Abstract
In budding yeast, the mitotic spindle is positioned in the neck between the mother and the bud so that both cells inherit one nucleus. The movement of the mitotic spindle into the neck can be divided into two phases: (1) Kip3p-dependent movement of the nucleus to the neck and alignment of the short spindle, followed by (2) dynein-dependent movement of the spindle into the neck and oscillation of the elongating spindle within the neck. Actin has been hypothesized to be involved in all these movements. To test this hypothesis, we disrupted the actin cytoskeleton with the use of mutations and latrunculin A (latrunculin). We assayed nuclear segregation in synchronized cell populations and observed spindle movements in individual living cells. In synchronized cell populations, no actin cytoskeletal mutant segregated nuclei as poorly as cells lacking dynein function. Furthermore, nuclei segregated efficiently in latrunculin-treated cells. Individual living cell analysis revealed that the preanaphase spindle was mispositioned and misaligned in latrunculin-treated cells and that astral microtubules were misoriented, confirming a role for filamentous actin in the early, Kip3p-dependent phase of spindle positioning. Surprisingly, mispositioned and misaligned mitotic spindles moved into the neck in the absence of filamentous actin, albeit less efficiently. Finally, dynein-dependent sliding of astral microtubules along the cortex and oscillation of the elongating mitotic spindle in the neck occurred in the absence of filamentous actin.
INTRODUCTION
For proper nuclear segregation upon cell division, the position of cytokinesis must be aligned with the position of the mitotic spindle. In most eukaryotes, the position of the mitotic spindle determines the position of cytokinesis. The spindle generally resides near the center of the cell, and cytokinesis produces two roughly equivalent daughters. However, certain developmental processes require that the spindle be either positioned asymmetrically or rotated so that cytokinesis produces cells that differ in size or contents (Hyman and White, 1987; Hyman, 1989; Dan and Tanaka, 1990; Allen and Kropf, 1992; Chenn and McConnell, 1995; Carminati and Stearns, 1997; Busson et al., 1998). In these cases, the mitotic spindle is positioned via the interaction of astral microtubules with the cell cortex at sites that contain filamentous actin (Lutz et al., 1988; Hyman, 1989; Waddle et al., 1994). The functions of the microtubule motor dynein and its regulator the dynactin complex are required for spindle rotation during germline cell divisions and oocyte differentiation in Drosophila and in early cell divisions of Caenorhabditis elegans development (McGrail and Hays, 1997; Skop and White, 1998). An attractive hypothesis is that dynein and dynactin complex are attached at the cortex to capture and pull on astral microtubules by attempting to move toward microtubule minus ends.
In the budding yeast Saccharomyces cerevisiae, the position of cytokinesis is determined early in the cell cycle by the site of bud growth, the mother–bud neck. The mitotic spindle is positioned so that it spans the mother–bud neck, ensuring that cytokinesis will produce two cells with one nucleus each. Positioning the mitotic spindle in the mother–bud neck appears to involve distinct processes (DeZwaan et al., 1997; Stearns, 1997). First, the nucleus moves to the nascent bud site; the mitotic spindle forms at this time. The short mitotic spindle remains at the mother–bud neck and becomes aligned along the mother–bud axis. These aspects of nuclear positioning—movement of the nucleus to the mother–bud neck, maintenance of the nucleus at the mother–bud neck, and orientation of the spindle along the mother–bud axis—all require the kinesin Kip3p (Cottingham and Hoyt, 1997; DeZwaan et al., 1997; Miller et al., 1998). Second, upon initiation of anaphase, the elongating spindle moves into the mother–bud neck. This movement depends on dynein (Dhc1p/Dyn1p) (Eshel et al., 1993; Li et al., 1993; Yeh et al., 1995; Carminati and Stearns, 1997).
Movements of the mitotic spindle in budding yeast are hypothesized to involve transient interactions of astral microtubules with the cell cortex (Carminati and Stearns, 1997; Shaw et al., 1997). The cortical attachment site in the bud for Kip3p-dependent movements involves the formin Bni1p, Bud6p, Kar9p, and probably filamentous actin (Lee et al., 1999; Miller et al., 1999; Theesfeld et al., 1999). The cortical attachment site for dynein-dependent movement of the mitotic spindle is less well defined.
The cortical attachment sites for astral microtubules during dynein-dependent movements have been hypothesized to involve filamentous actin, based largely on an influential study by Palmer et al. (1992). In those experiments, actin function was inhibited by shifting a conditional actin mutant, act1-4, to the restrictive temperature. At the restrictive temperature, the authors observed many large-budded cells with two nuclei contained in the mother cell, suggesting that the spindle failed to move into the mother–bud neck (Palmer et al., 1992). Large-budded cells with both nuclei in the mother are also seen in dynein null mutants (Eshel et al., 1993; Li et al., 1993; Yeh et al., 1995). Therefore, actin may participate in capturing microtubules by linking dynein or other accessory proteins to the cortex. Connection of microtubules from the spindle to the cortex is necessary for motors to pull the spindle into the neck. Actin patches are the predominant actin structures in the cortex of yeast, and cortical actin patches cluster at the bud tip; therefore, it has been widely hypothesized that cortical actin patches are the attachment sites in the bud for astral microtubules. This notion has been widely propagated based on other studies in which actin cytoskeleton mutants accumulate binucleate and multinucleate cells in asynchronous culture. For example, asynchronous cultures of fimbrin (sac6Δ) and tropomyosin (tpm1Δ) mutants contain binucleate and multinucleate cells (Adams et al., 1991; Wang and Bretscher, 1997).
