exams
A careful pharmacological anamnesis was collected. Three athletes declared they had not taken any drug, whereas two athletes declared they had taken an androgenic-anabolic steroid (AAS) regimen. They have reported the use of high dosage of several types of anabolic steroids simultaneously (‘staking’ therapy: testosterone, nandrolone decanoate, stanozolol, metandienone). The drugs were taken assumed in cycles: a drug free period was followed by an increased dosage to a maximum to anticipate peak performance. Biochemical and hormonal exams were collected in order to evaluate clinical conditions and drug abuse that was not declared. The two athletes that declared AAS abuse showed a hormonal profile of anabolic steroid-induced hypogonadotropic hypogonadism, due to the drug-free period of the cycling therapy. No indications of drug abuse were found in biochemical and hormonal exams of the three BB that declared they had not taken any drug.
Muscle biopsies
Muscle samples (about 50–100 mg) were obtained from the mid right vastus lateralis (VL) muscle of all subjects by needle biopsy (Bergstrom, 1962), 4 days after the last exercise involving quadriceps muscle. After the biopsy, muscle samples were put in skinning solution at ∼4.0°C, divided in small fibre bundles (∼100 fibres each) and finally stored at −20°C in skinning solution and 50% glycerol for up to 3 weeks before experiment. Bundles were mostly used for dissection of single muscle fibres for determination of CSA, force (Po) and maximum shortening velocity (Vo), for dissection of single muscle fibres for myosin extraction and in vitro motility assays experiments, and for determination of MHC isoform distribution by SDS-PAGE. Some small bundles of some biopsies were left after such experiments and were used for dissection of single muscle fibres to precisely determine the shape of the cross-section of single muscle fibres from control and BB subjects (see below).
Single fibre analysis
Cross-sectional area (CSA), force and maximum shortening velocity of single muscle fibres were analysed as previously described in detail (Bottinelli et al. 1994, 1996). The following solutions were prepared as previously described (Bottinelli et al. 1994, 1996): skinning solution: 150 mm potassium propionate, 5 mm KH2PO4, 5 mm magnesium acetate, 3 mm Na2ATP, 5 mm EGTA, pCa 9.0; relaxing solution: 100 mm KCl, 20 mm imidazole, 5 mm MgCl2, 5 mm Na2ATP, 5 mm EGTA, pCa 9.0; preactivating solution: 100 mm KCl, 20 mm imidazole, 5 mm MgCl2, 5 mm Na2ATP, 0.5 mm EGTA, 25 mm creatine phosphate, 300 U ml−1 creatine kinase, pCa 8.0; and activating solution: 100 mm KCl, 20 mm imidazole, 5 mm MgCl2, 5 mm Na2ATP, 5 mm EGTA, 25 mm creatine phosphate, 300 U ml−1 creatine kinase, pCa 4.5. The pH of all solutions was set at 7.0. Briefly, segments of single fibres were manually isolated from muscle bundles with the help of a stereomicroscope. The fibres were immersed for 1 h in a solution containing 1% Triton X-100 and afterward returned to the previous skinning solution. Using a dissecting microscope at 40–60× magnification, light aluminium clips were attached to both ends of the fibre segments (1.5–2 mm long), which were then transferred to the experimental set-up for mechanical measurements. The set-up enabled the determination of the CSA of muscle fibre segments, isometric force (Po) and unloaded shortening velocity (Vo) by the slack test technique. Experiments were performed at 12°C, in conditions of maximal activation (pCa 4.5) and at optimal sarcomere length for force developing (Bottinelli & Reggiani, 2000; D'Antona et al. 2003).
Determination of fibre cross-sectional area and of its shape
The set-up was placed on the stage of an inverted microscope (Axiovert 10, Zeiss, Germany) that enabled viewing of the fibre segment at 320× magnification. As previously described (Bottinelli et al. 1994; D'Antona et al. 2003), CSA was calculated from the mean of three diameters measured along the length of the fibre segment looking at it from below through the eyepieces of the inverted microscope. Skeletal muscle fibres did not always have a circular cross-section. In this latter case, due to the procedure used to mount the fibre in the apparatus, the larger of the two main diameters was that viewed from below. As CSA was calculated assuming a circular shape, an over-estimation of CSA could occur. The extent of such over-estimation was going to depend on the shape of the cross-section of the fibres: the less circular the shape, the larger could be the over-estimation. To make sure that variation in the shape of the cross-section of skinned muscle fibres between control subjects and body builders, and between slow and fast fibres did not introduce a systematic error in the measurements of CSA, two control experiments were performed. Such experiments assessed the ratio between the larger and smaller diameters of a population of fibres from both subject groups and suggested that such ratio, and therefore the shape of the CSA, was not different in CTRL and BB and between slow and fast fibres.
The first experiment was designed to assess very precisely the CSA of identified types of single muscle fibres in the same set-up and conditions used for functional analysis. Few fibre bundles were left after all analyses were performed and were used for this purpose. Such bundles enabled dissection of extra fibres from three control subjects and three BB (n= 50 from controls and n= 50 from BB). Fibre segments (1–2 mm long) were laid down straight at the bottom of a muscle chamber filled with preactivating solution. Ten diameters were determined at 320× magnification along the length of each segment turning it along its longitudinal axis to make sure that all the representative diameters could be measured. Fibres were thereafter characterized on the basis of MHC isoform composition. The larger diameter was calculated averaging the five larger diameters and the smaller diameter was calculated averaging the five smaller diameters. Both the larger and the smaller diameter were used to determine the CSA of the fibres assuming an elliptical shape (the approach that provides a more precise assessment of CSA), whereas the larger diameter only was used to determine the CSA of the fibres assuming a circular shape (the approach that for technical reasons is mostly used during mechanical experiments on skinned fibres). The whole procedure to determine the two CSAs was chosen on purpose to assess the largest error that can be possibly involved in CSA determination during mechanical experiments. The difference between the CSA calculated assuming an elliptical and circular shape was: 1.18 ± 0.15-fold for the slow fibres of controls (n= 28); 1.20 ± 0.17 for the fast fibres of controls (a pool of 2A, 2X and 2AX fibre, n= 22); 1.17 ± 0.15 for the slow fibres of BB (n= 20); and 1.20 ± 0.16 for the fast fibres of BB (n= 30).
The second experiment took advantage of some small fibre bundles that were frozen immediately after the biopsy and determined the ratio between the larger and smaller diameter of individual fibres on histological cross-sections. As discussed below, the size of the biopsy did not enable us to store muscle bundles of the appropriate size to perform a detailed immuno-histochemical analysis of the CSA of the fibres. However, at the beginning of the research project some bundles of three BB subjects and some bundles of one control subject were frozen after being skinned for 2 hours on the day of the biopsy. Such bundles turned out to be far too small to enable determination of CSA on a large number of fibres. Moreover, they could not be spared from all biopsies and subjects. Consequently, a systematic analysis of CSA was performed on isolated single fibres during mechanical experiments. However, some bundles could be immuno-stained with two monoclonal antibodies against MHC-1 (BA-F8; DSMZ – Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Germany) and MHC-2A (SC-71; DSMZ) and could be used for determination of the larger and smaller diameter of a population of 120 individual muscle fibres using freely available image analysis software (Scion Image, Scion Corp., USA). A Figure of the immunostained cross-sections is presented as on-line Supplemental material. As the bundles were skinned and very small, such determination should provide results qualitatively comparable to those obtained from the determination on isolated single skinned fibres (see above). The difference between the CSA calculated assuming an elliptical and circular shape using the diameters from immuno-stained cross-sections was: 1.20 ± 0.15 (n= 28) for the slow fibres of the control; 1.22 ± 0.13 (n= 30) for the fast fibres of the control subjects; 1.17 ± 0.13 (n= 30) for the slow fibres of the BB subjects; and 1.22 ± 0.17 (n= 32) for the fast fibres of BB subjects. Therefore, both experiments performed to check the possible error involved in determining CSA during a mechanical experiment indicate that, at the most, the overestimation of the CSA could be 20% and, more importantly, that the shape of the cross-section of the fibres was the same in CTRL and BB and in slow and fast fibres. Therefore, no systematic bias can be introduced by the way CSA has been routinely determined during mechanical experiments and the data of CSA and Po/CSA of single fibres could be safely used for comparative purposes between controls and BB.
As regards the error in determining the size of the fibres at 320× magnification, the diameter of the fibres was determined using a scale in the eyepiece of the microscope, having small divisions of 2.96 μm. The width of one division represents the largest possible error of the measurements. As the average diameter of the fibres ranged between 28 and 40 divisions, the largest possible error in CSA determination using the scale in the eyepiece ranged between 3.5 and 2.5% and was the same for all specimens.
