Abstract
Xenopus oocytes and eggs provide a dramatic example of how the consequences of p42 mitogen-activated protein kinase (p42 MAPK) activation depend on the particular context in which the activation occurs. In oocytes, the activation of Mos, MEK, and p42 MAPK is required for progesterone-induced Cdc2 activation, and activated forms of any of these proteins can bring about Cdc2 activation in the absence of progesterone. However, in fertilized eggs, activation of the Mos/MEK/p42 MAPK pathway has the opposite effect, inhibiting Cdc2 activation and causing a G2 phase delay or arrest. In the present study, we have investigated the mechanism and physiological significance of the p42 MAPK-induced G2 phase arrest, using Xenopus egg extracts as a model system. We found that Wee1-depleted extracts were unable to arrest in G2 phase in response to Mos, and adding back Wee1 to the extracts restored their ability to arrest. This finding formally places Wee1 downstream of Mos/MEK/p42 MAPK. Purified recombinant p42 MAPK was found to phosphorylate recombinant Wee1 in vitro at sites that are phosphorylated in extracts. Phosphorylation by p42 MAPK resulted in a modest (∼2-fold) increase in the kinase activity of Wee1 toward Cdc2. Titration experiments in extracts demonstrated that a twofold increase in Wee1 activity is sufficient to cause the delay in mitotic entry seen in Mos-treated extracts. Finally, we present evidence that the negative regulation of Cdc2 by Mos/MEK/p42 MAPK contributes to the presence of an unusually long G2 phase in the first mitotic cell cycle. Prematurely inactivating p42 MAPK in egg extracts resulted in a corresponding hastening of the first mitosis. The negative effect of p42 MAPK on Cdc2 activation may help ensure that the first mitotic cell cycle is long enough to allow karyogamy to be accomplished successfully.
INTRODUCTION
Immature, stage VI Xenopus laevis oocytes are arrested in a G2-like state. Upon exposure to progesterone, the cell is released from this arrest, progresses through meiosis I, and then spontaneously arrests in metaphase of meiosis II. Just before the resumption of meiosis I, the Mos/MEK/p42 mitogen-activated protein kinase (MAPK) cascade and the universal M phase regulator Cdc2/cyclin B become activated. Activation of the MAPK cascade can promote Cdc2 activation and oocyte maturation in the absence of added hormone (Yew et al., 1992; Gotoh et al., 1995; Haccard et al., 1995; Huang et al., 1995). Furthermore, interfering with p42 MAPK activation via expression of a MAPK-specific phosphatase (Gotoh et al., 1995), treatment with a pharmacological MEK inhibitor (Cross and Smythe, 1998), or microinjection of neutralizing MEK antibodies (Kosako et al., 1994, 1996) or Mos antisense oligonucleotides (Sagata et al., 1988) can prevent progesterone-induced maturation. Thus, p42 MAPK is both necessary and sufficient for Cdc2/cyclin B activation in Xenopus oocytes.
Recent work has shed light on how p42 MAPK exerts its positive effect on Cdc2 activation. Abrieu et al. (1997a) have presented evidence that p42 MAPK inhibits some negative regulator of Cdc2. In many vertebrate systems, including Xenopus eggs and egg extracts, Cdc2 is negatively regulated by both the Wee1 and Myt1 kinases via phosphorylation of Thr 14 and Tyr 15 and, conversely, activated by the actions of the dual specificity phosphatase Cdc25 (Dunphy and Kumagai, 1991; Gautier et al., 1991; Kumagai and Dunphy, 1991; Mueller et al., 1995a,b). However, Wee1 is absent from immature oocytes and only begins to accumulate during meiosis II (Murakami and Vande Woude, 1998). These findings suggest that p42 MAPK might exert its positive effect on Cdc2 activation by directly or indirectly inhibiting Myt1. Palmer et al. (1998) have shown that Rsk, a kinase phosphorylated and activated by p42 MAPK, can phosphorylate and partially inactivate Myt1. A mechanism thereby emerges to explain how the activation of p42 MAPK can bring about Cdc2 activation in the immature oocyte via the actions of Rsk.
Recent studies have shown that the activation of p42 MAPK in a very similar context results in a dramatically different outcome. In both cycling Xenopus egg extracts and activated eggs, activation of p42 MAPK during interphase inhibits Cdc2 activation and mitotic entry, without measurably affecting DNA synthesis (Abrieu et al., 1997a; Walter et al., 1997; Bitangcol et al., 1998; Murakami and Vande Woude, 1998). Thus, in eggs and extracts, p42 MAPK activation can cause a G2 delay or G2 arrest, rather than the release from G2 arrest that p42 MAPK activation causes in oocytes. Because Wee1 is present in eggs and absent from oocytes (Murakami and Vande Woude, 1998), it seemed plausible that Wee1 might mediate the negative effects of p42 MAPK on Cdc2. In agreement with this hypothesis, ectopic expression of Wee1 in oocytes at concentrations similar to those found normally in eggs prevents oocytes from maturing in response to progesterone (Murakami and Vande Woude, 1998), and depletion of Wee1 from cytostatic factor (CSF)-arrested egg extracts prevents the extracts from undergoing G2 arrest in response to Mos (Murakami et al., 1999).
In this report, we present evidence of how p42 MAPK and Wee1 cooperate to inhibit Cdc2. We found that the addition of Mos to cycling extracts increases the phosphorylation of catalytically inactive Wee1, implicating some enzyme downstream of Mos in the regulation of Wee1 phosphorylation. Moreover, p42 MAPK phosphorylated Wee1 in vitro at sites that are also phosphorylated in extracts, supporting the idea that p42 MAPK phosphorylates Wee1 directly. Incubation of Wee1 with p42 MAPK resulted in a modest (∼2-fold) increase in the activity of Wee1 toward Cdc2. The significance of this activity increase was tested via Wee1 titration experiments in egg extracts, which showed that p42 MAPK activation approximately doubles the G2-delaying activity of Wee1. These data indicate that p42 MAPK can directly affect the activity of Wee1, which may, in turn, result in important downstream effects on Cdc2.
In a previous report, we suggested that the persistence of p42 MAPK activity after fertilization might have a negative effect on Cdc2 activation, thereby delaying mitotic entry and allowing the first mitotic cell cycle to possess a G2 phase (G2 phases are absent from the next 11 cycles) (Walter et al., 1997). By supplementing CSF-arrested egg extracts with recombinant p42 MAPK to extend this period of high p42 MAPK activity, we showed that there was a direct relationship between the length of time p42 MAPK was active and the length of the G2 phase; the longer p42 MAPK remained active, the longer mitosis was delayed (Walter et al., 1997). We extend these studies here by showing that prematurely inactivating p42 MAPK shortens the duration of the first interphase and thus hastens Cdc2 activation. These findings establish the physiological relevance of the previously described p42 MAPK-induced G2 arrest; p42 MAPK is, at least in part, responsible for the unique length of the first mitotic cell cycle in X. laevis embryos.