Recent work by Theesfeld et al. (1999) has examined these questions more thoroughly with the use of the same mutants that Palmer et al. (1992) used, as well as latrunculin. Theesfeld et al. (1999) found that actin is required in small- and medium-budded cells for positioning the mitotic spindle at the bud neck but that large-budded cells do not require filamentous actin for proper positioning of the mitotic spindle. They proposed that actin cables function to establish asymmetric determinants in the bud that are used for positioning the spindle and that as the determinants mature the requirement for actin is lost. Using latrunculin A (latrunculin) and mutants lacking components of the actin cytoskeleton, we also find here in synchronized cell assays that actin is not needed late in the cell cycle. However, analysis of fixed synchronized cells does not provide information about how mitotic spindles become mispositioned. Live cell analysis is essential to understand how the spindle moves during mitosis. Therefore, we conducted fluorescence microscopy of live cells treated with latrunculin to examine short spindles as they progressed through mitosis in the absence of filamentous actin. We found that although short mitotic spindles require filamentous actin to be properly positioned and aligned at the bud neck, neither properly positioned nor mispositioned spindles require filamentous actin to move into the bud neck as they progress through mitosis. We conclude that filamentous actin is not a component of the cortical microtubule capture site for dynein-dependent movements and propose a model for how two separate attachment sites, actin dependent and actin independent, work coordinately to position the spindle in the bud neck.
MATERIALS AND METHODS
Reagents and Supplies
Yeast medium was from Bio101 (La Jolla, CA). Rhodamine-phalloidin was purchased from Molecular Probes (Eugene, OR). Frozen EZ transformation kit was from Zymo Research (Orange, CA). Oligonucleotides were from GIBCO/Life Sciences (Rockville, MD) or IDT (Coralville, IA). Latrunculin A was from Dr. Philip Crews (Department of Chemistry, UCSC, National Institutes of Health grant CA47135). KlenTaq was purchased from Dr. Wayne Barnes (Washington University). GFP-tubulin in plasmid pAFS92 was a gift from Aaron Straight and Andrew Murray (Straight et al., 1997). All other reagents were from Sigma (St. Louis, MO) or Fisher (St. Louis, MO). Yeast strains used are listed in Table 1.
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| yJC0078 | MAT a leu2 ura3-52 his4-539 cap2(orf1-1)∷URA3 | KT903 (Kelly Tatchell) |
| yJC0118 | MAT α sac6∷LEU2 his3 leu2 lys2 ura3 Gal+ rho+ TRP+ | AAY1046 (Alison Adams) |
| yJC0127 | MAT α his6 ura1 myo2-66 | GR663-13 (Rick Singer) |
| yJC0128 | MAT a abp1∷LEU2 ura3-52 leu2-3 112 lys2-801 | DDY262 (David Drubin) |
| yJC0160 | MAT a ura3 his3Δ ade2 lys2 CAP2 tpm1-Δ1∷URA3 met | This study |
| yJC0174 | MAT α ura3-52 lys2-801amb ade2-101och trp1-Δ63 his3-Δ200 leu2-Δ1 cap1∷TRP1 | This study |
| yJC0185 | MAT α myo3∷HIS3 his3-11 15 leu2-3 112 trp1 ura3-52 can1-100 | HU2a (Holly Goodson and Jim Spudich) |
| yJC1022 | MAT a sla2∶ URA3 ura3 leu2 | 3-7B (David Drubin) |
| yJC1160 | MAT a ura3-52 lys2-801amb ade2-101och trp1-Δ63 his3-Δ200 leu2-Δ1arp1∷HIS3 | Muhua et al., 1994 |
| yJC1162 | MAT a ura3-52 lys2-801amb ade2-101och trp1-Δ63 his3-Δ200 leu2-Δ1 | Muhua et al., 1994 |
| yJC1269 | MAT α myo1-Δ2∷LEU2 leu2 ura3 his4 | SBY3 (Susan Brown) |
| yJC1270 | MAT a smy1-Δ2∷LEU2 leu2 ura3 his4 | SBY55 (Susan Brown) |
| yJC1272 | MAT α myo4-Δ∷URA3 lys2 leu2 ura3 his4 trp1-289 | myo4ΔU5-2A (Susan Brown) |
| yJC1281 | MAT α myo5-Δ∷TRP1 can1-100 ade2-1 his3-11 leu2-3 112 trp1-1 ura3-1 | 1a-myo5Δ (Liza Pon) |
| yJC1284 | MAT α tpm2∷LEU2 ade2-101 ade3 ura3-52 leu2-3 112 his | ABY418 (Anthony Bretscher) |
| yJC1411 | MAT a/α leu2 ura3 his3-Δ200/leu2 ura3 his3-Δ200 | Karpova et al., 1998 |
| yJC1510 | MAT a ura3 trp1 his3-Δ200 leu2 lys2 | This study |
| yJC1527 | MAT a/α leu2 ura3 his3-Δ200 CAP1-GFP-HIS3/leu2 ura3 his3-Δ200 CAP1-GFP-HIS3 [p691∶GFP-TUB1-URA3] | This study |
| yJC1554 | MAT a myo1-Δ236∷GFP his3-Δ200 lys2-801 leu2 trp1 tyr1 | LR236 (Mark Johnston) |
| yJC1619 | MAT α leu2 ura3 his3-Δ200 | This study |
| yJC1623 | MAT α leu2 ura3 his3-Δ200 crn1∷HIS3 | Heil-Chapdelaine et al., 1998 |
| yJC1635 | MAT a leu2 ura3 his3-Δ200 aip2∷HIS3 | This study |
| yJC1637 | MAT a leu2 ura3 his3-Δ200 aip1∷HIS3 | This study |
| yJC1639 | MAT a leu2 ura3 his3-Δ200 axl1∷HIS3 | This study |
| yJC1640 | MAT a leu2 ura3 his3-Δ200 sla1∷HIS3 | This study |
| yJC1641 | MAT α leu2 ura3 his3-Δ200 sla1∷HIS3 | This study |
| yJC1650 | MAT a ura3 trp1 his3-Δ200 leu2 lys2 sac3∷HIS3 | This study |
Gene Disruption
The ORFs for AIP1 (GenBank No. 807975), AIP2 (GenBank No. 1431287), AXL1 (GenBank No. 1066471), KEL2 (GenBank No. 1323431), SAC3 (GenBank No. 899406), and SLA1 (GenBank No. 535990) were deleted via homologous recombination with the use of a PCR product composed of HIS3 with 45-base pair flanking regions immediately outside the coding regions (Baudin et al., 1993). To replace AIP1, AIP2, AXL1, KEL2, and SLA1, the HIS3 PCR product was transformed into the diploid yJC1411 and transformants were sporulated to obtain haploid mutants. To replace SAC3, the PCR product was transformed into yJC1510. Genomic DNA from transformants was tested by PCR for the appropriate deletion with the use of a forward primer upstream of the disruption and a reverse primer within the HIS3 gene.