Myosin isoforms identification
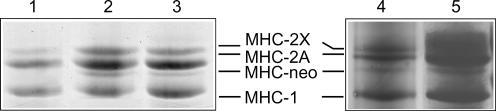
Separation and identification of myosin heavy chain (MHC) isoforms was performed as previously described (Bottinelli et al. 1996; D'Antona et al. 2003). Briefly, samples were dissolved in Laemmli solution (Laemmli, 1970) and loaded on 6% polyacrylamide gels. Electrophoresis was run for 20 h at 100 V. Samples were either the single muscle fibre segments used for mechanical experiments or fibre bundles obtained from the biopsy sample. To ensure that the latter samples were representative of the whole biopsy, cross-sections about 1 mm long of all the bundles of the biopsy were pooled. Electrophoresis of single muscle fibre segments were silver stained, as such staining, being more sensitive, is preferable when small amounts of protein are to be detected. Electrophoresis of fibre bundles were coomassie (Brilliant Blue R-250) stained, as such staining enables a more precise quantification of the protein bands on gels. In the region of MHC isoforms, three major bands were separated that corresponded, in order of migration from the fastest to the slowest, to MHC-1 or MHC-2A and MHC-2X. In relation to the presence of one or two bands in the MHC region, single fibres were classified in one of the following types: 1, 2A, 2X (pure fibres) and 1–2A, 2A–2X (mixed fibres) (D'Antona et al. 2003). In all BB a fourth, faint band was observed (Fig. 1, lanes 2–3). The myosin nature of the band was assessed by extracting myosin from the muscle bundles, using an extraction procedure previously described (Canepari et al. 1999), and loading pure myosin on gels (Fig. 1). The neonatal nature of the protein band was demonstrated loading in adjacent lanes (Fig. 1, lanes 4–5) the biopsy sample from BB with a muscle sample from an elderly immobilized subject, which was previously demonstrated to contain a fourth MHC corresponding to neonatal MHC (MHC-neo) (D'Antona et al. 2003). In the gel of Fig. 1 (lane 5), the BB sample was overloaded to make the MHC-neo band more evident. The latter indirect approach was necessary as the only available antibody against human MHC-neonatal (NCL-MHCn, Novocastra, Newcastle, UK) does not work on the denatured protein and therefore in Western blots. Densitometric scans of the four MHC bands were used to establish the relative proportion of the MHC isoforms identified in the biopsy samples (Harridge et al. 1996).
Figure 1. Electrophoretic (SDS-PAGE) separation of myosin heavy chain (MHC) isoforms in bioptic samples of vastus lateralis muscles from control subjects and body builders (BB).
All samples were pure myosin extracted from muscle bundles. Gels were coomassie stained. Lane 1: sample from a control subject; lane 2–3: samples from two BB; lane 4: sample from the vastus lateralis muscle of an elderly immobilized subject from D'Antona et al. (2003); lane 5: sample from a BB subject. The area of migration of MHC-1, 2A, 2X and MHC-neonatal (MHC-neo) is indicated in the middle. In lane 2 and 3 (BB) a fourth MHC band in addition of the three adult MHC is observed. In lanes 5 such an extra band is identified as MHC-neo as it comigrates with a MHC band previously identified as MHC-neo in the vastus lateralis sample loaded in lane 4 (D'Antona et al. 2003). Lane 5 is overloaded to make the faint band of MHC-neo more visible.
Myosin light chain (MLC) separation was performed as previously described (Bottinelli et al. 1994) on 10–20% linear polyacrylamide gradient slab gels. Two regulatory MLC isoforms, the slow isoform MLC2s and the fast isoform MLC2f, and three alkali MLC isoforms, the slow MLC1s and the two fast isoforms MLC1f and MLC3f, were separated.
Myosin extraction and in vitro motility assays (IVMA)
To be able to study actin sliding velocity on pure myosin isoforms, an approach based on extraction of myosin from single pure fibres was used (Canepari et al. 1999). As single fibres mostly contain only a type of MHC isoform they are a convenient source of pure myosin isoforms. However, due to the short length of the fibres in a needle biopsy sample, one fibre segment does not provide sufficient myosin to be used in IVMA. Therefore (i) single fibres were dissected, (ii) SDS-PAGE was performed (Bottinelli et al. 1994) to identify MHC isoform content, (iii) three to four fibres shown to contain the same MHC isoform were pooled, (iv) myosin was extracted from pooled fibres, and (v) this myosin was loaded in IVMA. The myosin samples were put as a drop on a coverslip with nitrocellulose, which was then used to construct the flow cell. The IVMA was carried out as previously described (Anson et al. 1995; Canepari et al. 1999). The sliding of actin filaments was studied in an assay buffer of the following composition: 25 mm Mops (pH 7.2), 25 mm KCl, 4 mm MgCl2, 1 mm EGTA, 1 mm DTT, 200 μg ml−1 glucose oxidase, 36 μg ml−1 catalase, 5 mg glucose and 2 mm ATP. The actin sliding velocity (Vf) on myosin samples was determinated at 25°C, 50 mm ionic strength and pH 7.2. The analysis of the velocity of each sample was done as described by Canepari et al. (1999). For each myosin sample the velocities of about 50 filaments were measured and their distribution characterized according to parametric statistics.
Anatomical CSA of vastus lateralis muscles
Muscle CSA and volume were measured from spin-echo, T1-weighted axial magnetic resonance images (MRI) recorded with a 0.3-tesla magnet (Siemens, Germany). To minimize the potential influence of fluid shifts on muscle size in the transition from the upright position, the subject remained supine for half an hour prior of the start of scanning (Tesch et al. 2004). Subjects also refrained from excessive muscle exercise for 4 days before the MRI. Seven axial slices (slice thickness 10 mm) interspaced by a distance of 1/10 femur length were obtained at 20, 30, 40, 50, 60, 70 and 80% of femur length. Anatomical CSA (ACSA) of the quadriceps muscle was determined in each axial image using public domain software package (Scion Image Beta 4.0.2 for Windows, Scion Corporation, Frederick, MD, USA). Volumes were calculated by the summation of successive CSA values, each multiplied by the respective slice thickness and interslice distance. In the present study, athletes showed a lack of distinct fasciae boundaries between the lateral and deep vastii (VL and DV) and between the medial (MV) and deep vastii at the proximal axial MRI scans (Aagaard et al. 2001). However, ACSA of the vastus lateralis muscle could be determined in several slices and the ACSA at the level of the biopsy site was used for comparison.
Mean fibre area
For the purpose of the present study, it was interesting to evaluate whether hypertrophy of individual muscle fibres could be sufficient to account for the larger size of the whole vastus lateralis muscle assessed by MRI. For the latter purpose mean fibre area (MFA) of vastus lateralis muscle was determined from CSA of the individual muscle fibres of CTRL and BB using the expression:
The CSA of individual fibres was measured during the mechanical experiments used for force and velocity determinations. The size of the muscles samples obtained by needle biopsy was just sufficient to enable dissection of the large number of fibres required for mechanical experiments and for myosin extraction for in vitro motility assays. No bundles of the appropriate size for CSA determination on immunostained cross-sections could be spared. It should be noted that, although muscle fibres swell upon skinning, i.e. their diameter increases ∼20% (Godt & Maughan, 1977), such a phenomenon does not limit the use of MFA for comparative purposes. The calculation of MFA assumes the same number of fibres in the muscles considered, i.e. it actually determines the CSA of 100 fibres of the vastus lateralis of BB and control subjects. MFA can provide a precise estimate of the percent increase in the CSA of the vastus lateralis muscle of BB expected on the basis of the differences in the CSA of individual muscle fibre types between BB and control subjects. However, MFA determination is not meant to provide a precise value of the actual whole muscle CSA. Since in humans for technical and ethical reasons it is not possible to determine the total number of fibres in a muscle, MFA has been widely used for similar purposes.
Statistical analysis
Data were expressed as means ±s.e.m. Statistical significance of the differences between means was assessed by Student's t test and ANOVA followed by the Student-Newman-Keuls test. A probability of less than 5% was considered significant (P < 0.05).
Results
As the body builders (BB) that took anabolic steroids and BB that did not take any drug were different only in a few of the parameters studied and all conclusions drawn from the data appeared independent from anabolic steroid misuse, the results of all BB subjects were pooled and reported together. The few significant differences are briefly reported at the end.
