Abstract
Oral streptococci utilize an F-ATPase to regulate cytoplasmic pH. Previous studies have shown that this enzyme is a principal determinant of aciduricity in the oral streptococcal species Streptococcus sanguis and Streptococcus mutans. Differences in the pH optima of the respective ATPases appears to be the main reason that S. mutans is more tolerant of low pH values than S. sanguis and hence pathogenic. We have recently reported the genetic arrangement for the S. mutans operon. For purposes of comparative structural biology we have also investigated the F-ATPase from S. sanguis. Here, we report the genetic characterization and expression in Escherichia coli of the S. sanguis ATPase operon. Sequence analysis showed a gene order of atpEBFHAGDC and that a large intergenic space existed upstream of the structural genes. Activity data demonstrate that ATPase activity is induced under acidic conditions in both S. sanguis and S. mutans; however, it is not induced to the same extent in the nonpathogenic S. sanguis. Expression studies with an atpD deletion strain of E. coli showed that S. sanguis-E. coli hybrid enzymes were able to degrade ATP but were not sufficiently functional to permit growth on succinate minimal media. Hybrid enzymes were found to be relatively insensitive to inhibition by dicyclohexylcarbodiimide, indicating loss of productive coupling between the membrane and catalytic subunits.
Streptococcus mutans and Streptococcus sanguis are oral microorganisms that are able to colonize the surfaces of teeth. Both bacteria survive by metabolizing dietary sugars such as sucrose into organic acids, particularly lactate. As salivary constituents, food particles, and bacteria accumulate on and around the tooth surface, the ecological niche known as dental plaque forms (45). Concomitant with the production of acid, localized plaque pH levels fall, resulting in the erosion of tooth enamel and the initiation of dental caries (40).
The relationship between S. mutans and S. sanguis in plaque appears to be an inverse one. Previous in vitro studies have shown that as mixed cultures of oral bacteria become acidified, S. mutans becomes a dominant member of the flora while the proportion of S. sanguis is greatly diminished (12, 13, 31). Supportive of the in vitro data, in vivo studies have repeatedly shown that S. mutans is a causative agent of dental disease (18). The ability of S. mutans to resist acidification appears to involve adaptive mechanisms which include a membrane-bound proton-translocating ATPase (F-ATPase, H+ ATPase) (11) and, potentially, other proteins (49).
The function of the F-ATPase in streptococci is to regulate internal pH by pumping protons out of the cell (11, 17, 36, 37). This movement of protons results in an internal pH which is more basic than that of the plaque environment, thereby protecting relatively acid-sensitive glycolytic enzymes (15). Previous studies have shown that the pH optima of the S. mutans ATPase is approximately 6.0 while that of S. sanguis is approximately 7.0 (55). Thus, the S. mutans enzyme is well positioned to continue pumping at pH values below levels at which S. sanguis can survive. Further, S. mutans is also apparently able to up-regulate synthesis of its ATPase (8, 9, 31). The participation of the ATPase in the acid-adaptive response of S. mutans explains, in part, why the bacterium is a major contributor to dental caries and why S. sanguis cannot effectively compete at low pH values.
In order to better understand the molecular mechanisms by which S. mutans up-regulates its ATPase, Smith et al. had previously undertaken the characterization of the S. mutans ATPase operon, including its cloning and nucleotide sequence determination (53). The deduced amino acid sequences for the eight structural genes of the S. mutans ATPase operon showed that this enzyme is homologous to the well-characterized Escherichia coli ATPase as well as those of other bacteria (53).
The enzyme's mechanism has been the subject of intensive investigation, and recent structural studies have provided insights into the spatial relationship of the subunits and ATPase activity versus proton conduction through the membrane. Functionally, the enzyme is organized into two distinct, but physically linked, domains; one is cytoplasmic (F1) and the other is membrane bound (F0) (60). The cytoplasmic domain consists of three subunits, α, β, and γ, in a stoichiometry of 3:3:1. The function of the cytoplasmic domain is to catalyze synthesis of ATP when protons move from the outside of cells into the cytoplasm, through the membrane-bound domain, or to cleave ATP when protons are pumped out of the cell, as in the case of the oral streptococci. The F1 subunit linking catalysis with proton conduction is the γ subunit, which functions as a rotor (6, 21, 46). The membrane-bound domain itself consists of five subunits, a, b, c, δ, and ɛ (1:2:9 to 10:1:1), and it functions as the membrane-bound, proton-specific channel (24, 27, 43, 54, 64).
The genetic organization of bacterial ATPase operons is typically similar to that of E. coli, in which the membrane subunit genes appear first, followed by the genes encoding the catalytic subunits (60). However, notable differences were observed in the genetic organization of the S. mutans operon. For example, the gene order for the F0 domain of the E. coli enzyme is atpBEF while that for S. mutans is atpEBF. Interestingly, the S. mutans operon did not contain an atpI gene equivalent upstream of the structural genes but rather contained an intergenic region of 239 bp. The functional significance of the DNA upstream of the structural genes remains to be elucidated; however, analysis of the region showed inverted repeats present in the DNA which are hypothesized to be involved in stem-loop structures (53).
Relatively little is known about the acid-adaptive abilities of S. sanguis or whether the regulatory mechanism for the ATPase operon is similar to that in S. mutans. Moreover, the availability of sequence data from the S. sanguis structural genes may be useful for comparison to the enzyme from the highly related S. mutans. As part of the effort towards understanding the role of the ATPase in the aciduricity of oral streptococci, we report here the cloning and expression in E. coli of the S. sanguis ATPase operon.
MATERIALS AND METHODS
Bacterial strains and media.
S. sanguis ATCC 10904 was grown on Todd-Hewitt broth (30 g per liter) (Difco, Detroit, Mich.) for chromosomal DNA isolation and in a modified Todd-Hewitt broth (per liter: 37 g of brain heart infusion, 1 g of Bacto peptone, 2 g of NaCl, 0.4 g of disodium phosphate, 2.5 g of sodium carbonate, 0.5% glucose) when grown in continuous culture in the chemostat. On solid medium, S. sanguis was grown at 37°C in an atmosphere of 5% (vol/vol) CO2. E. coli DH10B (Invitrogen, Carlsbad, Calif.) (30), GBE180 (48), or JP17 (38) was used for all cloning experiments, as indicated. E. coli strains were grown on Luria broth (10 g of tryptone [Difco], 5 g of yeast extract [Difco], 10 g of NaCl per liter) at 37°C (51). Selective antibiotics were added as follows: ampicillin, 100 μg/ml; chloramphenicol, 20 μg/ml. 5-Bromo-4-chloro-3-indolyl-β-d-galactosidase (X-Gal) was used at a concentration of 20 mg/ml in solid medium to facilitate screening of prospective clones.