Nuclear Segregation
To assay nuclear segregation, cells were synchronized with 200 mM hydroxyurea (HU) for 3–4 h and released. Samples were fixed in ethanol and stained with DAPI. More than 200 cells were examined per time point. The percentage of cells that did not move their spindles into the neck were compared among strains by dividing the integrated area under the graphs of large-budded cells with two nuclei in the mother cells during a 90-min interval centered on mitosis (Figure 1, ○) by the sum of this area plus the area under the curve of large-budded cells with one nucleus in the mother and one in the bud during the same 90-min interval (Figure 1, ▪).
Figure 1.
Examples of the time course of nuclear segregation in synchronized cells. Wild-type (wt) and arp1Δ cells were synchronized in HU, released, fixed at various times, and stained with DAPI to score nuclear segregation. (▪) Proper nuclear segregation, with one nucleus in the mother and one in the bud. (○) Improper nuclear segregation, with both nuclei in the mother. Two hundred cells were counted for each time point, and the data include two independent experiments.
Fluorescence Microscopy, Cell Staining, and Measurements
Cells (yJC1527) were grown to early log phase (OD600 ∼ 0.3) in YPD at 30°C, collected by centrifugation, resuspended in synthetic medium lacking methionine to induce the GFP-tubulin, and grown at 30°C for 2 h. Cells were then collected by centrifugation and resuspended in nonfluorescent complete medium (Waddle et al., 1996) containing 1% DMSO or 500 μM latrunculin. To verify loss of filamentous actin, samples were fixed and stained with rhodamine-phalloidin (Amatruda et al., 1990; Waddle et al., 1996). Live cells were mounted and viewed as described by Waddle et al. (1996) on agarose pads containing 1% DMSO or 500 μM latrunculin, respectively. Loss of Cap1p-GFP from patches in live cells further confirmed the loss of filamentous actin in <5 min. Twelve 0.5-μm Z-slices were collected at 1-min intervals and projected in two dimensions. Measurements were made with the use of NIH Image software, version 1.62. Twelve latrunculin-treated and 18 DMSO-treated cells were followed from the short-spindle stage to spindle breakdown. These cells were used to measure the distance of the short spindle from the neck 1 min before spindle elongation (range, 9–30 min after latrunculin addition; mean, 17.25 min), the timing of positioning of the elongating spindle in the bud neck, and to score oscillation of the elongating spindle in the neck. A subset of these cells (10 latrunculin treated and 10 DMSO treated) that we had recorded for a minimum of 6 min before spindle elongation was used to analyze spindle alignment (see Figure 4). Alignment of short spindles relative to a line drawn perpendicular to a tangent of the bud that intersected the bud neck was measured at 1-min intervals during the 6 min preceding spindle elongation. The mean angle during this 6 min was calculated for each spindle. To compare DMSO-treated cells with latrunculin-treated cells, the mean angle among spindles in each treatment group was determined. To determine the fraction of cells going into mitosis that positioned the spindle in the bud neck, we included cells that had drifted out of focus during mitosis or did not complete mitosis during filming in addition to the cells described above. Astral microtubules could be seen in only a few of the movies. Additional movies were made of both latrunculin-treated cells and DMSO-treated cells to analyze the orientation of astral microtubules during the 3 min before the movement of the spindle into the bud neck. In total, astral microtubules could be seen before movement of the spindle into the bud neck in 33 DMSO-treated cells and 29 latrunculin-treated cells.
Figure 4.
Short-spindle position in the absence of filamentous actin. Yeast expressing GFP-Cap1p (to visualize actin patches) and GFP-tubulin (to monitor spindle movements) was grown to early log phase and treated with DMSO (control) or 500 μM latrunculin (LatA) at 0 min. Spindle position is shown for the 6 min preceding spindle elongation. (○) Distance from the bud neck of the spindle pole body destined for the bud. (▴) Distance from the bud neck of the spindle pole body destined for the mother. Positive numbers are distances in the mother, and negative numbers are distances in the bud. The x axis represents minutes after treatment with latrunculin or DMSO.
RESULTS
Nuclear Segregation in Actin Cytoskeletal Mutants
We wanted to determine if filamentous actin is involved in dynein-dependent movement of the mitotic spindle into the mother–bud neck. First, we tested this hypothesis by examining a number of mutants with defects in their actin cytoskeleton for defective movement of the mitotic spindle into the mother–bud neck, a phenotype of dynein and dynactin complex mutants (DeZwaan et al., 1997).
