Abstract
Three endoglucanase genes, designated the rce1, rce2, and rce3 genes, were isolated from Rhizopus oryzae as the first cellulase genes from the subdivision Zygomycota. All the amino acid sequences deduced from the rce1, rce2, and rce3 genes consisted of three distinct domains: cellulose binding domains, linker domains, and catalytic domains belonging to glycosyl hydrolase family 45. The rce3 gene had two tandem repeated sequences of cellulose binding domains, while rce1 and rce2 had only one. rce1, rce2, and rce3 had various lengths of linker sequences.
Cellulose is the most abundant biological polymer on the earth and is degraded by cellulases in nature (20). It is thought that degradation of cellulose is achieved by the synergistic action of three types of cellulase components: endoglucanases (EC 3.2.1.4, endo-β-d-1,4-glucanases) (EG), cellobiohydrolases (EC 3.2.1.91) (CBH), and β-glucosidases (EC 3.2.1.21) (BGL) (9). Cellulases are known to be produced by a broad range of organisms including fungi (23), bacteria (5), plants (16) and insects such as termites (11). Among them, fungal cellulases have been studied extensively and have been effectively used for industrial purposes, since fungi produce large amounts of cellulases (10).
So far, fungal cellulases have been isolated mainly from the members of the subdivision Deuteromycotina (25), which have no sexual reproduction and which mainly reproduce by conidia. Generally, the cellulase systems of the subdivision Deuteromycotina contain various cellulase components belonging to different glycosyl hydrolase families. For example, Trichoderma reesei produces eight cellulase components belonging to seven different glycosyl hydrolase families (CBHI [family 7], CBHII [family 6], EGI [family 7], EGII [family 5], EGIII [family 12], EGV [family 45], BGLI [family 3], and BGLII [family 1]) (25). These cellulase components have been shown to degrade crystalline cellulose synergistically (9). The variety of cellulase components might arise mainly through multiple gene transfer events rather than gene duplication events, since it is thought that genes encoding cellulase components belonging to the different glycosyl hydrolase families evolved from different ancestral genes (13).
Although many fungal cellulase genes from members of the subdivision Deuteromycotina have been isolated and characterized, to our knowledge, the isolation of endoglucanase genes from members of the subdivision Zygomycota, which reproduce sexually by zygospores, has not been reported. To determine the cellulase system of the subdivision Zygomycota, we screened cellulase-producing fungi belonging to Zygomycota from soil. As a result, we obtained Rhizopus oryzae FERM BP-6889, a member of the subdivision Zygomycota, and purified two major endoglucanases, designated RCE1 and RCE2, from the culture supernatant of R. oryzae (14). RCE1 and RCE2 were shown to possess similar hydrolytic properties. Also, both RCE1 and RCE2 possessed homologous N-terminal and internal amino acid sequences, indicating that the endoglucanases were highly homologous, although their molecular masses were different (41 kDa for RCE1 and 61 kDa for RCE2) (14).
To understand the cellulase system of R. oryzae, the DNA sequences of rce1 and rce2, encoding RCE1 and RCE2, should provide important information. Therefore, in this study, we cloned and sequenced the rce1 and the rce2 genes. Also, we obtained the rce3 gene, whose derived protein had not been found in the supernatant of R. oryzae.
Cloning of rce1.
To obtain the partial DNA fragment of the rce1 gene, the primers were designed based on the N-terminal and internal amino acid sequences of the purified RCE1 from R. oryzae determined previously (14). PCR was performed with forward primer R1-F (5′-AARAAYTGGAAYGGXCCNAC-3′), corresponding to the N-terminal amino acid sequence of the native RCE1 (KNWNGPT), and with primer R1-R1 (5′-TTRAACCARTTRAANCG-3′) or R1-R2 (5′-TTRAACCARTTRAAYCT-3′), corresponding to one of the internal amino acid sequences (RFNWFK) determined previously (14). PCR with primers R1-F and R1-R1 and with R. oryzae chromosomal DNA as the template yielded no specific band, but PCR with primers R1-F and R1-R2 yielded an amplified band of about 800 bp. The amplified DNA was then subcloned into the pT7Blue-T vector designated pRD05. Nucleotide sequencing of the 800-bp fragment indicated the presence of an open reading frame encoding RCE1. The amino acid sequence deduced from the 800-bp fragment was in perfect agreement with the internal amino acid sequence determined from the purified RCE1 from R. oryzae (14).