Cloning of the F-ATPase operon.
A 5- to 10-kbp subgenomic library of S. sanguis 10904 was prepared, from which plasmid pSSA9, containing the entire ATPase operon except the c subunit, was isolated. The library was created in the EcoRI site of vector pSU20 (7) and screened by colony hybridization with a PCR-amplified fragment of the S. mutans β subunit (atpD) as the probe. Probe labeling, colony hybridization, and autoradiography were performed as previously reported (50). A second subgenomic library was created by digestion of the S. sanguis 10904 chromosomal DNA with Sau3AI and SstI. Fragments that were 1 to 4 kbp in size were cloned into pSU20. Southern hybridization of the clones to an atpB subunit probe from S. mutans indicated that plasmid pSSP19 contained the correct fragment. Sequencing confirmed that the insert carried the atpE and atpB subunits of the S. sanguis ATPase operon as well as the presumed promoter region.
To aid in sequencing the 6.2-kbp ATPase fragment, subclones were constructed by using EcoRI and HincII digestion products ligated into pGEM-5Zf(+) (Promega, Madison, Wis.). Sequence determinations were performed by using radioactive (Sequenase; USB Corp., Cleveland, Ohio) and fluorescence-based methods (LiCor, Lincoln, Nebr.). Alignment software packages (MacVector, AssemblyLign, and GCG) were utilized to analyze and assemble the contigs (Accelrys, Burlington, Mass.). Subunit homologies were estimated by using GCG software (Accelrys).
Primer extension analysis.
Primer extension was performed on RNA isolated from S. sanguis 10904 cells grown at steady state in a chemostat (BioFlo 2000; New Brunswick Scientific, Edison, N.J.). Cells in continuous culture were harvested at pH 7.0 and 6.0 after a minimum of 10 generations at each pH value. Dilution rates for the cells in continuous culture were D = 0.18 h−1, which is equivalent to a 3.8-h generation time. Cells (60 ml), at an optical density at 600 nm of 1.4 to 1.5, were collected at each pH value. Harvested cells were collected by centrifugation and frozen at −80°C. RNA was isolated according to the procedure outlined by Gagnon et al. (28) with the following modifications. Cell pellets were resuspended in 1 ml of RNase-free Tris-EDTA buffer (10 mM Tris-HCl, 1 mM EDTA [pH 7.5]) and mixed with 1 ml of a 1:1 phenol-chloroform-isoamyl alcohol (24:1) solution. A 20% (wt/vol) solution of sodium dodecyl sulfate (62.5 μl) was added, and the cell solution was divided into aliquots such that 1 ml of cell solution was added to 1 g of chloroform-treated glass beads (150 to 212 μm in diameter; Biospec Products, Bartlesville, Okla.). The cells were lysed in a Mini-Bead Beater homogenizer (Biospec Products) for 1 min (beat for 30 s, ice for 30 s, beat for 30 s). After lysis, the beads were collected by centrifugation at 10,300 × g for 20 min. Supernatant solutions were removed, pooled, and extracted 5 times with a 1:1 solution of phenol-chloroform-isoamyl alcohol (24:1). Nucleic acid was precipitated with a 1/10-volume of 10 M LiCl and 2 volumes of ethanol. RNA precipitates were resuspended in 100 μl of DNase buffer (20 mM Tris [pH 8.0], 50 mM KCl, 2 mM MgCl2) and treated with 30 to 50 U of RNase-free DNase (Invitrogen) for 15 min at room temperature. The digestion reaction was stopped by the addition of 2 μl of 0.5 M EDTA (pH 8.0) and extracted once with 1 volume of phenol and then once with 1 volume of chloroform-isoamyl alcohol (24:1). The Promega avian myeloblastosis virus reverse transcription primer extension kit (Madison, Wis.) was used to label oligonucleotide primers for use as probes. Primer (10 pmol) was end labeled with [γ-32P]ATP (6,000 Ci/mmol) (Perkin Elmer, Boston, Mass.). In the primer extension reactions, 35 μg of RNA from each sample and 100 fmol of labeled primer were used. The primer sequence was 5′-CCTTCAGCAATTGATACGCC-3′, which was located between bp 39 to 58 of the S. sanguis atpE gene (c subunit). A second primer with a sequence of 5′-ATTTCTGGCTGACGAGACGC-3′, located between bp 87 to 106 of the atpE gene, was used to confirm the start site. Extension and sequencing products were loaded and separated for 2.75 h at 73 W on 8% polyacrylamide gels.
Expression of enzymatic activity within ATPase-defective E. coli strains.
We examined the ability of the cloned ATPase genes to complement defects in E. coli strain JP17, an atpD deletion mutant (38). Strain JP17 was transformed with selected plasmids containing streptococcal DNA (Table 1); the resulting strains were then assayed for their ability to release inorganic phosphate from ATP. Plasmid pDP31 contains atpD and atpC and was used as a positive control (47). Cells from each of the strains were permeabilized for ATPase assays by using a previously described procedure (8) with the following modifications. Fifty-milliliter batch cultures were grown overnight in Luria broth supplemented with 10 mM MgSO4 and 0.1% glucose plus the appropriate antibiotic. Fifty milliliters of harvested cells was divided into 2 aliquots. Twenty-five milliliters was used for dry weight determinations, and 25 ml was permeabilized for assays. The cells to be assayed were washed once with membrane buffer (75 mM Tris [pH 7.0], 10 mM MgSO4) and then resuspended in a volume of 1 ml. One hundred-microliter aliquots of the permeabilized cells were frozen at −80°C and used within 1 month. A modified Fiske-Subbarow procedure (10, 56) was used to assay thawed aliquots of permeabilized cells for their ability to release phosphate from ATP (0.5 M ATP at pH 6.0; final concentration, 5 μM) (Sigma Diagnostics, St. Louis, Mo.).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Phenotype or description | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80ΔlacZdM15 ΔlacX74, deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ−rpsL nupG | Invitrogen |
| GBE180 | F−endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 φ80Δ(lacZ)M15 pcnB zad::Tn10(Tetr)λ− | 48 |
| JP17 | atpD (bp 60-998) argH pyrE endA recA (Tn10 from MV1193) Tetr | 38 |
| Plasmids | ||
| pSU20 | Cmr; intermediate-copy-number plasmid derived from pACYC184, contains multiple cloning sites in lacZ for X-Gal screening of insertions | 7 |
| pGEM-5Zf(+) | Apr; high-copy-number plasmid with multiple cloning sites in lacZ for X-Gal screening | Promega |
| pSSA9 | Cmr; a 6.2-kbp EcoRI fragment from S. sanguis 10904 chromosome-cloned into the EcoRI site of pSU20; this insert contains 7 out of 8 ATPase structural genes (lacking a portion of the c subunit) | This study |
| pClone 1.1 | Apr; pSSA9 subclone in pGEM-5 containing a 2.0-kbp EcoRI-HincII fragment containing the complete a, b, and δ subunits as well as 200 bp of the α subunit | This study |
| pClone 3.4 | Apr; pSSA9 subclone in pGEM-5 containing a 1.8-kbp HincII fragment containing the 3′ 1,313 bp of the α subunit and the 5′ 540 bp of the γ subunit | This study |
| pClone 1.3 | Apr; pSSA9 subclone in pGEM-5 containing a 568-bp HincII fragment containing the 3′ 341 bp of the γ subunit and the 5′ 187 bp of the β subunit | This study |
| pClone 1.8 | Apr; pSSA9 subclone in pGEM-5 containing a 1.4-kbp HincII fragment containing the 3′ 1,219 bp of the β subunit and the 5′ 198 bp of the ɛ subunit | This study |
| pClone 1.10 | Apr; pSSA9 subclone in pGEM-5 containing a 250-bp HincII fragment containing the rest (221 bp) of the ɛ subunit and 29 bp of downstream sequence | This study |
| pSSP19 | Cmr; a 2.1-kbp fragment of S. sanguis 10904 chromosome-cloned into pSU20 containing the carboxy terminus of the pmi gene, promoter sequences for the ATPase operon, the c and a subunits, and 458 bp of the b subunit | This study |
| pDP31 | Apr; contains the β and ɛ subunits of the E. coli ATPase operon | 47 |
DCCD inhibition studies.