MATERIALS AND METHODS
Recombinant Protein Production
A plasmid containing the cDNA for a Xenopus Mos–myelin basic protein fusion protein was obtained from George Vande Woude (Frederick Cancer Research and Development Center, Frederick, MD). The protein was expressed in bacteria and purified as described previously (Yew et al., 1992).
A plasmid containing the cDNA for a constitutively active version of human MEK (MEK R4F) was obtained from Natalie Ahn (University of Colorado, Boulder, CO) (Mansour et al., 1994, 1996a,b). The protein was expressed in bacteria as a hexahistidine-tagged fusion and purified as described (Wang et al., 1997).
A plasmid containing the cDNA for wild-type and kinase-minus (with Lys 57 changed to Arg) Xenopus p42 MAPK was obtained from Jonathan Cooper and Jim Posada (Fred Hutchinson Cancer Research Center, Seattle, WA) (Posada and Cooper, 1992). It was expressed as a hexahistidine fusion in Escherichia coli and purified by nickel chelate chromatography.
His6-tagged Xenopus Wee1 protein was expressed in Sf9 cells using baculovirus provided by Bill Dunphy (California Institute of Technology, Pasadena, CA). Kinase-minus Wee1 was constructed using the Stratagene (La Jolla, CA) QuikChange site-directed mutagenesis kit and the mutant oligonucleotide 5′-GTTTCTACGCCATTATACGCTCCAAGAAGCC-3′, which changed Lys 242 in the Wee1 ATP-binding pocket to Ile. Recombinant Wee1 proteins were purified as described (Mueller et al., 1995a).
Baculovirus encoding catalytically inactive Xenopus Cdc2 (with Thr 161 changed to Ala) was also provided by Bill Dunphy. Sf9 cells were coinfected with this virus and virus encoding a truncated version of Xenopus cyclin B1, denoted His6-Δ65 cyclin B1. This cyclin construct is missing the first 65 amino acids, including the destruction box, and contains an N-terminal hexahistidine tag. It was created by cutting cyclin B1/pGEM1, provided by Jeremy Minshull (Affymetrix, Santa Clara, CA), with BglI, digesting with T4 DNA polymerase to form a blunt end, and then excising the DNA fragment with HindIII. This portion of cyclin B1 was then subcloned into Fastbac Htc, cut previously with StuI and HindIII. The construct was then sequenced, and virus was made following the protocol of Life Technologies (Gaithersburg, MD). The Cdc2/His6-Δ65 cyclin B1 complexes were precipitated with nickel chelate resin.
Extract Preparation and Staining
Cycling egg extracts, cycloheximide-treated (150 μg of cycloheximide/ml of extract) interphase extracts, and CSF-arrested extracts were prepared as described (Murray and Kirschner, 1989; Murray, 1991). For one experiment (see Figure 7), the standard cycling extract preparation was modified slightly (eggs were allowed to sit for 30 min rather than 15 min between electrical activation and crushing, which increases the likelihood that the extract will perform two well-synchronized cell cycles) (Bhatt and Ferrell, 1999). Mos-treated extracts were produced by adding purified recombinant Mos to a final Mos concentration of 1 μM, which is sufficient to maximally activate the p42 MAPK within ∼30 min (Walter et al., 1997). Demembranated sperm chromatin was prepared as described (Smythe and Newport, 1991). Chromatin was routinely added to extracts at a concentration of 500 nuclei/μl to monitor cell-cycle progression. Samples were fixed with 11% formaldehyde, stained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml), and viewed by epifluorescence microscopy with a Zeiss (Thornwood, NY) Axioscop.
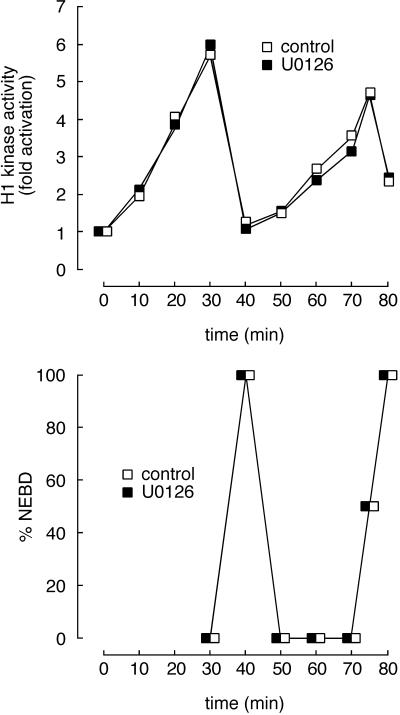
Figure 7.
Transient activation of p42 MAPK during mitosis has no measurable effect on the length of the subsequent interphase. Cycling extracts were incubated with U0126 (100 μM; ▪) to prevent mitotic activation of MAP kinase or with an equal volume of DMSO (1%; □), and samples were removed periodically for histone H1 kinase assays. This concentration of U0126 fully blocked the mitotic activation of p42 MAPK (our unpublished results).
Antibodies and Other Reagents
Antisera X15 and DC3 were raised against a C-terminal 12-amino-acid peptide from Xenopus p42 MAPK (Hsiao et al., 1994). Wee1 antibody (Walter et al., 1997) was obtained from Zymed Laboratories (South San Francisco, CA). Nonimmune serum was obtained from Sigma Chemical (St. Louis, MO). The MEK inhibitor U0126 was obtained from Promega (Madison, WI), reconstituted in DMSO at a concentration of 10 mM, and used at a final concentration of 100 μM.
Immunoblotting
Samples of egg extracts were run on SDS-PAGE, transferred to Immobilon P (Millipore, Bedford, MA), and then blocked with 3% milk. Antibodies were used at a concentration of 1:1000 for 2 h before detection with chemiluminescence.
Immunodepletion
Protein A Sepharose (Sigma Chemical) was bound to Wee1 or nonimmune antibodies for 1 h, washed with cycling extract buffer (100 mM KCl, 10 mM HEPES, 1 mM MgCl2, 0.1 mM CaCl2, and 50 mM sucrose), and then incubated with extract for 90 min. After centrifugation (1 min at full speed in an Eppendorf microcentrifuge, Brinkmann Instruments, Westbury, NY), the extract was removed from the pelleted resin and placed on ice before further manipulations.
Phosphorylation of Wee1 by p42 MAPK In Vitro
p42 MAPK (400 ng/10 μl reaction) was activated by incubation with MEK R4F (400 ng/10 μl reaction) in kinase buffer (100 mM NaCl, 50 mM Tris-HCl, pH 7.0, 10 mM MgCl2, 100 μM ATP, and 0.1% bovine serum albumin) for 30 min at 30°C. Recombinant His6-Wee1 (50 ng/reaction) was then added together with additional cold ATP (100 μM) and, when appropriate, [γ-32P]ATP, and the reaction (final volume of 15 μl) was continued for 30 min at room temperature.