These experiments have two complementary rationales. First, if a mutation is in a gene whose product is necessary for the attachment of microtubules to the cortex, then spindle movement into the neck should be inefficient, as seen in cells lacking dynein or dynactin complex (Figure 1, arp1Δ). Second, if the clustered actin patches at the bud tip are the sites where astral microtubules interact with the cortex, then depolarization of actin patches away from the bud should impair spindle movement through the neck.
We examined a number of mutants that lack components of cortical actin patches or that have depolarized actin patches. The mutants included abp1Δ, aip1Δ, cap1Δ, cap2Δ, crn1Δ, myo3Δ, myo5Δ, sac6Δ, sla1Δ, sla2Δ, tpm1Δ, and tpm2Δ. We analyzed mutants lacking cortical proteins that localize to a cap-like structure at the tip of the bud (myo2Δ, myo4Δ, smy1Δ, kel2Δ, and axl1Δ) (Lillie and Brown, 1994; Jansen et al., 1996; Philips and Herskowitz, 1998; Adames and Boone, personal communication). Finally, we examined three other mutants lacking proteins that interact with the actin cytoskeleton (myo1Δ, aip2Δ, and sac3Δ) but that do not localize to actin cables, patches, or the bud cap (Watts et al., 1987; Bauer and Kolling, 1996) (for Sac3p localization, see Yeast Protein Database). As a positive control, we used an arp1Δ mutant that lacks dynein function because Arp1, the core of the dynactin complex, is absent (Muhua et al., 1994).
In this assay, we used synchronized populations of cells progressing through mitosis. Large-budded cells with two nuclei within the mother cell were scored as failure of the spindle to move into the neck. Hydroxyurea treatment arrested cells as large budded and mononucleate, with short spindles positioned in the mother at the neck. Cells were released from the block and fixed at multiple time points.
Wild-type populations of cells efficiently completed mitosis in 90 min. Very few wild-type cells had two nuclei in the mother (Figure 1). arp1Δ cells, which lack dynein function, showed a substantial fraction of large-budded cells with two nuclei in the mother, indicating a failure of the spindle to move into the neck properly (Figure 1).
This assay was used to compare the various actin cytoskeletal mutants listed above with wild-type and arp1Δ strains. We examined a complete time course for each mutant. The number of mitotic events was calculated by integrating the area under a 90-min time interval centered on the peak of mitosis, based on graphs similar to those in Figure 1. The area under the curve for cells with two nuclei in the mother (Figure 1, ○) was divided by the sum of this area plus the area under the curve for cells with normal nuclear segregation (Figure 1, ▪). In the arp1Δ-positive controls, this fraction of abnormal mitoses was 21%. In wild-type cells, this fraction was 1.3% (Figure 2). The values for the experimental mutants were as follows: abp1Δ, 2.3%; aip1Δ, 0.0%; cap1Δ, 1.2%; cap2Δ, 4.6%; crn1Δ, 4.5%; myo3Δ, 2.9%; myo5Δ, 2.6%; sac6Δ, 5.5%; sla1Δ, 7.5%; sla2Δ, 1.9%; tpm1Δ, 2.4%; tpm2Δ, 1.5%; myo2-66Δ, 4.6%; myo4Δ, 4.2%; smy1Δ, 1.6%; axl1Δ, 4.5%; kel2Δ, 4.5%; myo1Δ, 0.6%; aip2Δ, 2.2%; and sac3Δ, 1.3% (Figure 2). No mutant had a defect as severe as arp1Δ cells lacking dynein function. Therefore, none of the mutations was in genes encoding components essential for nuclear segregation, suggesting that none was necessary for attachment of microtubules to the bud cortex late in the cell cycle. In addition, delocalization of patches did not cause mitosis to occur within the mother.
Figure 2.
Nuclear segregation in actin cytoskeleton mutants. Using the assay in Figure 1, we determined the frequency of improper nuclear segregation. To determine this value, the curve for improper nuclear segregation (Figure 1, ○) was integrated during a 90-min period centered on the peak of mitosis. The area under the curve of proper nuclear segregation (Figure 1, ▪) was calculated for the same 90-min period. The area for improper nuclear segregation was divided by the sum of the two areas (inset). Mutations were tested for genes whose products localize to actin cables, localize to cortical actin patches, localize to a cap in the bud tip, or have some other connection to the actin cytoskeleton. The error bars represent the SEM for repeated experiments. Wild type (wt), n = 7; arp1Δ, n = 5; sac6Δ, n = 2; sla1Δ, n = 2; and myo1Δ, n = 2.
Nuclear Segregation in the Absence of Filamentous Actin
As an alternative and more definitive test of the role of actin in nuclear segregation, we used latrunculin under conditions in which filamentous actin depolymerizes in 5 min (Ayscough et al., 1997). Because the effect is rapid, the results are more likely to represent the primary consequences of loss of filamentous actin than results obtained with mutants.
Cells were synchronized with HU as described above, released, and treated with latrunculin. If filamentous actin is necessary for the cortical attachment of astral microtubules and subsequent spindle movement into the mother–bud neck, then large-budded cells with two nuclei in the mother should arise as they do in cells lacking dynein function (Figure 1). Surprisingly, this was not the case (Figure 3). Latrunculin-treated cells initiated mitosis at ∼60 min after release from HU and segregated nuclei as efficiently as control cells (Figure 3). This result shows that dynein-dependent movement of the spindle into the neck after HU release does not require filamentous actin.
Figure 3.