For cloning the rce1 gene, the chromosomal DNA isolated from R. oryzae was partially digested with Sau3AI and separated on an 0.8% agarose gel. The DNA fragments in the molecular size range of 9 to 23 kbp were collected and ligated into a BamHI-treated Lambda DASH II vector to prepare the genomic library for cloning the rce1 gene. pRD05 was digested with BamHI, and a DNA fragment of 800 bp was collected by use of 0.8% agarose gel electrophoresis. Plaque hybridization was performed with this 800-bp fragment as a probe against the R. oryzae genomic library for rce1 by use of an ECL direct DNA/RNA labeling detection system (Amersham). Sixteen positive phage clones were obtained from about 50,000 phage plaques, and one of the strongly hybridized clones was isolated. The phage DNA extracted from the positive clone was digested with XbaI, and the resultant 3.5-kbp fragment was subcloned into the XbaI site of pUC119.
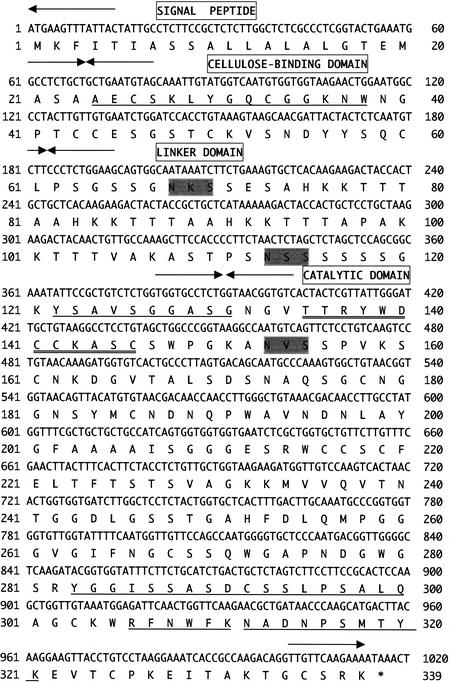
The nucleotide sequencing of the 3.5-kbp fragment indicated the presence of a single open reading frame encoding a predicted 338-residue protein. This open reading frame was designated the rce1 gene (Fig. 1). Residues 24 to 39 of the deduced amino acid sequence encoded by rce1 coincided perfectly with the N-terminal amino acid sequence of the purified RCE1 from R. oryzae (14), indicating that residues 1 to 23 constituted the signal peptide. Also, all four internal amino acid sequences of RCE1 determined previously were found in the amino acid sequence deduced from the rce1 gene (Fig. 1). Therefore, the rce1 gene was considered to encode RCE1. Based on the deduced amino sequence of RCE1, its mature molecular mass was 32,454 Da.
FIG. 1.
Nucleotide sequence of the rce1 gene from R. oryzae and deduced amino acid sequence. Arrows, border of each domain: signal peptide, CBD, linker domain, and catalytic domain. The N-terminal and internal amino acid sequences of the purified RCE1 from R. oryzae, determined previously (14), are underlined. The consensus amino acid residues of glycosyl hydrolase family 45 are double underlined. Potential N-linked glycosylation sites (N-X-S/T) are shaded.
Cloning of rce2 and rce3.
When the genomic DNA of R. oryzae digested with SacI was Southern blotted with the rce1 gene as a probe, one strong signal and two weak signals at about 10 and 3 kbp appeared (data not shown). These results suggested the presence of genes homologous with rce1. Therefore, we cloned the rce1-homologous genes, which were located on DNA fragments of about 10 and 3 kbp and which were designated the rce2 and rce3 genes, respectively.
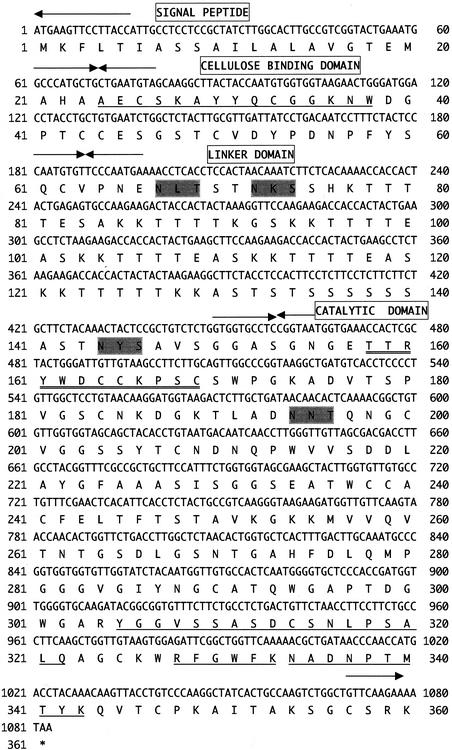
To clone the rce2 gene, we performed plaque hybridization with the rce1 gene as a probe against the genomic library by using the 10-kbp regions of DNA fragments from the R. oryzae genomic DNA digested with SacI. Two positive phage clones were obtained from about 10,000 phage plaques, and both of these clones were considered to contain the rce2 genes as judged by Southern hybridization. The phage DNA extracted from one of positive clones was digested with BamHI, and the resultant 2-kbp fragment was subcloned into the BamHI site of pUC118. The nucleotide sequencing of the 2-kbp fragment indicated the presence of a single open reading frame encoding a predicted 360-amino-acid protein (Fig. 2). Residues 24 to 38 of the amino acid sequence deduced from rce2 coincided perfectly with the N-terminal amino acid sequence of purified RCE2 from R. oryzae determined previously (14), indicating that residues 1 to 23 comprised the signal peptide. Also, all four internal amino acid sequences of RCE2 determined previously (14) were found in the amino acid sequence deduced from the rce2 gene (Fig. 2). These results suggested that the rce2 gene encoded RCE2 (14). The molecular mass of the mature RCE2 was calculated to be 35,097 Da.