Phosphorylation of Wee1 in Extracts
Kinase-minus Wee1 K242I was immobilized on nickel beads at a concentration of ∼0.4 nmol/μl of beads. Cycloheximide-treated interphase extracts were prepared and incubated for 30 min with or without Mos (1 μM). Control and Mos-treated extracts (200 μl) were added together with 4 μl (40 μCi) of [γ-32P]ATP to portions of the packed Wee1-coated beads (25 μl), and the mixtures were incubated at room temperature for 30 min.
Tryptic Peptide Mapping
Wee1 K242I was labeled with 32P in vitro or in extracts. Proteins were separated by SDS-PAGE and transferred to Immobilon P membranes. Without allowing the membranes to dry out, we wrapped the membranes in plastic wrap and subjected them to autoradiography. The Wee1 bands were identified, excised, and subjected to tryptic digestion in situ as described (Luo et al., 1991), omitting the performic acid oxidation step. The digested samples were subjected to thin-layer electrophoresis on cellulose TLC plates (E. M. Sciences, Darmstadt, Germany) at pH 8.9 (in 1% ammonium carbonate), 1000 V, for 30 min, followed by TLC in phosphochromatography buffer (750 ml of n-butanol, 500 ml of pyridine, 150 ml of glacial acetic acid, and 600 ml of water) as described (Boyle et al., 1991).
Kinase Assays
To determine Cdc2 kinase activity, we performed kinase assays using histone H1 as a substrate as described previously (Walter et al., 1997).
Wee1 kinase assays were performed using a complex of recombinant kinase-minus Cdc2 T161A and His6-Δ65 cyclin B1 as a substrate. Wee1 was incubated with p42 MAPK, MEK R4F, and albumin, as described above, or incubated with MEK R4F plus albumin, p42 MAPK plus albumin, or albumin alone. The reaction mixtures were then incubated with agarose-bound Cdc2 T161A–His6-Δ65 cyclin B1 and [γ-32P]ATP for 15 min at room temperature. After the samples were run on SDS-PAGE, the radioactive counts in the Cdc2 were quantified using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Wee1 Protein Is Necessary for the G2 Arrest
Activation of the p42 MAPK cascade in immature stage VI X. laevis oocytes promotes Cdc2 activation and G2/M progression. However, we and others have demonstrated that activation of p42 MAPK during interphase of the first mitotic cell cycle has the opposite effect, in that it inhibits Cdc2 activation and delays G2/M progression (Abrieu et al., 1997b; Walter et al., 1997; Bitangcol et al., 1998; Murakami and Vande Woude, 1998). We therefore set out to determine how p42 MAPK inhibits Cdc2 activation.
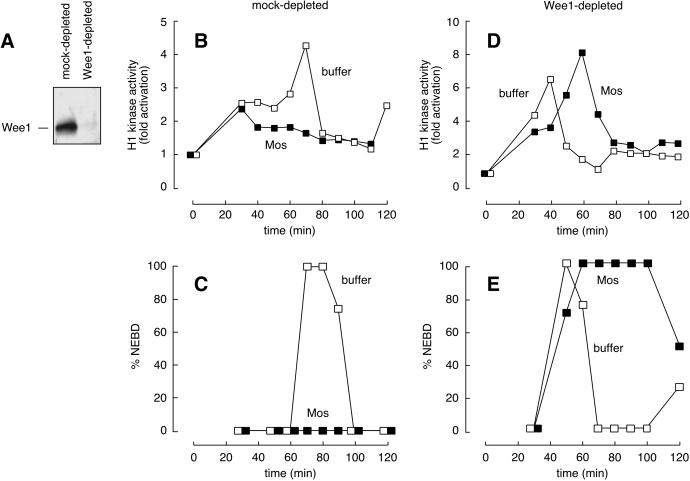
A clue came from the discovery that Wee1 is not translated in oocytes until meiosis II (Murakami and Vande Woude, 1998). The absence of this important negative regulator of Cdc2 might be the key difference between oocytes and eggs and explain how p42 MAPK activation can result in two very different outcomes in these two environments. Therefore, we examined whether Wee1 was required for the Mos-induced G2 arrest in egg extracts. Immunodepletion of Wee1 from a CSF-arrested extract was shown previously to prevent the p42 MAPK-induced interphase arrest (Murakami et al., 1999). We confirmed these results (our unpublished results) and extended them to cycling egg extracts (Figure 1). Extracts depleted of endogenous Wee1 (Figure 1A) entered mitosis before mock-depleted extracts did (Figure 1, compare E, □, mitotic entry at 50 min, with C, □, mitotic entry at 70 min). Furthermore, Wee1-depleted extracts failed to undergo a G2 phase arrest in response to Mos (Figure 1, D and E, ▪), whereas control extracts arrested in interphase with low levels of Cdc2 activity (Figure 1, B and C). These data indicate that Wee1 is required for Mos-mediated G2 arrest.
Figure 1.
p42 MAPK requires Wee1 to effect a G2 phase arrest in Xenopus egg extracts. Cycling extracts were prepared and incubated with protein A Sepharose bound to nonimmune serum or Wee1 antibody. (A) Wee1 immunoblot of mock-depleted and Wee1-depleted extracts. (B and C) Mos-induced G2 phase arrest in mock-depleted extracts. (D and E) Failure of Wee1-depleted extracts to arrest in response to Mos. (B and D) The histone H1 kinase activities of buffer-treated (□) and Mos-treated (1 μM; ▪) extracts. (C and E) Nuclear envelope breakdown (NEBD) in the same extracts.
Although Wee1-depleted extracts failed to undergo a G2 arrest in response to Mos, they still underwent a normal M phase arrest. That is, the extracts entered M phase (sometimes with a small delay [see Figure 1E], but sometimes without any delay [see Figure 5]) and remained in M phase for a protracted period of time (Figure 1E) (see also Abrieu et al., 1996, 1997b; Walter et al., 1997; Bitangcol et al., 1998; Bhatt and Ferrell, 1999; Chau and Shibuya, 1999). Thus, Wee1 is not required for the M phase–arresting activity of the Mos/MEK/p42 MAPK cascade.
Figure 5.