Nuclear segregation in the absence of filamentous actin. Cells were synchronized with HU and released. The cultures were treated with 1% DMSO (Control) at 0 min or with 500 μM latrunculin (Lat A) at 20 min (arrow). Samples were fixed and stained with DAPI. Nuclear segregation was determined for >200 total cells per time point. (▪) Large-budded cells with the nuclei properly segregated between the mother and the bud. (○) Large-budded cells with two nuclei within the mother cell. The percentage of large-budded cells with segregated nuclei remained high in the latrunculin-treated cells because cytokinesis is inefficient in latrunculin.
Cytokinesis is inefficient in latrunculin-treated cells (Bi et al., 1998). Therefore, in Figure 3, the percentage of large-budded cells with appropriately segregated nuclei remained high in latrunculin-treated cells because the cells did not divide within the time course of this experiment.
Direct Observation of Mitotic Spindle Movement in the Absence of Filamentous Actin
Although the experiments described above, and those of Theesfeld et al. (1999), show that actin is not required in large-budded cells to efficiently segregate nuclei, they are limited in that they are based on analysis of fixed cells and do not directly assess the positioning of the short spindle, the orientation of astral microtubules, or the movements of the mitotic spindle as it enters and completes anaphase. To assess the effect of latrunculin treatment on spindle movements directly, we conducted time-lapse analyses of spindle position in living cells by fluorescence microscopy with the use of GFP-tubulin. Loss of filamentous actin was verified in live cells by observing complete loss of Cap1p-GFP from cortical actin patches within 5 min.
If astral microtubules pull on the spindle and filamentous actin is necessary for the cortical attachment of astral microtubules, then loss of filamentous actin should result in misoriented astral microtubules and loss of spindle position at the bud neck. We examined the position and movement of the short mitotic spindle at the mother–bud neck, which depend on Kip3p (Cottingham and Hoyt, 1997; DeZwaan et al., 1997; Miller et al., 1998) and cortical attachment sites composed, in part, of Kar9p, Bni1p, Bud6p, and probably actin (Lee et al., 1999; Miller et al., 1999). We also examined movement of the elongating anaphase spindle in the neck, which requires dynein (Yeh et al., 1995; DeZwaan et al., 1997).
First, we asked whether latrunculin treatment caused cells to behave like kip3 mutants in terms of spindle position, spindle alignment at the neck, and astral microtubule orientation (DeZwaan et al., 1997; Miller et al., 1998). Cells expressing GFP-tubulin were grown to midlog phase, treated with latrunculin, and immediately observed by time-lapse microscopy. Latrunculin can activate the bud morphogenesis checkpoint, so only cells that had passed this checkpoint and were able to progress into mitosis were examined. The spindles were generally farther from the bud neck and misaligned during the 6 min before anaphase onset (Figure 4). The distance from the spindle to the neck at anaphase onset was 1.6 ± 0.23 μm (n = 18) for latrunculin-treated cells compared with 1.0 ± 0.15 μm (n = 12) for control cells (p = 0.029). The mean angle of the spindle relative to the mother–bud axis was 35 ± 6° for latrunculin-treated cells (n = 10) and 19 ± 5° for controls (n = 10). Similar findings for spindle position and alignment were reported previously for kip3 mutants (DeZwaan et al., 1997). After anaphase onset, immediately before movement into the bud neck, astral microtubules were oriented toward the mother cell in 76% of latrunculin-treated cells (n = 29), whereas only 12% of control cells (n = 33) displayed misoriented microtubules (Figure 5). These results are consistent with those reported for kip3Δ (Miller et al., 1998) and mutants lacking the cortical attachment molecule Kar9p (Miller et al., 1998). These results confirm that filamentous actin is important for Kip3p-dependent movement of the spindle and orientation of astral microtubules, presumably acting through Kar9p.
Figure 5.
Orientation of astral microtubules before movement of the spindle into the bud neck. Cells were treated as described for Figure 4 and examined during the 3 min preceding movement of the spindle into the neck. Cells were scored for orientation of astral microtubules from the spindle pole body destined for the bud. Astral microtubules were seen to project only into the bud, both into the bud and into the mother, or only into the mother. All cells oriented at least one astral microtubule into the bud before moving into the bud neck. n = 33 for DMSO-treated cells and n = 29 for latrunculin (LatA)-treated cells.
If a single type of cortical attachment site is used for both Kip3p-dependent and dynein-dependent movements, as suggested by Theesfeld et al. (1999), then misaligned and mispositioned short spindles lacking attachment sites for Kip3p-dependent movements should lack attachment sites for dynein-dependent movements and, therefore, lack dynein-based movements. Surprisingly, after orienting an astral microtubule into the bud, short, misaligned, and mispositioned spindles with misoriented astral microtubules moved into the bud neck after anaphase onset, a movement attributed to dynein (Yeh et al., 1995; DeZwaan et al., 1997). To examine further the presence of cortical attachment sites for dynein-dependent movements in latrunculin-treated cells, we examined two specific movements dependent on dynein: sliding of astral microtubules along the bud cortex (Adames and Cooper, unpublished data) and oscillation of the elongating spindle within the bud neck (Yeh et al., 1995; DeZwaan et al., 1997). Astral microtubules were seen to slide along the bud cortex during movement of the elongating spindle into the neck (Figure 6). Furthermore, we found that elongating spindles that spanned the mother–bud neck oscillated along the mother–bud axis in 17 of 18 latrunculin-treated cells (Figure 7), moving bidirectionally a minimum of 1 μm at least three times. Eleven of 12 control cells showed such oscillations (Figure 7). Therefore, dynein-dependent microtubule sliding along the cortex and spindle oscillations do not depend on filamentous actin, which is consistent with actin not being necessary for the cortical attachment of astral microtubules in these processes. That these movements occurred in cells that had mispositioned short spindles with misoriented astral microtubules suggests that dynein-based movements use cortical attachment sites that differ from those used for Kip3p-dependent movements.