Anatomical cross-sectional area of vastus lateralis muscle
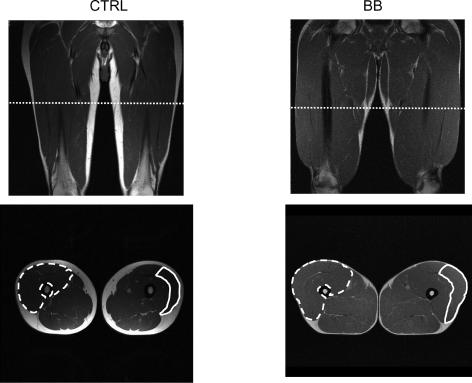
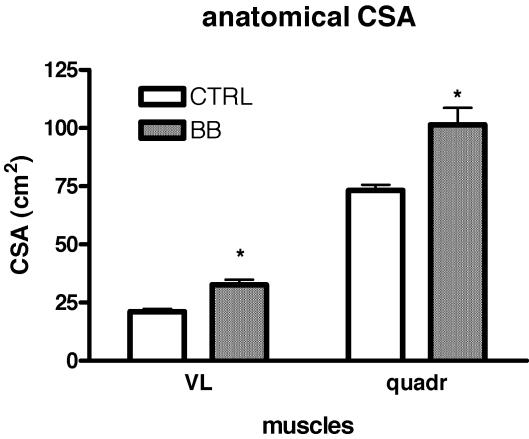
Figure 2 shows coronal and axial images of the thighs at 50% femur length, the level at which needle biopsy samples were obtained, of a CTRL and a BB subject. At this level not only the perimeter of the quadriceps, but also that of the vastus lateralis muscle could be identified and their anatomical cross-sectional areas could be determined. The mean values of the anatomical cross-sectional area of the quadriceps (ACSAqd) and of the vastus lateralis (ACSAvl) were significantly higher in BB than in CTRL subjects with BB having ACSAqd 34% larger and ACSAvl 54% larger than CTRL (Fig. 3). The volume of the quadriceps could be determined using the approach reported in the methods and was found to be 1541 ± 39 cm3 in CTRL and 2093 ± 172 cm3 in BB with a difference between CTRL and BB of 34%.
Figure 2. MRI images of the thigh of control subjects (CTRL) and body builders (BB).
Upper panels: coronal images of the thigh. Lower panels: axial images of the thigh. In the lower panels: the edges of quadriceps muscles of the right thigh are indicated by a dashed line; the edges of the vastus lateralis muscle of the left tight are indicated by a continuous line.
Figure 3. Mean values (±s.e.m.) of the anatomical cross-sectional area (ACSA) of the vastus lateralis (VL) and quadriceps (quadr) muscles of control subjects (CTRL) and body builders (BB).
CSAs were determined from MRI images and expressed in cm2. *Significantly different from CTRL (P < 0.05).
MHC isoform distribution
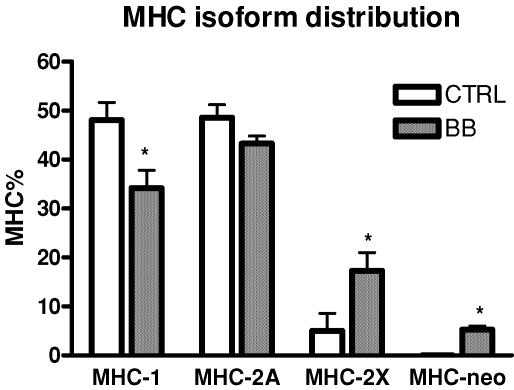
The relative content in MHC-2X of vastus lateralis muscle was significantly higher in BB than in controls, whereas the MHC-1 was significantly lower in BB (Fig. 4). In all BB, but in none of the controls, a small percentage (∼5%) of the neonatal isoform of MHC (MHC-neo) was observed.
Figure 4. Myosin heavy chain (MHC) isoform distribution of the vastus lateralis muscles of control subjects (CTRL) and body builders (BB).
MHC isoform distribution was determined from biopsy samples by SDS-PAGE and subsequent densitometric analysis of MHC bands. *Significantly different from CTRL (P < 0.05). Values are mean ±s.e.m.
Single muscle fibre analysis
A large population of individual muscle fibres was dissected from needle biopsy samples of the CTRL (n= 219) and the BB (n= 305) subjects and used for functional analysis.
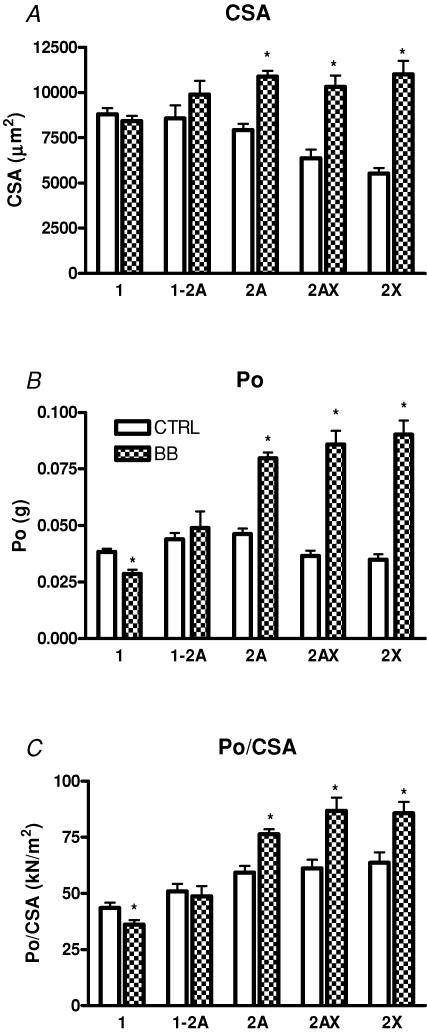
In CTRL subjects the CSA was significantly higher in slow fibres than in 2AX and 2X fibres, whereas type 2A fibres were intermediate (Fig. 5A). In contrast, in BB slow fibres were significantly smaller than fast (type 2A, 2AX and 2X) fibres. Type 2A, 2AX and 2X fibres were significantly larger in BB than in CTRL, whereas no significant difference was observed among type 1 and type 1–2A fibres. Among fast fibres, hypertrophy was significantly more pronounced in type 2X and type 2AX fibres than in type 2A fibres. Type 2X and 2AX fibres were 92% and 61% larger in BB than in CTRL, respectively, whereas type 2A fibres were 37% larger in BB than in CTRL.
Figure 5. Mean values (s.e.m.) of cross-sectional area (CSA) (A), absolute force (Po) (B) and specific force (Po/CSA) (C) of the single muscle fibre used for mechanical experiments from control subjects (CTRL) and from body builders (BB).
Experiments were performed at 12°C. CSA is expressed in μm2, Po in g, Po/CSA in kN m−2. Fibres were identified on the basis of their MHC isoform content by SDS-PAGE. The height of the bars represents the mean values (±s.e.m.). The numbers of fibres per type and per group were: 80 (CTRL) and 84 (BB) for type 1; 20 (CTRL) and 21 (BB) for type 1–2A; 85 (CTRL) and 143 (BB) for type 2A; 19 (CTRL) and 34 (BB) for type 2AX; 15 (CTRL) and 23 (BB) for type 2X fibres. *Significantly different from CTRL (P < 0.05).
To evaluate to what extent muscle fibre hypertrophy could account for VL hypertrophy, mean fibre area (MFA) of VL of BB and CTRL was determined combining MHC isoform distribution and CSA of individual muscle fibres as described in Methods. MFA was found to be 14% larger in BB ((940 ± 63) × 103μm2) than in CTRL ((826 ± 54) × 10 μm2). It should be pointed out that MFA enables just an estimate of the degree of whole muscle hypertrophy expected on the basis of single muscle fibre hypertrophy. MFA is not meant to provide the actual CSA of a muscle (see Methods).
Figure 5B shows that absolute force (Po) was significantly lower in type 1 and higher in type 2A and type 2X fibres of BB than in the corresponding fibre types of CTRL.
Figure 5C reports the mean values of specific force (Po/CSA) of the same fibre population. As expected on the basis of previous findings (Bottinelli et al. 1996; Bottinelli & Reggiani, 2000), in both CTRL and BB, type 1 fibres were weaker than type 2A, 2AX and 2X; type 1–2A fibres being intermediate. More interestingly, type 1 fibres were significantly weaker in BB than in CTRL, whereas type 2A, 2AX and 2X fibres were significantly stronger in BB than in CTRL.