FIG. 2.
Nucleotide sequence of the rce2 gene from R. oryzae and deduced amino acid sequence. Arrows, border of each domain: signal peptide, CBD, linker domain, and catalytic domain. The N-terminal and internal amino acid sequences of the purified RCE2 from R. oryzae, determined previously (14), are underlined. The consensus amino acid residues of glycosyl hydrolase family 45 are double underlined. Potential N-linked glycosylation sites (N-X-S/T) are shaded.
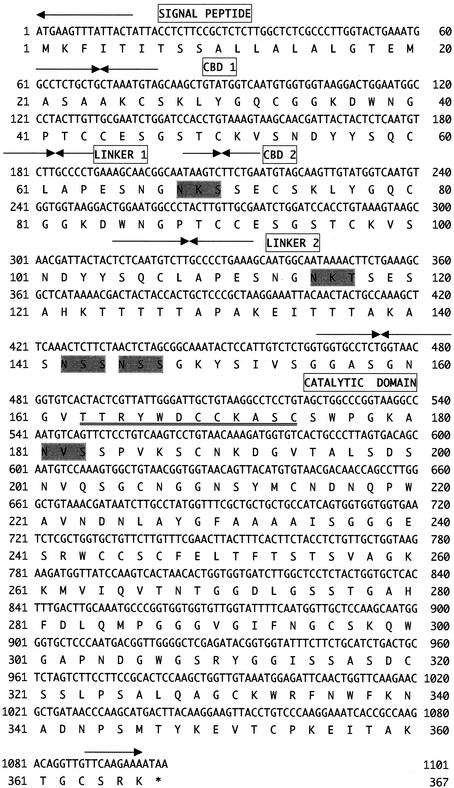
To clone the rce3 gene, plaque hybridization was performed with the rce1 gene as a probe against the genomic library by using the 3-kbp regions of DNA fragments from the R. oryzae genomic DNA digested with SacI. Three positive phage clones were obtained from about 10,000 phage plaques, and all these clones were considered to contain the rce3 genes detected by Southern hybridization. The phage DNA extracted from one of the positive clones was digested with SacI, and the resultant 3-kbp fragment was subcloned into the SacI site of pUC118. The nucleotide sequencing of the 3-kbp fragment indicated the presence of a single open reading frame encoding a predicted 366-amino-acid protein, designated RCE3 (Fig. 3). Since the amino acid sequence up to residue 24 of RCE3 was homologous with the N-terminal sequences of RCE1 and RCE2 (Fig. 4), residues 1 to 23 were considered to comprise the signal peptide (Fig. 3). The calculated molecular mass of mature RCE3 was 35,712 Da. Since we could not find the endoglucanase encoded by rce3 in the culture supernatant of R. oryzae (14), rce3 is considered to be expressed in only small amounts, if at all.
FIG. 3.
Nucleotide sequence of the rce3 gene from R. oryzae and deduced amino acid sequence. Arrows, border of each domain: signal peptide, CBDs (CBD 1 and CBD 2), linker domains (linker 1 and linker 2), and catalytic domain. The consensus amino acid residues of glycosyl hydrolase family 45 are double underlined. Potential N-linked glycosylation sites (N-X-S/T) are shaded.
FIG. 4.
Alignment of the amino acid sequences of the CBDs of RCE1, RCE2, and RCE3. Amino acids identical to those of the CBD of RCE1 are indicated by white letters in black boxes.
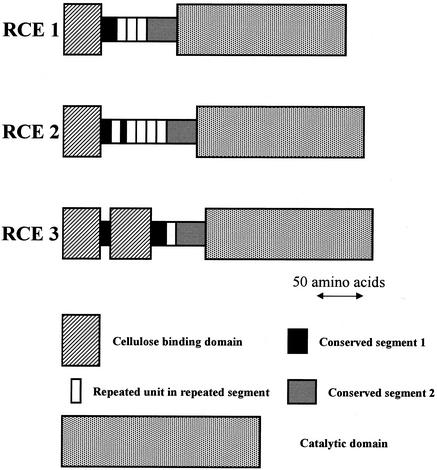
Domain structures of RCE1, RCE2, and RCE3.