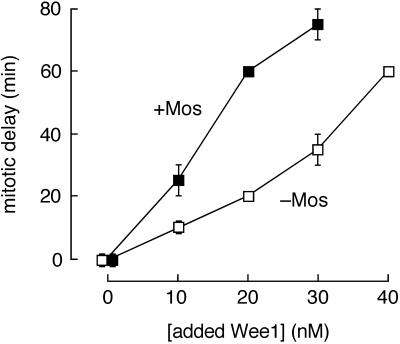
Mos potentiates the activity of Wee1 in cycling egg extracts. After immunodepleting extracts of endogenous Wee1, various amounts of recombinant Wee1 were added back in the presence (▪) or absence (□) of Mos protein. The Wee1-depleted extracts entered mitosis (assessed by nuclear envelope breakdown) 40 min after the initiation of cycling, 10 min before mock-depleted extracts (compare Figure 1). Data are expressed as the time of nuclear envelope breakdown in the Wee1-supplemented extracts relative to that of the Wee1-depleted extracts (“mitotic delay”). Data shown are from one (the 20 and 40 nM Wee1 data points) or two (the 0, 10, and 30 nM Wee1 data points) experiments. Error bars represent ranges.
p42 MAPK Can Phosphorylate Wee1 In Vitro
One simple hypothesis to account for the results described above would be that p42 MAPK exerted its inhibitory effects on Cdc2 by phosphorylating and activating Wee1. Although phosphorylation by mitotic kinases has been implicated in the negative regulation of Wee1 (McGowan and Russell, 1995; Mueller et al., 1995a; Watanabe et al., 1995), it is possible that the phosphorylation of different residues by p42 MAPK might exert a positive effect on Wee1 activity.
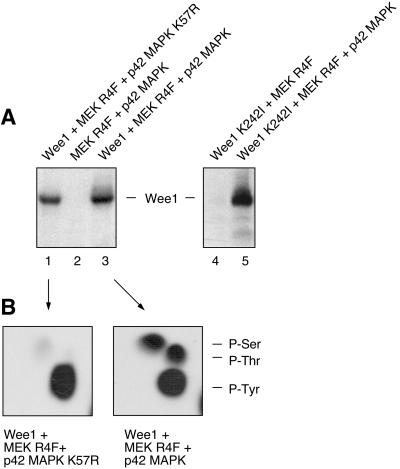
To test this hypothesis, we first determined whether p42 MAPK was capable of phosphorylating Wee1 in vitro. Recombinant p42 MAPK was activated with MEK R4F, a constitutively active form of MEK (Mansour et al., 1994, 1996a,b). The mixture of MEK R4F and activated p42 MAPK was then incubated with recombinant kinase-minus His6-Wee1 K242I and [γ-32P]ATP. As shown in Figure 2A, the combination of MEK R4F and p42 MAPK caused Wee1 to become heavily phosphorylated (lane 5), but MEK R4F alone did not (lane 4). We also incubated catalytically active Wee1 with [γ-32P]ATP in the absence of MEK R4F and p42 MAPK (Figure 2A, lane 1), MEK R4F and p42 MAPK with [γ-32P]ATP in the absence of Wee1 (lane 2), and MEK R4F, p42 MAPK, and Wee1 together (lane 3). As shown in Figure 2, Wee1 autophosphorylated (Figure 2A, lane 1) on tyrosine residues (Figure 2B, left). In the presence of MEK R4F and p42 MAPK, the phosphorylation of Wee1 increased by 72% (Figure 2A, lane 3), and Wee1 became phosphorylated at serine and threonine residues as well as tyrosine residues (Figure 2B, right). These data show that Wee1 can be phosphorylated in vitro by p42 MAPK at serine and threonine phosphorylation sites.
Figure 2.
Phosphorylation of Wee1 by p42 MAPK in vitro. (A) Autoradiogram of an in vitro phosphorylation reaction with purified, recombinant proteins and [γ-32P]ATP. Wild-type Wee1 was used in the experiment shown on the left; it exhibited substantial autophosphorylation (lane 1), with MEK R4F and p42 MAPK causing additional phosphorylation (lane 3). Kinase-minus Wee1 (Wee1 K242I) was used in the experiment shown on the right. It was phosphorylated in the presence of MEK R4F and p42 MAPK (lane 5), not in the presence of MEK R4F alone (lane 4). (B) Phosphoamino acid analysis of excised Wee1 bands from lanes 1 and 3 of A. Positions of ninhydrin-stained phosphoamino acid standards are indicated on the right.
Tryptic Analysis of Wee1 Phosphorylated In Vitro and in Extracts
Murakami et al. (1999) have shown that Mos can increase or extend the duration of tyrosine phosphorylation of Wee1, possibly by stimulating Wee1 autophosphorylation. We examined whether Mos could increase the phosphorylation of kinase-minus Wee1 K242I (which does not autophosphorylate; Figure 2A, lane 4) in extracts, as would be expected if p42 MAPK phosphorylated Wee1 directly. Recombinant Wee1 K242I (immobilized on nickel beads) and [γ-32P]ATP were added to control or Mos-treated interphase extracts. Mos addition resulted in an approximately twofold (194 ± 43%) increase in the incorporation of 32P into Wee1. These findings demonstrate that some factor downstream of Mos regulates the phosphorylation of Wee1.
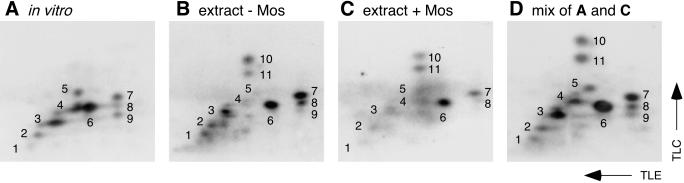
To test the hypothesis that p42 MAPK directly phosphorylates Wee1 in extracts, we compared the tryptic phosphopeptide map of Wee1 K242I phosphorylated by active p42 MAPK in vitro (Figure 3A) with maps of Wee1 phosphorylated in extracts (Figure 3B–D). The map of in vitro–phosphorylated Wee1 showed at least nine distinct phosphopeptide spots (Figure 3A, spots 1–9). All of these spots could be accounted for in the maps of Wee1 phosphorylated in extracts (Figure 3B–D). In addition, two new prominent phosphopeptide spots (Figure 3B–D, spots 10 and 11) were seen in the extract Wee1 maps, presumably corresponding to sites that p42 MAPK cannot phosphorylate. These data indicate that the peptides phosphorylated by p42 MAPK in vitro are phosphorylated in Wee1 in extracts, supporting the possibility that p42 MAPK directly phosphorylates Wee1 in extracts.
Figure 3.
Tryptic phosphopeptide maps of Wee1 K242I. Wee1 K242I was labeled by incubation with [γ-32P]ATP and activated p42 MAPK in vitro (A and D) or by incubation with [γ-32P]ATP in control interphase extracts (B) or in interphase extracts preincubated for 30 min with Mos (1 μM; C and D). Tryptic digests were performed, and equal numbers of counts were subjected to thin-layer electrophoresis (TLE; pH 8.9, anode to the left) followed by TLC in phosphochromatography buffer. (A) Wee1 K242I phosphorylated by p42 MAPK in vitro. (B) Wee1 K242I phosphorylated in interphase extracts, with no added Mos. (C) Wee1 K242I phosphorylated in Mos-treated interphase extracts. (D) Mix of the samples used in A and C.