Figure 6.
Misorientation of astral microtubules and astral microtubule sliding. A cell expressing GFP-Cap1p and GFP-tubulin treated at 0 min with 500 μM latrunculin. Astral microtubules can be seen oriented into the mother from the spindle pole body proximal to the bud at 8 min. An astral microtubule is then oriented into the bud at 9 min, and the astral microtubule can be seen sliding along the cortex (arrows) at 10 and 11 min as the spindle moves into the neck.
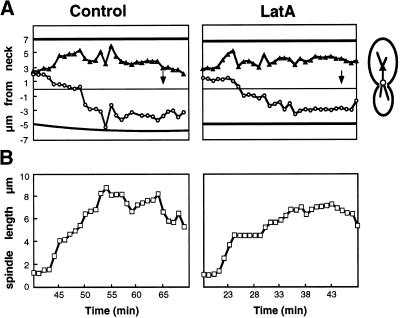
Figure 7.
Movement of anaphase mitotic spindles into the mother–bud neck and oscillation of the elongating spindles within the neck in the absence of filamentous actin. (A) Yeast expressing GFP-Cap1p to visualize actin patches and GFP-tubulin to monitor spindle movements was grown to early log phase and treated with DMSO (Control) or 500 μM latrunculin (LatA) at 0 min. (○) Position of the spindle pole destined for the bud. (▴) Position of the spindle pole destined for the mother. The 0-μm position on the y axis is the position of the mother–bud neck. Solid black lines indicate the edges of the cell. Arrows mark the time of spindle breakdown. (B) Spindle length over time for the spindles in A. The second phase of spindle elongation was variably affected among the lots of latrunculin used. Therefore, any effect on spindle elongation was interpreted as an artifact.
We found that filamentous actin is necessary for early Kip3p-dependent movements but, within the same latrunculin-treated cell, not for later dynein-dependent movements. Next, we examined more closely the role of filamentous actin in movement of the spindle into the neck, which reportedly depends on dynein (Yeh et al., 1995; DeZwaan et al., 1997). Midlog-phase cells expressing GFP-tubulin were treated with latrunculin and observed by time-lapse microscopy. Of 25 cells that went into mitosis, 22 spindles became positioned in the mother–bud neck and 3 spindles did not. Spindles always entered the neck in control cells. The mean time for spindles to move into the mother–bud neck after the onset of anaphase in latrunculin-treated cells was 11 ± 2.23 min (n = 18), which was more than twice the time in control cells (4.7 ± 0.56 min; n = 12). In addition, some of the anaphase spindles in latrunculin-treated cells (7 of 18 cells) moved into the neck and then back into the mother cell before permanently moving into the mother–bud neck (Figure 8). This never happened in control cells (0 of 12 cells). Overall, latrunculin slightly decreased the efficiency of movement and positioning of the mitotic spindle in the bud neck. Therefore, both actin-dependent and actin-independent mechanisms function coordinately to position the spindle in the neck.
Figure 8.
Aberrant movement of anaphase mitotic spindles into and out of the mother–bud neck in the absence of filamentous actin. Yeast expressing GFP-Cap1p to visualize actin patches and GFP-tubulin to monitor spindle movements was grown to early log phase and treated with 500 μM latrunculin at 0 min. Two examples are shown. (○) Position of the spindle pole destined for the bud. (▴) Position of the spindle pole destined for the mother. The 0-μm position on the y axis is the position of the mother–bud neck. Solid black lines indicate the edges of the cell.
DISCUSSION
We tested the hypothesis that filamentous actin is the cortical attachment site for astral microtubules during dynein-dependent movement of the mitotic spindle. We disrupted the actin cytoskeleton with mutations and latrunculin in synchronous populations of cells, allowing us to monitor the direct effect of these disruptions on spindle positioning during a single cell cycle. Consistent with Theesfeld et al. (1999), we found that actin is not required late in the cell cycle for proper positioning of the mitotic spindle. To better assess the acute effect of latrunculin on the different stages of spindle positioning within the same cell, we used digital fluorescence microscopy of live cells expressing GFP-tubulin. We have shown that actin is involved in maintaining the position and alignment of the preanaphase mitotic spindle at the mother–bud neck in yeast, that actin may play a role in positioning the mitotic spindle into the neck, and, surprisingly, that actin is not necessary for dynein-dependent movement of the mitotic spindle.
Actin and Kip3p-dependent Movements
Actin has been implicated in the attachment of microtubules involved in Kip3p-dependent movements. Mutants lacking either of two proteins that interact with the actin cytoskeleton, the formin Bni1p or the actin-interacting protein Bud6p, have defects in positioning the nucleus at the neck and in aligning the mitotic spindle along the mother–bud axis. These defects are similar to those of kip3 mutants (Lee et al., 1999; Miller et al., 1999). The severity of defects in nuclear position and spindle alignment correlates with the severity of Kar9p mislocalization from a spot at the bud tip, supporting the hypothesis that Kar9p is an attachment site for astral microtubules during Kip3p-dependent movements (Lee et al., 1999; Miller et al., 1999). Depolymerization of filamentous actin with latrunculin causes mislocalization of Kar9p and misorientation of short spindles in fixed cell analyses (Lee et al., 1999; Miller et al., 1999). Whether these effects are the direct result of depolymerizing filamentous actin or a secondary effect of inducing the bud morphogenesis checkpoint cannot be discerned from fixed cell analysis. Our analysis of individual live cells treated with latrunculin showed that spindles that later progressed into mitosis were positioned away from the neck, were misaligned from the mother–bud axis, and had misoriented astral microtubules. Therefore, depolymerizing filamentous actin directly affected the position and alignment of the short preanaphase spindle, providing strong support for the hypothesis that actin is part of the cortical attachment site for astral microtubules during Kip3p-dependent movements.