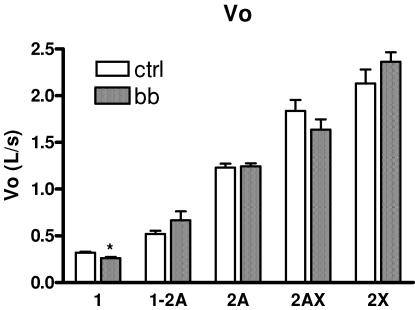
As expected on the basis of previous findings (Bottinelli & Reggiani, 2000), Vo of single muscle fibres increased in the order 1 → 2A → 2X in both CTRL and BB and hybrid fibres were intermediate between pure fibre types (Fig. 6). More interestingly, the comparison between corresponding fibre types of BB and CTRL showed that type 1 fibres had significantly lower Vo in BB than in CTRL, whereas no difference was observed among type 2A and 2X fibres. Analysis of myosin light chain (MLC) isoform composition of a subset (n= 20) of type 1 fibres of CTRL and BB have shown that all fibres contained only slow MLC isoforms (MLC1s and MLC2s) in both CTRL and BB. MLC isoform content could not explain therefore the lower Vo of BB type 1 fibres.
Figure 6. Mean values (±s.e.m.) of maximum shortening velocity (Vo) of the single muscle fibre used for mechanical experiments from control subjects (CTRL) and from body builders (BB).
Experiments were performed at 12°C. Symbols and fibre numbers are as in Fig. 5.
No fibres used for mechanical experiments were found to contain MHC-neo. This is probably due to the small size of fibres containing MHC-neo that makes the dissection of such fibres infrequent (D'Antona et al. 2003).
Function of isolated myosin
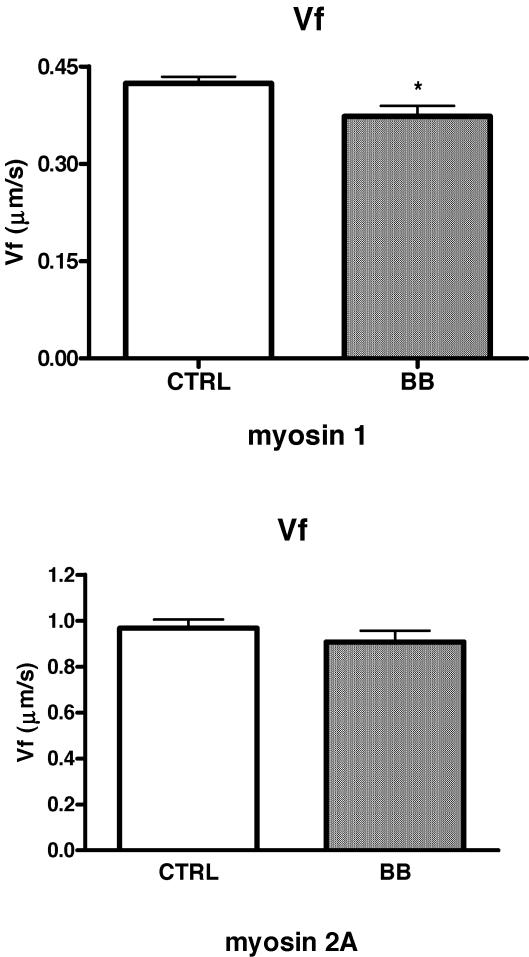
To clarify whether the lower Vo of type 1 fibres of BB depended on a change in the properties of the myosin molecule itself, velocity of sliding of actin on pure myosin isoforms was studied in in vitro motility assays (IVMA). As described in Methods, single muscle fibres (n= 180) were dissected, characterized and pooled together on the basis of MHC isoform content to enable extraction of sufficient amounts of pure myosin isoforms to load in IVMA.
As expected on the basis of previous findings (Canepari et al. 2000; D'Antona et al. 2003), the velocity of sliding of actin (Vf) was significantly lower with myosin isoforms 1 than with myosin isoforms 2A in both BB and controls. More interestingly, myosin 1 extracted from single muscle fibres of BB propelled actin filaments at a lower speed (Vf) than myosin 1 extracted from single muscle fibres of CTRL (Fig. 7). No difference in Vf was observed among myosin 2A of BB and CTRL, consistent with the lack of difference in Vo of type 2A fibres of CTRL and BB. It was not possible to obtain a sufficient number of samples to compare the functioning of myosin 2X.
Figure 7. Mean values (±s.e.m.) of actin sliding velocity (Vf) on pure myosin isoforms extracted from single muscle fibres of control subjects (CTRL) and body builders (BB).
Upper panel: myosin 1; lower panel: myosin 2A. Experiments were performed at 25°C. Vf is expressed in μm s−1. The numbers of samples studied per myosin isoform and per group were: 18 (CTRL) and 13 (BB) for myosin 1; 18 (CTRL) and 15 (BB) for myosin 2A. Each sample was obtained extracting myosin from a pool of 3–4 fibres with the same MHC content.
Anabolic steroid misuse
The only significant differences between the BB that took anabolic steroids (see Methods) and the BB that did not were found in the size of the muscles and of the muscle fibres. ACSAqd and ACSAvl were found to be significantly higher in the former (116 ± 7.8 cm2 and 38 ± 0.2 cm2, respectively) than in the latter (92 ± 6.6 cm2 and 29 ± 1.2 cm2, respectively). CSA of type 2A and 2X fibres were larger in BB that took anabolic drugs (12690 ± 387 μm2 and 11105 ± 543 μm2, respectively) than in BB that did not (8529 ± 355 μm2 and 8176 ± 698 μm2, respectively), whereas no difference was observed in CSA of type 1 fibres. No statistically significant differences between the two groups of BB were observed in all other parameters studied. Indeed, a trend for higher Po/CSA of fast fibres in BB that used anabolic steroids in comparison with BB that did not was observed consistently with the observation that an increase in Po/CSA paralleled fibre hypertrophy.
Discussion
Skeletal muscle hypertrophy
The values of CSA of the vastus lateralis and the volume of the quadriceps muscle of CTRL subjects here reported are fully consistent with recent determinations (Aagaard et al. 2001).
The hypertrophy of the quadriceps muscles of the BB subjects enrolled in the study was very pronounced especially in the vastus lateralis muscle chosen for tissue sampling. The degree of hypertrophy of quadriceps (+38%) and of vastus lateralis (+54%) muscles is consistent with the only previous comparison of muscle CSA in BB and controls performed on biceps brachii (+35–75%) (MacDougall et al. 1984). An increase in muscle mass following resistance training is largely expected (Booth & Thomason, 1991; Fluck & Hoppeler, 2003). However, longitudinal studies, which were necessarily of short (2–14 weeks) duration, found a much lower degree of hypertrophy (6–10%) (Aagaard et al. 2001; Tesch et al. 2004).
The hypertrophy of the quadriceps muscles of BB could be explained, to some extent, by the hypertrophy of single muscle fibres (Fig. 5A). Hypertrophy appeared to spare slow fibres, to selectively involve fast fibres and, among fast fibres, to be significantly more pronounced in the fastest 2X fibres (+72%) than in 2A fibres (+37%). Preferential hypertrophy of fast fibres following resistance training has been previously observed (Costill et al. 1979; Coyle et al. 1981; Sale et al. 1987; Sjostrom et al. 1988; Brown et al. 1990; Hather et al. 1991; Aagaard et al. 2001). However, due to the lower overall hypertrophies obtained in longitudinal studies the results were less clear than in the present study. Moreover, the lack of separation of type 2A and 2X fibres did not enable us to compare the relative hypertrophy of the two fast fibre types. The mechanism of such preferential hypertrophy is unclear. It might be that slow fibres are less susceptible to hypertrophic stimuli. Indeed, a significant hypertrophy of slow fibres has been rarely observed (Cadefau et al. 1990; Widrick et al. 2002). It could also be that the very powerful, but short contractions performed during hypertrophic heavy resistance exercise do not put the same stress on fast and slow fibres, as slow fibres can sustain maximum contraction with very little fatigue for a long time. The much larger increase in activity of fast than of slow motor units during heavy resistance exercise in comparison to everyday use, expected on the basis of Henneman's size principle (Henneman et al. 1974), might also play a role.
The observations that mean fibre area of VL was 14% larger in BB than in CTRL, whereas ACSA of VL determined by MRI was 54% larger in the former than in the latter subjects suggest that single muscle fibre hypertrophy cannot readily account for all the hypertrophy at whole muscle level. Interestingly, although a quantitative analysis was not performed, previous studies on BB showed very limited hypertrophy of muscle fibres in the presence of a very evident hypertrophy of the muscle (MacDougall et al. 1982; Tesch & Larsson, 1982). On the contrary, longitudinal studies on resistance training, which also combined determination of CSA of the muscle by MRI or computerized tomography (CT) and of CSA of single muscle fibres from biopsy samples, found a larger increase in MFA than in muscle CSA (Frontera et al. 1988; Aagaard et al. 2001; Esmarck et al. 2001), just the opposite of what was observed here. The latter observation suggests that other phenomena than the single muscle fibre hypertrophy that occurs in longitudinal studies might become evident in BB due to the high intensity and long duration of HHRE. Among such phenomena the following need to be considered: increase in non-contractile tissue; changes in muscle architecture; fibre hyperplasia.