Most of the spots present in the map of in vitro–phosphorylated Wee1 (Figure 3A) were also detectable in the map of Wee1 phosphorylated in extracts in the absence of Mos (Figure 3B). This finding suggests that there are kinases other than p42 MAPK in extracts that phosphorylate the same Wee1 peptides that p42 MAPK phosphorylates.
p42 MAPK Can Cause a Modest Increase in Wee1 Kinase Activity
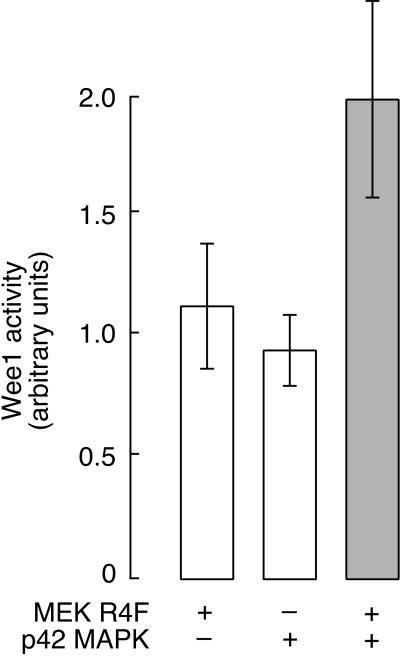
Next we asked whether p42 MAPK could activate Wee1. We prepared active recombinant p42 MAPK by incubation with ATP and MEK R4F and then supplemented the reaction with additional (unlabeled) ATP and purified His6-Wee1 protein. After a further incubation at room temperature, we assayed the resultant Wee1 kinase activity using [γ-32P]ATP and kinase-inactive Cdc2/cyclin B1 as a substrate. As shown in Figure 4, MEK R4F and p42 MAPK increased the activity of Wee1 to approximately twice the level seen with MEK R4F or (inactive) p42 MAPK alone. Thus, active p42 MAPK can produce a modest increase in Wee1 kinase activity in vitro.
Figure 4.
Phosphorylation of Wee1 by p42 MAPK results in a modest increase in kinase activity in vitro. Kinase activities are expressed in arbitrary units and shown as averages ± SD from three experiments. Open bars represent the activity of Wee1 in the presence of p42 MAPK or MEK R4F alone. The shaded bar shows the activity of Wee1 in the presence of both p42 MAPK and MEK R4F.
p42 MAPK Can Potentiate the Activity of Wee1 in Cycling Extracts
Having established that p42 MAPK can increase the kinase activity of Wee1, we set out to determine whether an approximately twofold increase in Wee1 activity would translate into a significant G2 phase delay. We prepared mock-depleted and Wee1-depleted cycling egg extracts. Wee1 depletion advanced the timing of mitosis by ∼10 min relative to that of mock-depleted extracts, and the timing of mitosis in the Wee1-depleted extracts was unaffected by the addition of Mos (Figure 5; however, also see Figure 1, where there was a small delay in the Mos-treated extract).
We then added increasing concentrations of recombinant Wee1 protein, in the presence or absence of Mos, and monitored cell-cycle progression. In the absence of Mos, the duration of G2 phase increased approximately linearly with the concentration of added Wee1 (Figure 5). The concentration of Wee1 required to restore normal mitotic timing was ∼10 nM, close to the estimated concentration of endogenous Wee1 (∼16 nM) (Mueller et al., 1995a). Each additional 10 nM increment of Wee1 caused an additional delay in the onset of mitosis (Figure 5). Thus, in cycling Xenopus egg extracts, modest increases in Wee1 concentration can cause a substantial G2 delay, consistent with previous reports (Mueller et al., 1995a). The Xenopus results are reminiscent of the demonstration of Russell and Nurse (1987a) that the length of fission yeast at mitosis is directly proportional to the dosage of the Wee1 gene.
Mos had no effect on the timing of mitosis in the absence of Wee1 but approximately doubled the effectiveness of added Wee1 in causing mitotic delay (Figure 5). For example, the same delay caused by 20 nM Wee1 in the absence of Mos (20 min) was caused by 10 nM Wee1 in the presence of Mos; the delay caused by 40 nM Wee1 in the absence of Mos (60 min) was caused by 20 nM Wee1 in the presence of Mos. The approximately twofold increase in Wee1 kinase activity observed in vitro in the presence of active p42 MAPK (Figure 4) was similar to this approximately twofold increase in G2-delaying activity (Figure 5). Thus, the modest increase in Wee1 activity that p42 MAPK causes in vitro may account for the G2-delaying activity of Mos.
Inactivation of MAP Kinase after Egg Activation Shortens the First Interphase
After fertilization, the egg does not divide for ∼90 min (see Figure 8B). The extended first mitotic cell cycle contrasts with the subsequent 11 divisions, which take 25–30 min each to complete (Hausen and Riebesell, 1991). p42 MAPK activity remains high for 30–40 min after fertilization (Ferrell et al., 1991; Hartley et al., 1994), unlike Cdc2 activity, which drops more rapidly (Watanabe et al., 1991; Hartley et al., 1996). We hypothesized that this elevated p42 MAPK activity after fertilization negatively affected Cdc2 and consequently was at least partly responsible for the extended first interphase. In support of this hypothesis, we demonstrated that artificially extending this period of p42 MAPK activation can further delay the onset of the first mitosis (Walter et al., 1997).
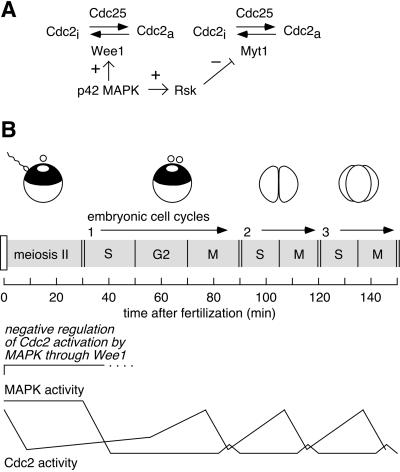
Figure 8.
Schematic view of the effects of p42 MAPK on Cdc2 activation. (A) p42 MAPK can directly phosphorylate and activate Wee1 (this work) and, via the intermediacy of Rsk, inactivate Myt1 (Palmer et al., 1998). (B) The postfertilization cell cycles (the conclusion of meiosis II and early embryogenesis) are shown. The present work indicates that the prolonged period of p42 MAPK activation before the first embryonic cell cycle contributes to the presence of a G2 phase in that cycle.