ATPase assays were performed as described above. One molar stock solutions of the ATPase inhibitor dicyclohexylcarbodiimide (DCCD; Sigma Diagnostics) were made and diluted for use in the assays. Various concentrations of DCCD were tested, but maximal inhibition was achieved with a 1 mM concentration in all strains. The inhibitor was added to 3 ml of ATPase buffer (50 mM Tris-Maleate [pH 6.0], 10 mM MgSO4) at the indicated concentrations prior to the addition of the permeabilized cells. The reactions were allowed to equilibrate at 37°C for 5 min and were initiated with the addition of 30 μl of 0.5 M ATP at pH 6.0. The amount of inorganic phosphate released was measured with a commercially available kit for the modified Fiske-Subbarow assay (Sigma Diagnostics). Assays were performed on the previously described strains JP17(pDP31) and JP17(pSSA9) (Table 1).
Nucleotide sequence accession number.
The GenBank accession number for the F-ATPase from S. sanguis 10904 is AF001955.
RESULTS
Cloning the S. sanguis 10904 ATPase operon.
A 930-bp atpD gene fragment from S. mutans was amplified by PCR and used as a probe to anneal to S. sanguis 10904 chromosomal DNA digested with various enzymes. The probe hybridized to an approximately 6-kbp EcoRI fragment, apparently containing at least part of the ATPase operon. A second Southern hybridization was performed with an atpA gene probe from S. mutans to verify that the same EcoRI fragment contained a second gene in the operon. The hybridizing fragment was then cloned into an intermediate-copy-number vector, pSU20, and screened by colony hybridization with the atpD probe. A putative clone, designated pSSA9, was chosen for further characterization. Due to the empirical observation that the clone was unstable in commonly used E. coli hosts, E. coli strain GBE180 was used to maintain plasmid pSSA9 (48). E. coli GBE180 is strain DH5α (Invitrogen) but with the addition of the pcnB allele, which has been shown to reduce the plasmid copy number (41). Reduction of the plasmid copy number resulted in the stabilization of the 6.2-kbp clone.
Nucleotide sequence determination of the insert in pSSA9 demonstrated that the 5′ end of the clone was homologous to the atpB (a subunit) of S. mutans (53) and other previously sequenced ATPase atpB genes. The 3′ end of the fragment was also sequenced, but it did not show significant homology to any previously identified genes as indicated via BLAST searching of the available databases. The 6-kbp fragment was subsequently digested with HincII and subcloned into pGEM-5Zf(+) linearized with EcoRV. Putative clones, possessing fragments of various lengths, were manually sequenced by the method of Tabor and Richardson (58) or automatically with a LiCor sequencing apparatus (42). Assembly of the resulting contigs revealed the following gene order: atpBFHAGDC. The only gene missing in pSSA9 was the atpE (c subunit), which was known to be the first gene in the S. mutans ATPase operon (53).
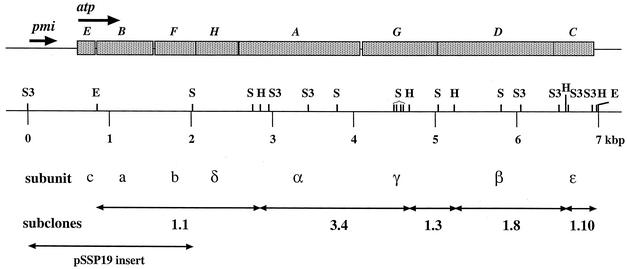
In order to sequence the rest of the operon, chromosome walking was performed by digesting S. sanguis chromosomal DNA with a panel of enzymes and isolating a fragment which hybridized to a PCR fragment of the S. sanguis atpB (a subunit). An approximately 2.3-kbp SstI/Sau3AI fragment was identified and cloned into pSU20. The resulting clone was named pSSP19. A BLAST search of the GenBank databases showed that the 5′ end of the insert in pSSP19 had significant homology to the mannose phosphate isomerase (pmi) gene previously identified in S. mutans (GenBank accession number D16594). Additional searches showed that the homology extended to other cloned pmi genes such as that from Bacillus subtilis (GenBank accession number Z99110). The 3′ end of the fragment aligned to the atpF gene in the S. sanguis ATPase operon as was expected from our restriction analysis. The gene order and size of the subunits of the operon is shown in Fig. 1. Complete sequencing of the contig revealed a 266-bp intergenic region between the pmi gene and the first structural gene of the ATPase operon (c subunit, atpE). The intergenic region is shown in more detail in Fig. 2.
FIG. 1.
Genetic organization of the S. sanguis ATPase operon. The map indicates the position and size of each of the genes with the atp designation in italics above and the subunit names below. The arrows indicate the direction of transcription for the operon as well as for the pmi gene. Restriction sites are shown on the kilobase pair scale with the following abbreviations: H, HincII; E, EcoRI; S, SstI; S3, Sau3AI. The organization and relative size of each of the subclones is shown below. The GenBank accession number for the F-ATPase from S. sanguis 10904 is AF001955.