Although some fatty tissue proliferation was found in skeletal muscle of BB (MacDougall et al. 1982), the relative content in connective and non-contractile tissue in muscles of BB and controls was not different (MacDougall et al. 1984), consistent with observations of a constant proportion of non-contractile tissue following resistance training in young healthy subjects (Mikesky et al. 1991; Roman et al. 1993).
It is well known now that the relation between the physiological CSA (PCSA), namely the sum of the CSAs of all individual muscle fibres of a muscle, and the anatomical CSA (ACSA), namely the CSA of a muscle determined in a plane axial to the long axis of a muscle, is not fixed. The latter relation, in fact, varies according to the pennation angle, which is the angle between the axis of the fibres and the axis of the muscle, and according to the length of the fibres (Narici et al. 1996; Reeves et al. 2004). Pennation angle has been seen to increase following resistance training (Kawakami et al. 1995) and to be larger in BB than in controls (Kawakami et al. 1993). As MRI assessed ACSA of VL whereas MFA is an index of PCSA of VL, an increase in pennation angle in BB must be considered as a possible source of discrepancy between the two estimates. Using a simple model of vastus lateralis architecture and known values of pennation angle (see Appendix), it can be shown that the large differences in ACSA and volume and the small difference in MFA between CTRL and BB can only be partially accounted for by differences in muscle architecture.
The hypothesis of an increase in fibre number in BB has to face the long-lasting (Morpurgo, 1879) and well supported belief (Gollnick et al. 1981, 1983) that in adult animals muscle hypertrophy is due solely to single muscle fibre hypertrophy. However, some evidence suggests that hyperplasia can actually occur in some animal (chicken and cat) and in some conditions (stretch-induced hypertrophy) (Antonio & Gonyea, 1993). In humans, for technical and ethical reasons the issue of hyperplasia versus hypertrophy can be addressed only indirectly, i.e. relating fibre CSA (determined in vitro) and muscle CSA (determined in vivo) in conditions in which significant hypertrophy occurs. The presence of MHC-neo in BB (Fig. 4) can be interpreted as an index of fibre regeneration (Sartore et al. 1982) following exercise damage consistent with its observation in several muscular diseases characterized by necrosis and regeneration (Marini et al. 1991). However, the formation of new fibres is also expected to determine a transient expression of developmental MHC isoforms as it is likely to recapitulate the formation of new fibres during development (Schiaffino & Reggiani, 1996). On this basis, fibre hyperplasia has been recently suggested in BB (Kadi et al. 1999). Finally, the possibility that some contribution to a possible increase in fibre number is given by fibre splitting cannot be ruled out (Vaughan & Goldspink, 1979).
MHC isoforms distribution
The analysis of MHC distribution shows that BB have significantly more MHC-2X isoform and less MHC-2A and MHC-1 isoforms in their vastus lateralis muscles than young active CTRL. That BB actually showed a bias towards a fast muscle phenotype is also suggested by the observation that their MHC isoform distribution (MHC-2A + MHC-2X = 61%) is similar to that of elite sprinters (MHC-2A + MHC-2X = 69%) (Sjostrom et al. 1988), the population that so far was found to have the fastest muscle phenotype. Moreover, as resistance training causes a MHC isoforms shift in the direction 2X → 2A the presence of a significant amount (18%) of MHC-2X in the vastus lateralis of BB that heavily trained for long time is noteworthy. It appears therefore that the muscle phenotype of BB is built to perform the short and powerful contractions required by HHRE.
The only previous analysis of MHC isoform distribution in BB in which three fibre types could be identified showed a lower MHC-2X content and a higher MHC-1 and MHC-2A content in BB than in CTRL (Klitgaard et al. 1990). The discrepancy might depend on the different technique used to compare MHC isoform content, on the muscle analysed (biceps brachii versus vastus studied here), and on the group used as control (sedentary versus physically active in the present study). Counting the number of fibres of different types in a muscle sample (Klitgaard et al. 1990) and determining the relative content of MHC isoforms by SDS-PAGE (present study) can provide different results especially if the size of the muscle fibres changes significantly (Harridge et al. 1996). A preferential hypertrophy of type 2X fibres can determine an increase in MHC-2X content in the muscle with no change in the number of type 2X fibres.
The inconsistency between longitudinal studies that show minor changes in the direction MHC-2X → MHC-2A (Campos et al. 2002; Fluck & Hoppeler, 2003; Hakkinen et al. 2003; Liu et al. 2003b) and cross-sectional studies on elite sprinters (Sjostrom et al. 1988) and on BB (this study) that show a bias towards fast fibres might be due to genetic predisposition. Indeed, a genetically determined higher type 2X content would give a significant advantage not only to sprinters, as type 2X are the fastest fibres, but also to BB, as type 2X fibres go through a larger hypertrophy than the other fibre types. Alternatively the duration of resistance training, years for elite athletes and generally not more than 12–14 weeks in longitudinal studies on sedentary subjects, and the kind of training, very intense and frequent in elite athletes and BB, might actually determine a MHC-2A → MHC-2X shift.
Finally, the possible contribution of the diet to muscle phenotype has been mostly overlooked when comparing longitudinal studies on controls and cross-sectional studies on athletes. Controls in longitudinal studies are on a normal diet whereas athletes are likely to modify their diet especially when muscle hypertrophy is desirable. A hypoproteic diet has been related to a decrease in MHC-IIX fraction in humans (Brodsky et al. 2004), whereas amino acid supplementation has been shown to determine a slow to fast shift in some muscles in mice (Pellegrino et al. 2005). It cannot be ruled out that the hyperproteic diet of BB bears some responsibility for their fast muscle phenotype.
Contractile properties of individual fibres
This study suggests that, although MHC isoform content remained the major determinant of force and velocity of muscle fibres (in both BB and CTRL Vo increased in the order 1 → 2A → 2X and Po/CSA was higher in type 2A and 2X than in type 1 fibres), long-lasting hypertorphic heavy resistance exercise training and extreme muscle hypertrophy can play a modulatory role.
Significant differences in Po/CSA between single muscle fibres of BB and controls were observed. Interestingly, the variations were in the opposite direction in slow and fast fibres. Whereas slow fibres had lower Po/CSA, fast fibres had higher Po/CSA in BB than in CTRL. Indeed fast fibres of BB had higher Po/CSA values than corresponding fibre types of any control population studied in our laboratory in the same experimental conditions (Bottinelli et al. 1996; D'Antona et al. 2003). Such variations in Po/CSA can be regarded as an adaptation of muscle fibres to the requirements of the very powerful contractions against high loads performed during HHRE.
As it is generally assumed that the packing of the myofibrils is constant, force of muscle fibres should be strictly related to CSA (Eisenberg, 1983) and Po/CSA should remain constant. However, a decrease in specific force of single skinned muscle fibres has been observed in ageing (Larsson et al. 1997; D'Antona et al. 2003) and following disuse (Larsson et al. 1996; Widrick et al. 1999; D'Antona et al. 2003). As regards young healthy subjects, no significant change in Po/CSA has been observed following resistance training (Widrick et al. 2002), and following a reduction in training volume (taper) in highly trained swimmers (Trappe et al. 2000). On the contrary, Po/CSA of single fibres of male cross-country runners was reported to fall below control values during endurance training at the beginning of the competitive season and recovered to normal values with interval training late in the season (Harber et al. 2004).
The lower Po/CSA of type 1 fibres of BB is unlikely to be due to a loss of myosin concentration in single fibres as it has been observed in ageing and disuse (D'Antona et al. 2003). Whereas in the latter conditions the loss of myosin concentration occurred in the presence of significant atrophy of muscle fibres (D'Antona et al. 2003), type 1 fibres of BB were not atrophic and surely not less used than type 1 fibres of controls. Moreover, there is evidence that the rate of protein synthesis decreases in ageing (Balagopal et al. 1997) and disuse (Ferrando et al. 1996), whereas just the opposite phenomenon occurs during resistance training (Hasten et al. 2000).
