Abstract
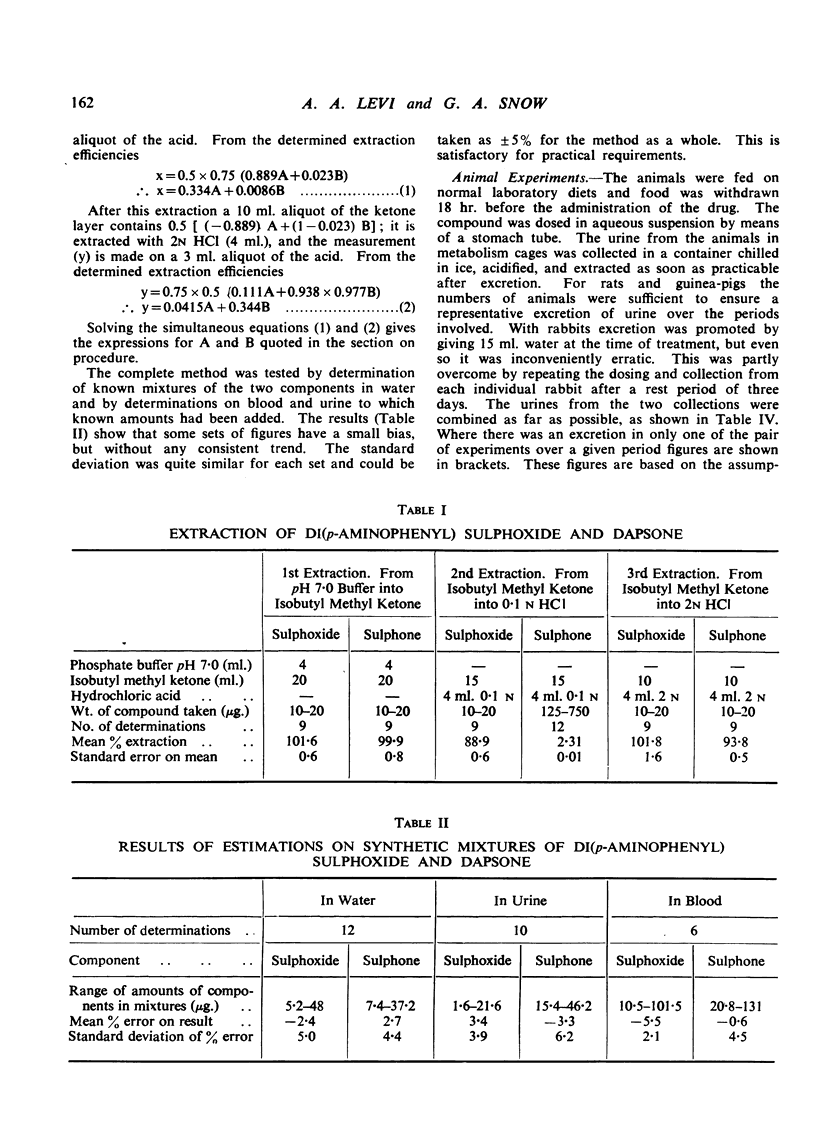
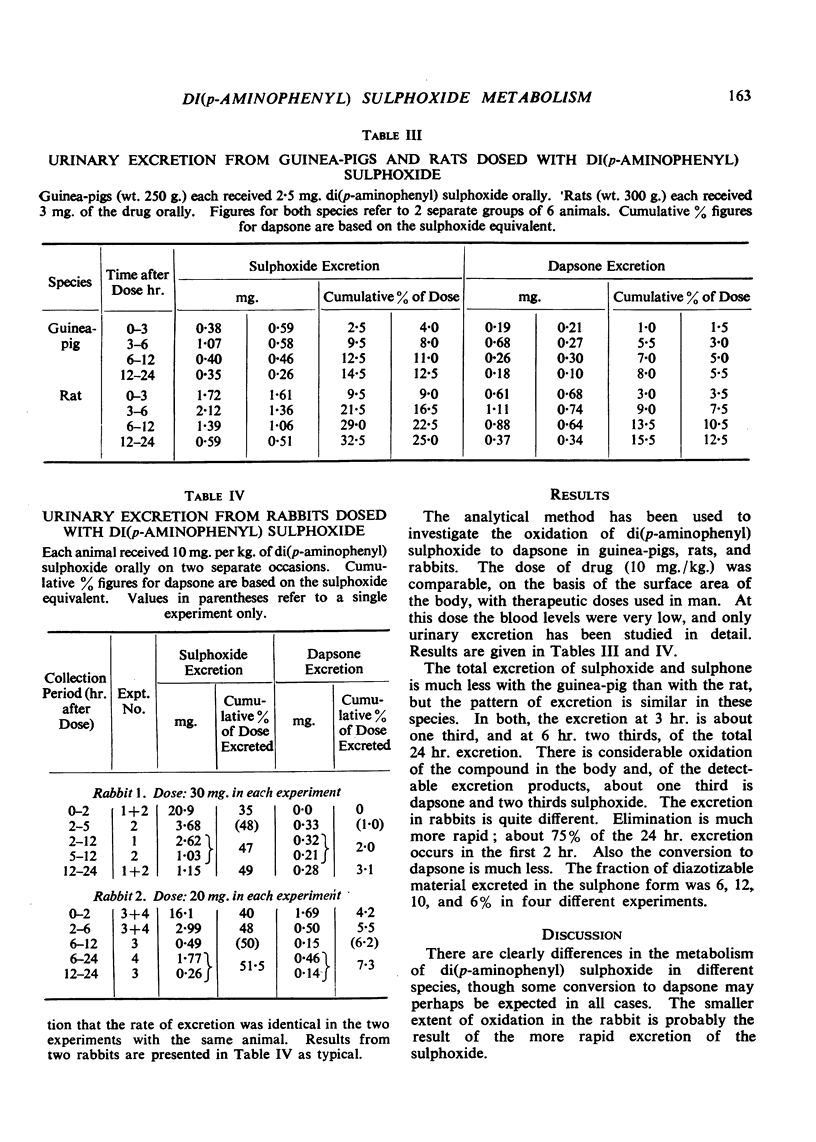
Rabbits, rats and guinea-pigs were treated with di(p-aminophenyl) sulphoxide and their urines examined by an analytical method which permits the simultaneous determination of this compound and of dapsone [di(p-aminophenyl) sulphone] which is a possible product of metabolic oxidation. The method gives for each drug the total of free compound plus acid-labile conjugates. All three species excreted unchanged drug together with dapsone. With rats and guinea-pigs about 33% of the excretion is dapsone, but with rabbits only 6 to 12%. The rate of combined excretion is much greater in rabbits than in the other two species. These results are discussed in relation to the significance of di(p-aminophenyl) sulphoxide as a drug in the treatment of leprosy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., SALZMAN N. P. Physiological disposition and fate of chlorpromazine and a method for its estimation in biological material. J Pharmacol Exp Ther. 1956 Sep;118(1):46–54. [PubMed] [Google Scholar]
- BURNS J. J., YU T. F., RITTERBAND A., PEREL J. M., GUTMAN A. B., BRODIE B. B. A potent new uricosuric agent, the sulfoxide metabolite of the phenylbutazone analogue, G-25671. J Pharmacol Exp Ther. 1957 Mar;119(3):418–426. [PubMed] [Google Scholar]