FASTA searches for sequences homologous to the amino acid sequences deduced from rce1, rce2, and rce3 revealed that RCE1, RCE2, and RCE3 consist of catalytic domains belonging to glycosyl hydrolase family 45, linker domains, and cellulose binding domains (CBDs). Interestingly, RCE3 had two CBDs (CBD1 and CBD2; Fig. 3) repeated at its N terminus; on the other hand, both RCE1 and RCE2 had only one CBD. The two CBDs of RCE3 were connected by a short linker sequence consisting of 10 amino acids (linker 1; Fig. 3). The schematic domain structures of RCE1, RCE2, and RCE3 are shown in Fig. 5.
FIG. 5.
Modular structures of RCE1, RCE2, and RCE3. CBDs (Fig. 4), conserved segment 1 (Fig. 7), repeated units in repeated segments (Fig. 7), conserved segment 2 (Fig. 7), and catalytic domains (Fig. 6) are shown as boxes to simplify the structure of each domain. Arrow, length of 50 amino acids.
The N-terminal CBDs of RCE1, RCE2, and RCE3 were highly homologous to the CBDs from fungi, such as CBDs of CBHII from Trichoderma reesei (24), CEL3 from Agaricus bisporus (2), and XYLB from Neocallimastix patriciarum (1) (data not shown). Also, the four CBDs of RCEs were highly homologous to each other as shown in Fig. 4. Especially, the CBD of RCE1 and both CBDs of RCE3 were highly homologous, with 94.6% homology. On the other hand, the homology between the CBD of RCE2 and the CBDs of RCE1 and RCE3 (67.5%) was lower than that between the CBD of RCE1 and both CBDs of RCE3.
The C-terminal catalytic domains of RCE1, RCE2, and RCE3 were homologous to the catalytic domains of endoglucanase type K from Fusarium oxysporum (21), EGV from Humicola insolens (3), EGL3 from Humicola grisea (22), EGI from Scopulariopsis brevicaulis (15), EGL4 from Humicola grisea (22), EG1 from Ustilago maydis (17), and endoglucanase B from Pseudomonas fluorescens (6) (data not shown). All these endoglucanases, which are homologous to RCEs, belong to glycosyl hydrolase family 45 (7, 8). Also, the deduced amino acid sequences of RCEs contained the consensus residues of glycosyl hydrolase family 45, (S or T or A)-T-R-Y-(F or Y or W)-D-X-X-X-X-X-(C or A) (7, 8) (Fig. 1 to 3). Therefore, the catalytic domains of the RCEs were considered to belong to family 45. Also, the catalytic domains of RCEs were highly homologous with each other as shown in Fig. 6. Especially, the catalytic domain of RCE1 and that of RCE3 were highly homologous, with 98.6% homology. The homology between the catalytic domain of RCE1 and that of RCE2 was 81.3%, and the homology between those of RCE2 and RCE3 was also 81.3%.
FIG. 6.
Alignment of the amino acid sequences of the catalytic domains of RCE1, RCE2, and RCE3. Amino acids identical to those of the catalytic domain of RCE1 are indicated by white letters in black boxes.
The deduced amino acid sequences of the RCEs suggested that the cellulolytic system of R. oryzae was comparatively simple and consisted mainly of glycosyl hydrolase family 45 endoglucanases, in contrast to the cellulase systems of Deuteromycotina, which contain various glycosyl hydrolase families of cellulases (25). Genes encoding different cellulases which belong to different glycosyl hydrolase families probably evolved from different ancestral genes. If so, the fungi belonging to Deuteromycotina should have obtained various kinds of cellulase genes by multiple gene transfer events (13). On the other hand, the CBD and catalytic domain sequences of RCEs were highly homologous, suggesting that the rce1, rce2, and rce3 genes evolved from the same ancestral rce gene by gene duplication rather than by multiple gene transfer events. Furthermore, the similarity between the CBDs of RCE1 and RCE3 was higher than that between the CBDs of RCE1 and RCE2, and the similarity between the catalytic domains of RCE1 and RCE3 was also higher than that between catalytic domains of RCE1 and RCE2. These results indicate that the rce1 and rce3 genes are evolutionarily nearer to each other than to the rce2 gene.
Comparison of linker domains of RCE1, RCE2, and RCE3.