Even less clear is the mechanism underlying the higher Po/CSA of fast fibres in BB than in controls. An increase in myosin concentration able to explain a ∼40% increase in Po/CSA is difficult to conceive as in young healthy subjects myofibrils occupy most (∼95%) of the cytoplasm of single muscle fibres and mitochondria occupy a small percentage of the intracellular space (Eisenberg, 1983). Whatever the underlying mechanisms, variations in Po/CSA in BB are particularly interesting for several reasons. They appear to parallel the degree of hypertrophy of muscle fibres, i.e. the larger the hypertrophy the larger Po/CSA, and to be absent when hypertrophy does not occur as in slow fibres. As they cannot be easily explained by a change in myosin concentration, they open the possibility that some still unknown mechanisms can modulate the force developed by a given amount of contractile material in skinned fibres, i.e. in conditions of maximal activation and in the absence of a possible modulatory role of excitation–contraction coupling (Renganathan et al. 1997).
Slow fibres of BB not only developed lower Po/CSA, but also shortened at lower Vo than slow fibres of controls, whereas no difference in Vo was observed in fast fibres. A change in Vo of a muscle fibre type without a change in MHC isoform is unexpected on the basis of the MHC isoform-based control of Vo (Bottinelli & Reggiani, 2000). However, exceptions were previously reported (Bottinelli, 2001). It has been shown that Vo of single fibres can decrease in ageing (Larsson et al. 1997; D'Antona et al. 2003), and increase following disuse (Widrick et al. 1999; D'Antona et al. 2003). In young healthy subjects, an increase in Vo was reported following a decrease in training volume (taper) in highly trained swimmers (Trappe et al. 2000), although in most training studies no change in Vo was observed (Harridge et al. 1998; Widrick et al. 2002).
To clarify whether variations in Vo were due to a change in the properties of the myosin molecule itself, pure myosin 1 and 2A isoforms were extracted from single fibres and studied in an in vitro motility assay (IVMA), a reconstituted contractile system in vitro in which the velocity of sliding of actin (Vf) on isolated myosin can be determined. Vf depends solely on the properties of the myosin molecule as sarcomere structure is lost and all other myofibrillar proteins are missing. The observation that Vf of myosin 1 of BB was lower than Vf of myosin 1 of controls, whereas no difference was observed between Vf of myosin 2A is consistent with the Vo findings and suggests that the lower Vo of type 1 fibres of BB is due to a change in the properties of the myosin molecule itself. A change in the properties of the myosin molecule without a change in myosin isoform has been observed before in ageing (D'Antona et al. 2003). The mechanisms underlying such change are unknown. In most studies and in the present study, the possible role of MLC isoforms in modulating velocity has been considered and ruled out. Among other possible candidates, post-translational modifications of myosin and the existence of yet unknown MHC isoforms appear the most likely.
Anabolic steroids misuse
Although the analysis of the impact of anabolic steroids on skeletal muscle was beyond the goal of the present work, as anabolic steroid misuse was reported during pharmacological anamnesis by two BB and confirmed by blood tests, the two populations of BB were compared. The larger hypertrophy is expected on the basis of the known effect of testosterone (Bhasin et al. 1996; Bhasin et al. 2001) and anabolic steroids misuse (Kadi et al. 1999; Hartgens et al. 2001) on muscle size. No difference in MHC distribution was found, consistent with previous observations in the rat suggesting that testosterone and nandrolone administration do not significantly modify MHC distribution (Noirez & Ferry, 2000). Indeed, none of the phenomena observed were found to depend on anabolic steroid misuse itself, and the conclusions of the study appear safely related to muscle hypertrophy.
Conclusions
Muscles of BB appear to be built to support the strong and powerful contractions performed during hypertrophic heavy resistance exercise. They show extreme hypertrophy (quantitative mechanism) and a bias towards the most powerful fibre type (qualitative mechanism). Fast fibres and especially the fastest 2X fibres do not only show a very significant (+76%) preferential hypertrophy, but they develop significantly higher specific force than corresponding fibre types of controls.
The mechanisms underlying such a characteristic phenotype are not fully understood. The extreme hypertrophy of vastus lateralis muscle could not be explained simply on the basis of muscle fibre hypertrophy as would be commonly expected. The selectively higher specific force of fast fibres has never been observed before and cannot be readily explained on the basis of previous findings. The bias towards MHC-2X is not expected on the basis of the shift 2X → 2A generally observed in longitudinal studies on resistance training, and is consistent with previous observations on elite sprinters. Collectively the data not only confirm the known mechanisms, but also suggest still undefined mechanisms able to shape muscle phenotype.
Acknowledgments
This work has been supported by a grant from Italian Space Agency (ASI) and by World Anti-Doping Organization (WADA). We are grateful to Professor Marco Narici for the enlightening discussions on muscle architecture. The authors wish to thank Dr Mauro Frascaroli for help with MRI images.
Appendix
In this appendix a simple model of muscle architecture is used to estimate whether the observed differences in mean fibre area (MFA), which are an index of the differences in physiological cross-sectional area (PCSA), in volume (V) and in anatomical cross-sectional area (ACSA) between the vastus lateralis muscle (VL) of control subjects (CTRL) and body builders (BB) can be explained by a change in muscle architecture (pennation angle θ and fibre length Lf) without any change in fibre number. The ratio between ACSA and PCSA is known to change with pennation angle (θ) and fibre length (Narici et al. 1996; Reeves et al. 2004). Moreover, θ is known to be higher in BB than in controls at least in the triceps muscle in which it has been measured (Kawakami et al. 1993).
It should be pointed out that the following calculations are not meant to provide exact values of the parameters, but mostly estimates to use for comparative purposes.
The experimental data that need to be fitted are as follows.
Besides the above experimental data, two parameters used in the model are derived from the literature. As the volume of VL could not be precisely measured, as the edges of the VL were ill-defined in some slices, it was estimated from the volume of the quadriceps on the basis of the relation VVL=VQD× 0.32 derived from Narici et al. (1992). Thus:
The pennation angle of the vastus lateralis of young controls reported in a number of findings (Henriksson-Larsen et al. 1992; Narici et al. 1992; Fukunaga et al. 1997) was used:
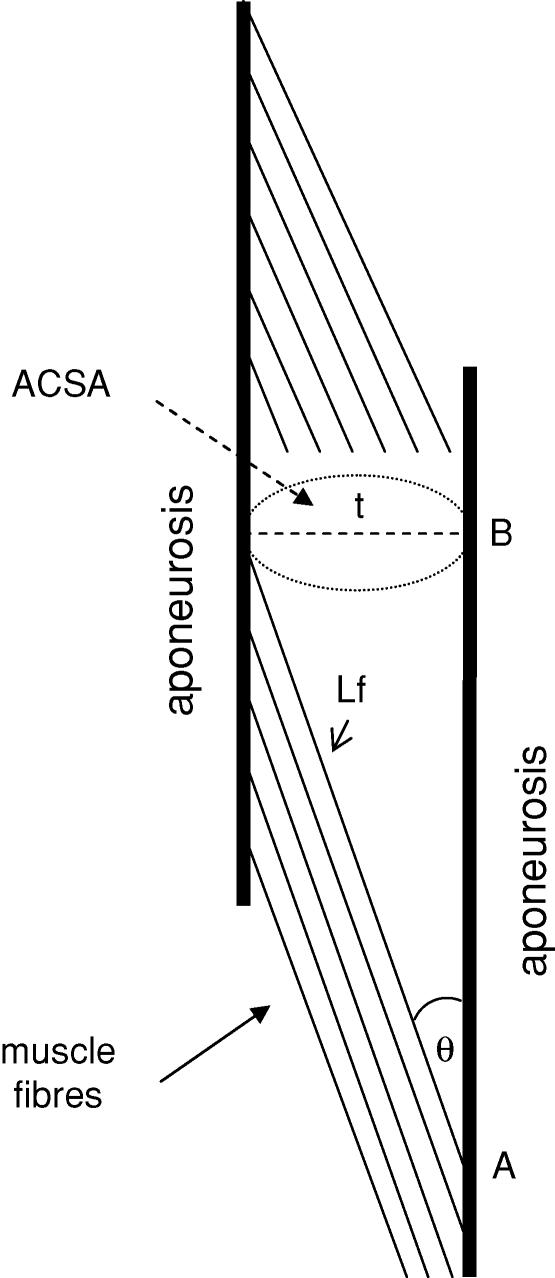
Finally, the linear model used is based on a widely accepted scheme of VL architecture previously described (Narici et al. 1992; Fukunaga et al. 1997; Aagaard et al. 2001) (Fig. 8). The calculations assume a circular CSA of the muscle and use basic trigonometric equations.
Figure 8. A model of vastus lateralis architecture.