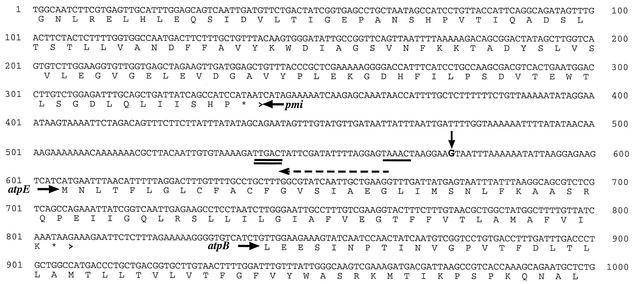
FIG. 2.
Partial nucleotide sequence of the S. sanguis ATPase operon including the 5′ upstream pmi gene as well as the intergenic region. The deduced amino acid sequence is shown in single-letter format, and the genes are indicated with bold italicized letters. An enlarged G with an arrow above it indicates the transcriptional start site at 575 bp. The single underline from bp 563 to 568 shows the presumptive −10 region, and the double underline from bp 540 to 545 indicates the presumptive −35 region. The dashed arrow above the sequence indicates the primer used in the primer extension reaction.
Comparison of ATPases.
Comparison of the S. mutans and S. sanguis ATPase operons showed various levels of homology within each of the subunits. The individual genes and their relative homologies to selected ATPases are shown in Table 2. In general, the subunits in the F1 (cytoplasmic) domain showed a higher level of homology than the F0 (membrane) domain. The data indicated that a high degree of homology existed at the amino acid level in the sequences of all of the ATPase genes and that, for the most part, the genetic organization of the operons has been maintained in the oral streptococci (Table 2).
TABLE 2.
Homology of the deduced S. sanguis F-ATPase subunit amino acid sequences compared to selected bacteria
| Subunita | atpb | % Identity (% similarity) toc:
|
||||
|---|---|---|---|---|---|---|
| S. mutans | Streptococcus pyogenes | E. hirae | B. megaterium | E. coli | ||
| a | E | 51 (66) | ND | 25 (37) | 30 (42) | 18 (33) |
| c | B | 57 (69) | ND | 44 (54) | 33 (44) | 26 (36) |
| b | F | 49 (63) | 50 (62) | 41 (52) | 38 (48) | 25 (42) |
| δ | H | 36 (48) | 36 (49) | 22 (40) | 25 (36) | 21 (32) |
| α | A | 86 (90) | 91 (94) | 81 (87) | 75 (80) | 52 (63) |
| γ | G | 68 (76) | 70 (78) | 67 (74) | 52 (62) | 35 (48) |
| β | D | 92 (94) | 93 (96) | 88 (92) | 78 (84) | 66 (76) |
| ɛ | C | 68 (81) | 68 (80) | 59 (73) | 40 (59) | 27 (42) |
It has been speculated that the large intergenic regions seen upstream of the S. mutans (53) and Enterococcus hirae (52) ATPase operons may be involved in the regulation of the operon. Sequence analysis of the region has suggested the presence of potential stem-loops (52, 53). Interestingly, a similar analysis did not yield predicted stem-loop structures for the S. sanguis operon (data not shown). However, further examination of the upstream region revealed that all three streptococci possess unusually high adenosine-thymine content in this area. For example, the S. sanguis putative promoter DNA has 18 A or T bases in a 20-bp section located approximately 86 bp upstream of the atpE gene (Fig. 2).
Transcriptional start site of the ATPase operon.
With the large intergenic region upstream of the operon, it was of interest to examine the transcriptional start site for the operon. The results of primer extension analysis identified a guanine at position −31 bp relative to the initial methionine for the atpE gene. We also evaluated whether the transcriptional start site was affected by the growth pH or by growth at steady state. To do this, RNA was isolated from S. sanguis grown at steady state at pH 7.0 and 6.0 in a chemostat. The results showed that the start site was unaffected by the pH of the culture (Fig. 3). Once the start site was identified, sequence analysis of the region immediately upstream suggested a putative Pribnow box with a sequence of TAACT, which was similar to the E. coli consensus sequence of TATAAT. A potential −35 sequence was also tentatively identified that was nearly identical to the canonical E. coli −35 region of TTGACA (Fig. 3).
FIG. 3.
Primer extension analysis of ATPase-specific RNA extracted from chemostat-grown S. sanguis 10904. The nucleotide ladder and extension products were generated with the same primer (see Materials and Methods). The lanes are marked for base content. Lanes with cDNA resulting from extension of the mRNAs are marked as pH 6.0 and 7.0, indicating the culture pH from which the RNA was prepared. The double-stranded nucleotide sequence from this region is shown above with an arrow indicating the site and direction of transcription initiation. Boxes are shown around the presumptive −10 region and the +1 nucleotide of the mRNA. An M above the ATG indicates the start codon for the c subunit (atpE).
Induction of ATPase activity under acidic growth conditions.
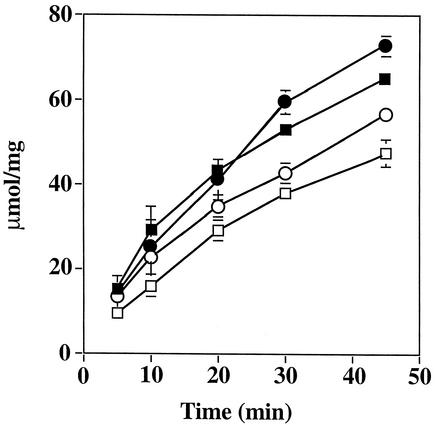
Previous data have shown that the S. mutans ATPase is up-regulated under acidic conditions (8). Maximal induction of activity was seen at pH 5, a level at which the nonpathogenic S. sanguis is not able to survive (8, 31). The ability of S. mutans to out-compete S. sanguis during growth in mixed cultures has raised questions about the acidurance of these organisms, that is, whether the up-regulation of F-ATPase contributes to the enhanced ability of S. mutans to survive at low pH values. To examine the issue more directly, S. sanguis was grown in a chemostat at steady state and the pH was dropped incrementally to 5.9; below this level the organism was unable to survive. ATPase levels from S. sanguis grown at pH 6.0 to 7.0 were compared to those of S. mutans grown at pH 5.0 to 7.0 (Fig. 4). The data show that ATPase activity was induced in both organisms; however, activity levels in S. sanguis did not reach the absolute levels seen in S. mutans at pH 5.0. Thus, it appeared that although both organisms possess acid-inducible enzymes, the S. mutans enzyme functions at a lower external pH and appears to have higher overall levels of activity, in agreement with related observations (55).
FIG. 4.
ATPase activity in S. sanguis and S. mutans as a function of growth pH values. Extracts were prepared from steady-state cultures of S. sanguis 10904 and S. mutans UA159 grown at the indicated pH values. Error bars represent the ranges from six determinations. The circles represent the UA159 extracts while the squares represent the S. sanguis 10904 extracts. Open symbols represent growth at pH 7 while filled symbols represent growth at lower pH values (pH 6 for S. sanguis or pH 5 for S. mutans).