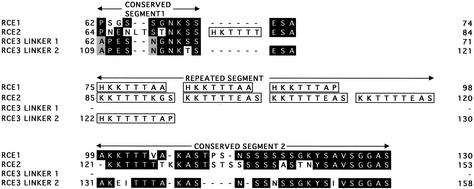
Figure 7 shows the alignment of the linker domains for RCE1, RCE2, and RCE3. This alignment suggests that the linker domains of RCEs were divided into three segments, designated conserved segment 1, repeated segment, and conserved segment 2 (Fig. 7).
FIG. 7.
Alignment of the amino acid sequences of the linker domains of RCE1, RCE2, and RCE3. Conserved segment 1, the repeated segment, and conserved segment 2 are shown. Boxed sequences, repeated units consisting of (H/K)K(K/T)TTT in the repeated segments. Conserved amino acids are indicated by letters in black or gray boxes.
Conserved segment 1 was located at the N termini of all linker domains and was highly conserved in the linkers of RCE1, RCE2, and RCE3 (Fig. 7). Conserved segment 2 was located at the C termini of the linkers of RCE1 and RCE2 and at the second linker of RCE3. The amino acid sequences of conserved segment 2 were also highly conserved in RCE1, RCE2 and RCE3. About 50% of the amino acids of conserved segment 2 consisted of serine or threonine.
The repeated segment was located between conserved segments 1 and 2. Interestingly, there were many (H/K)K(K/T)TTT sequences present in this segment as repeated units. The lengths of linkers of RCE1, RCE2, and RCE3 were different from each other because of different numbers of repeated units. The linker of RCE2, which was the longest of the linkers of RCEs, contained the KKTTTT(K/E)(G/A)S sequence four times. The linker of RCE1, which was the second longest, contained the HKKTTTA(A/P) sequence three times. The second linker of RCE3, which was the shortest, contained only one HKTTTTTAP sequence. The linker of RCE2 had another HKTTTT sequence between conserved segment 1 and the repeated segment. The linker domain sequence of xylanase B from the anaerobic fungus Neocallimastix patriciarum is known to contain 57 repeats of an octapeptide unit (XSKTLPGG, where X is S, K, or N) (1). The linker domain sequence of the xylanase A from Ruminococcus flavefaciens is also known to be composed of some reiteration of the octapeptide QQQNNDWN (28). Based on these facts, the duplication and/or deletion of the repeated peptides might be one of the common evolutionary processes producing diverse linker lengths of plant cell wall-degrading enzymes.
Expression of the rce1, rce2, and rce3 genes in Saccharomyces cerevisiae.
To express the rce1, rce2, and rce3 genes in S. cerevisiae, these rce genes were inserted between the GAP promoter and terminator on S. cerevisiae expression vector pY2831 (27). To facilitate cloning, BglII sites were incorporated upstream of the start codons and downstream of the stop codons of the rce1 and rce3 genes and the BamHI sites were those of the rce2 gene. Incorporation was performed by using a Mutagen M13 in vitro mutagenesis kit (Bio-Rad). The primers for mutagenesis were as follows: for rce1, 5′-GTAATAAACTTCATAGATCTATGTAAAAAGAATG-3′ (forward primer; underlining indicates the BglII site) and 5′-GGATGAGTATAAAAGATCTTATTTTCTTGAAC-3′ (reverse primer; underlining indicates the BglII site); for rce2, 5′-GCGGATCCATGAAGTTCCTTACCATTGCC-3′ (forward primer; underlining indicates the BamHI site) and 5′-GCGGATCCTTATTTTCTTGAACAGCCAGA-3′ (reverse primer; underlining indicates the BamHI site); for rce3, 5′-GTAATAAACTTCATAGATCTATGTAAAAAGAATG-3′ (forward primer; underlining indicates the BglII site) and 5′-CAAGAAAATAAGATCTTTTATACTCCTACT-3′ (reverse primer; underlining indicates the BglII site). The resultant plasmids were digested with BglII or BamHI, and the rce1, rce2, or rce3 fragment was subcloned into the BamHI site of pY2831 and placed between the GAP promoter and GAP terminator of S. cerevisiae. The resulting plasmids were designated pYRCE1, pYRCE2, and pYRCE3, respectively. S. cerevisiae strain MS161 (MATa trp1 ura3 Suc−) was transformed with pYRCE1, pYRCE2, or pYRCE3 by the lithium acetate method described by Ito et al. (12). S. cerevisiae transformants were selected on a selective medium containing 0.67% yeast nitrogen base without amino acids (Difco), 2% glucose, 0.005% uracil, and 1.5% purified agar (Sigma). For expression of the rce genes, the transformants were grown in a production medium containing 0.67% yeast nitrogen base without amino acids, 2% glucose, 0.005% uracil, and 2% Casamino Acids.