The thick vertical lines represent the aponeurosis of the muscle; the thin parallel lines represents a sample of the muscle fibres to show their pennation angle (θ) and length (Lf); t is the thickness of the muscle or distance between aponeurosis and is indicated by a dashed line; ACSA is anatomical cross-sectional area and is indicated by a dotted ellipse; the segment AB represents the projection of a fibre on the aponeurosis and is an index of the length of the muscle.
We can first of all calculate the diameter or thickness (t) of the VL of CTRL (tc) as:
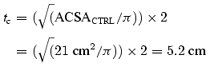 |
where π= 3.14.
Using a pennation angle of 18 deg, the length of the fibres of control subjects (Lf,c) and the projection (AB) of Lf on the aponeurosis can be estimated:
We can now estimate the θ that is compatible with the ACSABB we determined by MRI. In such calculation we consider that the segment AB must have the same length in CTRL and BB, as the length of the muscle was very similar in the two subject populations and cannot change during muscle hypertrophy.
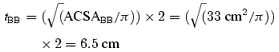 |
where tbb is the thickness of the VL of BB.
Now we can calculate the length of the fibres in BB (Lf, BB) from tBB and θ:
So far we have estimated that an ACSA of 21 cm2 in CTRL and of 33 cm2 in BB is compatible with differences in pennation angle of 3.8 deg (18 deg in CTRL and 21.8 deg in BB) and with a difference in Lf of 4.0% (Lf 16.8 cm in CTRL and 17.5 cm in BB). Such differences in θ and Lf seem compatible with the large values of θ observed in BB (Kawakami et al. 1993) and with the capacity of muscle fibres to increase in length (by gaining sarcomeres in series) following resistive training (Reeves et al. 2004).
We can now assess whether the calculated values of θ and Lf are consistent with an increase in PCSA of 1.14-fold as determined experimentally (see MFA).
PCSA of VL can be calculated knowing the volume of a muscle and the length of its fibres (PCSA =V/Lf).
| (1) |
Therefore, the estimated values for Lf and θ imply a PCSABB 1.3-fold larger than the PCSACTRL. The latter estimate is not consistent with MFA BB being merely 1.14-fold larger than MFACTRL.
We can confirm that this conclusion holds, using a slightly different approach. From eqn (1), we can estimate that the value of Lf,bb required to have PCSABB= PCSACTRL× 1.14 is 20.3 cm.
Now we can determine which θ is compatible with such value of Lf,bb with no change in muscle length (i.e. keeping the length of segment AB constant).
from which θ= 36.8 deg
A θ of 38.0 deg in BB seems conceivable as it is in agreement with values reported for the triceps muscle by Kawakami et al. (1993). Now we can estimate the ACSA compatible with θ= 36.8 deg and Lf,bb= 20.3 cm and see whether such estimated value is consistent with the experimental value.
The experimental values (33 cm2) is 3- to 4-fold smaller than the estimated value.
It appears therefore that changes in muscle architecture can only partly explain the large difference in volume (36%) and ACSA (55%) and the small difference in MFA between CTRL and BB (14%).
The above conclusions can be demonstrated to be largely independent from the values of θ (18 deg) and ratio between VQD and VVL (0.32) taken from the literature.
Supplemental material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2005.101642
http://jp.physoc.org/cgi/content/full/jphysiol.2005.101642/DC1 and contains supplemental material (Immunostained cross sections of muscle samples). This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol. 1993;74:911–915. doi: 10.1152/jappl.1993.74.2.911. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Klitgaard H, Bangsbo J, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of soccer players: effects of strength-training. Acta Physiol Scand. 1994;150:21–26. doi: 10.1111/j.1748-1716.1994.tb09655.x. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Schjerling P, Saltin B. Muscle, genes and athletic performance. Sci Am. 2000;283:48–55. doi: 10.1038/scientificamerican0900-48. [DOI] [PubMed] [Google Scholar]
- Anson M, Drummond DR, Geeves MA, Hennessey ES, Ritchie MD, Sparrow JC. Actomyosin kinetics and in vitro motility of wild-type Drosophila actin and the effects of two mutations in the Act88F gene. Biophys J. 1995;68:1991–2003. doi: 10.1016/S0006-3495(95)80376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio J, Gonyea WJ. Skeletal muscle fiber hyperplasia. Med Sci Sports Exerc. 1993;25:1333–1345. [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Baumann H, Jaggi M, Soland F, Howald H, Schaub MC. Exercise training induces transitions of myosin isoform subunits within histochemically typed human muscle fibres. Pflugers Arch. 1987;409:349–360. doi: 10.1007/BF00583788. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Muscle electrolytes in man. Scand J Clin Laboratory Investsupplement. 1962;68:1–110. [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol. 2001;170:27–38. doi: 10.1677/joe.0.1700027. [DOI] [PubMed] [Google Scholar]
- Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story. Pflugers Arch. 2001;443:6–17. doi: 10.1007/s004240100700. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994;478:341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- Brodsky IG, Suzara D, Hornberger TA, Goldspink P, Yarasheski KE, Smith S, Kukowski J, Esser K, Bedno S. Isoenergetic dietary protein restriction decreases myosin heavy chain IIx fraction and myosin heavy chain production in humans. J Nutr. 2004;134:328–334. doi: 10.1093/jn/134.2.328. [DOI] [PubMed] [Google Scholar]
- Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol. 1990;69:1725–1733. doi: 10.1152/jappl.1990.69.5.1725. [DOI] [PubMed] [Google Scholar]
- Cadefau J, Casademont J, Grau JM, Fernandez J, Balaguer A, Vernet M, Cusso R, Urbano-Marquez A. Biochemical and histochemical adaptation to sprint training in young athletes. Acta Physiol Scand. 1990;140:341–351. doi: 10.1111/j.1748-1716.1990.tb09008.x. [DOI] [PubMed] [Google Scholar]
- Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88:50–60. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- Canepari M, Rossi R, Pellegrino MA, Bottinelli R, Schiaffino S, Reggiani C. Functional diversity between orthologous myosins with minimal sequence diversity. J Muscle Res Cell Motil. 2000;21:375–382. doi: 10.1023/a:1005640004495. [DOI] [PubMed] [Google Scholar]
- Canepari M, Rossi R, Pellegrino MA, Reggiani C, Bottinelli R. Speeds of actin translocation in vitro by myosins extracted from single rat muscle fibres of different types. Exp Physiol. 1999;84:803–806. [PubMed] [Google Scholar]
- Costill DL, Coyle EF, Fink WF, Lesmes GR, Witzmann FA. Adaptations in skeletal muscle following strength training. J Appl Physiol. 1979;46:96–99. doi: 10.1152/jappl.1979.46.1.96. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Feiring DC, Rotkis TC, Cote RW, 3rd, Roby FB, Lee W, Wilmore JH. Specificity of power improvements through slow and fast isokinetic training. J Appl Physiol. 1981;51:1437–1442. doi: 10.1152/jappl.1981.51.6.1437. [DOI] [PubMed] [Google Scholar]
- D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, editor. Handbook of Physiology, section 10, Skeletal Muscle. Bethesda MD USA: American Physiological Society; 1983. pp. 77–112. [Google Scholar]
- Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535:301–311. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity—from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Ichinose Y, Ito M, Kawakami Y, Fukashiro S. Determination of fascicle length and pennation in a contracting human muscle in vivo. J Appl Physiol. 1997;82:354–358. doi: 10.1152/jappl.1997.82.1.354. [DOI] [PubMed] [Google Scholar]
- Godt RE, Maughan DW. Swelling of skinned muscle fibers of the frog. Experimental observations. Biophys J. 1977;19:103–116. doi: 10.1016/S0006-3495(77)85573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Parsons D, Riedy M, Moore RL. Fiber number and size in overloaded chicken anterior latissimus dorsi muscle. J Appl Physiol. 1983;54:1292–1297. doi: 10.1152/jappl.1983.54.5.1292. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Timson BF, Moore RL, Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol. 1981;50:936–943. doi: 10.1152/jappl.1981.50.5.936. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Alen M, Kraemer WJ, Gorostiaga E, Izquierdo M, Rusko H, Mikkola J, Hakkinen A, Valkeinen H, Kaarakainen E, Romu S, Erola V, Ahtiainen J, Paavolainen L. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol. 2003;89:42–52. doi: 10.1007/s00421-002-0751-9. [DOI] [PubMed] [Google Scholar]