The advent of the pharmacological MEK inhibitor U0126 made it possible to test this hypothesis further. U0126 rapidly inactivates MEK; after MEK is inactivated, the inactivation of p42 MAPK occurs rapidly because p42 MAPK's phosphates turn over rapidly even when p42 MAPK is fully active (t1/2 ≈ 5 min) (Sohaskey and Ferrell, 1999). Thus, using U0126, we could cause premature inactivation of p42 MAPK and determine whether that resulted in an acceleration of mitosis.
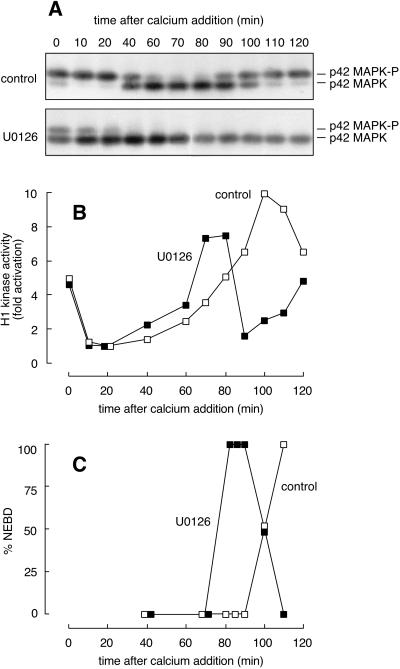
We treated CSF extracts with U0126 (100 μM) or an equal volume of DMSO (1%) for 40 min. The U0126 resulted in partial inactivation of p42 MAPK (Figure 6A) without diminishing the extract's H1 kinase activity (Figure 6B). Calcium was then added to drive the extracts into interphase. In the control extract, H1 kinase activity fell within 10 min (Figure 6B, □). This was followed by inactivation of p42 MAPK at 40–60 min (Figure 6A, top) and mitotic entry at 100–110 min (Figure 6, B and C, □). In the U0126-treated extract, H1 kinase activity still fell to basal levels within 10 min (Figure 6B, ▪). However, inactivation of p42 MAPK was advanced by ∼40 min (Figure 6A), resulting in an ∼30 min acceleration of mitotic H1 kinase activation (Figure 6B) and nuclear envelope breakdown (Figure 6C). These data support the hypothesis that p42 MAPK activity present in the egg after fertilization has important effects on the subsequent timing of Cdc2 activation and, consequently, the first mitosis.
Figure 6.
Entrance into mitosis is hastened by premature inactivation of p42 MAPK after parthenogenetic activation. A CSF-arrested extract was incubated with U0126 (100 μM) or an equal volume of DMSO (1%) for 40 min on ice. Calcium chloride (400 μM) was then added to each, and samples were removed for analysis. (A) Western blot analysis with the MAPK-specific antibody DC3. U0126 treatment (bottom) resulted in premature inactivation of p42 MAPK relative to that of the control extract (top). (B) Histone H1 kinase activities for control (□) and U0126-treated (▪) extracts. U0126 did not measurably alter the rapid inactivation of Cdc2 but did accelerate its reactivation. (C) NEBD in control and U0126-treated extracts.
Transient Activation of p42 MAPK during Mitosis Has No Effect on Cdc2 Activation
The effect that p42 MAPK exerts on Cdc2 activation in CSF extracts is pronounced, but the period of high p42 MAPK activity is also substantial, lasting between 30 and 40 min after calcium addition in a CSF-arrested extract. p42 MAPK also becomes transiently activated after Cdc2 during mitosis in Xenopus egg extracts (Takenaka et al., 1997; Guadagno and Ferrell, 1998). However, the mitotic activity of p42 MAPK is lower than that seen in CSF-arrested extracts and eggs, and the duration of p42 MAPK activation is shorter, lasting no more than 10 min. Moreover, in intact embryos the mitotic activation of p42 MAPK appears to be still smaller in magnitude and/or duration (Ferrell et al., 1991; Hartley et al., 1996). Thus we hypothesized that this transient MAPK activation might not be sufficient to affect the timing of Cdc2 activation in the second through eleventh mitotic cycles.
To test this hypothesis, we examined whether inhibiting the transient mitotic activation of p42 MAPK in cycling extracts had an effect on the timing of the subsequent mitosis. As shown in Figure 7, the MEK inhibitor did not measurably affect the timing of Cdc2 activation or mitotic entry. Thus p42 MAPK must be active for a period longer than that which normally occurs during mitosis in cycling extracts to produce a measurable delay in Cdc2 activation.
DISCUSSION
Previous work has demonstrated that activation of the Mos/MEK/p42 MAPK pathway during interphase in fertilized Xenopus eggs and cycling Xenopus egg extracts prevents normal Cdc2 activation and progression into mitosis (Abrieu et al., 1997b; Walter et al., 1997; Bitangcol et al., 1998; Murakami and Vande Woude, 1998; Murakami et al., 1999). Wee1 appeared to be critical for this effect of p42 MAPK, as indicated by two pieces of evidence. First, Wee1-depleted CSF extracts were found to be impervious to Mos treatment (Murakami et al., 1999), a finding that we have confirmed (our unpublished results) and extended to the cycling extract system (Figure 1), and adding back recombinant Wee1 restored the extracts' ability to respond to Mos. Second, Mos overexpression was found to prolong the duration of Wee1 autophosphorylation in fertilized eggs (Murakami et al., 1999). If we assume that Wee1 autophosphorylation is indicative of Wee1 activation, these results suggest that the Mos/MEK/p42 MAPK pathway directly or indirectly activates Wee1.
Here we provide evidence that p42 MAPK exerts its negative effect on Cdc2 by directly phosphorylating Wee1 (Figures 2–5). Addition of Mos to cycling extracts increases the phosphorylation of kinase-minus Wee1, placing Wee1 phosphorylation downstream of Mos. p42 MAPK can phosphorylate Wee1 in vitro (Figure 2), and the phosphorylation occurs at sites that are phosphorylated in extracts (Figure 3). Phosphorylation of Wee1 by p42 MAPK results in a modest (∼2-fold) increase in Wee1 activity (Figure 4). This twofold increase in Wee1 activity would be sufficient to account for the mitotic delay seen in Mos-treated extracts (Figure 5). Thus, we conclude that p42 MAPK inhibits Cdc2 activation at least in part by directly phosphorylating and activating Wee1. Our data do not exclude the possibility that p42 MAPK might also regulate some other aspect of Wee1 function, such as Wee1's nuclear localization, but it should be noted that p42 MAPK can cause a G2 delay even in extracts that contain no nuclei (Walter et al., 1997).
Physiological Significance of the p42 MAPK-induced G2 Phase Delay
After fertilization, a number of physical events must occur to set up the first mitotic cleavage properly. The sperm pronucleus must migrate toward and find the egg pronucleus and fuse with it. This process of karyogamy occurs ∼45 min after fertilization, and an additional 45 min elapses before mitosis occurs (Gerhart, 1980). In contrast, the subsequent 11 cleavages are quite rapid and require only ∼30 min each (Figure 8B).