Expression of enzymatic activity in ATPase-defective E. coli strains.
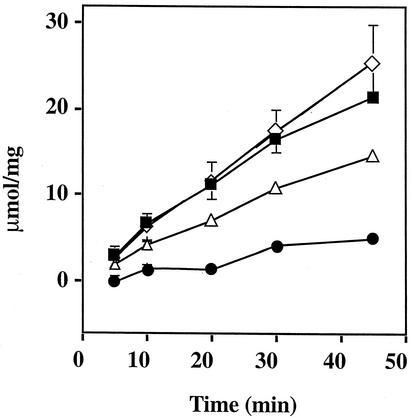
In order to determine whether streptococcal ATPase was actively expressed in E. coli, we transformed an ATPase-defective mutant, E. coli JP17, with the S. sanguis ATPase genes and measured the release of inorganic phosphate. The defective strain, JP17, had a deletion in the atpD gene (β subunit) of the operon and exhibited low levels of activity over time (Fig. 5). As a positive control, we transformed JP17 with a complementing E. coli plasmid, pDP31, which contains the complete atpD and atpC genes. The results show that the engineered strain can achieve approximately the same levels of activity seen in a wild-type E. coli strain, DH10B (Fig. 5). Strain JP17 containing plasmid pSSA9 showed ATPase activity at nearly wild-type levels. Interestingly, the ATPase activity seen when JP17 was complemented with the S. sanguis ATPase genes was typically higher than that observed in the strain containing pDP31. Since pSSA9 did not contain all of the genes, the extent of enzymatic activity could not have been due solely to the plasmid-borne subunits, suggesting the likelihood that hybrid enzymes had been formed.
FIG. 5.
Complementation of an E. coli atpD mutant strain with pDP31 (E. coli) and pSSA9 (S. sanguis) atpD genes expressed from plasmids. Plasmids and assay conditions are described in Materials and Methods. The extracts were prepared from overnight cultures of each strain. The error bars indicate six repeats with a minimum of two separate extract preparations. The filled squares represent the wild-type E. coli strain DH10B. The filled circles represent the E. coli atpD mutant JP17. The engineered strains are shown in by open diamonds for JP17(pSSA9) and open triangles for JP17(pDP31).
DCCD partially inhibits S. sanguis ATPase expressed in E. coli atp mutants.
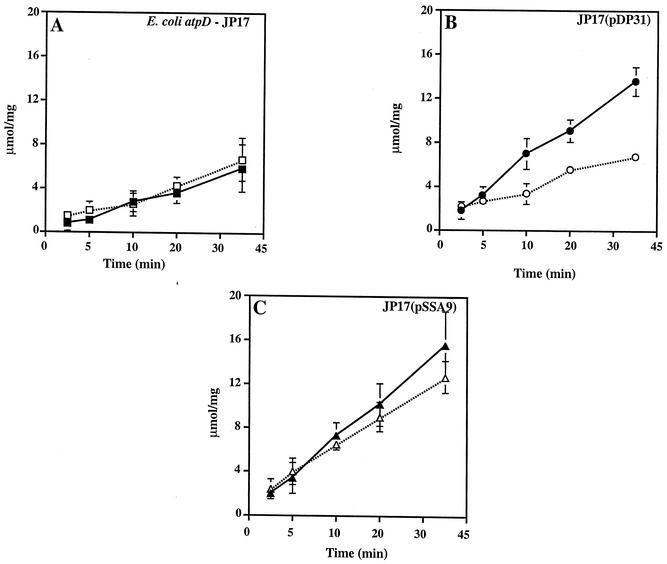
DCCD is known to inhibit the action of proton-translocating ATPases via binding to an aspartate residue at position 61 (in E. coli) of the atpE gene (c subunit). Enzymatic activity data indicated the strong possibility that hybrid enzymes were functional in the JP17 background. However, the presence of the S. sanguis genes was insufficient to support growth of JP17 on minimal succinate-containing medium, indicating that the enzyme was at least partially uncoupled from oxidative phosphorylation (data not shown). It was of interest then to determine the DCCD susceptibility of the strains containing cloned material. Strain JP17 was not affected greatly by the addition of 1 mM DCCD due to the fact that this strain exhibited only background levels of activity in the assay (Fig. 6). When the defect in JP17 was repaired by the addition of a functional E. coli atpD gene (β subunit) carried on plasmid pDP31, activity was inhibited by the addition of 1 mM DCCD. However, the S. sanguis-derived activity from JP17(pSSA9) was not inhibited to the same extent as that seen with the E. coli enzyme. Control reactions with extracts prepared from wild-type E. coli (Fig. 6) and S. sanguis (data not shown) showed significant inhibition of ATPase by DCCD. Thus, it seems that the lack of inhibition seen in JP17(pSSA9) was probably not due to a difference in the products of the S. sanguis ATPase genes themselves. Rather, it appears that hybrid enzymes had formed that were not capable of coupled ATP cleavage and proton translocation, in agreement with the observations on minimal succinate medium.
FIG. 6.
DCCD does not inhibit S. sanguis-E. coli ATPase hybrids in E. coli. Extracts were incubated with 1 mM DCCD for 5 min prior to the addition of ATP. Assay time points were as shown. (A) E. coli atpD strain JP17 with (▪) and without (□) 1 mM DCCD. (B) Strain JP17(pDP31) with (•) and without (○) 1 mM DCCD. (C) Strain JP17(pSSA9) with (▴) and without (▵) 1 mM DCCD.
DISCUSSION
Previously, Smith et al. reported the cloning and nucleotide sequence determination of the F-ATPase from S. mutans (53). The isolation and characterization of the homologous operon from S. sanguis reported here provides the necessary information for additional inquiry into the structural and functional differences of the F-ATPases from these organisms.
The genetic organization of the S. sanguis ATPase operon was identical to that seen previously in S. mutans (53), E. hirae (52), Streptococcus oralis, and Streptococcus pneumoniae (22). To date, all streptococci appear to possess an F0 gene order consisting of atpEBF in comparison to that of atpBEF, which is seen in most other bacteria. The significance of the altered gene order for the membrane-bound subunits is currently unclear.