- Harber MP, Gallagher PM, Creer AR, Minchev KM, Trappe SW. Single muscle fiber contractile properties during a competitive season in male runners. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1124–1131. doi: 10.1152/ajpregu.00686.2003. [DOI] [PubMed] [Google Scholar]
- Harridge SD, Bottinelli R, Canepari M, Pellegrino M, Reggiani C, Esbjornsson M, Balsom PD, Saltin B. Sprint training, in vitro and in vivo muscle function, and myosin heavy chain expression. J Appl Physiol. 1998;84:442–449. doi: 10.1152/jappl.1998.84.2.442. [DOI] [PubMed] [Google Scholar]
- Harridge SDR, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjornsson M, Saltin B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch. 1996;432:913–920. doi: 10.1007/s004240050215. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Van Marken Lichtenbelt WD, Ebbing S, Vollaard N, Rietjens G, Kuipers H. Body composition and anthropometry in bodybuilders: regional changes due to nandrolone decanoate administration. Int J Sports Med. 2001;22:235–241. doi: 10.1055/s-2001-18679. [DOI] [PubMed] [Google Scholar]
- Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand. 1991;143:177–185. doi: 10.1111/j.1748-1716.1991.tb09219.x. [DOI] [PubMed] [Google Scholar]
- Henneman E, Clamann HP, Gillies JD, Skinner RD. Rank order of motoneurons within a pool: law of combination. J Neurophysiol. 1974;37:1338–1349. doi: 10.1152/jn.1974.37.6.1338. [DOI] [PubMed] [Google Scholar]
- Henriksson-Larsen K, Wretling ML, Lorentzon R, Oberg L. Do muscle fibre size and fibre angulation correlate in pennated human muscles. Eur J Appl Physiol Occup Physiol. 1992;64:68–72. doi: 10.1007/BF00376443. [DOI] [PubMed] [Google Scholar]
- Hilber K, Galler S, Gohlsch B, Pette D. Kinetic properties of myosin heavy chain isoforms in single fibers from human skeletal muscle. FEBS Lett. 1999;455:267–270. doi: 10.1016/s0014-5793(99)00903-5. [DOI] [PubMed] [Google Scholar]
- Ingalls CP. Nature vs. nurture: can exercise really alter fiber type composition in human skeletal muscle. J Appl Physiol. 2004;97:1591–1592. doi: 10.1152/classicessays.00010.2004. [DOI] [PubMed] [Google Scholar]
- Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31:1528–1534. doi: 10.1097/00005768-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–2744. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Abe T, Kuno SY, Fukunaga T. Training-induced changes in muscle architecture and specific tension. Eur J Appl Physiol Occup Physiol. 1995;72:37–43. doi: 10.1007/BF00964112. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Richter EA. Myosin heavy chain composition of single fibres from m. biceps brachii of male body builders. Acta Physiol Scand. 1990;140:175–180. doi: 10.1111/j.1748-1716.1990.tb08989.x. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Berg HE, Frontera WR. Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflugers Arch. 1996;432:320–328. doi: 10.1007/s004240050139. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272:C638–C649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lormes W, Reissnecker S, Steinacker JM. Effects of high intensity resistance and low intensity endurance training on myosin heavy chain isoform expression in highly trained rowers. Int J Sports Med. 2003a;24:264–270. doi: 10.1055/s-2003-39509. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schlumberger A, Wirth K, Schmidtbleicher D, Steinacker JM. Different effects on human skeletal myosin heavy chain isoform expression: strength vs. combination training. J Appl Physiol. 2003b;94:2282–2288. doi: 10.1152/japplphysiol.00830.2002. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Sale DG, Alway SE, Sutton JR. Muscle fiber number in biceps brachii in bodybuilders and control subjects. J Appl Physiol. 1984;57:1399–1403. doi: 10.1152/jappl.1984.57.5.1399. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Sale DG, Elder GC, Sutton JR. Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur J Appl Physiol Occup Physiol. 1982;48:117–126. doi: 10.1007/BF00421171. [DOI] [PubMed] [Google Scholar]
- Marini JF, Pons F, Leger J, Loffreda N, Anoal M, Chevallay M, Fardeau M, Leger JJ. Expression of myosin heavy chain isoforms in Duchenne muscular dystrophy patients and carriers. Neuromuscul Disord. 1991;1:397–409. doi: 10.1016/0960-8966(91)90003-b. [DOI] [PubMed] [Google Scholar]
- Mikesky AE, Giddings CJ, Matthews W, Gonyea WJ. Changes in muscle fiber size and composition in response to heavy-resistance exercise. Med Sci Sports Exerc. 1991;23:1042–1049. [PubMed] [Google Scholar]
- Mizuno M. Human respiratory muscles: fibre morphology and capillary supply. Eur Respir J. 1991;4:587–601. [PubMed] [Google Scholar]
- Morpurgo B. Ueber actiitats-hypertrophie der willkuerlichen muskeln. Virchows Arch Pathol Anat Physiol. 1879;150:522–554. [Google Scholar]
- Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol. 1996;496:287–297. doi: 10.1113/jphysiol.1996.sp021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Landoni L, Minetti AE. Assessment of human knee extensor muscles stress from in vivo physiological cross-sectional area and strength measurements. Eur J Appl Physiol Occup Physiol. 1992;65:438–444. doi: 10.1007/BF00243511. [DOI] [PubMed] [Google Scholar]
- Noirez P, Ferry A. Effect of anabolic/androgenic steroids on myosin heavy chain expression in hindlimb muscles of male rats. Eur J Appl Physiol. 2000;81:155–158. doi: 10.1007/PL00013789. [DOI] [PubMed] [Google Scholar]
- Pellegrino MA, Brocca L, Dioguardi FS, Bottinelli R, D'Antona G. Effects of voluntary wheel running and amino acid supplementation on skeletal muscle of mice. Eur J Appl Physiol. 2005;93:655–664. doi: 10.1007/s00421-004-1237-8. [DOI] [PubMed] [Google Scholar]
- Pellegrino MA, D'Antona G, Lanfranconi F, Adami R, Todde F, Bottinelli R. Structure and function of single skeletal muscle fibres from hypertrophic muscle of body builders. J Muscle Res Cell Motil. 2003;24:338. [Google Scholar]
- Reeves ND, Narici MV, Maganaris CN. Effect of resistance training on skeletal muscle-specific force in elderly humans. J Appl Physiol. 2004;96:885–892. doi: 10.1152/japplphysiol.00688.2003. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol. 1997;157:247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- Roman WJ, Fleckenstein J, Stray-Gundersen J, Alway SE, Peshock R, Gonyea WJ. Adaptations in the elbow flexors of elderly males after heavy-resistance training. J Appl Physiol. 1993;74:750–754. doi: 10.1152/jappl.1993.74.2.750. [DOI] [PubMed] [Google Scholar]
- Sale DG, MacDougall JD, Alway SE, Sutton JR. Voluntary strength and muscle characteristics in untrained men and women and male bodybuilders. J Appl Physiol. 1987;62:1786–1793. doi: 10.1152/jappl.1987.62.5.1786. [DOI] [PubMed] [Google Scholar]
- Sartore S, Gorza L, Schiaffino S. Fetal myosin heavy chains in regenerating muscle. Nature. 1982;298:294–296. doi: 10.1038/298294a0. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Shoepe TC, Stelzer JE, Garner DP, Widrick JJ. Functional adaptability of muscle fibers to long-term resistance exercise. Med Sci Sports Exerc. 2003;35:944–951. doi: 10.1249/01.MSS.0000069756.17841.9E. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Johansson C, Lorentzon R. Muscle pathomorphology in m. quadriceps of marathon runners. Early signs of strain disease or functional adaptation. Acta Physiol Scand. 1988;132:537–541. doi: 10.1111/j.1748-1716.1988.tb08362.x. [DOI] [PubMed] [Google Scholar]
- Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J Physiol. 1996;493:299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch PA, Ekberg A, Lindquist DM, Trieschmann JT. Muscle hypertrophy following 5-week resistance training using a non-gravity-dependent exercise system. Acta Physiol Scand. 2004;180:89–98. doi: 10.1046/j.0001-6772.2003.01225.x. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Larsson L. Muscle hypertrophy in bodybuilders. Eur J Appl Physiol Occup Physiol. 1982;49:301–306. doi: 10.1007/BF00441291. [DOI] [PubMed] [Google Scholar]
- Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties. Med Sci Sports Exerc. 2000;32:48–56. [PubMed] [Google Scholar]
- Vaughan HS, Goldspink G. Fibre number and fibre size in a surgically overloaded muscle. J Anat. 1979;129:293–303. [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol. 1999;516:915–930. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Stelzer JE, Shoepe TC, Garner DP. Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol. 2002;283:R408–R416. doi: 10.1152/ajpregu.00120.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://jp.physoc.org/cgi/content/full/jphysiol.2005.101642/DC1 and contains supplemental material (Immunostained cross sections of muscle samples). This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com