To confirm the expression of recombinant RCE1, RCE2, and RCE3, the supernatants of S. cerevisiae transformants harboring the plasmid pY2831, pYRCE1, pYRCE2, or pYRCE3 were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using ready-made 10% polyacrylamide gels (Tefco). After electrophoresis, the gels were either silver stained or immunostained. For immunostaining, proteins in the gel were transferred to a polyvinylidene difluoride membrane and detected on the membrane by using the antibody against the purified RCE1 from R. oryzae (14) in combination with anti-rabbit (goat) antibodies conjugated with horseradish peroxidase as secondary antibodies. Culture supernatant of S. cerevisiae harboring the rce1, rce2, or rce3 gene showed broad bands around 100, 50, or 100 kDa by Western blotting with anti-RCE1 (data not shown); on the other hand, that of S. cerevisiae without the gene did not show any bands. These results indicated that the recombinant RCE1, RCE2, and RCE3 were expressed successfully. The positions of these bands indicated molecular weights much higher than the calculated molecular weights of RCE1, RCE2, and RCE3. The amino acid sequences of RCEs contained potential N-linked glycosylation sites (N-X-S/T) at positions 68, 113, and 153 for the RCE1 (Fig. 1); at 67, 72, 144, and 194 for RCE2 (Fig. 2); and at 68, 115, 142, 145, and 181 for RCE3 (Fig. 3). Therefore, the recombinant RCEs in S. cerevisiae were considered to be hyperglycosylated at some of these N-linked glycosylation sites, which are often found in recombinant proteins expressed by the yeast (26).
Characterization of the recombinant RCE1, RCE2, and RCE3.
The activities of culture supernatants of S. cerevisiae harboring the rce1, rce2, or rce3 gene against carboxymethylcellulose (Tokyo Kasei Kogyo Co., Ltd.; for endoglucanase), Avicel (Asahi Chemical Co.; for Avicelase), xylan from birchwood (Sigma), laminarin (Sigma), galactan (Lupin; Megazyme), linear arabinan (Megazyme), and mannan (ivory nut; Megazyme) were determined. Briefly, the activities were measured under standard conditions by using reaction mixtures that contained 10 mg of substrate in 1.0 ml of 50 mM sodium acetate buffer (pH 6.0) and that were incubated for 30 min at 50°C. For Avicelase activity, reaction mixtures were incubated for 24 h at 50°C. One unit of activity was defined as the amount of enzyme releasing 1 μmol of reducing sugar per min. All three supernatants of the recombinant RCEs showed much higher activities against soluble cellulose (endoglucanase) than against crystalline cellulose (Avicelase). The culture supernatants of S. cerevisiae harboring the rce1, rce2, or rce3 gene had endoglucanase activities of 245, 100, and 42 U/liter, respectively, and Avicelase activities of 0.240, 0.185, and 0.188 U/liter, respectively, whereas S. cerevisiae without any of the genes did not have any activity. The ratios of Avicelase activity to endoglucanase activity for the culture supernatants of S. cerevisiae harboring rce1, rce2, or rce3 genes were 0.00098, 0.00185, and 0.00449, respectively. Two tandem CBDs might be more effective for degradation of crystalline cellulose than one CBD, since the recombinant RCE3, which had two tandem CBDs, had higher relative Avicelase activity than the recombinant RCE1, which possessed only one CBD. The culture supernatants of the recombinant RCEs did not show any activities against hemicelluloses. The specific activities of endoglucanase of the native RCE1 and RCE2 purified from the culture supernatant of R. oryzae were 272.5 and 110.8 U/mg, respectively (14). Therefore, the calculated amounts of the recombinant RCE1 and RCE2 produced by S. cerevisiae harboring the rce1 or rce2 gene were 0.90 and 0.91 mg/liter, respectively.
The sugars generated upon hydrolysis of cellooligosaccharides by the recombinant RCEs were also determined by thin-layer chromatography. The products formed upon hydrolysis of cellotriose (3G), cellotetraose (4G), cellopentaose (5G), and cellohexaose (6G) with the recombinant RCEs were analyzed. The reaction mixtures (1 mg of substrate and 0.25 U of the culture supernatant of RCE1, RCE2, or RCE3 in 1 ml of 50 mM sodium acetate buffer, pH 6.0) were incubated at 50°C for 2 h. The reaction was terminated by boiling the reaction mixtures for 5 min. The hydrolytic products were developed and visualized as described previously (14). The three recombinant RCEs showed similar hydrolytic patterns. The 6G was completely hydrolyzed to produce 4G and cellobiose (2G) with very small amounts of 3G. The 5G was also completely degraded to form 3G and 2G. On the other hand, only small amounts of 4G were hydrolyzed to produce 2G with very faint spots of 3G. The recombinant RCEs did not act on 3G. These hydrolytic patterns were in good agreement with that of EGV from H. insolens, which also belongs to family 45 (18). The EGV from H. insolens is known to have six subsites in its active site, and the cleavage site is between the fourth and fifth subsites, as determined through substrate specificity studies (19) and crystal structure analysis (4). Based on the similarities of hydrolytic patterns and amino acid sequences between EGV and RCEs, the active sites of RCEs might be similar to that of EGV determined by crystal structure analysis (4).