After fertilization, p42 MAPK activity remains high for 30–40 min (Figure 8B). We hypothesized previously that this extended period of elevated p42 MAPK activity could exert a negative effect on the subsequent activation of Cdc2 (Walter et al., 1997). We showed that increasing the length of time p42 MAPK is active produces a corresponding delay in mitotic entry. Here we have demonstrated for the first time that decreasing the length of time p42 MAPK is active produces a corresponding advancement in mitotic entry (Figure 6). These findings argue that p42 MAPK-induced activation of Wee1 contributes to making the first mitotic cycle longer than the subsequent 11, allowing sufficient time for karyogamy.
Abrieu et al. (1997b) examined previously whether premature inactivation of p42 MAPK accelerates Cdc2 activation in Xenopus egg extracts and concluded that it does not, in conflict with the present results. We suspect that technical problems may account for this discrepancy. The methods used by Abrieu et al. (1997b) to inactivate p42 MAPK (partial immunodepletion of MAPK plus addition of the MAPK phosphatase Pyst1) destroyed the extract's ability to cycle, as did mock depletion. Therefore they were unable to assess mitotic progression directly. Instead they examined how rapidly the control and MAPK-depleted and/or -inhibited extracts generated active Cdc2 upon addition of sea urchin Δ90 cyclin B. Both types of extracts generated active Cdc2 very rapidly (within 20 min, implying that a high concentration of Δ90 cyclin B must have been used), and the authors concluded that inactivation of MAPK did not accelerate the Cdc2 activation. One potential problem with this interpretation is that the MAPK inactivation effect may have been negligible because the rate of Cdc2 activation was so fast. Indeed, Bitangcol et al. (1998) have shown that p42 MAPK activation markedly slows the rate at which low concentrations of Δ90 cyclin B produce active Cdc2 but has a negligible effect on the rate at which high concentrations do. Another possible problem is that, just as the depletion step eliminated the extract's ability to cycle, it may also have compromised the extract's Wee1 function. The advent of the MEK inhibitor U0126 allowed us to inactivate MAPK in extracts that were still able to cycle. Under these conditions, the acceleration of Cdc2 activation in MAPK-inhibited extracts is clearly apparent (Figure 6).
Positive and Negative Regulation of Wee1 by Phosphorylation
Phosphorylation of Wee1 by p42 MAPK in vitro increased the kinase activity of Wee1 toward its physiological substrate Cdc2. Although the activation was modest, the results were reproducible and were also observed using in vitro–translated Wee1 in place of purified recombinant protein (our unpublished results).
Previously, phosphorylation of Wee1 had only been correlated with negative regulation of the kinase. Genetic studies in Schizosaccharomyces pombe identified the gene cdr1/nim1 as a new inducer of mitosis on the basis of its ability to suppress a temperature-sensitive Cdc25 loss-of-function mutation (Russell and Nurse, 1987b; Feilotter et al., 1991). Biochemical analyses later demonstrated that Cdr1/Nim1 could phosphorylate and inactivate Wee1 in vitro (Coleman et al., 1993; Parker et al., 1993; Wu and Russell, 1993). Cdc2 has also been shown to phosphorylate Wee1 and reduce its ability to autophosphorylate and to phosphorylate Cdc2 in the Xenopus extract system (Mueller et al., 1995a). The present work shows that phosphorylation can also positively regulate Wee1 activity.
p42 MAPK is a proline-directed kinase, phosphorylating serines and threonines that lie directly N-terminal to a proline. There are 5 Ser-Pro and 6 Thr-Pro sites in Wee1, providing 11 candidate sites for phosphorylation by p42 MAPK. It will be of interest to determine which of these sites are phosphorylated by p42 MAPK and which are critical for p42 MAPK-induced Wee1 activation.
p42 MAPK Can Be Both a Positive and Negative Regulator of Cdc2 via Opposite Effects on Wee1 and Myt1
Xenopus oocytes and fertilized eggs provide a striking example of how two very similar cells can respond completely differently to the activation of p42 MAPK. In oocytes, p42 MAPK activation causes or facilitates Cdc2 activation; in fertilized eggs, it prevents it. The positive effects of p42 MAPK on Cdc2 activation have been proposed to result from inhibition of Myt1, a membrane-associated cytoplasmic protein kinase that can phosphorylate Cdc2 at both Thr 14 and Tyr 15 (Palmer et al., 1998). The Rsk protein kinase functions as an intermediate between p42 MAPK and Myt1 (Palmer et al., 1998). Here we have shown that the negative effects of p42 MAPK on Cdc2 activation in fertilized eggs can be attributed to the direct activation of Wee1, a nuclear protein kinase that phosphorylates Cdc2 at Tyr 15. Thus p42 MAPK has opposite effects on two key regulators of Cdc2 (Figure 8A).
The net effect of p42 MAPK on Cdc2 activation must depend on the balance of Wee1/Myt1. In oocytes, where Wee1 is absent, the positive effect is dominant. In contrast, in eggs, the Wee1-dependent negative effect of p42 MAPK on Cdc2 activation apparently outweighs any Myt1-mediated positive effect. In addition, because Myt1 and Wee1 are present in different cellular compartments (Myt1 associated with cytoplasmic membranes and Wee1 in the nucleus), p42 MAPK may exert opposite effects on different subpopulations of Cdc2.
Why Does p42 MAPK Not Inactivate Cdc2 in Unfertilized Eggs?
The effect of p42 MAPK on Cdc2 apparently depends not only on the balance of Wee1 and Myt1 but also on the order in which the various proteins are synthesized and activated. Thus, although p42 MAPK and Wee1 are able to block Cdc2 activation in fertilized eggs and in extracts from fertilized eggs, the same concentration of (fully) active p42 MAPK and the same total concentration of Wee1 do not reverse the activation of Cdc2 in unfertilized eggs; eggs remain arrested for long periods of time with fully active Cdc2, despite the presence of Wee1 and active p42 MAPK. Evidently it is easier for Cdc2 activation to be blocked than reversed.
We suggest two possible explanations for this apparent hysteresis in the response of Cdc2 to Wee1 and p42 MAPK. First, the hysteresis might arise from the fact that Cdc2 activation is reinforced by positive feedback. Cdc2 can (directly or indirectly) contribute to the activation of its activator Cdc25 and to the inactivation of its inactivators Myt1 and Wee1. Systems with sufficiently strong positive feedback exhibit the sort of hysteresis observed here; after the system is turned on, it tends to be maintained in the on state by the positive feedback. A second explanation for the hysteresis is that it might arise from the subcellular localization of Wee1. When a cell is in interphase, Wee1 is concentrated in the nucleus, and the high nuclear concentration of Wee1 may be more effective at blocking Cdc2 activation than the same amount and activity of Wee1 dispersed throughout the cytoplasm of an M phase cell.