The wealth of information regarding the structure of F-ATPases and the functional role of specific residues provide a basis for evaluating differences between the two oral streptococcal forms of the enzyme. The Walker-A sequence (61) found in E. coli is similar to domains found in other nucleotide binding proteins such as ras p21, adenylate cyclase (19, 20, 26, 29), and ercA (5). In E. coli, the domain is located at positions 149 to 156 of the atpD gene. The Gly-Gly-Ala-Gly-Val-Gly-Lys-Thr sequence is completely conserved in S. sanguis and S. mutans, although the position is modestly shifted to residues 155 to 162. Similarly, the nucleotide binding region sequence, Gly-Asp-Arg-Gln-Thr-Gly-Lys-Thr, established for the α subunit (atpA), is conserved in both position and sequence at residues 169 to 176 in both streptococci. The conservation of sequence in the catalytic domains extended well beyond the information in the nucleotide binding clefts, thereby providing additional evidence of the strong pressure in maintaining the ATPase structure in the streptococci. For example, coupling of proton conduction with ATP synthesis has been shown to involve, at least, the interaction of the β380DELSEEDβ386 sequence with the γM23 residue in the E. coli F-ATPase (E. coli F1F0) enzyme (1, 4, 35). The γM23 residue was conserved in both of the oral streptococci. The β subunit sequence, though somewhat rearranged as DELSDDE in S. sanguis and DELSDEE in S. mutans (53), still contains information appropriate for interaction with the γ subunit. Residues involved with the rotation of γ-ɛ have been identified via cysteine cross-linking mutagenesis (1, 3), two of which, αS411 and βE381, are conserved in the S. sanguis and S. mutans homologues. Two additional residues implicated in the rotational mechanism, γC87 and ɛS108, were not conserved in the oral streptococcal sequences (1-3). Glutamate residues were deduced at position 108 of the ɛ sequence for S. mutans and S. sanguis, such that it seems possible that the carboxyl OH might play a role similar to that of the serine residue in E. coli F1F0. It is difficult to reconcile the absence of cysteine in the γ subunit of both streptococcal forms. However, in both enzymes, position 87 is occupied by serine residues, suggesting the possibility of hydrogen bonding via the hydroxyl group.
Conservation of functionally important residues extends into the membrane-bound residues as well. Nuclear magnetic resonance data have shown that the c subunit (atpE) contains two helical regions connected by a polar loop of three amino acids: Arg-41, Gln-42, and Pro-43 (25). All three of the loop amino acids are conserved in the streptococcal enzymes. Proton-binding known to occur at site D61 in E. coli is also likely conserved in the c subunits of the streptococcal enzymes. The R210 residue in helix 4 of the a subunit is known to be physically close to cD61 in E. coli F1F0 (33, 59). S. sanguis and S. mutans enzyme forms have also retained the residue. Likewise, the highly conserved residue, βR36, which is necessary for the retention of coupling (16), has been similarly conserved in the streptococcal forms of the subunit. In the E. coli F1F0 enzyme, cQ42 is shielded from N-ethylmaleimide labeling by the presence of the γ and ɛ subunits (62), apparently by association with γY205 (63). The cQ42 residue has been conserved in S. sanguis, and a tyrosine residue at position 207 of the γ subunit was deduced from the nucleotide sequence. The corresponding position in S. mutans is an asparagine (53), and its ability to participate in bonding with cQ42 is an open question for the present.
It is clear from the strong conservation of relevant amino acids found in the oral streptococci that the mechanisms of function are likely to be identical to those described thus far for the E. coli F1F0 enzyme. What remains to be determined is the basis for the difference in pH optima for the two enzymes. Previous data have indicated that the kinetic parameters for the E. coli F1 enzyme are unaffected by the presence or absence of F0 (39). Results from studies with F1 and F1F0 preparations from S. sanguis and S. mutans indicated that pH profiles for F1 enzyme preparations were more alike than those using membrane-bound or membrane-free preparation of F1F0 (55). The conclusion derived from the experiments with the streptococcal forms was that the association of F1 with F0 led to enhanced acid tolerance of enzymatic activity. Mixing experiments have not yet been reported, so it is not yet possible to say that either the F1 or F0 domain of the S. mutans enzyme confers the pH advantage over the S. sanguis enzyme and which residues contribute to the difference.
Conservation of deduced amino acid sequences suggested the probability that the S. sanguis ATPase could be biochemically active in an E. coli strain lacking some or all of the ATPase subunits. Subsequent experiments with a strain defective in the atpD gene showed that cloned material from S. sanguis did confer on the mutant the ability to hydrolyze ATP. The constructs used in our experiments did not include the entire operon from S. sanguis, indicating the ability of the participating subunits to productively interact with those produced by E. coli as far as ATP cleavage was concerned. Our results were similar to those shown previously from experiments with clones from E. hirae (57) and Bacillus megaterium (32). DCCD sensitivity of the wild-type S. sanguis ATPase indicated a normally functioning enzyme, with ATP cleavage coupled to proton export such that in operon-lacking strains of E. coli, it might be possible to obtain completely functioning F1-F0 enzymes from oral streptococci. Importantly, it suggests the possibility that in future experiments, hybrid ATPase operons of S. mutans and S. sanguis genes might be constructed in a strain lacking ATPase subunits in E. coli, thereby permitting analyses of pH optima in a well-defined genetic background. Stable hybrids might then set the stage for evaluating the enzymes in streptococcal backgrounds for their effects on acid resistance and pathogenesis in the rodent model.
Additional analysis of the S. sanguis F-ATPase operon showed that, like S. mutans, F-ATPase activity increased as the growth pH decreased from a value of 7 to 6. Since the increase in specific activity was not due to a change in growth rate, we conclude that the means by which the regulation of ATPase transcription is controlled in S. sanguis differs somewhat from that seen for the E. coli operon (34).
In conclusion, we have identified, cloned, and determined the nucleotide sequence for the F-ATPase from the oral streptococcus S. sanguis. The enzyme, while exhibiting sequence homology to other sequenced ATPases, does possess unique elements. The streptococci lack the atpI gene and have an altered F0 gene order. Future experiments with more-defined contributions by streptococcal subunits will permit a clearer picture of whether catalytic and membrane subunits might productively interact with those from E. coli.
Acknowledgments
This work was supported by the National Institutes of Health/PHS (DE-01627). W.L.K. was supported by a predoctoral fellowship in the Rochester Cariology Training Program (NIH/PHS T32-DE07165).
We thank Roberta Faustoferri for assistance with the chemostat cultures and Robert E. Marquis, Alan Senior, and Joachim Weber for helpful discussions throughout.