The effect of temperature was measured under the standard conditions described above by varying the temperature from 30 to 70°C. The optimum temperatures of recombinant RCE1, RCE2, and RCE3 were 55, 50, and 50°C, respectively. The optimum temperatures of recombinant RCE1 and RCE2 were almost same as those of the native RCE1 and RCE2 from culture supernatants of R. oryzae (14).
Endoglucanase activity under several different pH conditions was also measured by using 50 mM sodium acetate (pH 3 to 6), sodium phosphate (pH 7 to 8), or glycine-NaOH (pH 9 to 11) buffer. The optimum pH for recombinant RCE1 was 7.0, whereas the optimum pH for the native RCE1 was 6.0. The optimum pH for the recombinant RCE2 was 5.0, and the activity remained at 90% of the highest activity between pH 4 and 7.7. Since the activity of purified RCE2 from culture supernatants of R. oryzae remained at 90% of the highest activity between pH 5 and 7 (14), recombinant RCE2 was considered to have higher pH tolerance than purified RCE2 from culture supernatants of R. oryzae. The optimum pH of recombinant RCE3 was 7.7, and the high activity was also maintained at a broad pH range (above 90% of the highest activity between pH 5 and 7.7).
Nucleotide sequence accession numbers.
The nucleotide sequences of the rce1 gene from R. oryzae FERM BP-6889 have been assigned accession no. AB047927 in the DDBJ database, and the nucleotide sequences of the rce2 and rce3 genes have been assigned accession no. AB056668.
Acknowledgments
We thank Roy H. Doi (UC Davis) for correction of the manuscript. We thank Don M. Carlson (UC Davis) for discussions about repeated sequences. We are grateful to C. Nojiri (Meiji Seika Kaisha, Ltd.) for performing TLC analysis.
REFERENCES
- 1.Black, G. W., G. P. Hazlewood, G. P. Xue, C. G. Orpin, and H. J. Gilbert. 1994. Xylanase B from Neocallimastix patriciarum contains a non-catalytic 455-residue linker sequence comprised of 57 repeats of an octapeptide. Biochem. J. 299:381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow, C. M., E. Yague, S. Raguz, D. A. Wood, and C. F. Thurston. 1994. The cel3 gene of Agaricus bisporus codes for a modular cellulase and is transcriptionally regulated by the carbon source. Appl. Environ. Microbiol. 60:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalboege, H., B. Diderichsen, T. Sandal, and S. Kauppinen. November 1997. Method of providing novel DNA sequence. World patent 9743409.
- 4.Davies, G. J., G. G. Dodson, R. E. Hubbard, S. P. Tolley, Z. Dauter, K. S. Wilson, C. Hjort, J. M. Mikkelsen, G. Rasmussen, and M. Shulein. 1993. Structure and function of endoglucanase V. Nature 365:362-364. [DOI] [PubMed] [Google Scholar]
- 5.Doi, R. H., J. S. Park, C. C. Liu, L. M. Malburg, Y. Tamaru, A. Ichiishi, and A. Ibrahim. 1998. Cellulosome and noncellulosomal cellulases of Clostridium cellulovorans. Extremophiles 2:53-60. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert, H. J., J. Hall, G. P. Hazlewood, and L. M. Ferreira. 1990. The N-terminal region of an endoglucanase from Pseudomonas fluorescens subspecies cellulosa constitutes a cellulose-binding domain that is distinct from the catalytic centre. Mol. Microbiol. 4:759-767. [DOI] [PubMed] [Google Scholar]
- 7.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrissat, B., and A. Bairoch. 1996. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 316:695-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrissat, B., H. Driguez, C. Viet, and M. Schulein. 1985. Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Bio/Technology 3:722-726. [Google Scholar]
- 10.Himmel, M. E., M. F. Ruth, and C. E. Wyman. 1999. Cellulase for commodity products from cellulosic biomass. Curr. Opin. Biotechnol. 10:358-364. [DOI] [PubMed] [Google Scholar]
- 11.Inoue, T., K. Murashima, J. I. Azuma, A. Sugimoto, and M. Slaytor. 1997. Cellulose and xylan utilization in the lower termite Reticulitermes speratus. J. Insect Physiol. 43:235-242. [DOI] [PubMed] [Google Scholar]
- 12.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungdahl, L. G., X.-L. Li, and H. Chen. 1998. Evidence in anaerobic fungi of transfer of genes between them from aerobic fungi, bacteria and animal hosts, p. 187-197. In J. Wiegel and W. W. Adams (ed.), Thermophiles: the keys to molecular evolution and the origin of life? Taylor and Francis Inc., Philadelphia, Pa.