Actin and Dynein-dependent Movements
Mitotic spindles in dynein mutants fail to oscillate in the neck, and astral microtubules fail to slide along the bud cortex as they do in wild-type cells (Yeh et al., 1995; DeZwaan et al., 1997; Adames and Cooper, unpublished data). We showed that both oscillation of the elongating mitotic spindle within the neck and sliding of astral microtubules along the bud cortex are unaffected by latrunculin treatment. This is consistent with dynein movements being actin independent. The spindles exhibiting oscillations in the neck and astral microtubule sliding were the same spindles that began the experiment positioned away from the neck, poorly aligned along the mother–bud axis, and with misoriented astral microtubules. This suggests that there are two populations of attachment sites within the bud, actin dependent and actin independent. A model in which Kip3p-dependent movements rely on an actin-dependent attachment site and dynein-dependent movements use actin-independent attachment sites is consistent with our data.
Actin and Movement of the Mitotic Spindle into the Neck
After initiating anaphase, the elongating mitotic spindle is positioned in the neck. In mutants lacking dynein, elongating anaphase spindles are not positioned in the neck as efficiently as in wild-type cells or kip3 mutants (Yeh et al., 1995; DeZwaan et al., 1997). Our data show that latrunculin also causes elongating anaphase spindles to move into the neck inefficiently. This is consistent with loss of the cortical attachment site for dynein-dependent movements. However, these same cells exhibited dynein-dependent astral microtubule sliding and spindle oscillations. How can latrunculin cause short spindles to lose Kip3p-dependent maintenance of short spindle position and alignment at the neck, followed by impaired movement into the neck, and yet have dynein-dependent astral microtubule sliding and spindle oscillations? One possible model is that Kip3p-dependent forces, using actin-dependent attachment sites, orient astral microtubules into the bud and bring the spindle close to the bud neck. The orientation of the astral microtubules into the bud by Kip3p increases the chance that astral microtubules will interact with actin-independent attachment sites at the cortex and slide in a dynein-dependent manner to pull the spindle into the bud neck. Astral microtubule sliding is transient. In this model, Kip3p and transient interactions with actin-dependent attachment sites impede movement of the spindle out of the neck until another sliding event pulls the spindle farther into the neck or until the spindle is too long to fit back through the neck. Once the spindle is long enough, Kip3p-dependent forces and actin-dependent sites are not needed to keep the spindle in the neck while dynein-dependent forces cause spindle oscillations (Figure 9). This model does not rule out the possibility that actin may function earlier to establish the attachment sites used for dynein-dependent movements, as suggested by Theesfeld et al. (1999).
Figure 9.
Model for mitotic spindle position and movement. First, the nucleus (○) moves to the nascent bud site. This movement requires the kinesin Kip3p and presumably attachment of astral microtubules (straight black lines) to the cortex of the bud (⋄). After forming a short mitotic spindle, the nucleus remains at the neck and the spindle is kept generally aligned along the mother–bud axis. Maintenance of nuclear position and spindle alignment also require Kip3p and presumably attachment of astral microtubules to the cortex of the bud. The cortical attachment site for astral microtubules during Kip3p-dependent movements depends, in part, on filamentous actin, the formin Bni1p, Bud6p, and Kar9p. After initiating spindle elongation (anaphase), the mitotic spindle moves into the neck concurrent with astral microtubules sliding along the cortex. Both actin-dependent (⋄) and actin-independent (angled lines) attachment sites participate in moving the spindle into the neck. Once in the neck, the elongating spindle exhibits dynein-dependent oscillations. The cortical attachment site for astral microtubules during dynein-dependent movements is not yet known.
Another possibility, more consistent with Theesfeld et al. (1999), is that actin is needed for maturation of a single type of attachment site. In cells that have progressed past the bud morphogenesis checkpoint, the buds have grown in an actin-dependent manner to a sufficient size that the cortical attachment site for astral microtubules may be partially formed. The partially mature attachment site is sufficient for oscillation of the elongating mitotic spindle in the neck but can only move the spindle into the neck in an impaired manner. However, it is difficult to imagine that an attachment site would retain sufficient function to move the elongating spindle into the neck and yet be insufficient to correctly position and align the spindle before anaphase. Furthermore, the astral microtubule behavior in latrunculin-treated cells is more consistent with the first model.
In conclusion, we have shown that filamentous actin is not essential for dynein-dependent movements. We have shown in these experiments that filamentous actin is involved in the Kip3p-dependent maintenance of the mitotic spindle at the neck, orienting astral microtubules into the bud and aligning the spindle along the mother–bud axis. Finally, our data support a model in which both actin-dependent and actin-independent cortical capture of astral microtubules are responsible for movement of the mitotic spindle into the mother–bud neck.