ACKNOWLEDGMENTS
We thank N. Ahn, J. Cooper, W. Dunphy, J. Minshull, P. Mueller, M. Murakami, J. Posada, and G. Vande Woude for providing cyclin B1, MEK, Mos, p42 MAPK, and Wee1-encoding plasmids and members of the Ferrell laboratory for helpful comments on this manuscript. This work was supported by National Institutes of Health grant GM-46383.
REFERENCES
- Abrieu A, Doree M, Picard A. Mitogen-activated protein kinase activation down-regulates a mechanism that inactivates cyclin B-cdc2 kinase in G2-arrested oocytes. Mol Biol Cell. 1997a;8:249–261. doi: 10.1091/mbc.8.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrieu A, Fisher D, Simon MN, Doree M, Picard A. MAPK inactivation is required for the G2 to M-phase transition of the first mitotic cell cycle. EMBO J. 1997b;16:6407–6413. doi: 10.1093/emboj/16.21.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrieu A, Lorca T, Labbe JC, Morin N, Keyse S, Doree M. MAP kinase does not inactivate, but rather prevents the cyclin degradation pathway from being turned on in Xenopus egg extracts. J Cell Sci. 1996;109:239–246. doi: 10.1242/jcs.109.1.239. [DOI] [PubMed] [Google Scholar]
- Bhatt RR, Ferrell JE., Jr The protein kinase p90 Rsk as an essential mediator of cytostatic factor activity. Science. 1999;286:1362–1365. doi: 10.1126/science.286.5443.1362. [DOI] [PubMed] [Google Scholar]
- Bitangcol JC, Chau AS, Stadnick E, Lohka MJ, Dicken B, Shibuya EK. Activation of the p42 mitogen-activated protein kinase pathway inhibits Cdc2 activation and entry into M-phase in cycling Xenopus egg extracts. Mol Biol Cell. 1998;9:451–467. doi: 10.1091/mbc.9.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Chau AS, Shibuya EK. Inactivation of p42 mitogen-activated protein kinase is required for exit from M-phase after cyclin destruction. J Biol Chem. 1999;274:32085–32090. doi: 10.1074/jbc.274.45.32085. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Tang Z, Dunphy WG. Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell. 1993;72:919–929. doi: 10.1016/0092-8674(93)90580-j. [DOI] [PubMed] [Google Scholar]
- Cross DA, Smythe C. PD 98059 prevents establishment of the spindle assembly checkpoint and inhibits the G2-M transition in meiotic but not mitotic cell cycles in Xenopus. Exp Cell Res. 1998;241:12–22. doi: 10.1006/excr.1998.4023. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Feilotter H, Nurse P, Young PG. Genetic and molecular analysis of cdr1/nim1 in Schizosaccharomyces pombe. Genetics. 1991;127:309–318. doi: 10.1093/genetics/127.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gerhart JC. Mechanisms regulating pattern formation in the amphibian egg and early embryo. In: Goldberger RF, editor. Biological Regulation and Development. Vol. 2. New York: Plenum Press; 1980. pp. 133–316. [Google Scholar]
- Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Guadagno TM, Ferrell JE., Jr Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Lewellyn AL, Maller JL. MAP kinase is activated during mesoderm induction in Xenopus laevis. Dev Biol. 1994;163:521–524. doi: 10.1006/dbio.1994.1168. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Rempel RE, Maller JL. In vivo regulation of the early embryonic cell cycle in Xenopus. Dev Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- Hausen P, Riebesell M. The Early Development of Xenopus laevis: An Atlas of the Histology. Berlin: Springer-Verlag; 1991. [Google Scholar]
- Hsiao K-M, Chou S-y, Shih S-J, Ferrell JE., Jr Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc Natl Acad Sci USA. 1994;91:5480–5484. doi: 10.1073/pnas.91.12.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Akamatsu Y, Tsurushita N, Lee KK, Gotoh Y, Nishida E. Isolation and characterization of neutralizing single-chain antibodies against Xenopus mitogen-activated protein kinase kinase from phage display libraries. Biochemistry. 1996;35:13212–13221. doi: 10.1021/bi960956f. [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J. 1994;13:2131–2138. doi: 10.1002/j.1460-2075.1994.tb06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- Luo K, Hurley TR, Sefton BM. Cyanogen bromide cleavage and proteolytic peptide mapping of proteins immobilized to membranes. Methods Enzymol. 1991;201:149–152. doi: 10.1016/0076-6879(91)01014-s. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Candia JM, Gloor KK, Ahn NG. Constitutively active mitogen-activated protein kinase kinase 1 (MAPKK1) and MAPKK2 mediate similar transcriptional and morphological responses. Cell Growth Differ. 1996a;7:243–250. [PubMed] [Google Scholar]
- Mansour SJ, Candia JM, Matsuura JE, Manning MC, Ahn NG. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochemistry. 1996b;35:15529–15536. doi: 10.1021/bi961854s. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- McGowan CH, Russell P. Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995a;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995b;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murakami MS, Copeland TD, Vande Woude GF. Mos positively regulates Xe-Wee1 to lengthen the first mitotic cell cycle of. Xenopus. Genes Dev. 1999;13:620–631. doi: 10.1101/gad.13.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami MS, Vande Woude GF. Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development. 1998;125:237–248. doi: 10.1242/dev.125.2.237. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Palmer A, Gavin AC, Nebreda AR. A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, Walter SA, Young PG, Piwnica-Worms H. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature. 1993;363:736–738. doi: 10.1038/363736a0. [DOI] [PubMed] [Google Scholar]
- Posada J, Cooper JA. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992;255:212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987a;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987b;49:569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Smythe C, Newport JW. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- Sohaskey ML, Ferrell JE., Jr Distinct, constitutively active MAPK phosphatases function in Xenopus oocytes: implications for p42 MAPK regulation in vivo. Mol Biol Cell. 1999;10:3729–3743. doi: 10.1091/mbc.10.11.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K, Gotoh Y, Nishida E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J Cell Biol. 1997;136:1091–1097. doi: 10.1083/jcb.136.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter SA, Guadagno TM, Ferrell JE., Jr Induction of a G2-phase arrest in Xenopus egg extracts by activation of p42 MAP kinase. Mol Biol Cell. 1997;8:2157–2169. doi: 10.1091/mbc.8.11.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Zhai Y, Ferrell JE., Jr A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J Cell Biol. 1997;137:433–443. doi: 10.1083/jcb.137.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Hunt T, Ikawa Y, Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991;352:247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- Wu L, Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature. 1993;363:738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]
- Yew N, Mellini ML, Vande Woude GF. Meiotic initiation by the mos protein in Xenopus. Nature. 1992;355:649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]