REFERENCES
- 1.Aggeler, R., and R. A. Capaldi. 1996. Nucleotide-dependent movement of the epsilon subunit between alpha and beta subunits in the Escherichia coli F1F0-type ATPase. J. Biol. Chem. 271:13888-13891. [DOI] [PubMed] [Google Scholar]
- 2.Aggeler, R., G. Gruber, and R. A. Capaldi. 1998. Trapping of conformations of the Escherichia coli F1 ATPase by disulfide bond formation. A state of the enzyme with all three catalytic sites of equal and low affinity for nucleotides. FEBS Lett. 426:37-40. [DOI] [PubMed] [Google Scholar]
- 3.Aggeler, R., I. Ogilvie, and R. A. Capaldi. 1997. Rotation of a gamma-epsilon subunit domain in the Escherichia coli F1F0-ATP synthase complex. The gamma-epsilon subunits are essentially randomly distributed relative to the alpha3beta3delta domain in the intact complex. J. Biol. Chem. 272:19621-19624. [DOI] [PubMed] [Google Scholar]
- 4.Al Shawi, M. K., and R. K. Nakamoto. 1997. Mechanism of energy coupling in the FOF1-ATP synthase: the uncoupling mutation, gammaM23K, disrupts the use of binding energy to drive catalysis. Biochemistry 36:12954-13960. [DOI] [PubMed] [Google Scholar]
- 5.Amano, T., M. Yoshida, Y. Matsuo, and K. Nishikawa. 1994. Structural model of the ATP-binding domain of the F1-beta subunit based on analogy to the RecA protein. FEBS Lett. 351:1-5. [DOI] [PubMed] [Google Scholar]
- 6.Arechaga, I., and P. C. Jones. 2001. The rotor in the membrane of the ATP synthase and relatives. FEBS Lett. 494:1-5. [DOI] [PubMed] [Google Scholar]
- 7.Bartolome, B., Y. Jubete, E. Martinez, and F. de la Cruz. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. [DOI] [PubMed] [Google Scholar]
- 8.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belli, W. A., and R. E. Marquis. 1994. Catabolite modification of acid tolerance of Streptococcus mutans GS-5. Oral Microbiol. Immun. 9:29-34. [DOI] [PubMed] [Google Scholar]
- 10.Bencini, D. A., M. S. Shanley, J. R. Wild, and G. A. O'Donovan. 1983. New assay for enzymatic phosphate release: application to aspartate transcarbamylase and other enzymes. Anal. Biochem. 132:259-264. [DOI] [PubMed] [Google Scholar]
- 11.Bender, G. R., S. V. Sutton, and R. E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowden, G. H., and I. R. Hamilton. 1987. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and “S. mitior” growing in continuous culture. Can. J. Microbiol. 33:824-827. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw, D. J., P. D. Marsh, C. Allison, and K. M. Schilling. 1996. Effect of oxygen, inoculum composition and flow rate on development of mixed-culture oral biofilms. Microbiology 142:623-629. [DOI] [PubMed] [Google Scholar]
- 14.Brusilow, W. S., M. A. Scarpetta, C. A. Hawthorne, and W. P. Clark. 1989. Organization and sequence of the genes coding for the proton-translocating ATPase of Bacillus megaterium. J. Biol. Chem. 264:1528-1533. [PubMed] [Google Scholar]
- 15.Casiano-Colon, A., and R. E. Marquis. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl. Environ. Microbiol. 54:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caviston, T. L., C. J. Ketchum, P. L. Sorgen, R. K. Nakamoto, and B. D. Cain. 1998. Identification of an uncoupling mutation affecting the b subunit of F1F0 ATP synthase in Escherichia coli. FEBS Lett. 429:201-206. [DOI] [PubMed] [Google Scholar]
- 17.Dashper, S. G., and E. C. Reynolds. 1992. pH regulation by Streptococcus mutans. J. Dent. Res. 71:1159-1165. [DOI] [PubMed] [Google Scholar]
- 18.De Stoppelaar, J., J. Van Houte, and D. O. Backer. 1969. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 3:190-199. [DOI] [PubMed] [Google Scholar]
- 19.Duncan, T. M., and R. L. Cross. 1992. A model for the catalytic site of F1-ATPase based on analogies to nucleotide-binding domains of known structure. J. Bioenerg. Biomembr. 24:453-461. [DOI] [PubMed] [Google Scholar]
- 20.Duncan, T. M., and A. E. Senior. 1985. The defective proton-ATPase of uncD mutants of Escherichia coli. Two mutations which affect the catalytic mechanism. J. Biol. Chem. 260:4901-4907. [PubMed] [Google Scholar]
- 21.Elston, T., H. Wang, and G. Oster. 1998. Energy transduction in ATP synthase. Nature 391:510-513. [DOI] [PubMed] [Google Scholar]
- 22.Fenoll, A., R. Munoz, E. Garcia, and A. G. de la Campa. 1994. Molecular basis of the optochin-sensitive phenotype of pneumococcus: characterization of the genes encoding the F0 complex of the Streptococcus pneumoniae and Streptococcus oralis H(+)-ATPases. Mol. Microbiol. 12:587-598. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. M. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillingame, R. H., M. E. Girvin, W. Jiang, F. Valiyaveetil, and J. Hermolin. 1998. Subunit interactions coupling H+ transport and ATP synthesis in F1F0 ATP synthase. Acta Physiol. Scand. Suppl. 643:163-168. [PubMed] [Google Scholar]
- 25.Fillingame, R. H., M. E. Girvin, and Y. Zhang. 1995. Correlations of structure and function in subunit c of Escherichia coli F0F1 ATP synthase. Biochem. Soc. Trans. 23:760-766. [DOI] [PubMed] [Google Scholar]
- 26.Fry, D. C., S. A. Kuby, and A. S. Mildvan. 1986. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc. Natl. Acad. Sci. USA 83:907-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Futai, M., H. Omote, Y. Sambongi, and Y. Wada. 2000. Synthase (H(+) ATPase): coupling between catalysis, mechanical work, and proton translocation. Biochim. Biophys. Acta 1458:276-288. [DOI] [PubMed] [Google Scholar]
- 28.Gagnon, G., C. Vadeboncoeur, L. Gauthier, and M. Frenette. 1995. Regulation of ptsH and ptsI gene expression in Streptococcus salivarius ATCC 25975. Mol. Microbiol. 16:1111-1121. [DOI] [PubMed] [Google Scholar]
- 29.Garboczi, D. N., A. H. Fox, S. L. Gerring, and P. L. Pedersen. 1988. Beta subunit of rat liver mitochondrial ATP synthase: cDNA cloning, amino acid sequence, expression in Escherichia coli, and structural relationship to adenylate kinase. Biochemistry 27:553-560. [DOI] [PubMed] [Google Scholar]
- 30.Grant, S. G., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton, I. R., and N. D. Buckley. 1991. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol. Immunol. 6:65-71. [DOI] [PubMed] [Google Scholar]
- 32.Hawthorne, C. A., and W. S. Brusilow. 1986. Complementation of mutants in the Escherichia coli proton-translocating ATPase by cloned DNA from Bacillus megaterium. J. Biol. Chem. 261:5245-5248. [PubMed] [Google Scholar]