- BUSHBY S. R., WOIWOD A. J. The identification of the major diazotizable metabolite of 4:4'-diaminodiphenyl sulphone in rabbit urine. Biochem J. 1956 Jul;63(3):406–408. doi: 10.1042/bj0630406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUU-HOI N. P., NGUYEN-BA-KHUYEN, NGUYEN-DAT-XUONG Six mois de chimiothérapie antilépreuse au Sud-Vietnam avec le 4,4'-diaminodiphénylsulfoxyde et le 4,4'-diéthoxythiocarbanilide. Bull Acad Natl Med. 1955 May 3;139(15-16):275–280. [PubMed] [Google Scholar]
- DAVEY T. F., KISSAUN A. M., MONETA G. The treatment of leprosy with diaminodiphenyl sulphoxide: a progress report. Lepr Rev. 1957 Apr;28(2):51–59. doi: 10.5935/0305-7518.19570006. [DOI] [PubMed] [Google Scholar]
- FRANCIS J., SPINKS A. Antibacterial action and metabolism of five sulphones. Br J Pharmacol Chemother. 1950 Dec;5(4):565–583. doi: 10.1111/j.1476-5381.1950.tb00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVIRON P., LAURET L., KERBASTARD P., JARDIN C. Le 4, 4'-diaminodiphénylsulfoxyde dans le traitement de la lèpre. Bull Acad Natl Med. 1957 Mar 5;141(9-10):195–204. [PubMed] [Google Scholar]
- SNOW G. A. The metabolism of compounds related to ethanethiol. Biochem J. 1957 Jan;65(1):77–82. doi: 10.1042/bj0650077. [DOI] [PMC free article] [PubMed] [Google Scholar]