ACKNOWLEDGMENTS
We are grateful to Neil Adames, Tatiana Karpova, and Dorothy Schafer for helpful discussions. This work was supported by National Institutes of Health grant GM47337 to J.A.C
REFERENCES
- Adams AEM, Botstein D, Drubin DG. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991;354:404–408. doi: 10.1038/354404a0. [DOI] [PubMed] [Google Scholar]
- Allen VW, Kropf DL. Nuclear rotation and lineage specification in Pelvetia embryos. Development. 1992;115:873–883. [Google Scholar]
- Amatruda JF, Cannon JF, Tatchell K, Hug C, Cooper JA. Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature. 1990;344:352–354. doi: 10.1038/344352a0. [DOI] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A, Ozierkalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A, Kolling R. The SAC3 gene encodes a nuclear protein required for normal progression of mitosis. J Cell Sci. 1996;109:1575–1583. doi: 10.1242/jcs.109.6.1575. [DOI] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Cottingham FR, Hoyt MA. Mitotic spindle positioning in Saccharomyces cerevisiae is accomplished by antagonistically acting microtubule motor proteins. J Cell Biol. 1997;138:1041–1053. doi: 10.1083/jcb.138.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan K, Tanaka Y. Attachment of one spindle pole to the cortex in unequal cleavage. Ann NY Acad Sci. 1990;582:108–119. doi: 10.1111/j.1749-6632.1990.tb21672.x. [DOI] [PubMed] [Google Scholar]
- DeZwaan TM, Ellingson E, Pellman D, Roof DM. Kinesin-related Kip3 of Saccharomyces cerevisiae is required for a distinct step in nuclear migration. J Cell Biol. 1997;138:1023–1040. doi: 10.1083/jcb.138.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel D, Urrestarazu LA, Vissers S, Jauniaux JC, vanVliet-Reedijk JC, Planta RJ, Gibbons IR. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci USA. 1993;90:11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil-Chapdelaine RA, Adames NR, Cooper JA. Formin connection between microtubules and the cell cortex. J Cell Biol. 1999;144:809–811. doi: 10.1083/jcb.144.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil-Chapdelaine RA, Tran NK, Cooper JA. The role of Saccharomyces cerevisiae coronin in the actin and microtubule cytoskeletons. Curr Biol. 1998;8:1281–1284. doi: 10.1016/s0960-9822(07)00539-8. [DOI] [PubMed] [Google Scholar]
- Hyman AA. Centrosome movement in the early divisions of Caenorhabditis elegans: a cortical site determining centrosome position. J Cell Biol. 1989;109:1185–1193. doi: 10.1083/jcb.109.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, White JG. Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J Cell Biol. 1987;105:2123–2135. doi: 10.1083/jcb.105.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R-P, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- Karpova TS, McNally JG, Moltz SL, Cooper JA. Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J Cell Biol. 1998;142:1501–1517. doi: 10.1083/jcb.142.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Klee SK, Evangelista M, Boone C, Pellman D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J Cell Biol. 1999;144:947–961. doi: 10.1083/jcb.144.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Yeh E, Hays T, Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci USA. 1993;90:10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz DA, Hamaguchi Y, Inoue S. Micromanipulation studies of the asymmetric positioning of the maturation spindle in Chaetopterus sp. oocytes. I. Anchorage of the spindle to the cortex and migration of a displaced spindle. Cell Motil Cytoskeleton. 1988;11:83–96. doi: 10.1002/cm.970110202. [DOI] [PubMed] [Google Scholar]
- McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Miller RK, Heller KK, Frisen L, Wallack DL, Loayza D, Gammie AE, Rose MD. The kinesin-related proteins, kip2p and kip3p, function differently in nuclear migration in yeast. Mol Biol Cell. 1998;9:2051–2068. doi: 10.1091/mbc.9.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Matheos D, Rose MD. The cortical localization of the microtubule orientation protein, Kar9p, is dependent upon actin and proteins required for polarization. J Cell Biol. 1999;144:963–975. doi: 10.1083/jcb.144.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhua L, Karpova TS, Cooper JA. A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration. Cell. 1994;78:669–679. doi: 10.1016/0092-8674(94)90531-2. [DOI] [PubMed] [Google Scholar]
- Palmer RE, Sullivan DS, Huffaker T, Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast. Saccharomyces cerevisiae. J Cell Biol. 1992;119:583–593. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips J, Herskowitz I. Identification of Kel1p, a kelch domain-containing protein involved in cell fusion and morphology in Saccharomyces cerevisiae. J Cell Biol. 1998;143:375–389. doi: 10.1083/jcb.143.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL, Yeh E, Bloom K, Salmon ED. Imaging green fluorescent protein fusion proteins in Saccharomyces cerevisiae. Curr Biol. 1997;7:701–704. doi: 10.1016/s0960-9822(06)00299-5. [DOI] [PubMed] [Google Scholar]
- Skop AR, White JG. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr Biol. 1998;8:1110–1116. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T. Motoring to the finish: kinesin and dynein work together to orient the yeast mitotic spindle. J Cell Biol. 1997;138:957–960. doi: 10.1083/jcb.138.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Marshall WF, Sedat JW, Murray AW. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- Theesfeld CL, Irazoqui JE, Bloom K, Lew DJ. The role of actin in spindle orientation changes during the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1999;146:1019–1032. doi: 10.1083/jcb.146.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddle JA, Cooper JA, Waterston RH. Transient localized accumulation of actin in Caenorhabditis elegans blastomeres with oriented asymmetric divisions. Development. 1994;120:2317–2328. doi: 10.1242/dev.120.8.2317. [DOI] [PubMed] [Google Scholar]
- Waddle JA, Karpova TS, Waterston RH, Cooper JA. Movement of cortical actin patches in yeast. J Cell Biol. 1996;132:861–870. doi: 10.1083/jcb.132.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Bretscher A. Mutations synthetically lethal with tpm1Δ lie in genes involved in morphogenesis. Genetics. 1997;147:1595–1607. doi: 10.1093/genetics/147.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts FZ, Shiels G, Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987;6:3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Skibbens RV, Cheng JW, Salmon ED, Bloom K. Spindle dynamics and cell cycle regulation of dynein in the budding yeast Saccharomyces cerevisiae. J Cell Biol. 1995;130:687–700. doi: 10.1083/jcb.130.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]