- 14.Murashima, K., T. Nishimura, Y. Nakamura, J. Koga, T. Moriya, N. Sumida, T. Yaguchi, and T. Kono. 2002. Purification and characterization of new endo-1,4-β-D-glucanases from Rhizopus oryzae. Enzyme Microb. Technol. 30:319-326. [Google Scholar]
- 15.Nakatani, F., T. Kawaguchi, G. Takada, J. I. Sumitani, Y. Moriyama, and M. Arai. 2000. Cloning and sequencing of an endoglucanase gene from Scopulariopsis brevicaulis TOF-1212, and its expression in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 64:1238-1246. [DOI] [PubMed] [Google Scholar]
- 16.Ohmiya, Y., M. Samejima, M. Shiroishi, Y. Amano, T. Kanda, F. Sakai, and T. Hayashi. 2000. Evidence that endo-1,4-beta-glucanases act on cellulose in suspension-cultured poplar cells. Plant J. 24:147-158. [DOI] [PubMed] [Google Scholar]
- 17.Schauwecker, F., G. Wanner, and R. Kahmann. 1995. Filament-specific expression of a cellulase gene in the dimorphic fungus Ustilago maydis. Biol. Chem. Hoppe-Seyler 376:617-625. [DOI] [PubMed] [Google Scholar]
- 18.Schou, C., G. Rasmussen, M. B. Kaltoft, B. Henrissat, and M. Schulein. 1993. Stereochemistry, specificity and kinetics of the hydrolysis of reduced cellodextrins by nine cellulases. Eur. J. Biochem. 217:947-953. [DOI] [PubMed] [Google Scholar]
- 19.Schulein, M., D. F. Tikhomirov, and C. Schou. 1993. Humicola insolens alkaline cellulases. Found. Biotech. Ind. Ferment. Res. Publ. 8:109-116. [Google Scholar]
- 20.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634-649. [DOI] [PubMed] [Google Scholar]
- 21.Sheppard, P. O., F. J. Grant, P. J. Oort, C. A. Sprecher, D. C. Foster, F. S. Hagen, A. Upshall, G. L. McKnight, and P. J. O'Hara. 1994. The use of conserved cellulase family-specific sequences to clone cellulase homologue cDNAs from Fusarium oxysporum. Gene 150:163-167. [DOI] [PubMed] [Google Scholar]
- 22.Takashima, S., H. Iikura, A. Nakamura, M. Hidaka, H. Masaki, and T. Uozumi. 1999. Comparison of gene structures and enzymatic properties between two endoglucanases from Humicola grisea. J. Biotechnol. 67:85-97. [DOI] [PubMed] [Google Scholar]
- 23.Teeri, T. T. 1997. Crystalline cellulose degradation: new insight into the function of cellobiohydrolases. Trends Biotechnol. 15:160-167. [Google Scholar]
- 24.Teeri, T. T., P. Lehtovaara, S. Kauppinen, I. Salovuori, and J. Knowles. 1987. Homologous domains in Trichoderma reesei cellulolytic enzymes: gene sequence and expression of cellobiohydrolase II. Gene 51:43-52. [DOI] [PubMed] [Google Scholar]
- 25.Tomme, P., R. A. Warren, and N. R. Gilkes. 1995. Cellulose hydrolysis by bacteria and fungi. Adv. Microb. Physiol. 37:1-81. [DOI] [PubMed] [Google Scholar]
- 26.Van Arsdell, J. N. 1987. Cloning, characterization, and expression in Saccharomyces cerevisiae of endoglucanase I from Trichoderma reesei. Bio/Technology 5:60-64. [Google Scholar]
- 27.Yanai, K., A. Nakane, A. Kawate, and M. Hirayama. 2001. Molecular cloning and characterization of the fructooligosaccharide-producing beta-fructofuranosidase gene from Aspergillus niger ATCC 20611. Biosci. Biotechnol. Biochem. 65:766-773. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, J. X., and H. J. Flint. 1992. A bifunctional xylanase encoded by the xynA gene of the rumen cellulolytic bacterium Ruminococcus flavefaciens 17 comprises two dissimilar domains linked by an asparagine/glutamine-rich sequence. Mol. Microbiol. 6:1013-1023. [DOI] [PubMed] [Google Scholar]