- 33.Jiang, W., and R. H. Fillingame. 1998. Interacting helical faces of subunits a and c in the F1F0 ATP synthase of Escherichia coli defined by disulfide cross-linking. Proc. Natl. Acad. Sci. USA 95:6607-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasimoglu, E., S. J. Park, J. Malek, C. P. Tseng, and R. P. Gunsalus. 1996. Transcriptional regulation of the proton-translocating ATPase (atpIBEFHAGDC) operon of Escherichia coli: control by cell growth rate. J. Bacteriol. 178:5563-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketchum, C. J., M. K. Al Shawi, and R. K. Nakamoto. 1998. Intergenic suppression of the gammaM23K uncoupling mutation in F0F1 ATP synthase by betaGlu-381 substitutions: the role of the beta380DELSEED386 segment in energy coupling. Biochem. J. 330:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi, H. 1985. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J. Biol. Chem. 260:72-76. [PubMed] [Google Scholar]
- 37.Kobayashi, H., T. Suzuki, and T. Unemoto. 1986. Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J. Biol. Chem. 261:627-630. [PubMed] [Google Scholar]
- 38.Lee, R. S., J. Pagan, S. Wilke-Mounts, and A. E. Senior. 1991. Characterization of Escherichia coli ATP synthase beta-subunit mutations using a chromosomal deletion strain. Biochemistry 30:6842-6847. [DOI] [PubMed] [Google Scholar]
- 39.Lobau, S., J. Weber, and A. E. Senior. 1998. Catalytic site nucleotide binding and hydrolysis in F1F0-ATP synthase. Biochemistry 37:10846-10853. [DOI] [PubMed] [Google Scholar]
- 40.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopilato, J., S. Bortner, and J. Beckwith. 1986. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol. Gen. Genet. 205:285-290. [DOI] [PubMed] [Google Scholar]
- 42.Middendorf, L. R., J. C. Bruce, R. C. Bruce, R. D. Eckles, D. L. Grone, S. C. Roemer, G. D. Sloniker, D. L. Steffens, S. L. Sutter, J. A. Brumbaugh, et al. 1992. Continuous, on-line DNA sequencing using a versatile infrared laser scanner/electrophoresis apparatus. Electrophoresis 13:487-494. [DOI] [PubMed] [Google Scholar]
- 43.Nakamoto, R. K. 1996. Mechanisms of active transport in the FOF1 ATP synthase. J. Membr. Biol. 151:101-111. [DOI] [PubMed] [Google Scholar]
- 44.Needleman, S. B., and C. D. Wunsch. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48:443-453. [DOI] [PubMed] [Google Scholar]
- 45.Nikiforuk, G. 1985. Understanding dental caries, vol. 1. Etiology and mechanisms. Karger, Basel, Switzerland.
- 46.Noji, H., R. Yasuda, M. Yoshida, and K. J. Kinosita. 1997. Direct observation of the rotation of F1-ATPase. Nature 386:299-302. [DOI] [PubMed] [Google Scholar]
- 47.Parsonage, D., M. S. Wilke, and A. E. Senior. 1987. Directed mutagenesis of the beta-subunit of F1-ATPase from Escherichia coli. J. Biol. Chem. 262:8022-8026. [PubMed] [Google Scholar]
- 48.Pierais, V. L., and G. J. Barcak. 1999. Development of E. coli host strains tolerating unstable DNA sequences on ColE1 vectors. Focus 21:18-19. [Google Scholar]
- 49.Quivey, R. G., Jr., W. L. Kuhnert, and K. Hahn. 2000. Adaptation of oral streptococci to low pH. Adv. Microbial Physiol. 42:239-274. [DOI] [PubMed] [Google Scholar]
- 50.Quivey, R. G., Jr., R. C. Faustoferri, W. A. Belli, and J. S. Flores. 1991. Polymerase chain reaction amplification, cloning, sequence determination and homologies of streptococcal ATPase-encoding DNAs. Gene 97:63-68. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 52.Shibata, C., T. Ehara, K. Tomura, K. Igarashi, and H. Kobayashi. 1992. Gene structure of Enterococcus hirae (Streptococcus faecalis) F1F0-ATPase, which functions as a regulator of cytoplasmic pH. J. Bacteriol. 174:6117-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, A. J., R. J. Quivey, and R. C. Faustoferri. 1996. Cloning and nucleotide sequence analysis of the Streptococcus mutans membrane-bound, proton-translocating ATPase operon. Gene 183:87-96. [DOI] [PubMed] [Google Scholar]
- 54.Stock, D., C. Gibbons, I. Arechaga, A. G. Leslie, and J. E. Walker. 2000. The rotary mechanism of ATP synthase. Curr. Opin. Struct. Biol. 10:672-679. [DOI] [PubMed] [Google Scholar]
- 55.Sturr, M. G., and R. E. Marquis. 1992. Comparative acid tolerances and inhibitor sensitivities of isolated F-ATPases of oral lactic acid bacteria. Appl. Environ. Microbiol. 58:2287-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sutton, S. V., and R. E. Marquis. 1987. Membrane-associated and solubilized ATPases of Streptococcus mutans and Streptococcus sanguis. J. Dent. Res. 66:1095-1098. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki, T., C. Shibata, A. Yamaguchi, K. Igarashi, and H. Kobayashi. 1993. Complementation of an Enterococcus hirae (Streptococcus faecalis) mutant in the alpha subunit of the H(+)-ATPase by cloned genes from the same and different species. Mol. Microbiol. 9:111-118. [DOI] [PubMed] [Google Scholar]
- 58.Tabor, S., and C. C. Richardson. 1987. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc. Natl. Acad. Sci. USA 84:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valiyaveetil, F. I., and R. H. Fillingame. 1997. On the role of Arg-210 and Glu-219 of subunit a in proton translocation by the Escherichia coli F0F1-ATP synthase. J. Biol. Chem. 272:32635-32641. [DOI] [PubMed] [Google Scholar]
- 60.Walker, J. E., M. Saraste, and N. J. Gay. 1984. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim. Biophys. Acta 768:164-200. [DOI] [PubMed] [Google Scholar]
- 61.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watts, S. D., and R. A. Capaldi. 1997. Interactions between the F1 and F0 parts in the Escherichia coli ATP synthase. Associations involving the loop region of C subunits. J. Biol. Chem. 272:15065-15068. [DOI] [PubMed] [Google Scholar]
- 63.Watts, S. D., C. Tang, and R. A. Capaldi. 1996. The stalk region of the Escherichia coli ATP synthase. Tyrosine 205 of the gamma subunit is in the interface between the F1 and F0 parts and can interact with both the epsilon and c oligomer. J. Biol. Chem. 271:28341-28347. [DOI] [PubMed] [Google Scholar]
- 64.Weber, J., and A. E. Senior. 1997. Catalytic mechanism of F1-ATPase. Biochim. Biophys. Acta 1319:19-58. [DOI] [PubMed] [Google Scholar]